Abstract
Introduction
To compare fracture prevalence in oligo-amenorrheic athletes (AA), eumenorrheic athletes (EA), and non-athletes (NA) and determine relationships with bone density, structure and strength estimates.
Methods
175 females (100 AA, 35 EA, and 40 NA) 14–25 yo were studied. Lifetime fracture history was obtained through participant interviews. Areal BMD was assessed by DXA at the spine, hip and whole body (WB). Bone structure was assessed by HRpQCT at the radius and tibia, and strength by finite element analysis.
Results
AA, EA, and NA did not differ in age, sexual maturity, or height. AA had lower BMI, and older menarchal age than EA and NA (p≤0.001). BMD Z-scores were lower in AA vs. EA at the total hip, femoral neck, spine, and whole body (p≤0.001). Lifetime fracture risk was higher in AA than EA and NA (47%, 25.7%, 12.5%, p≤0.001), largely driven by stress fractures in AA vs. EA and NA (32% vs. 5.9% vs. 0%). In AA, those who fractured had lower lumbar and WB BMD Z-scores, vBMD of outer trabecular region in radius and tibia, and trabecular thickness of the radius (p≤0.05). In AA, those who had 2 stress fractures had lower lumbar and WB BMD Z-scores, total cross-sectional area, trabecular vBMD, stiffness and failure load at radius; and lower stiffness and failure load at tibia versus those with <2 stress fracture (p≤0.05).
Conclusion
Weight-bearing athletic activity increases BMD, but may increase stress fracture risk in those with menstrual dysfunction. Bone microarchitecture and strength differences are more pronounced in AA with multiple stress fractures. This is the first study to examine fractures in relation to bone structure in adolescent female athletes.
Keywords: Female Athlete Triad, Stress Fracture, Amenorrhea, Bone Microarchitecture, Bone Mineral Density
Introduction
Many female athletes are at risk of developing the female athlete triad (Triad), the interrelationship of decreased energy availability, menstrual dysfunction, and poor bone health.(42) Low energy availability has independent negative effects on reproductive function (19) and bone, and low levels of gonadal steroids are also detrimental to bone. (17) Furthermore, low energy availability has negative effects on other metabolic hormones known to influence bone, including IGF-1, leptin and peptide YY. (19) A recent, prospective, multisite study demonstrated a higher incidence of bone stress injuries in athletes with specific Triad risk factors.(4) Of importance, the Triad may be particularly detrimental during adolescence, a time characterized by maximal increases in bone accrual towards attainment of peak bone mass.(26, 50, 52) Few studies have examined determinants of stress and other fractures in adolescent athletes, and particularly in those who are oligoamenorrheic.
Athletes in general are more prone to injuries including fractures. Stress fractures are fatigue fractures of bone caused by repeated submaximal stress and can delay return to sport by weeks to months.(24) These fractures are common in endurance athletes and often involve the foot, tibia, and fibula in long distance runners, track and field athletes, and dancers.(10) Stress fractures are reported in up to 10% of female athletes and 22% of female track and field athletes.(7, 15) Weight-bearing activity stimulates bone modeling and remodeling during childhood and adolescence and increases bone mineral density (BMD), (34, 55) which is also determined by genetics, body habitus, nutritional status, hormonal milieu, medications, and lifestyle choices (28, 45, 47). Although BMD is an important determinant of the ability of bone to withstand loading, (39) it does not always correlate with fracture risk in athletes.(38, 46) Given the debility associated with fractures, it is important to have a better understanding of factors that contribute to the risk for stress and other fractures in athletes.
Dual energy x-ray absorptiometry (DXA) is the clinical gold standard used to measure BMD. However, DXA assesses areal and not volumetric BMD, and thus underestimates BMD in short individuals while overestimating BMD in tall individuals. In addition, it cannot distinguish between cortical and trabecular bone.(36) In contrast, high-resolution peripheral quantitative computed tomography (HR-pQCT) provides measures of volumetric BMD (vBMD) of cortical and trabecular bone, and of bone microarchitecture.(5) We have previously reported characteristic differences in vBMD, bone microarchitecture and strength estimates at the distal radius and tibia (sites of non-weight bearing and weight bearing bone respectively) using HRpQCT and microfinite element analysis (μFEA) in oligoamenorrheic and eumenorrheic weight-bearing endurance athletes and non-athletes.(1, 2) Our data overall suggest that while repetitive weight-bearing activity improves microarchitecture and strength of the tibia in adolescent athletes with a normal hormonal milieu, this effect is lost in those with menstrual dysfunction. Of note, studies thus far have not examined associations of bone structure and strength estimates with fracture history in adolescent amenorrheic athletes.
The purpose of this study was to examine fracture prevalence in adolescent and young adult athletes and non-athletes in relation to menstrual status, and bone density, structure and strength estimates. We hypothesized that in addition to menstrual dysfunction and lower measures of areal BMD (aBMD), impaired microarchitectural parameters (using HRpQCT) and reduced strength estimates (using μFEA) would predict risk for fracture (particularly stress fracture) in adolescent and young adult athletes.
