Abstract
Glia of the central nervous system (CNS) help to maintain homeostasis in the brain and support efficient neuronal function. Microglia are innate immune cells of the brain that mediate responses to pathogens and injury. They have key roles in phagocytic clearing, surveying the local microenvironment and propagating inflammatory signals. An interruption in homeostasis induces a cascade of conserved adaptive responses in glia. This response involves biochemical, physiological and morphological changes and is associated with the production of cytokines and secondary mediators that influence synaptic plasticity, cognition and behavior. This reorganization of host priorities represents a beneficial response that is normally adaptive but may become maladaptive when the profile of microglia is compromised. For instance, microglia can develop a primed or pro-inflammatory mRNA, protein and morphological profile with aging, traumatic brain injury and neurodegenerative disease. As a result, primed microglia exhibit an exaggerated inflammatory response to secondary and sub-threshold challenges. Consequences of exaggerated inflammatory responses by microglia include the development of cognitive deficits, impaired synaptic plasticity and accelerated neurodegeneration. Moreover, impairments in regulatory systems in these circumstances may make microglia more resistant to negative feedback and important functions of glia can become compromised and dysfunctional. Overall, the purpose of this review is to discuss key concepts of microglial priming and immune-reactivity in the context of aging, traumatic CNS injury and neurodegenerative disease.
Introduction on Microglia in the Healthy Brain
Microglia are innate immune cells of the brain that mediate responses to pathogens and injury. In addition, microglia provide support, synaptic pruning and immunological activities within the central nervous system (CNS) (Schafer and Stevens, 2013). At one time, microglia were described as being in a ‘quiescent’ or ‘resting’ state. Recent evidence, however, indicates that microglia are constantly surveying their microenvironment (Davalos et al., 2005; Nimmerjahn et al., 2005). Moreover, it is clear that microglia are actively involved in maintaining homeostasis in the CNS. For instance, microglia have an important role in brain development and are critical in synaptic pruning and clearing debris (Schafer et al., 2012). Overall, emerging evidence indicates that microglia work to support normal CNS functions (Salter and Beggs, 2014).
Microglia are myeloid derived cells and have many immune functions related to innate immunity (Ransohoff and Perry, 2009). These immune related activities include key roles in immune surveillance and the interpretation and propagation of inflammatory signals that are initiated in the periphery (Dantzer et al., 2008). These responses are pivotal in the coordinated communication between the immune system and the brain. Microglia actively survey the brain microenvironment for disruptions in homeostasis. For example, in infection or disease, microglia become ‘activated’ and function as inflammatory cellular mediators. Activated microglia rapidly alter their transcriptional profile and produce inflammatory cytokines and chemokines. Active microglia also undergo cytoskeletal rearrangements that alter the pattern of receptor expression. This facilitates cytokine communication between cells. In addition, these alterations allow for microglia to migrate towards sites of injury or infection and to potentially increase their phagocytic efficiency (Davalos et al., 2005). In general, microglia activation and the increased expression of cytokines are aimed to be protective to the CNS and beneficial to the host organism. This is represented in their role in mediating the behavioral symptoms of sickness following innate immune challenge (Corona et al., 2012). Moreover, a recent study shows that repeated injection of lipopolysaccharide (LPS) moves microglia towards a novel profile in which they migrate to the synapses of inhibitory neurons displacing them from cortical neurons (Chen et al., 2014). This action of microglia was associated with neuroprotection and reduced lesion size after cryogenic brain injury. Nonetheless, when microglia profiles are altered or key regulatory systems are impaired, microglia activation may be maladaptive. Depending on the context, exaggerated microglial activation can lead to prolonged neuroinflammation and neurobehavioral complications. In this review, we will highlight changes that occur in microglia of aging, CNS trauma and neurodegenerative disease and the influence of these changes on secondary inflammatory insult.
Evidence of Increased Brain Inflammation with Age
There is significant clinical and experimental evidence that the inflammatory status of the brain increases as a function of normal aging. Hallmarks of brain aging include increased oxidative stress, lipid peroxidation and DNA damage (Norden and Godbout, 2013). Consistent with this premise, microarray studies indicate that there is an overall increase in inflammatory and pro-oxidant genes with a reduction in growth and anti-oxidant genes in the brain of older rodents compared to adults (Godbout et al., 2005b; Lee et al., 1999). In addition, two potent pro-inflammatory cytokines interleukin (IL)-1β and IL-6 are increased in the brain of aged rodents and humans (Fenn et al., 2013; Godbout et al., 2005b; Henry et al., 2009; Sheng et al., 1998). In addition, there are reductions in several regulatory molecules and anti-inflammatory cytokines including IL-10 and IL-4 (Maher et al., 2005; Ye and Johnson, 2001). Because of these changes in the balance of pro- and anti-inflammatory profiles, microglia are implicated as the source of this inflammation.
Microglia are long-lived cells that have limited turnover from myeloid cells from the bone marrow during the course of a lifetime (Ajami et al., 2007; Ginhoux et al., 2010). Microglia develop early in embryogenesis from myeloid precursor cells in the embryonic yolk sac and migrate to the area of the CNS around embryonic day 8.5 (Ginhoux et al., 2010). Although microglia are not replaced from the bone marrow, a recent study shows that turnover may be from a progenitor source within the CNS (Elmore et al., 2014). In this study, intervention with a colony stimulating factor 1 receptor (CSF1R) antagonist effectively depleted the majority of microglia in the CNS. The removal of the CSF1R antagonist allowed for the robust repopulation of microglia from a previous unidentified progenitor source within the CNS (Elmore et al., 2014). It is still unclear however, the rate at which microglia would normally be turned over in the absence of CSF1R blockade. Overall, microglia appear to have a relatively low turnover rate. This stability and longevity of microglia makes them particularly sensitive to oxidative stress and inflammatory exposure over time.
Evidence of Microglial Priming with Age
As innate immune cells of the CNS, the increased pro-inflammatory phenotype of the brain that occurs with aging is linked to changes in microglia. With aging, microglia may develop an altered profile consistent with a more inflammatory state. This is also referred to as a primed profile. An inflammatory or primed microglia profile is defined by 1) higher baseline expression of markers of inflammation and inflammatory mediators, 2) a lower threshold to be activated and ‘switch’ to a pro-inflammatory state (Lull and Block, 2010), and 3) an exaggerated inflammatory response following immune activation. This primed phenotype of microglia is detected in models of aging, neurodegenerative disease and traumatic brain injury. This phenomena of primed microglia was first described in a model of prion disease where microglia from prion infected mice produced exaggerated IL-1β following both central and systemic immune challenges (Cunningham et al., 2005). Overall, in this review priming is defined as microglia that have an increased inflammatory state at baseline and also produce an exaggerated response to immune challenge. First, we will describe priming of microglia that occurs with aging. In the following sections, we will discuss similar phenotypes of primed microglia in the brain following TBI and with neurodegenerative disease. Although there are similarities between these disease models, no single source has been identified as the cause of priming. Therefore, microglial priming towards an inflammatory state is likely influenced by exposure to immune challenges, stressors and injury over time.
In the aged brain, microglial priming has been characterized by increased mRNA and protein expression of various inflammatory markers and alterations in morphology. For example, expression of the antigen presenting molecule major histocompatibility complex (MHC) II and complement receptor 3 (CD11b) were increased in the aged brain of rodents and humans (Frank et al., 2006; Streit and Sparks, 1997; VanGuilder et al., 2011). MHC II is increased specifically on microglia of the aged brain (Henry et al., 2009). MHC II is commonly used as a marker of microglial priming both in models of aging and injury because primed MHC II positive microglia produce exaggerated IL-1β following activation (Henry et al., 2009). Consistent with heightened MHC II expression, several other inflammatory markers are also increased in models of aging (for a review see (Norden and Godbout, 2013). In addition to immune markers, baseline expression of inflammatory cytokines tumor necrosis factor (TNF)-α, IL-1β and IL-6 are also increased in the brains of aged rodents and humans (Hickman et al., 2013; Sheng et al., 1998; Sierra et al., 2007; Youm et al., 2013).
These results are paralleled by morphological alterations detected using Iba-1 labeling. Microglia from non-diseased aged brains have shorter and less branched dendritic arbors than microglia of young adults (Streit et al., 2004). Microglia from the brains of aged gerbils (Choi et al., 2007) and dogs (Hwang et al., 2008) also have a shift towards a more activated morphology (thickened and de-ramified processes) compared to young adults. This de-ramified morphology is comparable to the activated morphology of microglia. Moreover, the de-ramified morphology of microglia in aged rats corresponded with higher MHC II expression (VanGuilder et al., 2011). These data further support the hypothesis that MHC II is a marker of primed microglia. Recent studies have also used positron emission tomography (PET) to evaluate microglial activation in humans. The ligand PK [11C](R)PK11195 binds to translocator protein (TSPO) receptors that are expressed in mitochondria of activated microglia. PET imaging using this compound showed increased TSPO in older individuals, indicating that microglial activation was elevated with age (Schuitemaker et al., 2012). Taken together, the increase of inflammatory mediators and altered phenotype of microglia with age indicates that microglia develop a primed phenotype and this may contribute to the heightened inflammatory status of the aged brain.
