Abstract
Background
Remodeling of cardiac repolarizing currents, such as the downregulation of slowly activating K+ channels (IKs), could underlie ventricular fibrillation (VF) in heart failure (HF). We evaluated the role of I ks remodeling in VF susceptibility using a tachypacing HF model of transgenic rabbits with Long QT Type 1 (LQT1) syndrome.
Methods and Results
LQT1 and littermate control (LMC) rabbits underwent three weeks of tachypacing to induce cardiac myopathy (TICM). In vivo telemetry demonstrated steepening of the QT/RR slope in LQT1 with TICM (LQT1-TICM; pre: 0.26±0.04, post: 0.52±0.01, P<0.05). In vivo electrophysiology showed that LQT1-TICM had higher incidence of VF than LMC-TICM (6 of 11 vs. 3 of 11, respectively). Optical mapping revealed larger APD dispersion (16±4 vs. 38±6 ms, p<0.05) and steep APD restitution in LQT1-TICM compared to LQT1-sham (0.53±0.12 vs. 1.17±0.13, p<0.05). LQT1-TICM developed spatially discordant alternans (DA), which caused conduction block and higher-frequency VF (15±1 Hz in LQT1-TICM vs. 13±1 Hz in LMC-TICM, p<0.05). Ca2+ DA was highly dynamic and preceded voltage DA in LQT1-TICM. Ryanodine abolished DA in 5 out of 8 LQT1-TICM rabbits, demonstrating the importance of Ca2+ in complex DA formation. Computer simulations suggested that HF remodeling caused Ca2+-driven alternans, which was further potentiated in LQT1-TICM due to the lack of IKs.
Conclusions
Compared with LMC-TICM, LQT1-TICM rabbits exhibit steepened APD restitution and complex DA modulated by Ca2+. Our results strongly support the contention that the downregulation of IKs in HF increases Ca2+ dependent alternans and thereby the risk of VF.
Introduction
Ventricular arrhythmia is a significant cause of mortality in heart failure (HF) patients [1–3]. Electrical and structural remodeling associated with HF have been proposed to increase vulnerability to ventricular fibrillation (VF) [2–4]. The hallmark of electrical remodeling in HF is a decrease in repolarization reserve that prolongs action potential duration (APD) [5–7], which is thought to promote triggered activity such as early and delayed afterdepolarizations and APD alternans, thereby enhancing reentry formation [8, 9].
Voltage-dependent K+ channels are critical in cardiac repolarization, and their downregulation is thought to play a major role in HF-related APD prolongation. The most consistently downregulated K+ channels in HF are the transient outward potassium current (Ito) [10] and slowly activating delayed rectifier potassium current (IKs) [6, 11, 12]. Since Ito rapidly inactivates during the plateau phase of action potentials, it is thought to have minimal impact on APD in large mammals [13, 14]. In contrast, IKs can play an essential role as repolarization reserve [15–18] in action potential repolarization when other repolarization currents are reduced [19–21]. Besides downregulation of repolarizing currents such as Ito and IK1, several depolarizing currents are upregulated, including late Na+ current [22–24] and Na+/Ca2+ exchanger current (INCX) [25–28]. Hence, IKs downregulation in HF in conjunction with other ion channel remodeling may further accentuate APD prolongation and promote arrhythmogenesis.
Alternatively, IKs downregulation in HF may not necessarily be arrhythmogenic. Due to slow deactivation [29], the amplitude of IKs becomes larger during fast heart rates [19, 20, 30, 31]. As a result, IKs contributes APD shortening at short diastolic intervals to form the characteristic APD restitution curve [32]. Steep APD restitution has been linked to susceptibility to APD alternans [33–36], and the blockade of IKs may be effective against repolarization shortening in the setting of fast heart rate and flattened APD restitution, thereby protecting against reentry formation [37, 38]. In addition, due to its slow deactivation, IKs can promote post-repolarization refractoriness and enhance wavebreaks in VF [39]. Therefore, it is possible that the effect of IKs in HF remodeling may be compensatory and beneficial by preventing repolarization shortening and post-repolarization refractoriness. Overall, the reduction of IKs in HF can have either a pro-arrhythmic or an anti-arrhythmic effect depending on its amplitude and relative contribution to repolarization and restitution. Intracellular Ca2+ could also play a role in modulating IKs current density, as higher levels of intracellular Ca2+ increase IKs current [40].
Here we investigated the role of IKs in HF-related arrhythmias by inducing HF by a tachypacing protocol [41] in a transgenic rabbit model of LQT1 [42] and comparing that to HF induced in littermate control rabbits (LMC). We then compared susceptibility to developing alternans and VF induction in LQT1 rabbits that completely lack IKs vs. their LMCs in which tachypacing induced ~55% downregulation of IKs [43]. We found that total lack of IKs significantly increases arrhythmogenesis by increasing APD dispersion and promoting spatially discordant alternans (DA). This finding emphasizes the importance of IKs remodeling in promoting arrhythmogenesis in failing hearts.
Methods
All animal experiments were performed in accordance with the local guidelines of the institutions and only after approval by the Institutional Animal Care and Use Committee (IACUC) at Rhode Island Hospital, in accordance with the Institute for Laboratory Animal Research (ILAR) Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication #85–23; Revised 1996). Adult male New Zealand white rabbits were selected from LMC and LQT1 lines and age-matched.
Tachypacing-induced Cardiomyopathy (TICM) Protocol
Tachypacing-induced cardiomyopathy [41] was used to model dilated, non-ischemic cardiomyopathy. A programmable right ventricular (RV) pacemaker (Medtronic) was implanted in adult male LQT1 and LMC rabbits in a sterile surgical suite. Rabbits were anesthetized with IM ketamine/xylazine (25 mg/kg; 3.75 mg/kg body weight) and buprenorphine (0.03 mg/kg subcutaneously), followed by intubation and ventilation with inhaled isoflurane (1–2%, FiO2 0.5). A neck dissection was performed and the external jugular vein was isolated and cannulated. A 5F Micropuncture Peel-Away Sheath was advanced into the vein retrogradely to the RV apex over a 4F deflectable catheter under fluoroscopic guidance. A 4F bipolar lead (Medtronic 3830 Secure Select, active fixation, exposed screw) was then advanced through the sheath to the RV apex and screwed in place into the interventricular septum. The lead was connected to a sterilized pulse generator, and both were implanted subcutaneously.
Following a week recovery period, the rabbits underwent a three-week rapid pacing protocol (350 bpm for 1 week, then 370 bpm for 2 weeks; see Fig 1A). The two-step stimulation protocol was necessary due to the long refractoriness characteristic of LQT1 rabbits. Following the pacing period, the pacemaker was reprogrammed to OVO nonpacing mode (baseline HR = 240 bpm). The pacemaker implementation was carried out in 34 rabbits; 11 LMC and LQT1 rabbits were paced to induce HF (LMC-TICM and LQT1-TICM), and the remaining 6 LMC and LQT1 rabbits were not paced (sham pacing; SH-P).
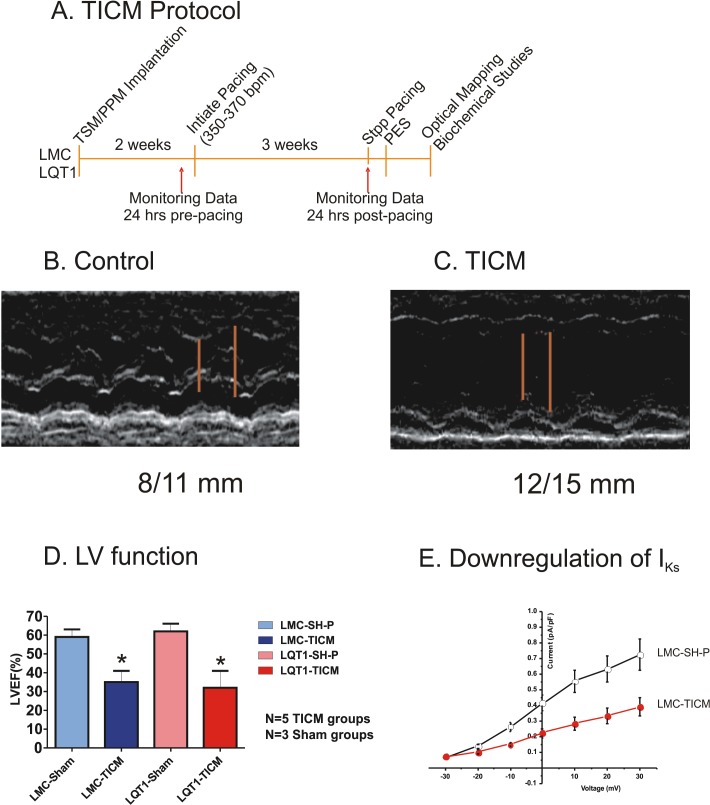
Fig 1. TICM Protocol.