Subjects and Methods
Subjects
We cross-sectionally studied 175 females between the ages of 14 and 25 years: 100 oligoamenorrheic athletes (AA), 35 eumenorrheic athletes (EA), and 40 non-athletes. Enrolled athletes ran at least 20 miles every week or were engaged in weight-bearing aerobic activity for at least 4 hours/week for at least 6 months preceding the study. Cyclists, swimmers, rowers and gymnasts were excluded because of variable weight-bearing potentially confounding BMD and microarchitecture results. Non-athlete participants were not engaged in any organized sports and exercised for less than 2 hours/week. All athletes and non-athletes had a BMI between the 10th and 90th percentiles. We defined oligoamenorrhea (for AAs) as the absence of menses for at least 3 months within a period of oligomenorrhea (cycle length >6 weeks) for at least 6 months preceding enrollment, or absence of menarche at 15 years or older. We defined eumenorrhea (for EA and non-athletes) as at least 9 menses (cycle length 21–35 days) in the preceding year with no oral contraceptive (OCP) use in the preceding 3 months. Subjects were recruited through advertisements in the Partners HealthCare system, medical clinics, local newspapers, and colleges. Exclusion criteria included conditions other than exercise-induced amenorrhea and use of medications other than calcium and vitamin D supplements that may affect bone metabolism, and other causes of amenorrhea such as premature ovarian failure, hyperprolactinemia, thyroid dysfunction, and hyperandrogenism, which were ruled out with a history, physical examination, and screening laboratory tests.
The Institutional Review Board of Partners HealthCare approved the study. Informed consent was obtained from subjects ≥18 years and parents of subjects <18 years old. Informed assent was obtained from subjects <18 years. DXA, HRpQCT, and FEA results from a subset of this population were previously published without reference to fracture histories.(1, 2)
Experimental Protocol
Subjects were studied at the Clinical Research Center of our institution. Anthropometric measurements were obtained on the same electronic scale (to the nearest 0.1 kg) and wall-mounted stadiometer (to the nearest 0.1 cm). A study physician recorded lifetime fracture and menstrual history, as well as details regarding exercise/athletic activity for the preceding 12 months during participant interviews. Tanner staging was determined by a study endocrinologist. Hand radiographs were obtained to determine bone age by the standards of Greulich and Pyle.(27) We used a chemiluminescent immunoassay to measure fasting 25-hydroxyvitamin D [25(OH)D] (sensitivity, 4 ng/ml; intraassay coefficient of variation, 2.9%–5.5%; DiaSorin, Stillwater, Minnesota). Calcium levels were assessed by Labcorp using standard methods. Resting energy expenditure (REE) values were obtained from measures of carbon dioxide production and oxygen consumption during rest using indirect calorimetry.
Bone Density Assessment
DXA (Hologic QDR-Discovery A, Apex software version 13.3; Hologic Inc, Waltham, Massachusetts) was used to assess total hip, femoral neck, spine and whole body BMD and body composition. The coefficients of variation for BMD, fat mass, and lean mass for our institution are 0.8% to 1.1%, 2.1%, and 1.0%, respectively. The same scanner and software version were used for all participants.
Bone Microarchitecture Measurement and Finite Element Analysis
HRpQCT was used to measure volumetric density, morphology, and microarchitecture at the ultradistal radius and tibia (XtremeCT; Scanco Medical AG, Bassersdorf, Switzerland) with an isotropic voxel size of 82 μm3.(8) Measurements were performed at the non-dominant wrist and leg unless there was a history of fracture at those sites, in which case the non-fracture side was measured. Outcome variables computed by automated analysis included area (mm2) and volumetric bone mineral density (vBMD) (mgHA/cm3) for total, trabecular, and cortical regions; cortical thickness (21) and perimeter; and trabecular number (1/mm), thickness (21), and spacing (21). The precision is 0.7–1.5% for densities and 2.5–4.4% for trabecular and cortical microarchitecture.
In addition to the standard evaluation protocol provided by the HRpQCT manufacturer, we also performed detailed cortical bone analysis by a semi-automated segmentation technique as previously described.(2, 11–13, 43). We used the 3D HRpQCT images to perform linear μFEA and calculate apparent biomechanical properties under uniaxial compression, as previously described, specifically stiffness and failure load.(2, 9, 13, 32, 37, 53) Micro FEA-derived estimates of failure load using these methods are strongly correlated (r2 = 0.75) with experimentally measured failure loads that produce Colles’ fractures in human cadaveric radii.(44) We also calculated the proportion of load carried by the cortical and trabecular compartments (%) at the distal and proximal ends of the region of interest. All HR-pQCT data were acquired on a single instrument by one operator, who performed standard evaluations (periosteal contouring). All finite element analyses (endosteal contouring) were also performed by one study investigator blinded to study groups.
Statistical Analysis
We used JMP (version 10; SAS Institute, Inc., Cary, NC) for all analyses and report data as means ±SD. For three-group comparisons, we performed an overall ANOVA for normally distributed data, followed by the Dunnett’s analysis to assess differences between AA vs. EA and AA vs. non-athletes. For two-group comparisons, we used the Student t-test for normally distributed data. For non-normally distributed variables we used the Kruskal-Wallis or Wilcoxon tests. The Fisher’s exact test was used to analyze differences among groups for categorical variables, and the Bonferroni correction was used to adjust for multiple comparisons as and when necessary. Fracture incidence rates were calculated by dividing the number of AA, EA, or non-athlete controls with at least one fracture after age 12.5 years by person-years of observation time; 12.5 years was chosen because it is the average age of menarche in U.S. girls.(3). For stress fractures analysis, as having more than one stress fracture often becomes concerning clinically, raising questions about Triad risk factors(19), we divided the AA group into those who had <2 stress fractures versus those who had had 2 or more stress fractures and compared these subgroups. Multivariate analysis was used to determine whether differences in bone density and structural parameters persisted after controlling for menarchal age.