As stated above, the source of the glial priming in the aged brain is unclear. A hallmark of brain aging is increased oxidative stress and free radical damage that may affect the profile of microglia. In addition, changes in microRNA regulation of multiple genes may play a role in brain aging (Fenn et al., 2013). Moreover, recent evidence indicates that immune sensors, such as inflammasomes, are involved in increased inflammatory status with age. The NLRP3 inflammasome is an immune sensor that is activated in response to a diverse array of signals. When the NLRP3 inflammasome was deleted, CNS inflammation was reduced in mice that were allowed to age (Youm et al., 2013). For example, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), IL-1β, interferon, and complement pathways were all markedly increased in the brains of aged wildtype mice compared to adult wildtype mice. In aged NLRP3 knockout mice, however, these pathways were significantly attenuated compared to aged wildtype mice (Youm et al., 2013). Thus, increases in oxidative stress, changes in microRNA regulation and increased inflammasome activity with age could drive mediators of inflammation.
Evidence of Microglial Priming after Traumatic Brain Injury (TBI)
While it is unclear what causes priming as a function of normal aging, emerging evidence indicates that traumatic brain injury (TBI) causes prolonged activation of microglia and the development of a primed profile. Myriad studies indicate that both focal (penetrating) and diffuse (non-penetrating) TBI induce significant inflammatory processes in the brain mediated, in part, by resident microglia and astrocytes (Lifshitz et al., 2007b; McCrea et al., 2003; Tang et al., 1997; Woodcock and Morganti-Kossmann, 2013). There is evidence of long lasting changes in microglia and astrocytes after penetrating TBI caused by cortical impact injury (CCI). For example, there was increased OX6 (MHC II) expression in the brains of rats 16 days after moderate CCI (Holmin et al., 1995). Moreover, a recent study showed that CCI increased the inflammatory profile of microglia that persisted (Loane et al., 2014). Microglia in the lesion border had higher expression of several inflammatory mediators including MHC II, CD68 and NADPH oxidase (NOX2) in the cortex and thalamus that persisted 12 months after injury. In the same brain regions, YM-1, an alternative activation marker (M2) was transiently up-regulated 1 week later, but was reduced over time and was undetected at 3 and 12 months after injury. This suggests that in the midst of heightened inflammatory status and progressing gliosis, reparative mechanisms are down-regulated. Furthermore, this inflammation initiated by the CCI continued over time with increased inflammatory marker expression on microglia/macrophages and this was associated with lesion volume expansion and loss of neurons in the hippocampus (Loane et al., 2014). These features of tissue damage and neuroinflammation mediated by microglia/macrophages that persist over time are consistent with other models of focal or penetrating brain injury (Huang et al., 2014; Shultz et al., 2013).
There is also evidence that a primed or pro-inflammatory profile of microglia persists after diffuse head injury (non-penetrating). Experimental models of diffuse injury include midline fluid percussion injury (mFPI), blast injury and closed head impact (CHI) injuries (Xiong et al., 2013). For instance, mFPI is administered by a fluid-filled pulse directly to the brain at the midline causing equally distributed undulation of the brain and leaves the dura intact. This causes mild neuronal pathology including diffuse axonal injury (Lifshitz et al., 2007a) and transient neurological deficits (Morales et al., 2005) that recapitulate complications after mild to moderate concussive head injuries in humans (Lifshitz, 2009). A moderate mFPI in rodents caused activated microglia and an altered phenotype of microglia that persisted up to 1 month after injury. For instance, mFPI in rats was associated with increased expression of OX6 (MHC II) and CD68 on microglia that was still detected 1 week later (Ziebell et al 2012). Moreover, these microglia had a unique rod-like morphology and train arrangement along axon tracks and were predominantly detected in the primary sensory barrel fields of rats (Ziebell et al 2012). A related study in mice showed that mFPI increased mRNA and protein expression of MHC II on microglia in the brain 30 days post injury (dpi) (Fenn et al 2013). In addition, there were subtle changes in microglial morphology in the parietal cortex and hippocampus, both of which are brain regions affected by percussive injury. These increases in MHC II expression on microglia 30 dpi were detected in mice that had returned to baseline behavior. Overall, these data provide evidence that there are long-term changes in microglial profiles after diffuse brain injury.
Findings of an inflammatory profile of glia that persists after TBI are paralleled in humans after moderate or severe TBIs. For instance, evaluation of microglia at immediate, short and long-term time points following blunt head trauma in humans showed increased expression of CR3 (CD11b) and CD68 in the parenchyma 16 years after injury (Gentleman 2004). In another study, post mortem brain tissue showed extensive, densely packed, reactive microglia (CR3/43+ and/or CD68) in 28% of cases 1–18 years after a single, moderate to severe TBI (Johnson et al., 2013). Recent developments in magnetic resonance imaging (MRI) techniques have been used to examine microglial activation in human brains after TBI in vivo. As described above, PK [11C](R)PK11195 ligand is expressed on activated microglia. Higher PK binding was detected in TBI patients in thalami, occipital lobes, putamen and posterior limb of internal capsule (Ramlackhansingh et al., 2011). Persistent microglial activation was relatively sparse within and around focal lesions, more prominent in adjacent normal appearing gray and white matter, and most prominent within subcortical structures (thalami and putamen). The time frame after injury ranged from 11 months to 17 years, but there was no correlation between PK binding and the time interval since head trauma. Overall, these findings indicate that there are chronic levels of microglia immune activity and priming that persist months to years after TBI in humans.
Microglial Priming with Neurodegenerative Disease
In addition to aging and following TBI, there is also increased priming of microglia in pre-symptomatic neurodegenerative disease. Several neurodegenerative diseases including Alzheimer’s disease (AD) increase in risk as a function of aging and may also be influenced by history of head injury (Nemetz et al., 1999). Therefore, it is not surprising that microglia in the aged brain and in neurodegeneration share similar phenotypes. For example, both prion disease (transmissible spongiform encephalopathies) and AD show marked microglial priming associated with an increased inflammatory status of the CNS. In a mouse model of prion disease, microglia have an activated morphology and increased expression of immune markers including MHC II (Betmouni et al., 1996; Cunningham et al., 2005). In AD, microglia also acquire an activated morphological profile (McGeer et al., 1987). Furthermore, in aging combined with AD, microglia become even more activated. Microglia in aged AD mice were significantly more activated compared to wildtype controls, and these changes in microglia morphology were most evident in regions of high beta-amyloid plaque pathology (Koenigsknecht-Talboo et al., 2008). In AD, microglia have similar or exacerbated increases in inflammatory surface markers (such as MHC II and CD68) as in aging (Cameron and Landreth, 2010). Importantly, these changes in microglial activation within the hippocampus of AD patients correlated to a precipitous loss in cognitive function and memory (Cagnin et al., 2001). Immune changes that occur in the brain with AD have been linked to such functional impairments.
The most prominent pathology during AD is impaired clearance of neurotoxic AB plaques, which leads to impaired neuronal signaling and ultimately cognitive decline. The contribution of neuroinflammation to impaired clearance of AB has been extensively studied, unfortunately no conclusive results have been reported. It is well understood, however, that microglial clearance of AB plaques is impaired in AD (Floden and Combs, 2011). Recent genomic studies indicate that several AD risk genes such as TREM2 and CD33 are implicated in microglial phagocytosis (Griciuc et al., 2013; Jiang et al., 2013; Jonsson et al., 2013), supporting the idea that microglia in the aged or diseased brain have impaired phagocytosis functions. TREM2 was recently identified as a gene that is significantly down-regulated in microglia from aged mice (Hickman et al., 2013). This result indicates that loss of TREM2 expression is involved in both normal aging and neurodegenerative aging and may be a potential target of therapeutics for treatment of age-associated CNS complications. Another association between aging and AD is the immune modulator NLP3. As discussed above, recent evidence highlight the detrimental role of NLPR3 in normal, non-pathological aging (Youm et al., 2013). Similarly, in mouse model of AD, the NLPR3 inflammasome also had detrimental effects. NLPR3 was activated in both human and mouse AD brains. Genetic deletion of NLPR3 reduced plaque burden and mice were protected from loss of spatial memory (Heneka et al., 2013). These recent studies indicate that NLPR3 contributes both to again and to pathology in AD. Overall, these studies suggest common pathways for the development of primed microglia in both aging and neurodegeneration.