(A) Three-week pacing protocol. TSM/PPM = transmitter/ pacemaker, PES = programmed electrical stimulation. (B) TICM groups show dilated left ventricle and reduced ejection fraction. The red bars indicate end-systolic and end-diastolic LV internal dimensions (8 and 11 mm for LMC sham pacing and 12 and 15 mm for TICM). (C) Post-pacing protocol LV function presented as left ventricular ejection fraction in LMC-TICM, LQT1-TICM, and their sham controls. TICM rabbits show statistically significant differences in LV ejection fraction compared with sham; *P<0.05. (D) Quantification of IKs in LMC rabbits. Current amplitude was normalized to cell capacitance. Compared with LMC-SH-P (n = 12), significant downregulation of IKs was seen in LMC-TICM (n = 11).
Echocardiography
To assess LV function following the three-week pacing protocol, we performed transthoracic echocardiography in sedated animals (LMC-TICM, LQT1-TICM, n = 5; LMC-SH-P, LQT1-SH-P, n = 3). After anesthesia with ketamine and xylazine, the chests of rabbits were shaved and ECG leads were attached for simultaneous recording of ECG and echocardiography. Two-D echocardiogram images (Hewlett Packard 5500) were obtained using a 7.5 mHz probe, and both long- and short-axis views were used, similar to human echocardiograms. The M-mode was obtained from the short-axis view. Analysis of LV and RV dimensions, left atrium, wall thickness, valve function, and LV ejection fraction (by Simpson’s planimetry method) were performed by a blinded echocardiographer. Fig 1B shows typical examples of 2D echocardiogram images from sham and TICM rabbits. The TICM protocol induced dilated cardiac myopathy after three weeks of pacing.
Telemetric ECG monitoring, QT/RR ratio
LQT1 and LMC rabbits were monitored using telemetric ECG devices to calculate QT/RR ratios (LMC-TICM, LMC-SH-P, LQT1-SH-P, LQT1-TICM, n = 5) [42]. Telemetric ECG signals were acquired by Dataquest A.R.T. data-acquisition software and analyzed with Ponemah ECG analysis software (both Data Sciences International). QT and RR intervals were measured and averaged over 5 seconds every 20 minutes over 48-hour monitoring periods prior to and following the pacing protocol. Linear regressions of the QT/RR relationships were then performed for each animal both before and after the pacing protocol, and the resulting regressions were then averaged per experimental cohort.
Minimally invasive in vivo electrophysiological studies (EPS)
The EPS protocol was modified from a previously established protocol from our lab [44]. Studies were performed in the animal electrophysiology (EP) laboratory with a two-channel computer-based programmable stimulator (EP Med systems) and an EP digital recording system (Prucka). LMC-TICM, LQT1-TICM, and their respective sham rabbits were anesthetized, intubated, and monitored as described for pacemaker implantation. A decapolar transvenous 4F electrophysiology catheter (Inquiry, Boston Scientific, Natick, MA) was inserted into the right femoral vein through a 4F sheath and advanced toward the right ventricle, with guidance by fluoroscopy and pacing thresholds. During the procedure, 12-lead surface and five intra-cardiac ECG signals were recorded continuously using EP-Bard-System software OS2/warp (kindly provided by Bard, Lowell, MA, USA). EPS were performed at basic cycle length (CL) of 240 ms. Ventricular effective refractory periods in RV apex and RV septal base position (VERPapex, VERPbase) were analyzed by progressively shortening the S2-interval in 10-ms steps after 10-beat S1 trains. Programmed ventricular stimulation was performed with one, two, and three extra stimuli in apical and basal positions to investigate inducibility of sustained VF. VF frequency was determined by the inverse of the averaged cycle lengths measured during the last five seconds of each VF episode.
Patch Clamping
Isolation of cardiomyocytes by standard enzymatic techniques and patch-clamp recordings were performed as described previously [42]. Apical ventricular myocytes were isolated from hearts (n = 3 each from LMC-SH-P, LMC-TICM, LQT1-SH-P, and LQT1-TICM groups). Whole-cell recordings (of 11–18 cardiomyocytes per group) were obtained with an Axopatch-200B amplifier (Axon Instruments) with standard patch-clamp techniques. The methods for K+ current recording were the same as before [42]. ICa,L was obtained in Tyrode solution before K+ current recording; holding potential was—50 mV, and test potentials were—40 to +40 mV with 10-mV steps lasting 250 ms. ICa,L was defined as the difference of peak and steady-state current at the end of the pulse. E-4031 (5 μM) and chromanol 293B (30 μM) were used for isolating IKr and IKs, respectively. Tetrodotoxin (20 μM) and CdCl2 (0.2 mM) were added as needed to block Na+ and Ca2+ currents.
Optical Mapping
Rabbits were injected with buprenorphene (0.03 mg/kg IM), acepromazine (0.5 mg.kg-1 IM), xylazene (15 mg.kg-1 IM), ketamine (60 mg.kg-1 IM), pentothal (35 mg.kg-1 IV), and heparin (200 U.kg-1). After an appropriate level of anesthesia was achieved, rabbits were euthanized via beating-heart harvest. The hearts were retrogradely perfused through the aorta with (in mmol.L-1) 130 NaCl, 24 NaHCO3, 1.0 MgCl2, 4.0 KCl, 1.2 NaH2PO4, 5 Dextrose, 25 Mannitol, 1.25 CaCl2, at pH 7.4, gassed with 95% O2 and 5% CO2. Hearts were placed in a chamber to maintain temperature, and 5 μmol.L-1 blebbistatin was added to the perfusate to reduce movement artifact.
The optical apparatus for simultaneous Vm and Ca2+ recording has been previously described [45]. Sampling rate was set to 1000 frames.s-1 with 2x2 cm2 field of view. Hearts were stained with the voltage-sensitive dye PGH1 [46] (from Dr. Salama at University of Pittsburgh) and calcium-sensitive dye Rhod-2/AM (Invitrogen, Carlsbad).
Hearts were stimulated using a ramp pacing protocol [42, 47] starting from the basic cycle length (CL) of 350 ms to shorter CL with 10-ms steps until either loss of 1:1 capture or VF induction. Ryanodine (2 μM) was perfused for 30 minutes, and the standard stimulation protocol was repeated.
Data Analysis
The activation and repolarization time-points at each site were determined from fluorescence (F) signals by calculating (dF/dt)max and (d2F/dt2)max. Data was filtered using a spatial Gaussian filter (3×3 pixel), and first/second derivatives (dF/dt, d2F/dt2) were calculated using a polynomial filter (3rd order, 13 points). Pixels with low signal-to-noise ratio determined by (dF/dt)max (lower than 3×σ of baseline) and outliers of pixels determined by Grubbs’ test were removed from the analysis (typically less than 1% of total pixels) [48]. APD dispersion was defined as APDmax-APDmin [42].
Alternans analysis of APD and Ca2+ duration was performed by comparing odd and even beats as described in [49, 50]. The nodal lines of DA, where alternans phase shifts occurred, were identified by detecting pixels with negligible APD difference between odd and even beats as described in [51]. Briefly, the local temporal periodicity of fluorescence signals was recognized using the following equation,
| 1 |
where is fluorescence and τ is the pacing cycle length. The pseudo-color images were reconstructed for visualizing . The larger value (or brighter color) of denotes the region exhibiting alternans, while (or darker color) means no alternans, which displays as nodal lines [51].