Results
Clinical Characteristics
Most subjects classified themselves as Caucasian (n= 134), followed by Asian American (n= 18), more than one race (n= 15), African American (n= 6), and Native American (n= 1). The race distribution did not differ across groups. Sixty-three percent of the athletes were runners, 21% participated in weight-bearing team sports (such as basketball, soccer, lacrosse, hockey and tennis), 6% were dancers, and 10% were involved in a variety of weight-bearing activities, including cardio machine training. The distribution of the different varieties of weight-bearing activities did not differ across groups. Age, bone age, Tanner stage, and height did not differ among AA, EA and non-athlete groups. Age of menarche was greater, and BMI, percent ideal body weight, and fat mass lower in AA than the other two groups. Lean mass was lower in AA versus EA, and body fat percentage was lower in AA versus non-athletes. Resting energy expenditure was lower in AA versus EA. Vitamin D levels were higher in AA compared to the other two groups. Twenty-six percent of AA, 5.7% of EA and none of the non-athletes had a history of disordered eating behavior. Average hours of exercise per week, and the percentage of athletes whose main exercise activity was running did not differ between AA and EA (Table 1).
Table 1.
Clinical Characteristics of Amenorrheic Athletes (AA), Eumenorrheic Athletes (EA) and Non-athletes (NA)
| AA (n=100) | EA (n=35) | NA (n=40) | ANOVA | AA vs EA | AA vs NA: P | |
|---|---|---|---|---|---|---|
| P | P | P | ||||
| Age (years) | 19.7±2.5 | 18.9±2.5 | 19.8±2.1 | 0.22 | - | - |
| Bone age (years) | 17.5±1.1 | 17.4±1.1 | 17.6±1.0 | 0.52 | - | - |
| Age of menarche (years) | 13.8±1.9 | 12.5±1.5 | 12.4±1.2 | <0.0001 | 0.0004 | <0.0001 |
| Duration since last menses (months) | 8.9±12.7 | - | - | - | - | - |
| Tanner Stage | 4.7±0.6 | 4.8±0.5 | 4.9±0.4 | 0.17 | - | - |
| Height (cm) | 165.0±6.2 | 164.7±7.2 | 162.3±6.6 | 0.07 | - | - |
| BMI (kg/m2) | 20.4±2.3 | 22.6±2.3 | 22.1±2.3 | <0.0001 | <0.0001 | 0.0003 |
| % Ideal BMI | 95.9±10.3 | 107.5±12.6 | 103.4±11.6 | <0.0001 | <0.0001 | 0.001 |
| Fat mass (kg) | 13.3±4.7 | 15.4±4.0 | 17.0±5.0 | <0.0001 | 0.04 | <0.0001 |
| Percent body fat | 22.9±5.7 | 24.3±4.0 | 28.6±5.8 | <0.0001 | 0.33 | <0.0001 |
| Lean mass (kg) | 41.8±5.2 | 45.3±6.4 | 40.0±4.3 | <0.0001 | 0.002 | 0.13 |
| REE (calories) | 1216±173 | 1363±216 | 1223±189 | 0.0006 | 0.0003 | 0.98 |
| Vitamin D (ng/mL) | 38.4±13.5 | 30.3±13.1 | 25.1±13.2 | <0.0001 | 0.006 | <0.0001 |
| Calcium (mg/dL) | 9.3±0.4 | 9.1±0.7 | 9.1±0.5 | 0.04 | 0.15 | 0.04 |
| Hours/week of exercise | 10.5±5.8 | 10.0±4.2 | 1.7±2.5 | <0.0001 | 0.84 | <0.0001 |
| Type of exercise (%) | ||||||
| Running | 66.0 | 57.1 | 0 | 0.27 | - | - |
| Other | 34.0 | 42.9 | 0 | |||
| History of eating disorders (%) | 26.0 | 5.7 | 0 | 0.0001 | 0.13 | 0.01 |
AA: oligoamenorrheic athletes, EA: eumenorrheic athletes, NA: non-athletes Data presented as means ± standard deviations or as percentage where noted.
ANOVA used for 3 group comparisons followed by Dunnett’s testing when ANOVA was significant with AA as the comparison group. Significant p values are in bold.
REE: resting energy expenditure
Bone Density and HRpQCT findings
Results for DXA and HRpQCT are shown in Table 2. While EA had significantly greater femoral neck, total hip, lumbar spine, and total body BMD Z-scores than AA, AA did not demonstrate a similar benefit from exercise, as they did not significantly differ from non-athletes for BMD at any measured site. Differences in BMD among groups persisted after controlling for menarchal age, a factor known to impact pubertal bone accrual.
Table 2.