A Link between Head Trauma and Neurodegenerative Disease: Potential Role of Prolonged Microglia Activation
There is significant interest in the degree to which head injury can precipitate the increased risk for neurodegenerative disease including chronic traumatic encephalopathy (CTE) (McKee et al., 2013). CTE is a progressive degenerative disease of the brain typically found in individuals with a history of brain trauma, especially those with repeated injuries. CTE pathology in the brain is close to that of AD characterized by tau build up (i.e. neurofibrillary tangles), multifocal axonal varicosities and memory deficits. Overall, there is mounting evidence of a connection between head injury and the risk of developing CTE and other neurodegenerative pathologies (Smith et al., 2013). For instance, recent post-mortem analyses of brain tissue of patients surviving more than a year after a single moderate to severe TBI showed that neurofibrillary tangles (NFTs) were present at higher density and wider distribution in TBI cases. This was significantly different in younger cases (<60yo) of TBI versus uninjured controls (Johnson et al., 2012). Additionally, a trend of more extensive and fibrillary amyloid pathology was evident. In a subsequent study using the same tissue, researchers investigated neuroinflammation and associated white matter degeneration. They found evidence of extensive axonal pathology, predominantly found in all acute cases (75%) following TBI, 45% in subacute cases, and 8% in long-term cases (~50% displayed moderate pathology), whereas age-matched controls exhibited only ~20% of extensive or moderate pathology (Johnson et al., 2013). A population study of over 1,200 TBI survivors demonstrated that the time to onset of AD is significantly accelerated compared to age matched controls. In fact, the median time to onset from TBI to development of AD was 10 years (Nemetz et al., 1999). Consistent with this premise, repeated mild head injury in rodents amplified neuroinflammation and accelerated cognitive decline (Mouzon et al., 2014; Weil et al., 2014). Overall it is likely that TBI accelerates onset of age-related cognitive decline and frequency of neurodegenerative pathologies. The role of primed microglia in linking head trauma to neurodegenerative disease is still largely unknown, but is an important area of study.
Primed Microglia and Increased Reactivity to Secondary Challenge
A functional consequence of microglial priming in aging, CNS trauma and neurodegenerative disease is an exaggerated neuroinflammatory response following a peripheral or central immune challenge (Cunningham, 2013; Fenn, 2013; Norden and Godbout, 2013). This idea is illustrated in Fig. 1. This hypothesis of microglia priming and immune reactivity is well supported in research using models of aging. For instance, mixed glial cultures and coronal brain sections derived from the brains of aged mice had an exaggerated response to LPS stimulation and produced more IL-1β and IL-6 compared to cultures established from adults (Xie et al., 2003; Ye and Johnson, 2001). In addition, microglia cultured ex vivo from the brains of aged mice had increased IL-6 and TNF-α levels compared to microglia from adults (Njie et al., 2010). In vivo studies demonstrate that stimulation of the peripheral innate immune system by injection of LPS caused a prolonged and exaggerated neuroinflammatory cytokine response (IL-1β and IL-6) in aged BALB/c mice compared to young adults (Godbout et al., 2005b; Wynne et al., 2010). Similarly, peripheral injection of Escherichia coli (E. coli) promoted higher and prolonged levels of IL-1β in the hippocampus of aged rats compared to young adults (Barrientos et al., 2009a; Barrientos et al., 2006).
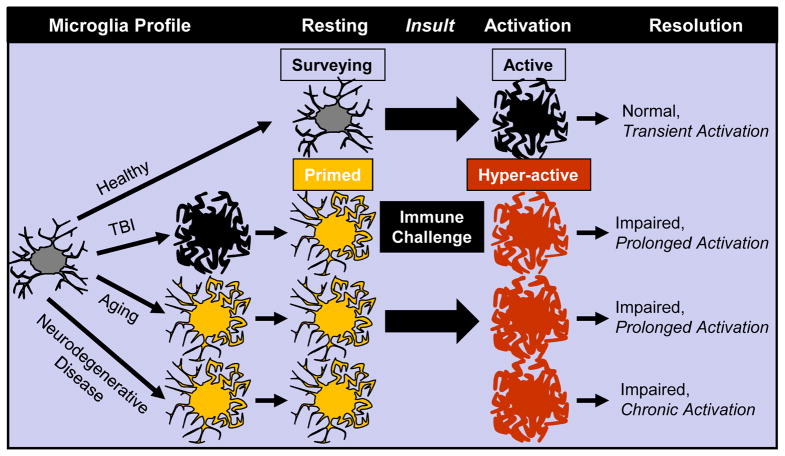
Figure 1. Conceptualization of Microglia Priming and Reactivity to Immune Challenge in Aging, Traumatic CNS Injury and Neurodegenerative Disease.
This diagram illustrates that microglia in the healthy brain are surveying their local microenvironment. Microglia become active with immune challenge and secrete cytokines but return to a surveying state with the resolution of the challenge. In the healthy brain, microglial activation is transient, adaptive and beneficial to the host organism. In aging, after TBI or in neurodegenerative disease, there is evidence that microglia develop an altered morphology and primed or immune-reactive phenotype. Compared to the healthy brain, primed microglia have an intermediate morphological profile with increased expression of several inflammatory receptors and mediators. In the rodent models where this priming profile of microglia is detected, immune challenge promotes amplified and prolonged inflammatory responses that are maladaptive. In models of neurodegenerative disease, there is evidence that these events promote chronic and unresolved inflammation.
It is relevant to note that while a peripheral injection of LPS or E. coli induced higher levels of IL-1β in the aged brain compared to adult, plasma levels of IL-1β were not amplified (2009a; Barrientos et al., 2006; Godbout et al., 2005b). The disconnection between peripheral IL-1β and brain IL-1β induction has been interpreted to suggest that an amplification of the immune response occurs within the brain. Indeed, central activation of the innate immune system by intracerebroventricular (i.c.v.) administration of LPS or GP120, a viral envelope glycoprotein, caused amplified mRNA expression of inflammatory cytokines, IL-1β, IL-6 and TNF-α in aged (22–24 mo) mice (Abraham et al., 2008; Huang et al., 2008). These studies provide evidence that increased pro-inflammatory cytokine production in the aged brain is related to an amplified response from the resident glia population.
This exaggerated cytokine production is attributed to the microglia. The priming effect in microglia of the CNS was first demonstrated in a mouse model of prion disease (Cunningham et al., 2005). In this study, microglia from pre-symptomatic prion disease mice had exaggerated IL-1β production compared to non-prion mice following both central and systemic LPS challenges. This study provided direct evidence of heightened inflammatory response to secondary inflammatory challenge. There are several studies showing exaggerated activation of primed microglia in models of aging. For instance, ex vivo stimulation of microglia from aged mice with LPS (Frank et al., 2010) or Pam3CSK4, a toll-like receptor-2 (TLR2) agonist (Njie et al., 2010), resulted in exaggerated production of IL-1β, IL-6 and TNF-α compared to adults. When microglia were isolated from whole brain homogenates after a peripheral LPS injection, microglia-specific mRNA levels of TLR2 and IL-1β were increased in aged compared to adult mice (Henry et al., 2009). It is important to note that aged microglial reactivity increased production of both pro-inflammatory cytokines and anti-inflammatory cytokines. For example, aged mice had amplified microglial specific mRNA induction and intracellular protein expression of both IL-1β and IL-10 compared with adult mice following LPS injection (Henry et al., 2009; Sierra et al., 2007). Furthermore, a key point was that the reactive (MHC II+) microglia of aged mice had the most prominent IL-1β induction following immune challenge compared to non-reactive (MHC II−) microglia (Henry et al., 2009). These studies indicate that after a peripheral immune stimulus primed, MHC II+ microglia had an exaggerated and prolonged production of inflammatory cytokines.
Reactivity of microglia to a secondary immune challenge or insult is mirrored in models of TBI and pre-symptomatic neurodegenerative disease. In fact, the priming effect in microglia of the CNS was first demonstrated in a mouse model of prion disease (Cunningham et al., 2005). In addition, there is significant evidence that TBI increases the pro-inflammatory profile of microglia, and these alterations are long-lasting (Fenn, 2013; Johnson et al., 2013; Loane et al., 2014). This low level inflammation is similar to what is detected in the aged brain and is consistent with the concept of a primed or sensitized microglial phenotype. For example, in an optic nerve crush model of CNS injury, there is evidence of microglial priming with increased expression of CD68+ resident microglia that persisted 28 days after injury. When these mice were challenged peripherally with LPS 28 days after injury, there was exaggerated IL-1β, TNF-α and IL-6 expression in the brain compared to the controls (Palin et al., 2008). The authors interpreted these data to indicate that the more inflammatory profile was associated with increased phagocytosis and axonal degeneration. In another recent example of priming, moderate and diffuse mFPI caused transient inflammation with increased cytokine expression, edema and evidence of macrophage trafficking and microglia activation (Fenn, 2013). The acute neuroinflammation that occurred on a cellular level was resolved within 72 hours coinciding with return of baseline coordination and behavior. In the post-acute phase, microglia retained or developed a primed profile with increased MHC II mRNA and protein that was detected 1 month after injury. Furthermore, a peripheral LPS challenge in sham and TBI mice 1 month after injury induced exaggerated expression of IL-1β and TNF-α in microglia from the TBI mice. Thus, CNS injury is a “priming event” sufficient to induce persistent microglial activation and transient activation of the immune system to cause an amplified glia response.