Computer Simulation Methods
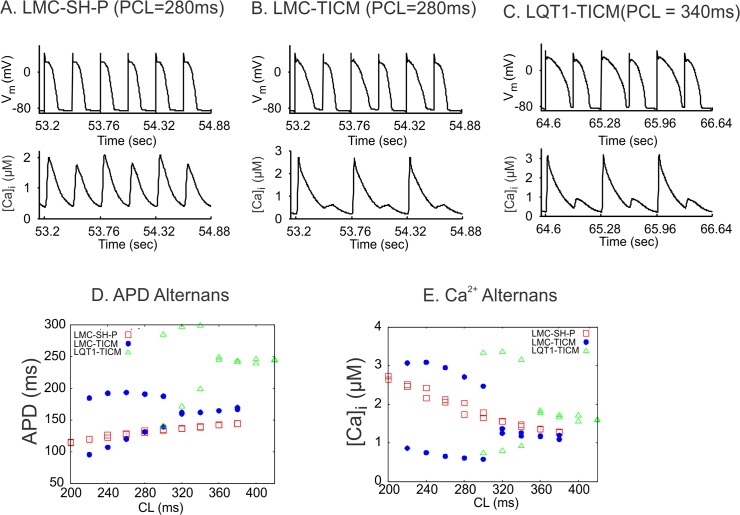
Computer simulations were carried out using a ventricular myocyte model modified from the model developed by Restrepo et al.[52]. The sarcolemmal ionic currents were adopted from the model by Mahajan et al. [53] with the L-type Ca2+ channels and Na+-Ca2+ exchange properly distributed in space. The ryanodine receptors and L-type Ca2+ channels were simulated using stochastic algorithms. To model LMC-TICM, we reduced IKs by 50% (see Fig 1E); reduced the maximum SERCA activity by 33% [54, 55] but increased RyR leakiness by doubling the rate constant from the closed state to the open state [54], and doubled the Na+-Ca2+ exchange activity [26]. To model LQT1-TICM, we further reduced IKs to zero. Details of the model and computer simulation methods are presented in S1 File.
Statistical Analysis
For normally distributed values, we used Student’s t-test (paired and unpaired) to compare the means of two groups and the Mann-Whitney test to compare values not normally distributed. Fisher’s exact test was used for categorical variables. All data are presented as means ± standard deviation, and a p value < 0.05 was considered significant.
Results
Experiments were carried out in the following sequence: 1) TICM protocol to create HF, 2) echocardiographic studies to verify TICM phenotype in different genotypes, 3) in vivo ECG monitoring to examine restitution kinetics from free-moving animals, 4) in vivo EPS for VF inducibility, and 5) optical mapping of isolated hearts to investigate VF mechanisms (Fig 1A).
Rabbit Model of HF
Four cohorts of rabbits were studied: LMC sham pacing (LMC-SH-P), LMC-TICM, LQT1 sham pacing (LQT1-SH-P), and LQT1-TICM. After three weeks of tachypacing, we verified HF phenotypes in TICM rabbits based on LV ejection fraction. TICM rabbits exhibited a significant reduction in mean LV ejection fraction (LVEF (%), n = 5; LMC-SH-P: 59±7; LMC-TICM: 35±6, LQT1-SH-P; 62±7, LQT1-TICM: 32±9, see Fig 1C). Furthermore, the development of HF in tachypaced rabbits was also indicated by ascites, pleural effusions, pericardial effusions, weight increase, and significant reduction in activity.
Downregulation of IKs and VF induction during in vivo EPS
Cellular electrophysiological study demonstrated that IKs was significantly downregulated in LMC-TICM (Fig 1C ) in line with previous studies that showed ~55% downregulation of IKs [43]. To assess the impact of TICM on cardiac repolarization, we recorded ECGs from free-moving animals and compared the QT/RR ratios before and after the TICM pacing protocol. Free-moving telemetry demonstrated significant steepening of the QT/RR slope post-tachypacing, which was most notable in LQT1-TICM rabbits (pre: 0.26 ± 0.04, post: 0.52 ± 0.02, P<0.05) (Fig 2). Although higher QT/RT slope trends were observed in LMC-TICM (0.34 ± 0.01 vs. 0.38 ± 0.01), the results were not statistically significant.
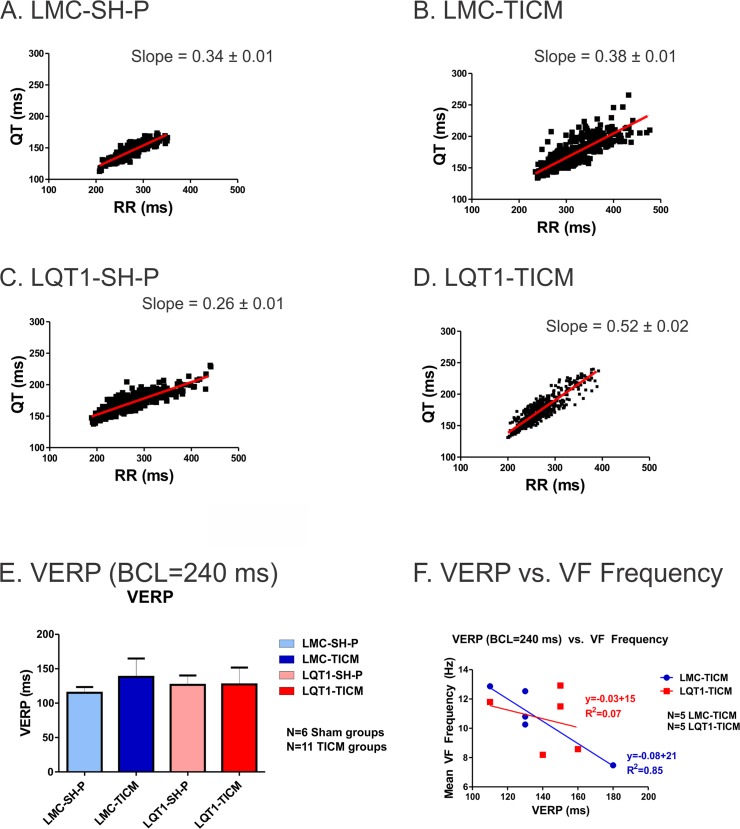
Fig 2. Free-Moving Telemetry: QT-RR Ratios.
(A-D): QT/RR relationship in awake, free-moving rabbits, recorded approximately every 20 minutes for 24 hours in each group. Lines indicate linear regression derived from the mean of all individual regression lines per genotype. Note that LQT1-TICM demonstrates steeper QT/RR slope post-pacing compared to pre-pacing. (E): VERP at BCL = 240 ms in LMC-TICM, LQT1-TICM, and sham animals. No significant difference was found among the four groups (F): VERP (BCL = 240 ms) vs. VF frequency in 5 LMC-TICM and LQT1-TICM animals. Unlike LMC-TICM, LQT1-TICM did not show a correlation between VERP and VF frequency.
We investigated refractoriness and vulnerability to VF under in vivo EPS using programmed stimulation. VERP in vivo did not reveal significant differences across cohorts (panel E) regardless of progress in deterioration of LV function and the reduction of IKs in LMC-TICM. Due to the Iks-blocking properties of inhaled isoflurane (the anesthetic used for sedation in EPS), VERP measurements here should be interpreted with caution. Both LMC-TICM (3 of 11) and LQT1-TICM (6 of 11) animals were found to be inducible for VF during the programmed stimulation protocol, while sham-operated animals were not inducible (Table 1).
Table 1. In vivo VF Inducibility using Pen protocol (S1S2S3S4).
| Group | + | - |
|---|---|---|
| LQT1-TICM | 6 | 5 |
| n = 11 | ||
| LQT1-SH-P | 0 | 6 |
| n = 6 | ||
| LMC-TICM | 3 | 8 |
| n = 11 | ||
| LMC-SH-P | 0 | 6 |
| n = 6 |
P<0.05 LQT1-TICM vs. LQT1-SH-P; all other comparisons, P = NS.