Areal Bone Density (DXA), HRpQCT and Finite Element Analyses for the Radius and Tibia in Amenorrheic Athletes (AA), Eumenorrheic Athletes (EA) and Non-athletes (NA)
| AA | EA | NA | ANOVA | AA vs EA | AA vs NA | |
|---|---|---|---|---|---|---|
| p-value | p-value | p-value | ||||
| DXA (Areal BMD Z-scores) | N = 100 | N =35 | N =40 | |||
| Femoral Neck | −0.17±1.06 | 0.38±0.93 | −0.41±0.83 | 0.003a | 0.01a | 0.35 |
| Total Hip | 0.05±1.01 | 0.80±0.87 | −0.06±0.78 | <0.0001a | 0.0001 a | 0.79 |
| Lumbar Spine | −0.77±1.21 | 0.00±0.88 | −0.40±0.93 | 0.002 a | 0.001 a | 0.15 |
| Whole Body | −0.64±1.02 | 0.19±1.05 | −0.71±0.96 | 0.0001 a | 0.0001 a | 0.92 |
|
| ||||||
| HRpQCT and FEA: Radius | N=87 | N=34 | N=38 | |||
| Total area (mm2) | 263.7±45.0 | 272.2±42.1 | 256.7±40.7 | 0.32 | - | - |
| % Ct. area | 18.4±5.9 | 19.3±5.1 | 21.7±6.6 | 0.02 | 0.69 | 0.008 |
| Ct. thickness (mm) | 0.70±0.20 | 0.75±0.16 | 0.83±0.25 | 0.008 | 0.41 | 0.004 |
| Ct. porosity (%) | 0.012±0.008 | 0.008±0.004 | 0.008±0.005 | 0.006a | 0.05 | 0.007a |
| Ct. vBMD (mg HA/cm3) | 816.3±67.5 | 824.6±54.6 | 845.2±72.8 | 0.09 | - | - |
| Tb. vBMD (mg HA/cm3) | 165.4±31.6 | 177.0±37.0 | 174.4±35.8 | 0.16 | - | - |
| Outer Tb. vBMD (mg HA/cm3) | 223.3±30.7 | 234.1±35.4 | 231.7±35.2 | 0.19 | - | - |
| Inner Tb. vBMD (mg HA/cm3) | 125.3±32.9 | 137.4±38.9 | 134.8±37.2 | 0.16 | - | - |
| Total vBMD (mg HA/cm3) | 299.7±56.5 | 314.2±51.5 | 333.4±63.6 | 0.01 | 0.37 | 0.006 |
| Stiffness (kN/m) | 72.5±14.0 | 79.5±12.3 | 77.9±14.3 | 0.02 | 0.03 | 0.09 |
| Failure load (kN) | 3.7±0.7 | 4.0±0.6 | 4.0±0.7 | 0.02 | 0.03 | 0.09 |
| (Tb.F/TF) distal (%)* | 53.4±8.1 | 55.7±9.0 | 50.2±9.4 | 0.03 | 0.33 | 0.12 |
| (Tb.F/TF) proximal (%)* | 20.8±7.3 | 22.5±7.4 | 20.3±7.3 | 0.43 | - | - |
|
| ||||||
| HRpQCT and FEA: Tibia | N=87 | N=34 | N=38 | |||
| Total area (mm2) | 669.8±102.8 | 698.7±91.5 | 615.8±99.0 | 0.002a | 0.33a | 0.01 |
| Tb. area (mm2) | 547.6±106.2 | 568.6±94.4 | 494.3±101.0 | 0.006 | 0.57 | 0.02 |
| % Ct. area | 18.69±4.78 | 18.94±4.10 | 20.05±4.97 | 0.33 | - | - |
| Ct. thickness (mm) | 1.22±0.25 | 1.27±0.23 | 1.25±0.24 | 0.52 | - | - |
| Ct. porosity (%) | 0.019±0.011 | 0.017±0.009 | 0.014±0.010 | 0.03a | 0.61 | 0.01a |
| Ct. vBMD (mg HA/cm3) | 867.4±37.0 | 874.4±36.2 | 893.0±40.51 | 0.003 | 0.63 | 0.001 |
| Tb. vBMD (mg HA/cm3) | 203.1±28.4 | 208.4±34.6 | 192.5±33.2 | 0.08 | - | - |
| Outer Tb. vBMD (mg HA/cm3) | 266.7±31.1 | 273.0±36.1 | 255.1±36.2 | 0.07 | - | - |
| Inner Tb. vBMD (mg HA/cm3) | 159.9±28.3 | 164.5±34.7 | 149.9±32.9 | 0.11 | - | - |
| Total vBMD (mg HA/cm3) | 328.1±46.9 | 334.8±52.3 | 335.1±58.2 | 0.69 | - | - |
| Stiffness (kN/m) | 227.9±30.5 | 242.1±36.8 | 211.1±33.7 | 0.0005a | 0.07 | 0.02a |
| (Tb.F/TF) distal (%)* | 59.6±7.0 | 59.6±5.8 | 53.7±6.7 | <0.0001a | 1.00 | <0.0001a |
| (Tb.F/TF) proximal (%)* | 38.7±6.9 | 39.0±6.1 | 33.5±6.2 | 0.0002a | 0.96 | 0.0002a |
| Tb VM (N/mm2)** | 63.7±4.6 | 63.1±5.3 | 60.9±6.3 | 0.03a | 0.84 | 0.02a |
AA: oligoamenorrheic athletes, EA: eumenorrheic athletes, NA: non-athletes Data presented as means ± standard deviations or as percentage where noted.
ANOVA used for 3 group comparisons followed by Dunnett’s testing when ANOVA was significant with AA as the comparison group.
P value < 0.05 after controlling for age of menarche
Ct: Cortical; Tb: Trabecular
(Tb.F/TF) distal or proximal: percent load carried by trabecular bone at most distal (or proximal) slice.