TBI may also be classified as a secondary insult that causes microglial reactivity. For instance, aged rodents and older individuals have exacerbated inflammation, increased neurological damage and poorer recovery following TBI, stroke, spinal cord injury and other neurological injuries (Kumar et al., 2013; Pelinka et al., 2004). For example, TBI induced by CCI in aged mice caused greater neuronal damage and tissue loss than adult mice (Kumar et al., 2013). This was associated a higher inflammatory or M1 profile (and reduced M2a repair prolife) in the brains of aged mice (Kumar et al., 2013). Another study showed that markers of microglia (CD11b and Iba1) and astrocyte activation (GFAP and S100b mRNA) were higher in aged mice for all time points up to 28 days after CCI (Sandhir et al., 2008). As expected, the presence of neuroinflammation persisted longer in the brain of aged mice compared to the adults.
Repeated exposure to head injury may also sensitize or prime the glia of the CNS. In this manner, the initial hit provides the priming event and the secondary and tertiary hits result in an exaggerated inflammatory response and progressive pathology (Aungst et al., 2014; Mouzon et al., 2014; Weil et al., 2014). This idea has not been investigated specifically, however, it is clear across the literature that even a single TBI has long-lasting glial affects and repeated TBI exacerbates the neuroinflammatory response. For example, Mouzon et al. (2014) showed morphologically reactive astrocytes and microglia (thick processes and hypertrophied cell soma) were evident in cortices and hippocampal regions 6 months after single and repeated closed head TBI (5 hits with 48h interval). This increased to a greater extent by 12 months after repeated TBI. The pathology at 18 months following single and repeated TBI is still undergoing analysis. The time frame allotted in between injuries may also be an important factor. Another study looked at pathological outcomes after a single and repeated closed head TBI (2 hits) with a 3 or 20 day interval (Weil et al., 2014). One month after the last hit, repeated TBI with a shorter interval induced an enhanced neuroinflammatory response, more robust axonal degeneration and poorer performance in spatial learning and memory task. These data provide evidence that pathological changes evolve months following initial injury, and multiple injuries augment this process.
In the context of neurodegenerative disease, the hyper-inflammatory response to secondary challenges is similar to those observed in aging and CNS trauma. In support of this idea, challenges that activate the immune response worsen cognitive impairments and disease progression in murine models of prion disease and AD. For example, both central and peripheral immune challenge exacerbated brain inflammation (IL-1β and iNOS) and neuronal death in a model of prion disease (Cunningham et al., 2009; Cunningham et al., 2005; Murray et al., 2012). In addition, systemic immune challenge caused a significant increase in CNS IL-1β in transgenic AD mice compared to non-transgenic controls (Sly et al., 2001) and these increases in immune activity contributed significantly to pathology in AD mice (Krstic et al., 2012). Similarly, in a mouse model of Parkinson’s disease, central LPS challenge exacerbated neurodegeneration and increased microglial IL-1β production (Pott Godoy et al., 2008). Importantly, systemic expression of IL-1β also increased neurodegeneration and microglial activation. Similar to AD, iNOS expression and nitric oxide release was indicated as a mediator for neuronal death (Pott Godoy et al., 2008)
Because early life infection can alter microglial phenotypes, it is possible that early exposure to inflammation is a priming event for microglia (Bilbo, 2010) that sets the stage for predisposition to neurodegenerative disease (Krstic et al., 2012). For instance, a recent study showed that systemic inflammatory challenge provided first in utero and then provided later in adulthood predisposed wild-type mice to develop AD-like pathology and cognitive impairments later in life. Wild-type mice subjected to two systemic immune challenges had similar phenotypes (elevated IL-1β, increased AB peptides and increased Tau phosphorylation) to transgenic AD mice that received a single systemic immune challenge. These mice also had activated microglia based on CD68 labeling and these changes in pathology were coupled with impairments in working memory in the Y-maze (Krstic et al., 2012). These results were interpreted to indicate that inflammation itself is an initial priming event and that subsequent activation promotes a similar neuropathology profile that is normally associated with AD (Krstic et al., 2012). Furthermore, repeated exposure to inflammation and increased reactivity may not have to be as spread out over a lifetime. For example a recent study showed that repeated injections of LPS in adult mice induced prolonged activation of microglia and increased expression of complement-phagosome pathway genes. This increase in microglial activation was associated with loss of dopaminergic neurons. In complement C3 deficient mice, neurodegeneration was rescued. These data indicate a direct effect of microglial complement expression in loss of dopaminergic neurons with repeated inflammatory exposure (Bodea et al., 2014). Overall, priming of microglia and their hyper-activation under inflammatory conditions contributes to and amplifies the neurodegenerative processes, making microglial priming an important research focus in the field of neurodegeneration (Lucin and Wyss-Coray, 2009; Perry et al., 2007). Last, these data are consistent with the clinical data that peripheral infections exaggerate cognitive decline in AD patients (Perry et al., 2007).
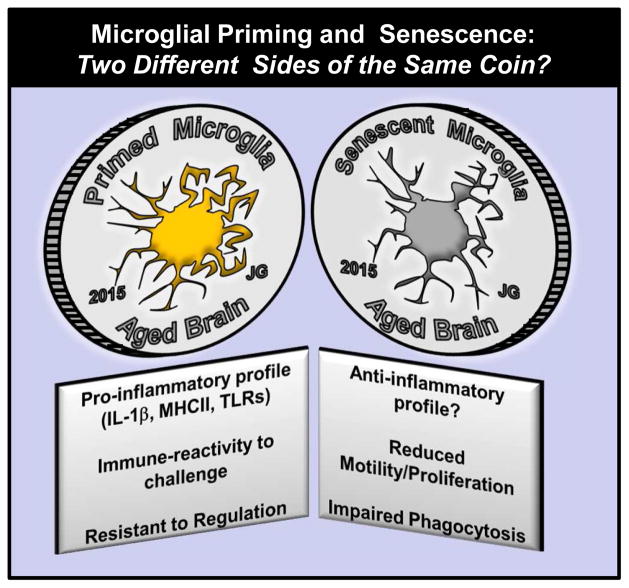
Senescent versus Primed Microglia: Two Sides of the Same Coin?
In this review, long-term changes in microglia profiles are described as primed or pro-inflammatory. This is because there are increased markers of inflammation (IL-1β, MHC II, CD68) on microglia with age, injury and neurodegenerative disease and when these cells are activated by an innate immune challenge (i.e., LPS) they have an amplified activation profile (Cunningham et al., 2009; Fenn, 2013; Henry et al., 2008). Nonetheless, several other studies have described microglia of the aged brain to be anti-inflammatory or neuroprotective, and also as senescent (Streit and Xue, 2009). For example, a recent study using RNA sequencing shows a microglial profile interpreted as an anti-inflammatory or neuroprotective profile (Hickman et al., 2013). The transcriptional profile of microglia from aged mice had decreased expression of proteins involved in sensing endogenous ligands whereas transcripts for sensing immune pathogens were elevated. There was also a significant increase of alternative or neuroprotective pathways including IL-4. In the same mice, however, elevated expression of pro-inflammatory mediators such as IL-1β and TNF-α remained. As discussed above, increased activation of NF-κB, IL-1β, and NLP3 inflammasome pathways is consistent with other studies (Godbout et al., 2005a; Youm et al., 2013) and these data are interpreted to show that aging is associated with a more inflammatory profile. Therefore, it is difficult to categorize the activation of glia based on RNA analysis alone.