VF cycle length has been shown to provide mechanistic insight into VF behaviors [56]. Thus, we measured VF cycle length and correlated baseline VERP measurements to VF. We observed a linear relationship in LMC-TICM, but surprisingly, no correlation between VERP and VF frequency in LQT1-TICM (r = 0.92 vs. r = 0.28, p<0.05, Fig 2F).
APD dispersion increases in LQT1-TICM
To further understand the mechanisms underlying the increased arrhythmogenesis in the TICM group, we performed optical mapping experiments. Baseline APD measurements at 350 ms CL revealed significant APD prolongation in LQT1 cohorts as expected (APD in ms, LMC-SH-P: 212±13, LQT1-SH-P: 235±5; n = 5; LMC-TICM: 208±16, LQT1-TICM: 238±13, Fig 3C). Although there was a tendency toward increasing APD dispersion in both TICM models, we found that only LQT1-TICM hearts showed a statistically significant increase in APD dispersion (ΔAPD in ms, LMC-SH-P: 18.6±8.9, LQT1-SH-P: 16.6±4.9; LMC-TICM: 22.0±6.0, LQT1-TICM: 38.0±6.3, Fig 3D).
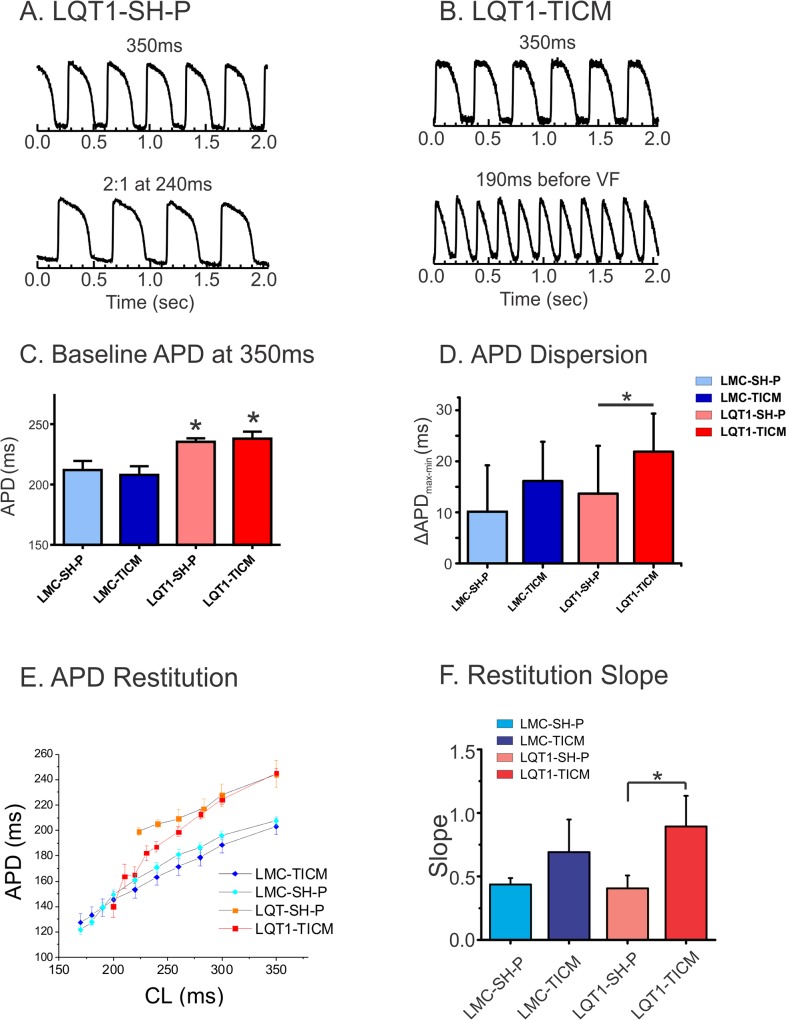
Fig 3. APD dispersion and restitution in TICM rabbits.
(A&B): Typical raw data of action potential traces from optical mapping. LQT1-SH-P shows 2:1 block at 240 ms CL, while LQT1-TICM was paced as low as 190 ms CL with marked shortening of APD. (C) Mean APD in each group at basic cycle length of 350 ms. LQT1 rabbits show statistically significant differences in APD compared with LMC; *P<0.05. (D) APD dispersion increased in LQT1-TICM. (E) APD restitution curves from four groups. (F): Maximum slopes of the APD restitution curves. LQT1-TICM demonstrates increase in APD restitution slopes (*P<0.05).
Steep APD restitution and alternans in TICM groups
Fig 3E shows the APD restitution curves from four hearts, one representing each group. APD restitution slope was markedly increased in LQT1-TICM (panel F, Slopes; LMC-SH-P: 0.43±0.05, LQT1-SH-P: 0.40±0.26; LMC-TICM: 0.69±0.25; LQT1-TICM: 0.89±0.24, n = 5 in sham and n = 9 in TICM groups, *P<0.05). As predicted by the restitution hypothesis [36], alternans was frequently observed in LQT1-TICM (8 of 9 hearts), which had the steepest restitution curve. In addition, alternans were spatially discordant in LQT1-TICM (7 of 8 hearts), i.e., one location had short-long phase while another had long-short phase. However, the LMC-TICM showed relatively rare cases of DA (2 of 10 hearts).
Fig 4 shows a typical example of DA in LQT1-TICM. Space-time plots of Vm and Ca2+ in panel B show that Vm repolarization (see dotted white line in panel B) changed gradually in space, while Ca2+ transient changed more abruptly. The alternation of Ca2+ transient recovery between odd and even beats was greater than that of APD (see panel C). This effect can also be seen in panel A, middle trace (b), where APD alteration between odd and even beats are not prominent, while Ca2+ exhibits marked alternation between odd and even beats.
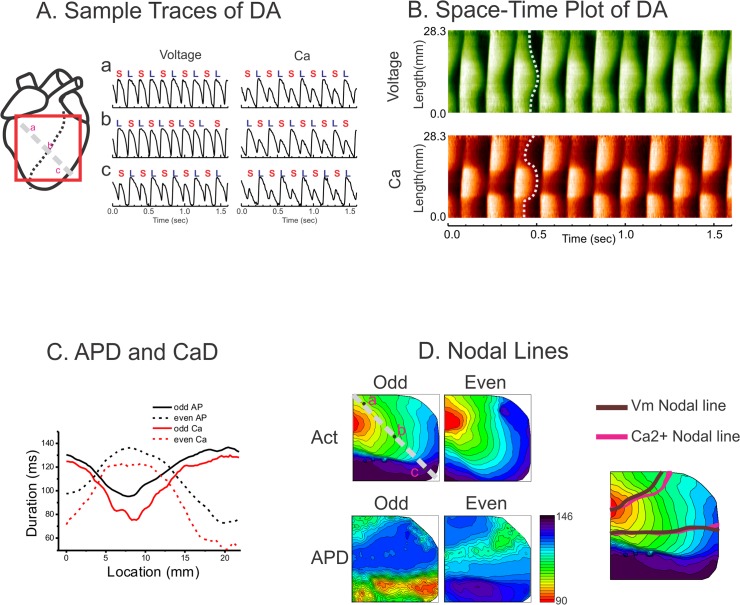
Fig 4. Prevalent discordant alternans in LQT1-TICM.
(A) Sample traces of DA from three locations. (B) Space-time plot of DA along the line a-c in panel A. (C) APD (black) and Ca2+ duration (red) along the line a-c. Alternation between odd and even beats was larger in Ca2+. (D) Maps of activation and nodal lines of DA. Note that activation in odd beats shows markedly slowing conduction toward the apex region. However, the nodal lines were not associated with the alternating activation pattern.