Tb VM: Trabecular von Mises stress (amount of stress the trabecular compartment can withstand before permanently deforming)
HRpQCT measurements at the radius showed lower % cortical area and cortical thickness, greater cortical porosity, and lower total vBMD in AA than non-athletes. Percent cortical porosity trended higher in AA versus EA. Micro-FEA analysis demonstrated lower stiffness and failure load at the radius in AA versus EA. At the tibia, total and trabecular cross-sectional area were greater in the AA versus non-athletes, suggesting greater moment of inertia at weight bearing bone. However, cortical porosity was higher and cortical vBMD lower in the AA compared with non-athletes. Stiffness and failure load trended lower in AA than EA, but were higher in AA than non-athletes. Percent load carried by trabecular bone at the most proximal and the most distal tibial slices was greater in AA versus non-athletes. Unlike areal bone density, some differences in bone structure and strength parameters were no longer evident after controlling for menarchal age using multivariate analysis.
Fracture Comparisons across Groups
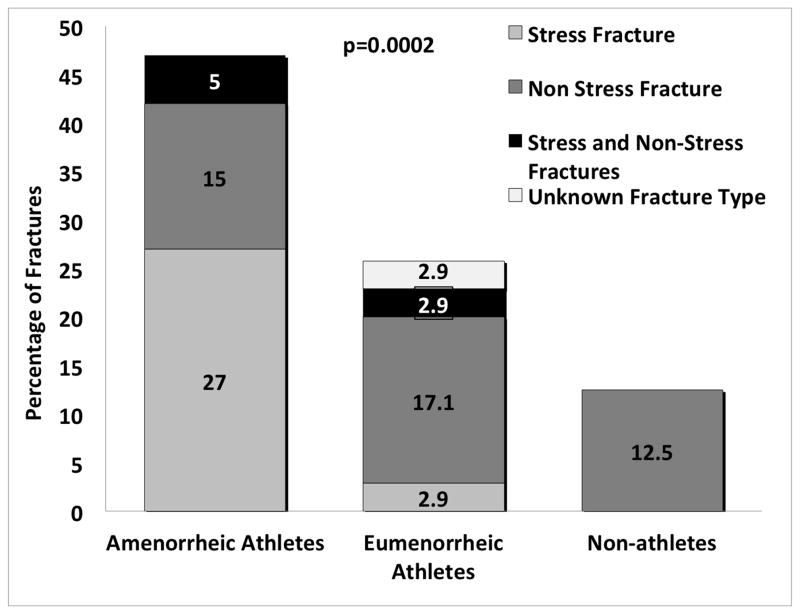
A larger proportion of AA than EA and non-athletes reported a history of fracture (stress and non-stress) (47 % vs. 25.7 % vs. 12.5 %) (Table 3). This was driven mostly by stress fractures, as 32% of AA, 5.9% of EA, and none of the controls had ever had stress fractures in their lifetime. The majority of stress fractures occurred after the average age of menarche in U.S. girls, i.e. 12.5 years, when amenorrhea would be expected to exert a significantly negative impact on bone metabolism (Table 3). The incidence rate (cases per 10,000 person-years) for all types of fractures after age 12.5 years was calculated in AA (558.2, 95% CI: 398.8 – 760) and EA (312.4, 95% CI: 125.6 – 643.6), but there was no significant difference in the rates between the two groups (p=0.15). The incidence rate of stress fractures after age 12.5 years was also calculated, yielding a significant different incidence in AA (432.6, 95% CI: 293.9 – 614) versus EA (89.3, 95% CI 10.8 – 322.4), (p=0.017). No non-athlete sustained fractures after 12.5 years of age. Because many subjects experienced more than one lifetime fracture, Figure 1 shows the percentage of AA, EA, and non-athletes who experienced only stress fractures, only non-stress fractures, or both at any time of their lives. Of note, differences among groups for fractures persisted after excluding patients with a history of eating disorders. The AA group had the largest number of subjects with a history of disordered eating behavior. After excluding subjects with eating disorders, the proportion of AAs with any fracture, stress fractures, non-stress fractures, stress fractures after 12.5 years and non-stress fractures after 12.5 years was 50.0%, 35.1%, 18.9%, 33.8% and 10.8% respectively compared with 47.0%, 32.0%, 20.0%, 31.0% and 10.0% when subjects with eating disorders were included. Only two eumenorrheic athletes and no non-athlete had a history of disordered eating.
Table 3.
Percentage of Amenorrheic Athletes (AA), Eumenorrheic Athletes (EA) and Non-athletes (NA) who Experienced Stress and Non-stress Fractures and Fracture Location
| AA | EA | NA | Fisher’s Exact Test | AA vs EA | AA vs NA | |
|---|---|---|---|---|---|---|
| N=100 | N=35 | N=40 | P | P | P | |
| Fracture (%) | 47.0 | 25.7 | 12.5 | <0.0001a | 0.04 a | 0.0004 a |
| Stress fracture (%) | 32.0 | 5.9 | 0 | 0.01 a | 0.004 a | <0.001 a |
| Lower Extremity (%) | 27.0 | 5.9 | 0 | <0.0001 a | 0.02 a | <0.0001 a |
| Upper Extremity (%) | 4.0 | 0 | 0 | 0.50 | - | - |
| Non-extremity or spine (%) | 3.0 | 0 | 0 | 0.58 | - | - |
| Non-stress fracture (%) | 20.0 | 20.6 | 12.5 | 0.56 | - | - |
| Lower Extremity (%) | 8.0 | 8.8 | 2.5 | 0.51 | - | - |
| Upper Extremity (%) | 12.0 | 14.3 | 7.5 | 0.61 | - | - |
| Non-extremity or spine (%) | 3.0 | 0 | 2.5 | 0.82 | - | - |
| Stress fracture after 12.5 yrs (%)* | 31.0 | 5.9 | 0 | <0.0001 a | 0.005 a | <0.001 a |
| Non-stress fracture after 12.5 yrs (%)* | 10.0 | 14.7 | 0 | 0.04 a | 0.54 | 0.12 |
AA: oligoamenorrheic athletes, EA: eumenorrheic athletes, NA: non-athletes
Data presented as percentage of each group. Fisher’s exact test was used for both 2 and 3 group comparisons.