Consistent with a senescence profile of microglia, microglia from older rodents have functional impairments in proliferation, motility and phagocytosis (Hefendehl et al., 2014). For example, there was reduced phagocytosis of beta-amyloid by microglia from older AD transgenic mice (Hickman et al., 2008; Lee et al., 2010). Recent genomic studies indicate that several AD risk genes such as TREM2 and CD33 are implicated in microglial phagocytosis (Griciuc et al., 2013; Jiang et al., 2013; Jonsson et al., 2013), supporting the idea that microglia in the aged brain have impaired phagocytosis functions. In addition, microglia from aged rats showed delayed recruitment of phagocytic cells and less clearance of myelin after a toxin-induced demyelination lesion (Zhao et al., 2006). In a focal laser injury model, microglia from aged mice migrated at a slower velocity towards the site of injury and also aggregated at the injury site for a longer duration than that of adult mice (Damani et al., 2011). Microglia may also appear ‘senescent’ in the context of anti-inflammatory feedback. For instance, microglia from aged mice are less sensitive to specific regulatory feedback from anti-inflammatory mediators including IL-4 and transforming growth factor beta (TGFβ). Active microglia from aged mice failed to up-regulate the expression IL-4Ra after LPS injection or spinal cord injury (SCI). In both cases this was associated with reduced sensitivity to IL-4 dependent programming towards an M2a or repair profile of microglia in vivo (Fenn et al., 2014) and ex vivo (Fenn et al., 2012). In addition, microglia isolated from aged mice had reduced expression of TGFβ receptor compared to adults (Hickman et al., 2013) and aged microglial cultures were less sensitive to the anti-inflammatory effects of TGFβ (Rozovsky et al., 1998). Finally, when microglia were isolated from LPS-injected adult and aged mice, only microglia from adult mice were responsive to TGFβ treatment ex vivo (Tichauer et al., 2013). Although functional impairments may be considered indicators of microglial senescence, these same cells may be highly inflammatory when activated by immune challenge or injury. Therefore, the terminology of microglial priming or microglial senescence both reflect age-related differences in microglia function, but are related to the context in which they are examined (Fig. 2).
Figure 2. Microglial Priming and Senescence in the Aged Brain: Two Different Sides of the Same Coin?
This diagram illustrates that microglia in the aged brain have been described as either primed (left side) or senescent (right side). While the two are often considered to be contrasting issues, it is more likely that they are related issues and that it depends on the context in which the function of aged microglia is being evaluated. On one hand, aged microglia have a more inflammatory profile and this is exaggerated after a transient immune challenge (peripheral or central). On the other hand, aged microglia have a reduction in proliferation, motility and ability to clear debris. In both sides of the coin, regulation of these microglia is impaired.
Behavioral Consequences of Microglia Priming and Immune Reactivity
Sickness and depressive-complications following innate immune challenges
The primed profile of microglia leads to an exaggerated response to a secondary challenge or insult (Fig. 1). Importantly this exaggerated neuroinflammation negatively affects behavior and cognition and increases CNS pathology (Fig. 3). One potential consequence of this is the induction of prolonged sickness and depressive-like behaviors. While the induction of cytokine-mediated sickness behavior is a necessary and beneficial response to systemic infection, an amplified or prolonged response affects behavioral and cognitive processes (Jurgens and Johnson, 2010). In the studies discussed above, peripheral or central immune stimulation with LPS caused protracted neuroinflammation in the brain of aged rodents. This was paralleled by a prolonged sickness response with protracted anorexia, lethargy and social withdrawal (Abraham et al., 2008; Godbout et al., 2005b; Huang et al., 2008). An amplified sickness response was also detected in older rats that were infected subcutaneously with E. coli. The aged rats displayed an altered febrile response including a blunted and delayed increase of core body temperature followed by a significant and prolonged increase of inflammatory cytokines (Barrientos et al., 2009b). Similar to the extended sickness behaviors in aged BALB/c mice, the increase of inflammatory cytokines were likely driven by exaggerated microglial IL-1β (Henry et al., 2009). In support of this notion, i.c.v. infusion of IL-1 receptor antagonist (IL-1RA) reversed the prolonged LPS-induced sickness behavior in aged mice (Abraham and Johnson, 2008). These findings indicate that the exaggerated sickness response in aged rodents was likely caused by the exaggerated and prolonged production of IL-1β by primed microglia.
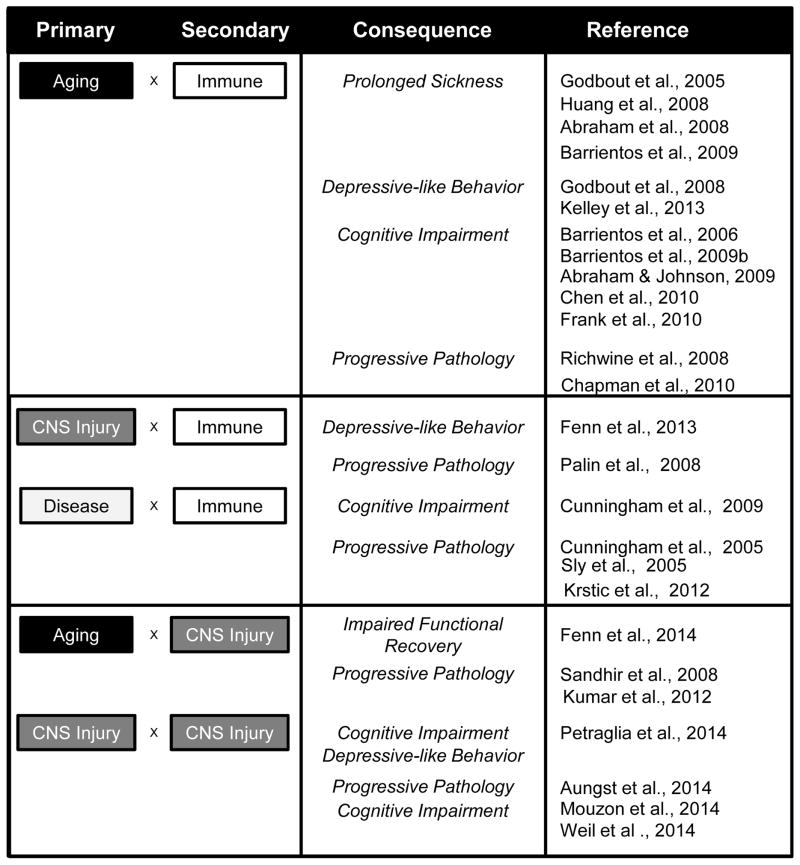
Figure 3. Behavioral and Cognitive Consequences of Exaggerated Response to Secondary Insult.
This diagram represents the interactions between the primary insult aging, CNS trauma or disease and the secondary insult (transient immune challenge or traumatic CNS injury). There is clear interaction between these events on the level of neuroinflammation. In each case, the cited references provide evidence for amplified and prolonged neuroinflammation mediated by microglia that is associated with cognitive impairment, behavioral deficits and progressive pathology.
Related to prolonged sickness behavior, there is also evidence of development of depressive-like complications in aged rodents following an immune challenge. Depressive-like complications in rodent models are reflected by increased resignation behavior (i.e. immobility) in the forced swim test, or tail suspension test. These behavioral assays are intended to model the aspect of despair displayed by depressed human patients (Cryan et al., 2005). In aged BALB/c mice, peripheral stimulation of the innate immune system with LPS caused prolonged depressive-like behavior 72 h after injection in aged mice after acute (Godbout et al., 2008) and chronic (Kelley et al., 2013) immune challenge. In one study aged mice showed increased resignation behavior in the forced-swim and tail-suspension tests even after the acute effects of LPS on lethargy and food intake were resolved. These protracted depressive-like behaviors are likely a direct consequence of the impaired regulatory mechanisms of microglia. As mentioned previously, microglia from aged BALB/c mice show extended down regulation of fractalkine receptor (CX3CR1) on microglia that may be an underlying cause of prolonged microglial activation (Corona et al., 2010; Wynne et al., 2010). Consistent with this finding, adult CX3CR1-deficient mice displayed prolonged social withdrawal and depressive-like behaviors 48 and 72 h after an LPS injection. The depressive-like behavior in CX3CR1-deficient mice was paralleled by a prolonged activated morphology of microglia in the absence of apparent neuronal death (Corona et al., 2010).
TBI elicits immediate neuroinflammatory events that cause acute cognitive, motor and behavioral disturbances (Lifshitz et al., 2007b; McCrea et al., 2003; Tang et al., 1997; Woodcock and Morganti-Kossmann, 2013). Despite resolution of these acute complications, depression can develop and persist years after TBI (Fleminger, 2008; Jorge et al., 1993; Kreutzer et al., 2001). Indeed, individuals who suffer a TBI are 5–10 times more likely to develop symptoms of depression compared to the general population (Gualtieri and Cox, 1991). Depressive symptoms are diagnosed in 30–40% of individuals within the first year of TBI (Jorge et al., 1993; Jorge et al., 2004), in 60% of individuals within 8 years of TBI (Hibbard et al., 1998) and 50 years after TBI patients continue to report higher rates of depression (Holsinger et al., 2002). Therefore, these individuals are at risk for a lifetime of depressive complications that negatively affect quality of life and life-span. In rodents, multiple closed head injuries provided over time are associated with the development of depressive like behavior (Petraglia et al., 2014). In addition, it is plausible that the second insult does not have to be an additional head injury. In fact, a recent study showed that transient activation of the immune system 30 days after diffuse TBI in mice caused amplified and prolonged inflammatory cytokine response from microglia in TBI mice compared to shams (Fenn, 2013). This was associated with prolonged social withdrawal and the development of depressive-like behavior with increased resignation like behavior and reduced sucrose preference. Thus, a challenge to the immune system that is unrelated to the initial head injury is sufficient to promote the onset of depressive complications. These findings paralleled the consequences of secondary immune challenge in aged mice with a primed microglia profile (Godbout et al., 2008). These data can be interpreted to indicate that the depression associated with head injury is influenced by the inflammatory status of the brain.