DA can be created by two competing mechanisms: conduction alternans or tissue heterogeneities such as heterogeneous Ca2+ handling [57]. Theoretical studies [57] have proposed that the cause of DA can be determined by investigating the behavior of nodal lines (between regions with APD alternans out of phase from each other). The activation maps in Fig 4D indicate that conduction is alternating between odd and even beats. However, the nodal lines superimposed over the activation maps (panel D) are perpendicular to the activation isochronal lines and precede the conduction delay in the odd beats. The conduction delay most likely occurred when the activation front encountered enhanced APD dispersion near nodal lines. This finding suggests that tissue heterogeneities may play an important role in the formation of DA.
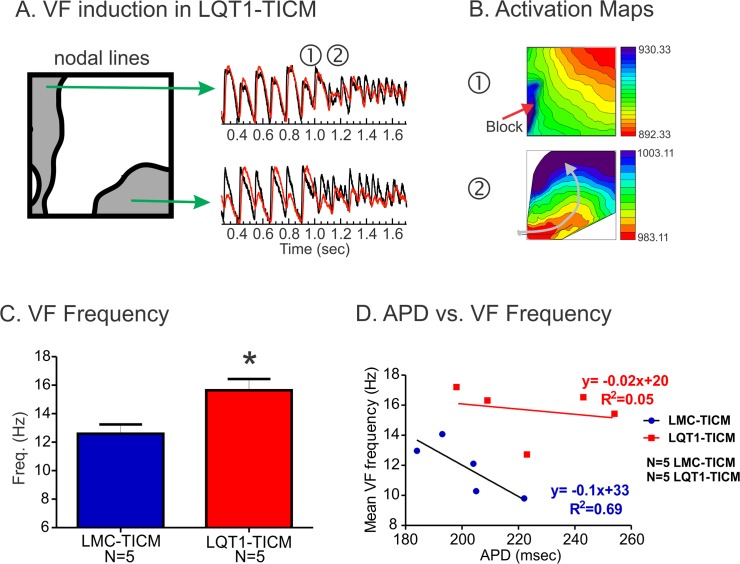
Characteristics of VF in LQT1-TICM
As in in vivo experiments, LMC-TICM and LQT1-TICM both demonstrated increased propensity to VF under ramp pacing protocol during optical mapping (6 of 9 LMC-TICM and 7 of 9 LQT1-TICM were inducible; see Table 2). Interestingly, DA often preceded VF induction in LQT1-TICM hearts. Fig 5 shows an example of DA that preceded VF induction. The initiation of VF was due to a conduction block on the left side of the nodal lines (➀) that formed a rotating wave (➁, arrow). This result demonstrates direct link between DA and VF induction in LQT1-TICM.
Table 2. VF inducibility in optical mapping.
| Group | VF | No VF |
|---|---|---|
| LQT1-TICM | 7 | 2 |
| n = 9 | ||
| LQT1-SH-P | 1 | 4 |
| n = 5 | ||
| LMC-TICM | 6 | 3 |
| n = 9 | ||
| LMC-SH-P | 2 | 3 |
| n = 5 |
LQT1-TICM vs. LQT1-SH-P: p = 0.09
Fig 5. VF induction and VF frequency in LQT1-TICM.
(A) Sample traces of Vm and Ca2+ from 190 ms during VF induction. DA was prominent before VF. (B) Activation maps of VF induction. Conduction block occurred near the nodal line and formed a rotating wave (grey arrow). (C) VF frequency. VF frequency was higher in LQT1-TICM despite the prolongation of APD at basic CL (Fig 3C (D) Correlation between APD and VF frequency. The baseline APD is no longer a predictor of VF frequency as in in vivo EPS in Fig 2F.
VF frequency in LQT1-TICM was significantly higher in LQT1-TICM than in LMC-TICM (Frequency (Hz), n = 5; LMC-TICM: 12.59±1.47, LQT1-TICM: 15.65±1.75, *P<0.05, panel C). The basic relationship between baseline APD and VF frequency was lost (r = 0.92 vs. r = 0.28, p<0.05, panel D) as in in vivo EPS (Fig 2F).
Lack of IKs leads to greater APD alternans in computer simulation of HF
Fig 6 shows the results of a computer model of ventricular myocytes, which includes a detailed spatiotemporal Ca2+ cycling system (details are provided in S1 File). Under the control condition, both APD and Ca2+ exhibited very small amplitudes of alternans at rapid pacing rates. However, under HF conditions, large-amplitude alternans of APD and Ca2+ occurred when the pacing cycle length was shorter than 320 ms. Alternans in the HF condition was caused mainly by changes in Ca2+ cycling properties from the control condition, i.e., Ca2+ cycling was the major origin of alternans [58]. With further reduction of IKs from the HF condition to mimic the LQT1-TICM condition, alternans occurred at pacing cycle length shorter than 360 ms, and the amplitudes of APD and Ca2+ alternans were further increased. In other words, alternans was further potentiated by the lack of IKs in addition to remodeling of Ca2+ cycling in HF. This supports the experimental observation that LQT1-TICM rabbits had a higher propensity to alternans and arrhythmias than LMC-TICM rabbits.
Fig 6. Computer simulations of APD and Ca2+ alternans under different conditions.
(A) Vm (upper) and whole-cell Ca2+ concentration (lower) versus time for the control condition at CL = 280 ms. (B) Vm and whole-cell Ca2+ concentration versus time for the HF condition. (C) Vm and whole-cell Ca2+ concentration versus time for the same condition as in B but with zero IKs. (D) Peak whole-cell Ca2+ concentrations of two consecutive beats versus CL for the three conditions. (E) APD of the same two consecutive beats as in D versus CL for the three conditions.
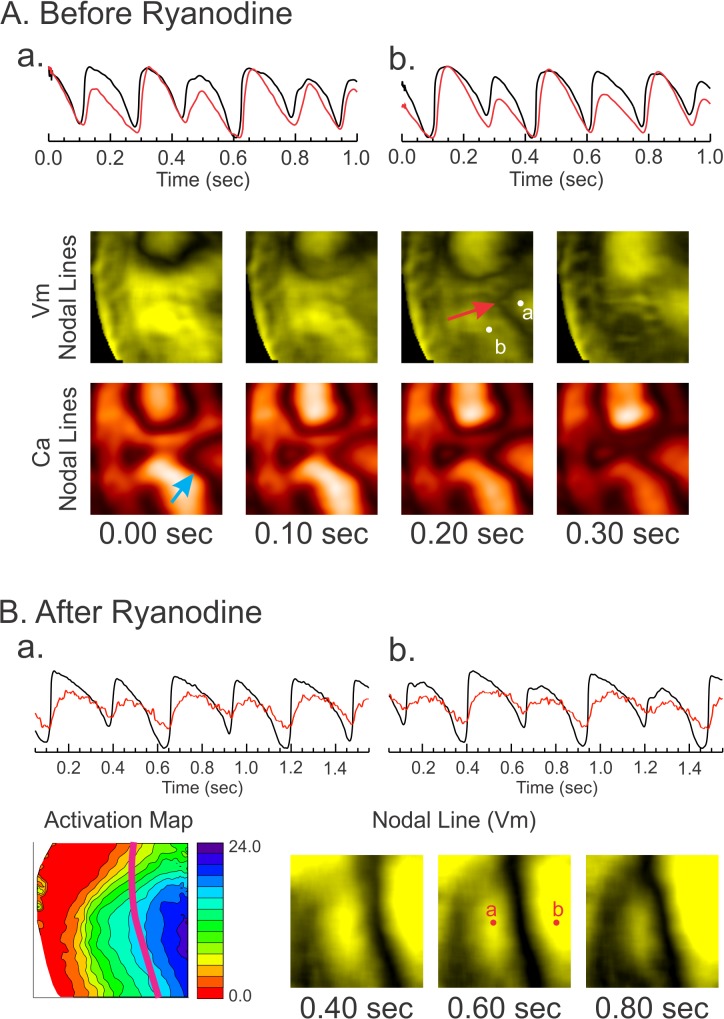
Effects of ryanodine on the behavior of DA in LQT1-TICM
In an effort to investigate the potential role of Ca2+ underlying the increased propensity to DA in LQT1-TICM, Ca2+ transients were abolished by 2 μM ryanodine, and the ramp pacing protocol was repeated. Before ryanodine administration, the behavior of DA was complex, dynamically appearing and disappearing (see Fig 7). Importantly, Ca2+ nodal lines were independent of APD nodal lines, often appearing without them. In panel A, additional Ca2+ nodal lines appear alone (1st column, blue arrow), followed by formation of the APD nodal line (3rd column), suggesting that Ca2+ instability promotes complex Vm DA. As expected, abolishing Ca2+ transient with ryanodine reduced the incidence of DA (6 of 9) and VF induction in LQT1-TICM. Some LQT1-TICM hearts still demonstrated DA under ryanodine (3 of 9 hearts), though their dynamics were markedly different before ryanodine perfusion. The nodal lines of Vm DA under ryanodine were closely related to the activation pattern (Fig 7B) and slowly moved toward the pacing site, which was predicted by computer modeling studies when the conduction velocity restitution causes DA. Overall, our data provide strong evidence that the lack of IKs combined with abnormal Ca2+ handling increase voltage instability and arrhythmia risk in failing hearts.