Stress or non-stress fractures that occurred after the average age of menarche, 12.5 years.
P value <0.05 after excluding subjects with eating disorders
Figure 1.
Percentage of Fractures in Amenorrheic Athletes (AA), Eumenorrheic Athletes (EA) and Non-athletes (NA)
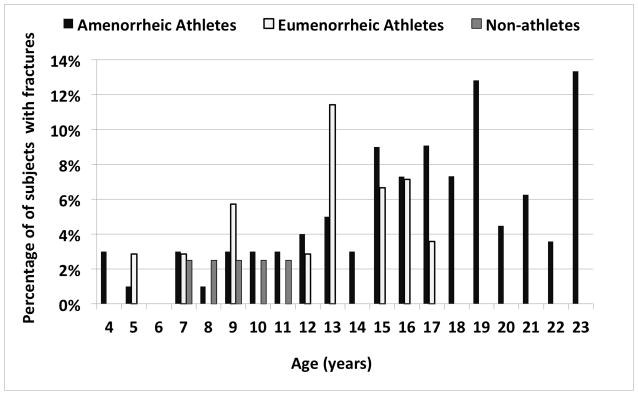
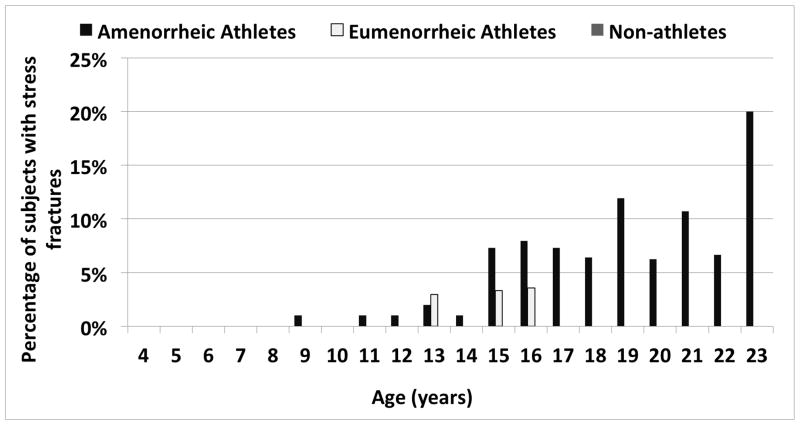
Figure 2a illustrates the proportion of subjects in each the three groups who sustained one or more fractures at any particular age. Whereas the non-athletes only experienced fractures between the ages of 7 and 12 in this cohort, the two athlete groups continued to experience fractures during adolescence, when they were presumably more active than the non-athletes. In addition, fractures continued to occur in AA (but not EA) with further increases in age. A similar, but even more striking pattern was observed when examining the proportion of subjects with stress fractures in the three groups according to age (Figure 2b). None of the non-athletes experienced stress fractures, and a greater proportion of AA than EA had stress fractures at nearly every age. Table 3 shows the location and type of fracture incurred by subjects. Stress fractures of the lower extremity were more common in AA versus the other two groups.
Figure 2.
Proportion of Amenorrheic Athletes (AA), Eumenorrheic Athletes (EA) and Non-athletes (NA) who Fractured Each Year Between 0 and 25 Years
Figure 2a. All Types of Fractures
Figure 2b. Stress Fractures
We next examined the individual groups (AA, EA, and non-athletes) to determine whether there were differences in the clinical characteristics of those who had a history of fracture versus those who did not (data not shown). There were no significant differences in fat mass, percent body fat, lean mass, average hours of exercise, or types of exercise between those who had fractured in the AA group versus those who had not. When examining the EA subgroup, we found that those who had fractured were older (20.3±2.6 years versus 18.4±2.3 years, p=0.045), and had higher fat mass (18.5±3.9 versus 14.4±3.5 kg, p=0.006) and percent body fat (26.7±3.4 versus 23.5±3.9, p=0.03) compared to those who had never fractured. Similarly, among non-athletes, those with a history of fracture were older (21.9±2.2 versus 19.5±2.0 years, p=0.01), had higher BMIs (24.4±2.6 versus 21.8±2.0 kg/m2, p=0.01), fat mass (22.9±6.4 versus 16.2±4.2 kg, p=0.003) and percent body fat (36.4±5.4 versus 27.4±5.0%, p<0.001).
Bone Parameters in Fracture versus Non-fracture Subjects
Table 4 shows pertinent DXA and HRpQCT results for AA based on fracture history. Whole body and spine BMD Z-scores were lower in those AA who had fractured versus those who had not. Volumetric BMD of the outer portion of the trabecular region was lower at both the radius and tibia in AA with a history of fracture versus those without a history of fracture. At the radius, trabecular thickness was lower and trabecular von Mises stress (the amount of stress the trabecular compartment can withstand before permanently deforming) trended lower in the fracture versus non-fracture groups. No differences were noted in tibial microarchitecture in these AA subgroups.