Cognitive complications following innate immune challenges
As mentioned, increased cytokine expression is important in the behavioral response to inflammation in order to mount an adaptive sickness response. These transient increases in neuroinflammation also effect cognitive performance even in healthy rodents. For example, IL-1β administered into the hippocampus caused hippocampal-dependent memory deficits (contextual fear conditioning) but not deficits to hippocampal-independent memory tasks (auditory fear conditioning) (Rachal Pugh et al., 2001). Furthermore, a recent study in rats assessed neural circuit activity in the hippocampus in relation to context discrimination memory retrieval after systemic administration of LPS (Czerniawski and Guzowski, 2014). They showed that along with eliciting an acute neuroinflammatory response in the hippocampus, LPS abolished retrieval of discrimination memory by disrupting cellular pattern separation processes within the hippocampus. Together these studies show that acute and transient increases in neuroinflammation can lead to cognitive impairments in healthy mice. Similar immune challenges, however, lead to amplified and exaggerated cognitive impairments in conditions where microglia are primed.
There are multiple studies indicating that increased cytokine production in the aged brain after peripheral innate immune challenge is associated with impaired cognitive function. For example, injection of LPS caused an amplified cytokine response in the hippocampus of older mice that was paralleled by impaired hippocampal-dependent spatial memory (Chen et al., 2008). Moreover, infection by E. coli led to prolonged production of IL-1β in the hippocampus of aged rats (Barrientos et al., 2009a) and reduced long-term contextual memory examined by context-dependent fear conditioning and Morris water maze (Barrientos et al., 2009a; Barrientos et al., 2006). When aged mice were fed a diet supplemented with resveratrol, a potent anti-oxidant, LPS-induced neuroinflammation and working memory deficits were attenuated (Abraham and Johnson, 2009). In the absence of an immune stimulus there is not a significant effect of age on the acquisition of memory tasks. There is, however, evidence of age-associated memory problems in the reversal task of the Morris water maze (Jang et al., 2010). Nonetheless, age-related cognitive impairment is exaggerated when a secondary immune challenge is provided.
This exaggeration or acceleration of cognitive decline after immune challenge has also been reported in models of preclinical prion disease. In these mice with prion disease, prior to the onset of pathology, both central and peripheral immune challenges exacerbated brain inflammation and neuronal death (Cunningham et al., 2005). Furthermore, in prion diseased mice, an LPS injection caused acute cognitive deficits in hippocampal-dependent learning task (Cunningham et al., 2009). Furthermore, even after recovery from the transient immune challenge, these mice showed accelerated onset of progressive and permanent loss of neurological function. Also in pre-symptomatic prion disease, systemic immune challenge induced acute working memory deficits and increased CNS inflammation (Murray et al., 2012). These studies give a clear indication that there cognitive deficits can be triggered by activation of primed microglia in the degenerating brain.
Cognitive issues also develop after TBI and may be exaggerated by secondary insults. These may also represent a progression in CNS pathology associated with CTE. In a neuropsychological evaluation, human TBI subjects had significantly lower performance on tests for processing speed than uninjured controls (Ramlackhansingh et al., 2011). However, no differences were detected between groups in tests for executive function (inhibition, cognitive flexibility, word generation fluency, set-shifting), memory (working, logical, associative) and other measures of intellect (reading, nonverbal). In this study, higher PK binding of activated microglia inversely correlated with speed of processing complex information (measured by time to complete the conflicting task, stoop inhibition). While this method has not yet been applied to cases of multiple head trauma, meta-analyses of CTE records in the literature (verified by postmortem pathology) confirms that chronic neuropathology is correlated with the progressive onset of a memory impairments and emotional instability (McKee et al., 2009; Stern et al., 2011). This is important because currently post-mortem examination is the only method by which CTE is diagnosed (Stern et al., 2011). As a result, biomarker studies in humans are highly relevant to improve and validate clinical evaluation of CTE (Jeter et al., 2012; Pelinka et al., 2004). Similarly, rodent models of multiple head injury have been used and report similar pathological and behavioral characteristics of CTE (Mouzon et al., 2014; Petraglia et al., 2014; Weil et al., 2014). For example, mice perform worse than sham in learning and memory tasks at 6, 12 and 18 months after a single injury, though the more pronounced deficits are apparent earlier and persist after repeated TBI (Mouzon et al., 2014). These studies confirm that multiple mild head injuries augment CNS pathologies compared to a single TBI. Moreover, the enhanced inflammation after multiple hits in rodents is associated with accelerated cognitive decline. It is important to note that the idea of acute cognitive deficits from post-inflammatory insult and long-term cognitive decline occurring after such insults are separate issues. These kinds of studies are of high interest and future investigations should address if a single inflammatory insult will lead to cognitive decline even after the initial acute phase following the challenge. Such studies would give insight into the degree to which a secondary insult to primed microglia cause a precipitous decline in cognitive function.
Age-related alterations of neuroplasticity following innate immune challenges
The mechanisms by which depressive-like and cognitive complications develop and persist in these models is unclear. A potential explanation is that neuroinflammatory pathways can impact neuronal plasticity (e.g, neurogenesis, long-term potentiation and dendritic restructuring). For example, when neuroinflammation was prolonged in aged mice, increased dendritic atrophy was detected in the CA1 region of the hippocampus (Richwine et al., 2008). In addition, neurogenesis steadily decreases throughout life in mouse models of aging (Ben Abdallah et al., 2010) and may be further disrupted by inflammation (Ekdahl et al., 2003; Monje et al., 2003). It is expected that age-related decreases in neurogenesis would be exaggerated during an inflammatory challenge, but to our knowledge, this has not been determined. Nevertheless, it is possible that impaired microglial regulatory processes in the aged brain would negatively impact neurogenesis. For example, Bachstetter et al. (2011) have shown that CX3CR1-deficient mice show profound deficits in neurogenesis. Furthermore, infusion of recombinant CX3CL1 into the brain of the aged rats reversed this decrease (Bachstetter et al., 2011). Therefore, it is plausible that a prolonged impairment of CX3CR1 on the microglia of aged mice after an LPS injection (Corona et al., 2010) may cause impaired neuroplasticity, leading to depressive-like and cognitive complications.
Increased pro-inflammatory cytokines and other neuroinflammatory pathways also suppress long-term potentiation (LTP) (Griffin et al., 2006; Kelly et al., 2001; Murray and Lynch, 1998; Vereker et al., 2000). LTP is a key mechanism involved in memory formation and can have different manifestations, including early and late-phase LTP. A recent study examined different types of LTP in hippocampal slices prepared from young or aged rats after recovery from E. coli infection or no infection. Early-phase LTP was not different with age, but late-phase LTP was significantly suppressed in aged rats 4 days after E. coli infection in hippocampal area CA1 (Chapman et al., 2010). These electrophysiological data correspond with observed deficits in long-term memory with age. Suppression of LTP is likely caused by enhanced IL-1β expression as i.c.v. administration of IL-1RA reversed the E. coli induced suppression of late-phase LTP in aged rats (Chapman et al., 2010). Furthermore, IL-1RA also prevented E. coli- induced suppression of Arc expression, an immediate early gene that is essential for LTP, and long-term memory consolidation in contextual fear conditioning in aged rats (Frank et al., 2010). Taken together, these data show that impaired regulation of the neuroimmune response results in inflammatory cytokine-mediated suppression of neuronal plasticity to cause cognitive deficits.
Evidence of Astrocyte Changes with Age, Injury and Disease
This review focuses on evidence of priming and reactivity of microglia. It is, however, important to discuss that astrocytes have increased inflammatory profiles with age, trauma and diseases associated with increased expression of glial fibrillary acidic protein (GFAP) and vimentin. Up-regulation of GFAP and vimentin filaments is associated with numerous functions, including increased astrocyte motility and vesicle trafficking (Lepekhin et al., 2001). In aging, there are several reports showing that astrocyte inflammatory markers GFAP and vimentin are increased in the brains of aged rodents and humans (Cotrina and Nedergaard, 2002; Finch, 2003; Godbout et al., 2005b; Nichols et al., 1993; Porchet et al., 2003; Unger, 1998; VanGuilder et al., 2011). Similar to microglia, astrocytes have an increased hypertrophic morphology with a shift from resting/stellate to active in the brains of aged rats (VanGuilder et al., 2011).