Fig 7. The effect of ryanodine on DA.
(A) Complex Ca2+ DA in LQT1-TICM. Top: Vm and Ca2+ traces from three locations. Note that the phase shift from short-long to long-short occurs in Ca2+, which was followed by Vm. Bottom: Series of nodal line images. Initially, a single nodal line was observed in Vm, while an additional nodal line was seen in Ca2+ (blue arrow). Several beats later, a nodal line appeared in Vm, which is in close proximity to the Ca2+ nodal line (red arrow). (B) Nodal line behavior after 2 μM ryanodine. DA were observed in only one third of LQT1-TICM hearts with ryanodine (3 of 9 hearts). The series of nodal line images shows that the beat-to-beat changes in DA were minimal and closely related to the activation pattern.
Discussion
We evaluated the effects of IKs remodeling on arrhythmogenesis in HF using a transgenic rabbit model of LQT1 and their littermate controls. Our major findings are that while the HF phenotype is very similar between LMC-TICM and LQT1-TICM, the complete lack of IKs in LQT1-TICM produces a more arrhythmogenic substrate due to complex APD and Ca2+ DA, ultimately causing conduction block and VF.
Steep restitution and alternans in TICM
We previously reported that rabbit LQT1 hearts have smaller APD dispersion and a flatter restitution curve than LMC [42]. Ramp pacing and programmed stimulation were less effective in inducing alternans and VF in LQT1 rabbits, suggesting that lack of IKs alone does not promote alternans reentry [42, 50]. In contrast, TICM protocol steepened APD restitution curves in LQT1 both in vivo QT/RR plot (Fig 2) and ex vivo optical mapping studies (Fig 3). In line with APD restitution theory, LQT1-TICM demonstrated higher risk of DA and VF induction under programmed stimulation (Table 1). VF initiation (Fig 5) indicates that conduction block occurred in the region of the large repolarization gradient caused by DA, verifying alternans as a major factor behind arrhythmogenesis in LQT1-TICM.
It is well known that APD restitution is steep in animal models and human HF [8, 59–61], but our result is quite surprising, because the lack of IKs in LQT1-TICM is supposed to prevent arrhythmogenic repolarization shortening and protect against reentry formation [37, 38]. Since electrical remodeling in LQT1-TICM is relatively minor (see S2 File for ionic current remodeling; no statistical significance was found), our results suggest that the combination of IKs downregulation and remodeling in Ca2+ handling in the failing heart plays a major role in inducing alternans and reentry formation.
IKs downregulation accentuates Ca2+-mediated alternans in TICM
Abnormal Ca2+ handling in HF has been well documented and is thought to play a key role in arrhythmogenesis [4, 25, 62, 63]. Action-potential clamp studies showed that Ca2+ transients can still alternate in isolated myocytes from failing hearts under non-alternating action potential clamp conditions [64], indicating that Ca2+ alternans are independent from electrophysiological remodeling of repolarizing currents in HF. Ca2+ alternans in our data was much larger than APD alternans (Fig 4A) and also changes its phase abruptly across nodal lines (see Fig 4B & 4C), while APD alternans gradually changes across nodal lines. In addition, Ca2+ alternans was still present in certain locations even when APD alternans was not prominent (Fig 4A and 4B and S4 File and S5 File), indicating that abnormal Ca2+ handling is a major driver of APD alternans in HF. Ryanodine significantly reduced the incidence of DA and VF in the LQT1-TICM group, demonstrating that Ca2+ is a major driver of DA in the failing heart.
Ca2+ alternans in HF can promote APD alternans through Ca2-depedendent ionic currents. For example, INCX is upregulated in HF [25–28], which can enhance APD alternans via alternating depolarizing currents during Ca2+ alternans. Our experimental and computer simulation data suggest that IKs downregulation is also an important factor to accentuate APD alternans. Since IKs is Ca2+ dependent and is augmented by Ca2+ transients, the reduced IKs in HF may increase the influence of Ca2+ on Vm through INCX, resulting in enhanced APD alternans.
Complex DA dynamics in TICM
In the present study, LQT1-TICM lacking IKs demonstrated highly dynamic Ca2+ DA, changing from its phase of alternans (S3 File and S1 Movie). Detailed analysis shows that the phase transition of Ca2+ alternans often precedes that of APD alternans (see S4 File). As a result, more complex APD nodal lines were readily formed in LQT1-TICM hearts. The complex nodal lines increased the risk of conduction block due to increased dispersion of repolarization. In this study, complex nodal line dynamics were observed almost exclusively in LQT1-TICM (see Fig 7 and S3 and S4 Files for LQT1-TICM vs. S5 File for LMC-TICM), suggesting the role of IKs downregulation along with abnormal Ca2+ handling in HF.
It is important to point out that a small number of LQT1-TICM hearts (3 of 9 LQT1-TICM) still exhibited DA after ryanodine perfusion. The nodal lines after ryanodine administration indicate abnormal conduction, as they are aligned with wave fronts and move towards the pacing sites, unlike the case in LQT1-TICM (Fig 7). Abnormal conduction is another well-known phenomenon in HF due to fibrosis, gap junction remodeling, and downregulation of INa [4, 62, 65]. Our result shows the complex nature of HF and demonstrates that multiple arrhythmia mechanisms, including Ca2+ alternans and slow conduction, contribute to overall arrhythmia risk in HF.
High-frequency VF in LQT1-TICM
Our study demonstrated that VF in LQT1-TICM exhibited significantly higher VF frequency despite prolonged APD. Since LQT1-TICM hearts lack IKs, it is surprising to see even higher-frequency VF in LQT1-TICM compared to LMC-TICM. The higher VF frequency in LQT1-TICM may be related to its steep APD restitution. As a result, refractoriness and wavelength can be substantially shortened at short CLs such as in VF, allowing high-frequency VF in LQT1-TICM. The cause of APD shortening at rapid heart rate is not clear. Harada et al.[8] provided mechanistic insight into APD shortening in the failing heart, linking it to downregulation of ICaL. Further studies are needed to understand the mechanism underlying APD shortening and high-frequency VF in LQT1-TICM.
Conclusions
Our results strongly support the contention that lack of repolarization reserve in HF, particularly reduction in IKs, is highly arrhythmogenic. Abnormal Ca2+ handling in failing hearts promotes Ca2+ alternans, and lack of IKs enhances the effect of Ca2+ alternans on APD alternans, complicating the dynamics of DA and VF. This study emphasizes that the repolarization reserve provided by IKs is an important modulator of Ca2+-driven DA and VF.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Top panels: Vm and Ca2+. Bottom panels: Vm and Ca2+ nodal lines. The nodal lines of LQT1-TICM demonstrated complex patterns including genesis, vanishing, wiggling, and moving. The spatiotemporal patterns of nodal lines of action potential and Ca2+ transient were also complex. This movie corresponds to Fig 7A in the manuscript.