Table 4.
DXA Z-Scores and HRpQCT Data from the Radius and Tibia in Amenorrheic Athletes according to Fracture History [no fracture versus ≥1 fracture (stress and non-stress), and <2 stress fractures versus ≥ 2 stress fractures]
| No Fractures | ≥1 Fractures | P | < 2 Stress Fractures | ≥2 Stress Fractures | P | |
|---|---|---|---|---|---|---|
| DXA (BMD Z-scores) | n=53 | n = 47 | n=84 | n=16 | ||
| Femoral Neck | −0.01±1.06 | −0.33±1.05 | 0.14 | −0.09±1.07 | −0.55±0.96 | 0.11 |
| Total Hip | 0.16±1.03 | −0.09±0.97 | 0.21 | 0.12±1.02 | −0.33±0.86 | 0.10 |
| Lumbar Spine | −0.54±1.28 | −1.02±1.08 | 0.045 | −0.61±1.20 | −1.58±0.87 | 0.003 |
| Whole Body | −0.40±1.10 | −0.90±0.87 | 0.01 | −0.55±1.02 | −1.09±0.94 | 0.05 |
|
| ||||||
| HRpQCT Radius | n =45 | n =42 | n=71 | n=13 | ||
| Total area (mm2) | 263.3±45.7 | 264.1±44.8 | 0.94 | 267.9±45.8 | 240.7±32.9 | 0.045 |
| % Ct. area | 18.5±6.8 | 18.2±4.7 | 0.77 | 18.4±6.2 | 18.4±3.9 | 0.99 |
| Ct. thickness (mm) | 0.71±0.23 | 0.70±0.15 | 0.76 | 0.71±0.21 | 0.68±0.12 | 0.61 |
| Ct. porosity (%) | 1.3±0.9 | 1.1±0.7 | 0.29 | 1.2±0.9 | 0.8±0.5 | 0.07 |
| Ct. vBMD (mg HA/cm3) | 813.8±79.0 | 819.1±52.2 | 0.72 | 814.9±71.5 | 823.8±41.0 | 0.67 |
| Tb. thickness (mm) | 0.073±0.011 | 0.067±0.009 | 0.03 | 0.071±0.011 | 0.067±0.009 | 0.25 |
| Tb. vBMD (mg HA/cm3) | 170.8±4.7 | 159.1±27.9 | 0.09 | 168.5±32.2 | 148.1±21.2 | 0.03 |
| Outer Tb. vBMD (mg HA/cm3) | 230.0±31.9 | 215.7±27.8 | 0.03 | 226.8±31.1 | 204.5±21.0 | 0.02 |
| Inner Tb. vBMD (mg HA/cm3) | 129.8±35.8 | 120.0±28.8 | 0.18 | 128.3±33.8 | 109.0±21.9 | 0.05 |
| Total vBMD (mg HA/cm3) | 305.9±64.9 | 292.5±44.6 | 0.28 | 302.2±59.1 | 285.9±38.4 | 0.34 |
| Stiffness (kN/m) | 74.4±14.2 | 70.4±13.5 | 0.19 | 74.3±13.7 | 63.0±12.1 | 0.007 |
| Failure load (kN) | 3.79±0.70 | 3.58±0.69 | 0.17 | 3.78±0.68 | 3.18±0.60 | 0.004* |
| (Tb.F/TF) distal (%)* | 54.3±7.2 | 52.3±8.9 | 0.26 | 54.0±8.1 | 49.6±7.2 | 0.07 |
| (Tb.F/TF) proximal (%)* | 21.4±7.1 | 20.1±7.6 | 0.44 | 21.3±7.6 | 18.0±5.0 | 0.13 |
| TbVM (N/mm2)** | 52.9±6.9 | 49.8±7.3 | 0.049 | 51.9±7.1 | 49.0±7.6 | 0.19 |
|
| ||||||
| HRpQCT Tibia | n =45 | n = 42 | n=73 | n=14 | ||
| Total area (mm2) | 668.3±108.0 | 671.4±98.3 | 0.89 | 674.3±104.0 | 646.5±96.6 | 0.36 |
| % Ct. area | 19.2±5.2 | 18.2±4.3 | 0.32 | 18.9±4.9 | 17.8±3.9 | 0.46 |
| Ct. thickness (mm) | 1.25±0.28 | 1.19±0.22 | 0.27 | 1.24±0.26 | 1.15±0.19 | 0.23 |
| Ct. porosity (%) | 2.0±1.2 | 1.8±0.9 | 0.45 | 1.9±1.1 | 2.0±1.0 | 0.79 |
| Ct. vBMD (mg HA/cm3) | 865.8±43.8 | 869.1±28.6 | 0.68 | 867.4±4.4 | 867.3±28.1 | 1.00 |
| Tb. vBMD (mg HA/cm3) | 208.4±33.3 | 197.5±20.7 | 0.07 | 204.6±30.0 | 195.1±15.8 | 0.25 |
| Outer Tb. vBMD (mg HA/cm3) | 273.9±35.2 | 258.9±24.1 | 0.02 | 268.6±33.0 | 256.6±15.7 | 0.19 |
| Inner Tb. vBMD (mg HA/cm3) | 163.9±33.7 | 155.7±20.6 | 0.18 | 161.2±29.7 | 153.3±18.9 | 0.34 |
| Total vBMD (mg HA/cm3) | 335.1±53.6 | 320.5±37.7 | 0.15 | 330.5±49.0 | 315.4±32.4 | 0.27 |
| Stiffness (kN/m) | 230.9±31.3 | 224.7±29.6 | 0.35 | 230.7±30.3 | 213.8±28.0 | 0.05* |
| Failure load (kN) | 11.5±1.5 | 11.2±1.5 | 0.35 | 11.5±1.5 | 10.7±1.4 | 0.048* |
| (Tb.F/TF) distal (%)** | 59.6±7.3 | 59.5±6.7 | 0.96 | 59.5±7.1 | 60.1±6.2 | 0.75 |
| (Tb.F/TF) proximal (%)** | 38.6±7.3 | 38.8±6.7 | 0.89 | 38.6±7.1 | 39.9±6.5 | 0.88 |
| TbVM (N/mm2)*** | 63.94±5.01 | 63.40±4.25 | 0.60 | 63.68±4.72 | 63.66±4.37 | 0.99 |
Data presented as means ± standard deviations.