In addition to aging, there are robust changes in astrocytes after TBI and spinal cord injury. Research in spinal cord injury has extensively characterized the glial scar that forms as rapidly proliferating astrocytes accumulate around the damaged tissue. In this way, astrocytes serve as a mechanical and chemical barrier, walling off areas of damage to protect surrounding tissue (White and Jakeman, 2008). Therefore, it is not surprising that focal models of TBI, with overt tissue cavitation, cause a glial scar around the site of impact (Kabadi et al., 2014). However, in diffuse models of TBI, as well as regions non-adjacent to site of impact in focal trauma, there is long-lasting increase in reactive astrocytes populations (Browne et al., 2006; Fenn, 2013). In more critical cases of human TBI, increased serum levels of GFAP is an accurate predictor of TBI severity and prognosis (Pelinka et al., 2004). Similar to aging and trauma, activated astrocytes in AD have increased GFAP and vimentin expression. Recently, these reactive astrocytes were implicated in improved clearance of AB plaques in a mouse model of AD (Kraft et al., 2013).
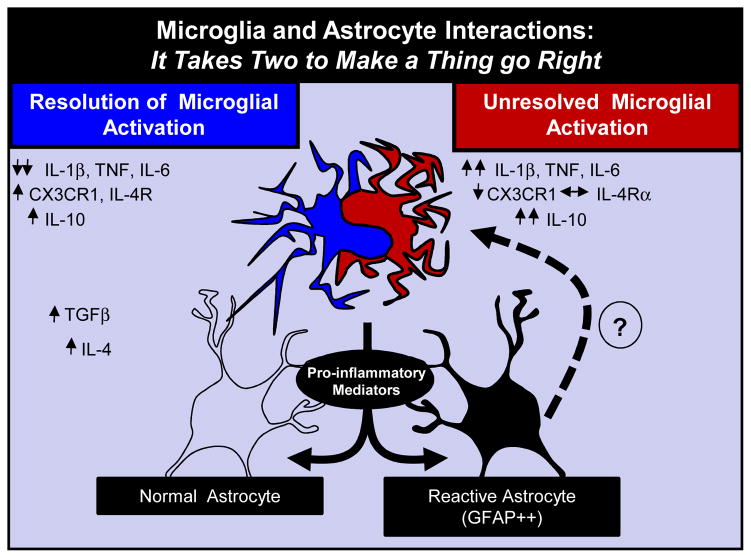
More recent findings also reveal that astrocytes provide several important immunological functions (Kimelberg, 2010; Kimelberg and Nedergaard, 2010). For example, astrocytes express several immune receptors including scavenging receptors, toll-like receptors and complement receptors and produce inflammatory cytokines when activated. Under homeostatic conditions, astrocytes maintain an anti-inflammatory environment by producing mediators that regulate inflammation. Upon immune challenge, disease, or injury, astrocytes can be activated directly by cytokines produced by microglia or cytokines reaching the brain from the periphery. Activated astrocytes are also involved in propagating cytokine production and producing factors that resolve microglial activation (Tichauer et al., 2007). For example, astrocytes produce TGFβ, which down-regulates inflammatory cytokine expression in microglia (Norden et al., 2014; Ramirez et al., 2005) (Fig. 4). While it is known that astrocytes have a more ‘reactive’ profile with higher GFAP expression in aging trauma and neurodegenerative disease, the consequence of this reactive profile is less clear. We propose that this these altered profiles of astrocytes effect the dynamic interactions between microglia and influences the ability to regulate microglia activation (Fig. 4). These features of astrocytes should open up new areas of research aimed at understanding the specific role of astrocytes and their dynamic interactions with microglia.
Figure 4. Primed Glia and Impaired Regulation of Active Microglia by Reactive Astrocytes.
Astrocytes also have a more ‘reactive’ profile with higher GFAP expression after TBI or in neurodegenerative disease. The long-term consequence of this reactive astrocyte profile in the brain is not well understood. One idea that this these altered profiles of astrocytes affects the dynamic interaction with active microglia. In this scenario, astrocytes help to regulate microglia activation. Thus it takes the appropriate interactions between these two glia cells types to make things go right.
Conclusion
In conclusion, there are inflammatory alterations in microglia biology with aging, traumatic CNS injury and neurodegenerative diseases. These changes represent a profile of microglia that are more reactive to secondary insults. This shift towards priming is associated with a prolonged and amplified response to an immune challenge. In each of the models examined in the review, this prolonged neuroinflammation mediated by microglia is associated with cognitive and behavioral deficits and progressive pathologies. What causes these impairments and gives rise to a primed microglial population are not well understood. Head injury may provide the most direct evidence of a primed and pro-inflammatory profile that persist long after the injury. This is particularly relevant because of an increased frequency of neuropsychiatric disorders and increased risk for the development of neurodegenerative disease after TBI. Two key questions that remain elusive are, to what degree can theses long term changes in microglia profiles be prevented and can priming be reversed once it has been established? Further characterization of microglia, astrocyte and neuronal interactions is needed in all of these examples discussed. It is important to highlight that these concepts of microglia priming and reactivity are also relevant to psychological stressors (Wohleb et al., 2011; Wohleb et al., 2014) and early life insults (Bilbo, 2010). Overall, a better understanding of the pathways that cause long term changes in glia profiles is needed to improve therapies and interventions for neuropsychiatric disorders.
Highlights.
Discuss basics of glial biology and heightened inflammatory state of microglia
“Microglial priming” in aging, traumatic brain injury and neurodegenerative disease
A consequence of priming is increased immune-reactivity to secondary insult
Relevance of amplified response to secondary insult within these models
Evidence of microglial priming as a trigger for neuropsychiatric problems
Acknowledgments
This work is supported by NIH grants R01-AG-033028 to JPG.
Footnotes
Competing interests
The authors of this manuscript declare that there are no actual or potential conflicts of interest. The authors affirm that there are no financial, personal or other relationships with other people or organizations that have inappropriately influenced or biased their research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiology of aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain, behavior, and immunity. 2008 doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1223–1232. doi: 10.1038/jcbfm.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiology of aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain, behavior, and immunity. 2009a;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of the sickness response in young and aging rats following E. coli infection. Brain, behavior, and immunity. 2009b;23:450–454. doi: 10.1016/j.bbi.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiology of aging. 2010;31:151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Betmouni S, Perry VH, Gordon JL. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74:1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiology of learning and memory. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodea LG, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, Kaoma T, Muller A, Vallar L, Di Monte DA, et al. Neurodegeneration by activation of the microglial complement-phagosome pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:8546–8556. doi: 10.1523/JNEUROSCI.5002-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne KD, Iwata A, Putt ME, Smith DH. Chronic ibuprofen administration worsens cognitive outcome following traumatic brain injury in rats. Experimental neurology. 2006;201:301–307. doi: 10.1016/j.expneurol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. The Lancet. 2001;358:461. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiology of disease. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain, behavior, and immunity. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Hu W, Park HJ, Gale JT, Kidd GJ, Bernatowicz R, Gossman ZC, Chen JT, Dutta R, Trapp BD. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nature communications. 2014;5:4486. doi: 10.1038/ncomms5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, Lee HS, Lee IS. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J Vet Med Sci. 2007;69:1131–1136. doi: 10.1292/jvms.69.1131. [DOI] [PubMed] [Google Scholar]
- Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7:7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. Journal of neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Astrocytes in the aging brain. Journal of neuroscience research. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Guzowski JF. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:12470–12480. doi: 10.1523/JNEUROSCI.0542-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Fifshitz J, Godbout JP. Immune activation promotes depression one month after diffuse brain injury: a role for primed microglia. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 Signaling Drives a Unique Arginase+/IL-1beta+ Microglia Phenotype and Recruits Macrophages to the Inflammatory CNS: Consequences of Age-Related Deficits in IL-4Ralpha after Traumatic Spinal Cord Injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, behavior, and immunity. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Smith KM, Lovett-Racke AE, Guerau-de-Arellano M, Whitacre CC, Godbout JP. Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiology of aging. 2013;34:2748–2758. doi: 10.1016/j.neurobiolaging.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiology of aging. 2003;24(Suppl 1):S123–127. doi: 10.1016/s0197-4580(03)00051-4. discussion S131. [DOI] [PubMed] [Google Scholar]
- Fleminger S. Long-term psychiatric disorders after traumatic brain injury. Eur J Anesth. 2008;25:123–130. doi: 10.1017/S0265021507003250. [DOI] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-beta fibrils. Journal of Alzheimer’s disease : JAD. 2011;25:279–293. doi: 10.3233/JAD-2011-101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiology of aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain, behavior, and immunity. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. Journal of neuroimmunology. 2005a;169:97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005b;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, JOC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. Journal of neurochemistry. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Gualtieri T, Cox DR. The delayed neurobehavioural sequelae of traumatic brain injury. Brain Injury. 1991;5:219–232. doi: 10.3109/02699059109008093. [DOI] [PubMed] [Google Scholar]
- Hefendehl JK, Neher JJ, Suhs RB, Kohsaka S, Skodras A, Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging cell. 2014;13:60–69. doi: 10.1111/acel.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. Journal of neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehab. 1998;13:24–39. doi: 10.1097/00001199-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nature neuroscience. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta neurochirurgica. 1995;132:110–119. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- Holsinger T, Steffens DC, Phillips C, Helms MJ, Havlik RJ, Breitner JC, Guralnik JM, Plassman BL. Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry. 2002;59:17–22. doi: 10.1001/archpsyc.59.1.17. [DOI] [PubMed] [Google Scholar]