(MP4)
Left panel: Vm, Right panel: Vm nodal line in LQT1-TICM heart after ryanodine. This movie corresponds to Fig 7B in the manuscript.
(MP4)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Heart, Lung and Blood Institute Grants R01-HL-096669-04 to BC. and R01-HL-046005-18A1 to GK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kjekshus J. Arrhythmias and mortality in congestive heart failure. Am J Cardiol. 1990;65(19):42I–8I. Epub 1990/05/22. PubMed . [DOI] [PubMed] [Google Scholar]
- 2. Chakko S, de Marchena E, Kessler KM, Myerburg RJ. Ventricular arrhythmias in congestive heart failure. Clin Cardiol. 1989;12(9):525–30. Epub 1989/09/01. PubMed . [DOI] [PubMed] [Google Scholar]
- 3. Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–171. Epub 2006/12/30. CIRCULATIONAHA.106.179918 [pii] 10.1161/CIRCULATIONAHA.106.179918 PubMed . [DOI] [PubMed] [Google Scholar]
- 4. Ebinger MW, Krishnan S, Schuger CD. Mechanisms of ventricular arrhythmias in heart failure. Curr Heart Fail Rep. 2005;2(3):111–7. Epub 2005/09/06. PubMed . [DOI] [PubMed] [Google Scholar]
- 5. Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61(2):208–17. Epub 2004/01/23. S0008636303007272 [pii]. PubMed . [DOI] [PubMed] [Google Scholar]
- 6. Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol. 2002;283(3):H1031–41. Epub 2002/08/16. 10.1152/ajpheart.00105.2002 PubMed . [DOI] [PubMed] [Google Scholar]
- 7. Nuss HB, Kaab S, Kass DA, Tomaselli GF, Marban E. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Physiol. 1999;277(1 Pt 2):H80–91. Epub 1999/07/17. PubMed . [DOI] [PubMed] [Google Scholar]
- 8. Harada M, Tsuji Y, Ishiguro YS, Takanari H, Okuno Y, Inden Y, et al. Rate-dependent shortening of action potential duration increases ventricular vulnerability in failing rabbit heart. Am J Physiol Heart Circ Physiol. 2011;300(2):H565–73. Epub 2010/12/15. ajpheart.00209.2010 [pii] 10.1152/ajpheart.00209.2010 PubMed . [DOI] [PubMed] [Google Scholar]
- 9. Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93(7):638–45. Epub 2003/08/23. 10.1161/01.RES.0000092248.59479.AE 01.RES.0000092248.59479.AE [pii]. PubMed . [DOI] [PubMed] [Google Scholar]
- 10. Kaab S, Nuss HB, Chiamvimonvat N, O'Rourke B, Pak PH, Kass DA, et al. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78(2):262–73. Epub 1996/02/01. PubMed . [DOI] [PubMed] [Google Scholar]
- 11. Li GR, Lau CP, Leung TK, Nattel S. Ionic current abnormalities associated with prolonged action potentials in cardiomyocytes from diseased human right ventricles. Heart Rhythm. 2004;1(4):460–8. Epub 2005/04/27. S1547-5271(04)00292-9 [pii] 10.1016/j.hrthm.2004.06.003 PubMed . [DOI] [PubMed] [Google Scholar]
- 12. Tsuji Y, Opthof T, Kamiya K, Yasui K, Liu W, Lu Z, et al. Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc Res. 2000;48(2):300–9. Epub 2000/10/31. S0008-6363(00)00180-2 [pii]. PubMed . [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Hill JA. Electrophysiological remodeling in heart failure. J Mol Cell Cardiol. 2010;48(4):619–32. 10.1016/j.yjmcc.2010.01.009 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42(2):270–83. PubMed . [DOI] [PubMed] [Google Scholar]
- 15. Roden DM, Yang T. Protecting the heart against arrhythmias: potassium current physiology and repolarization reserve. Circulation. 2005;112(10):1376–8. Epub 2005/09/08. 112/10/1376 [pii] 10.1161/CIRCULATIONAHA.105.562777 PubMed . [DOI] [PubMed] [Google Scholar]
- 16. Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259(1):59–69. Epub 2005/12/13. JIM1589 [pii] 10.1111/j.1365-2796.2005.01589.x PubMed . [DOI] [PubMed] [Google Scholar]
- 17. Roden DM. Repolarization reserve: a moving target. Circulation. 2008;118(10):981–2. Epub 2008/09/04. 118/10/981 [pii] 10.1161/CIRCULATIONAHA.108.798918 PubMed . [DOI] [PubMed] [Google Scholar]
- 18. Roden DM. Taking the "idio" out of "idiosyncratic": predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21(5):1029–34. PubMed . [DOI] [PubMed] [Google Scholar]
- 19. Jost N, Papp JG, Varro A. Slow delayed rectifier potassium current (IKs) and the repolarization reserve. Ann Noninvasive Electrocardiol. 2007;12(1):64–78. Epub 2007/02/09. ANEC140 [pii] 10.1111/j.1542-474X.2007.00140.x PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112(10):1392–9. Epub 2005/09/01. CIRCULATIONAHA.105.550111 [pii] 10.1161/CIRCULATIONAHA.105.550111 PubMed . [DOI] [PubMed] [Google Scholar]
- 21. Silva J, Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005;112(10):1384–91. Epub 2005/09/01. CIRCULATIONAHA.105.543306 [pii] 10.1161/CIRCULATIONAHA.105.543306 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maltsev VA, Sabbah HN, Undrovinas AI. Late sodium current is a novel target for amiodarone: studies in failing human myocardium. J Mol Cell Cardiol. 2001;33(5):923–32. 10.1006/jmcc.2001.1355 PubMed . [DOI] [PubMed] [Google Scholar]
- 23. Undrovinas AI, Maltsev VA, Kyle JW, Silverman N, Sabbah HN. Gating of the late Na+ channel in normal and failing human myocardium. J Mol Cell Cardiol. 2002;34(11):1477–89. PubMed . [DOI] [PubMed] [Google Scholar]
- 24. Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38(3):475–83. 10.1016/j.yjmcc.2004.12.012 PubMed . [DOI] [PubMed] [Google Scholar]
- 25. Bers DM, Pogwizd SM, Schlotthauer K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol. 2002;97 Suppl 1:I36–42. Epub 2002/12/14. PubMed . [DOI] [PubMed] [Google Scholar]
- 26. Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88(11):1159–67. PubMed . [DOI] [PubMed] [Google Scholar]
- 27. Reinecke H, Studer R, Vetter R, Holtz J, Drexler H. Cardiac Na+/Ca2+ exchange activity in patients with end-stage heart failure. Cardiovasc Res. 1996;31(1):48–54. PubMed . [PubMed] [Google Scholar]
- 28. Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, et al. Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ Res. 1994;75(3):443–53. PubMed . [DOI] [PubMed] [Google Scholar]
- 29. Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96(1):195–215. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: a simulation study. Circulation. 1999;99(18):2466–74. Epub 1999/05/11. PubMed . [DOI] [PubMed] [Google Scholar]
- 31. Zeng J, Laurita KR, Rosenbaum DS, Rudy Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formulation and their role in repolarization. Circ Res. 1995;77(1):140–52. Epub 1995/07/01. PubMed . [DOI] [PubMed] [Google Scholar]
- 32. Decker KF, Heijman J, Silva JR, Hund TJ, Rudy Y. Properties and ionic mechanisms of action potential adaptation, restitution, and accommodation in canine epicardium. Am J Physiol Heart Circ Physiol. 2009;296(4):H1017–26. 10.1152/ajpheart.01216.2008 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karma A. Electrical alternans and spiral wave breakup in cardiac tissue. Chaos. 1994;4(3):461–72. PubMed . [DOI] [PubMed] [Google Scholar]
- 34. Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98(10):1244–53. Epub 2006/05/27. 98/10/1244 [pii] 10.1161/01.RES.0000224540.97431.f0 PubMed . [DOI] [PubMed] [Google Scholar]
- 35. Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112(8):1232–40. Epub 2005/08/24. 112/8/1232 [pii] 10.1161/CIRCULATIONAHA.104.529545 PubMed . [DOI] [PubMed] [Google Scholar]
- 36. Gilmour RF Jr, Chialvo DR. Electrical restitution, critical mass, and the riddle of fibrillation. J Cardiovasc Electrophysiol. 1999;10(8):1087–9. PubMed . [DOI] [PubMed] [Google Scholar]