- Huang EY, Tsai TH, Kuo TT, Tsai JJ, Tsui PF, Chou YC, Ma HI, Chiang YH, Chen YH. Remote effects on the striatal dopamine system after fluid percussion injury. Behavioural brain research. 2014;267:156–172. doi: 10.1016/j.bbr.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IK, Lee CH, Li H, Yoo KY, Choi JH, Kim DW, Suh HW, Won MH. Comparison of ionized calcium-binding adapter molecule 1 immunoreactivity of the hippocampal dentate gyrus and CA1 region in adult and aged dogs. Neurochem Res. 2008;33:1309–1315. doi: 10.1007/s11064-007-9584-6. [DOI] [PubMed] [Google Scholar]
- Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–1898. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CB, Hergenroeder GW, Ward NH, 3rd, Moore AN, Dash PK. Human traumatic brain injury alters circulating L-arginine and its metabolite levels: possible link to cerebral blood flow, extracellular matrix remodeling, and energy status. Journal of neurotrauma. 2012;29:119–127. doi: 10.1089/neu.2011.2029. [DOI] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Tan L. TREM2 in Alzheimer’s disease. Molecular neurobiology. 2013;48:180–185. doi: 10.1007/s12035-013-8424-8. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain : a journal of neurology. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain pathology. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. The New England journal of medicine. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge R, Robinson R, Arndt S, Starkstein S, Forrester A, Geisler F. Depression following traumatic brain injury: a 1 year longitudinal study. J Affect Disorders. 1993;27:233. doi: 10.1016/0165-0327(93)90047-n. [DOI] [PubMed] [Google Scholar]
- Jorge R, Robinson R, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental neurology. 2010 doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabadi SV, Stoica BA, Loane DJ, Luo T, Faden AI. CR8, a novel inhibitor of CDK, limits microglial activation, astrocytosis, neuronal loss, and neurologic dysfunction after experimental traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:502–513. doi: 10.1038/jcbfm.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, O’Connor JC, Lawson MA, Dantzer R, Rodriguez-Zas SL, McCusker RH. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guerin. Brain Behav Immun. 2013;32:63–69. doi: 10.1016/j.bbi.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, O’Neill LA, Lynch MA. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. The Journal of biological chemistry. 2001;276:45564–45572. doi: 10.1074/jbc.M108757200. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Functions of mature mammalian astrocytes: a current view. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2010;16:79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer, Jeffrey S, Seel, Ronald T, Gourley, Eugene The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Injury. 2001;15:563–576. doi: 10.1080/02699050010009108. [DOI] [PubMed] [Google Scholar]
- Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, Manalastas A, Hilfiker M, Pfister S, Schwerdel C, et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. Journal of neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiology of aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. The American journal of pathology. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M. Intermediate filaments regulate astrocyte motility. Journal of neurochemistry. 2001;79:617–625. doi: 10.1046/j.1471-4159.2001.00595.x. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Fluid percussion injury model. In: Chen J, Xu ZC, Xu X-M, Zhang JH, editors. Animal Models of Acute Neurological Injuries. Humana Press; 2009. p. 369. [Google Scholar]
- Lifshitz J, Kelley BJ, Povlishock JT. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J Neuropathol Exp Neurol. 2007a;66:218–229. doi: 10.1097/01.jnen.0000248558.75950.4d. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Witgen B, Grady M. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response. Beh Brain Res. 2007b;177:347. doi: 10.1016/j.bbr.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. Journal of neuropathology and experimental neurology. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiology of aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: The NCAA concussion study. JAMA. 2003;290:2556. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. Journal of neuropathology and experimental neurology. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain : a journal of neurology. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morales D, Marklund N, Lebold D, Thompson H, Pitkanen A, Maxwell W, Longhi L, Laurer H, Maegele M, Neugebauer E, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, Crawford F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Annals of neurology. 2014;75:241–254. doi: 10.1002/ana.24064. [DOI] [PubMed] [Google Scholar]
- Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiology of aging. 2012;33:603–616. e603. doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. The Journal of biological chemistry. 1998;273:12161–12168. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. American journal of epidemiology. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiology of aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and applied neurobiology. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K, Cunningham C, Forse P, Perry VH, Platt N. Systemic inflammation switches the inflammatory cytokine profile in CNS Wallerian degeneration. Neurobiology of Disease. 2008;30:19. doi: 10.1016/j.nbd.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. Journal of neurotrauma. 2004;21:1553–1561. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. Journal of neurotrauma. 2014;31:1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porchet R, Probst A, Bouras C, Draberova E, Draber P, Riederer BM. Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics. 2003;3:1476–1485. doi: 10.1002/pmic.200300456. [DOI] [PubMed] [Google Scholar]
- Pott Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain : a journal of neurology. 2008;131:1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neuroscience and biobehavioral reviews. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Ramirez G, Toro R, Dobeli H, von Bernhardi R. Protection of rat primary hippocampal cultures from A beta cytotoxicity by pro-inflammatory molecules is mediated by astrocytes. Neurobiology of disease. 2005;19:243–254. doi: 10.1016/j.nbd.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Annals of neurology. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annual review of immunology. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Chen J, Johnson RW. Peripheral LPS injection causes apical dendritic atrophy of hippocampal pyrimidal neurons in aged mice 2008 [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiology of aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Salter MW, Beggs S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Experimental neurology. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Current opinion in neurobiology. 2013;23:1034–1040. doi: 10.1016/j.conb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW, Lammertsma AA, et al. Microglial activation in healthy aging. Neurobiology of aging. 2012;33:1067–1072. doi: 10.1016/j.neurobiolaging.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol (Berl) 1998;95:229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Weaver LC, Cain DP, Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. Journal of neuroinflammation. 2013;10:26. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, Chin JE. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res Bull. 2001;56:581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nature reviews. Neurology. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM & R : the journal of injury, function, and rehabilitation. 2011;3:S460–467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75:130–138. doi: 10.1007/s001090050097. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Tang Y, Noda Y, Hasegawa T, Nabeshima T. A concussive-like brain injury model in mice (I): impairment in learning and memory. J Neurotrauma. 1997;14:851–862. doi: 10.1089/neu.1997.14.851. [DOI] [PubMed] [Google Scholar]
- Tichauer J, Saud K, von Bernhardi R. Modulation by astrocytes of microglial cell-mediated neuroinflammation: effect on the activation of microglial signaling pathways. Neuroimmunomodulation. 2007;14:168–174. doi: 10.1159/000110642. [DOI] [PubMed] [Google Scholar]
- Tichauer JE, Flores B, Soler B, Eugenin-von Bernhardi L, Ramirez G, von Bernhardi R. Age-dependent changes on TGFbeta1 Smad3 pathway modify the pattern of microglial cell activation. Brain, behavior, and immunity. 2013 doi: 10.1016/j.bbi.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JW. Glial reaction in aging and Alzheimer’s disease. Microsc Res Tech. 1998;43:24–28. doi: 10.1002/(SICI)1097-0029(19981001)43:1<24::AID-JEMT4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. Journal of neuroinflammation. 2011;8:138. doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. The Journal of biological chemistry. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Gaier KR, Karelina K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiology of disease. 2014;70:108–116. doi: 10.1016/j.nbd.2014.06.016. [DOI] [PubMed] [Google Scholar]
- White RE, Jakeman LB. Don’t fence me in: harnessing the beneficial roles of astrocytes for spinal cord repair. Restorative neurology and neuroscience. 2008;26:197–214. [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:2583–2591. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain, behavior, and immunity. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Experimental neurology. 2003;182:135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nature reviews Neuroscience. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell metabolism. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li WW, Franklin RJ. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiology of aging. 2006;27:1298–1307. doi: 10.1016/j.neurobiolaging.2005.06.008. [DOI] [PubMed] [Google Scholar]