- 37. Islam MA. Pharmacological modulations of cardiac ultra-rapid and slowly activating delayed rectifier currents: potential antiarrhythmic approaches. Recent patents on cardiovascular drug discovery. 2010;5(1):33–46. PubMed . [DOI] [PubMed] [Google Scholar]
- 38. Varro A, Biliczki P, Iost N, Virag L, Hala O, Kovacs P, et al. Theoretical possibilities for the development of novel antiarrhythmic drugs. Curr Med Chem. 2004;11(1):1–11. PubMed . [DOI] [PubMed] [Google Scholar]
- 39. Munoz V, Grzeda KR, Desplantez T, Pandit SV, Mironov S, Taffet SM, et al. Adenoviral expression of IKs contributes to wavebreak and fibrillatory conduction in neonatal rat ventricular cardiomyocyte monolayers. Circ Res. 2007;101(5):475–83. Epub 2007/07/14. CIRCRESAHA.107.149617 [pii] 10.1161/CIRCRESAHA.107.149617 PubMed . [DOI] [PubMed] [Google Scholar]
- 40. Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990;258(4 Pt 2):H1200–7. PubMed . [DOI] [PubMed] [Google Scholar]
- 41. Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29(4):709–15. Epub 1997/03/15. S073510979600592X [pii]. PubMed . [DOI] [PubMed] [Google Scholar]
- 42. Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118(6):2246–59. PubMed 10.1172/JCI33578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsuji Y, Zicha S, Qi XY, Kodama I, Nattel S. Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia: discrete arrhythmogenic consequences related to differential delayed-rectifier changes. Circulation. 2006;113(3):345–55. 10.1161/CIRCULATIONAHA.105.552968 PubMed . [DOI] [PubMed] [Google Scholar]
- 44. Odening KE, Hyder O, Chaves L, Schofield L, Brunner M, Kirk M, et al. Pharmacogenomics of anesthetic drugs in transgenic LQT1 and LQT2 rabbits reveal genotype-specific differential effects on cardiac repolarization. Am J Physiol Heart Circ Physiol. 2008;295(6):H2264–72. Epub 2008/10/07. 00680.2008 [pii] 10.1152/ajpheart.00680.2008 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi B-R, Salama G. Simultaneous optical maps of early afterdepolarizations (EADs) and intracellular Ca2+ (Cai) in drug-induced long QT syndrome (LQTS). Circulation. 1999;100:I51. [Google Scholar]
- 46. Salama G, Choi BR, Azour G, Lavasani M, Tumbev V, Salzberg BM, et al. Properties of new, long-wavelength, voltage-sensitive dyes in the heart. J Membr Biol. 2005;208(2):125–40. Epub 2006/04/29. 10.1007/s00232-005-0826-8 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayashi H, Kamanu SD, Ono N, Kawase A, Chou CC, Weiss JN, et al. Calcium transient dynamics and the mechanisms of ventricular vulnerability to single premature electrical stimulation in Langendorff-perfused rabbit ventricles. Heart Rhythm. 2008;5(1):116–23. Epub 2008/01/09. S1547-5271(07)00877-6 [pii] 10.1016/j.hrthm.2007.08.020 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi BR, Jang W, Salama G. Spatially discordant voltage alternans cause wavebreaks in ventricular fibrillation. Heart Rhythm. 2007;4(8):1057–68. PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol. 2000;529(Pt 1):171–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ziv O, Morales E, Song YK, Peng X, Odening KE, Buxton AE, et al. Origin of complex behaviour of spatially discordant alternans in a transgenic rabbit model of type 2 long QT syndrome. J Physiol. 2009;587(Pt 19):4661–80. Epub 2009/08/14. jphysiol.2009.175018 [pii] 10.1113/jphysiol.2009.175018 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim TY, Woo SJ, Hwang SM, Hong JH, Lee KJ. Cardiac beat-to-beat alternations driven by unusual spiral waves. Proc Natl Acad Sci U S A. 2007;104(28):11639–42. Epub 2007/07/04. 10.1073/pnas.0704204104 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Restrepo JG, Weiss JN, Karma A. Calsequestrin-mediated mechanism for cellular calcium transient alternans. Biophys J. 2008;95(8):3767–89. PubMed 10.1529/biophysj.108.130419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie L-H, et al. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J. 2008;94(2):392–410. 10.1529/biophysj.106.98160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97(12):1314–22. 10.1161/01.RES.0000194329.41863.89 PubMed . [DOI] [PubMed] [Google Scholar]
- 55. Shannon TR, Wang F, Bers DM. Regulation of cardiac sarcoplasmic reticulum Ca release by luminal [Ca] and altered gating assessed with a mathematical model. Biophys J. 2005;89(6):4096–110. 10.1529/biophysj.105.068734 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lammers WJ, Allessie M, Rensma PL, Schalij MJ. The use of fibrillation cycle length to determine spatial dispersion in electrophysiological properties and to characterise the underlying mechanism of fibirllation. New Trends in Arrhythmias. 1986;II. [Google Scholar]
- 57. Hayashi H, Shiferaw Y, Sato D, Nihei M, Lin SF, Chen PS, et al. Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J. 2007;92(2):448–60. Epub 2006/10/31. S0006-3495(07)70845-7 [pii] 10.1529/biophysj.106.091009 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qu Z, Nivala M, Weiss JN. Calcium alternans in cardiac myocytes: Order from disorder. J Mol Cell Cardiol. 2013;58(0):100–9. 10.1016/j.yjmcc.2012.10.007. 10.1016/j.yjmcc.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benoist D, Stones R, Drinkhill MJ, Benson AP, Yang Z, Cassan C, et al. Cardiac arrhythmia mechanisms in rats with heart failure induced by pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2012;302(11):H2381–95. 10.1152/ajpheart.01084.2011 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lou Q, Janks DL, Holzem KM, Lang D, Onal B, Ambrosi CM, et al. Right ventricular arrhythmogenesis in failing human heart: the role of conduction and repolarization remodeling. Am J Physiol Heart Circ Physiol. 2012;303(12):H1426–34. 10.1152/ajpheart.00457.2012 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Watanabe T, Yamaki M, Yamauchi S, Minamihaba O, Miyashita T, Kubota I, et al. Regional prolongation of ARI and altered restitution properties cause ventricular arrhythmia in heart failure. Am J Physiol Heart Circ Physiol. 2002;282(1):H212–8. PubMed . [DOI] [PubMed] [Google Scholar]
- 62. Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95(8):754–63. Epub 2004/10/16. 95/8/754 [pii] 10.1161/01.RES.0000145047.14691.db PubMed . [DOI] [PubMed] [Google Scholar]
- 63. Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol. 2009;297(4):H1235–42. Epub 2009/08/04. 01320.2008 [pii] 10.1152/ajpheart.01320.2008 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, et al. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6(2):251–9. Epub 2009/02/04. S1547-5271(08)01091-6 [pii] 10.1016/j.hrthm.2008.11.008 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jeong EM, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52(2):454–63. Epub 2011/10/08. S0022-2828(11)00420-2 [pii] 10.1016/j.yjmcc.2011.09.018 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Top panels: Vm and Ca2+. Bottom panels: Vm and Ca2+ nodal lines. The nodal lines of LQT1-TICM demonstrated complex patterns including genesis, vanishing, wiggling, and moving. The spatiotemporal patterns of nodal lines of action potential and Ca2+ transient were also complex. This movie corresponds to Fig 7A in the manuscript.
(MP4)
Left panel: Vm, Right panel: Vm nodal line in LQT1-TICM heart after ryanodine. This movie corresponds to Fig 7B in the manuscript.
(MP4)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.