The Student t-test was used for normally distributed 2 group comparisons. The Wilcoxon Rank Sum test was used for data not normally distributed (*).
Ct.: cortical; Tb: trabecular
(Tb.F/TF) distal or proximal: percent load carried by trabecular bone at most distal (or proximal) slice.
Tb VM: Trabecular von Mises stress (amount of stress the trabecular compartment can withstand before permanently deforming)
When comparing BMD and HRpQCT data of EA who had fractured versus those who had not, EA with fractures had lower trabecular number (1.8±0.29 versus 2.03±0.25/mm, p=0.04), greater trabecular spacing (0.49±0.11 versus 0.43±0.06 mm, p=0.03), with lesser percent load carried by trabecular bone at the most distal slice of the radius (0.51±0.10 versus 0.58±0.08 %, p=0.04). There were no differences found at the tibia in EA who had fractured versus those who had not (data not shown). In non-athletes who had fractured versus those who had not, no differences in BMD or HRpQCT data at the radius or tibia were found except that those with a history of fracture had lower percent load carried by trabecular bone at the most distal slice of the tibia (0.48±0.05 versus 0.55±0.07 %, p=0.02) as well as the most proximal slice of the tibia (0.29±0.05 versus 0.34±0.06 %, p=0.049) (data not shown).
Finally, we divided the AA group into those who had <2 stress fractures versus those who had had ≥ 2 stress fractures, as having more than one stress fracture often becomes concerning clinically and raises questions about Triad risk factors.(19) Clinical characteristics were similar in both groups, except that those with ≥ 2 stress fractures had less fat mass (10.6±3.1 versus 13.8±4.8 kg, p=0.01) and lower percent body fat (19.5±4.9 versus 23.5±5.7 %, p=0.009).
Table 4 shows DXA and microarchitecture comparisons in AA with <2 stress fracture versus AA with ≥ 2 stress fractures. The group with ≥ 2 stress fractures had significantly lower lumbar spine BMD Z-scores and their whole body BMD Z-scores trended lower than those with fewer stress fractures. At the radius, total cross-sectional area, trabecular vBMD and vBMD of the outer portion of the trabecular region were lower in the group with ≥ 2 stress fractures, and inner trabecular vBMD trended lower. In addition, stiffness and failure load were lower in AA with ≥ 2 stress fractures. Similarly, at the tibia, stiffness and failure load were lower in those with ≥ 2 stress fractures versus those with fewer fractures.
Discussion
This is the first study to examine bone microarchitecture and bone strength estimates in female adolescent and young adult athletes according to menstrual and fracture history.
Age and Fractures
The incidence of fractures, especially at the radius, peaks during early adolescence (18, 33, 35) from a dissociation between peak statural bone growth and peak mineralization, as well as increased cortical porosity.(32, 54) In our study, a larger proportion of adolescent and young adult AA had fractures compared to EA and non-athletes, and this difference was mostly driven by a higher prevalence of stress fractures in AA. We also found that AA experienced fractures later in adolescence compared to EA and non-athletes, with a later peak than reported in healthy children (early adolescence).(18)
Menstrual Status and Fractures
Few studies have evaluated associations between menstrual dysfunction and stress and non-stress fractures in athletes, and findings are not consistent. In a study of 18–26 year old female distance runners, Kelsey et al. reported a non-significant increased risk for stress fractures in those with irregular periods, (31) while Barrack et al. showed that an accumulation of Triad risk factors, but not oligoamenorrhea alone, increased the odds of developing a stress injury in young athletes.(4) In contrast, Nattiv, et al. did report greater severity of stress fracture (by MRI staging) in collegiate athletes with oligo-amenorrhea versus eumenorrhea.(41) Menstrual irregularity was noted in 75% of female athletes with stress injuries at predominantly trabecular bone sites, compared to only 12.5% of those with stress injuries at cortical sites. However, the study did not report comparisons of menstrual status in those who did or did not sustain stress injuries.(41) Our results of increased prevalence and incidence of stress fracture, particularly of the lower extremity, in AA versus EA and non-athletes are consistent with findings in other retrospective studies of female athletes, although these did not assess fracture risk in non-athletes.(6, 14, 21, 40) These studies also reported menstrual status in athletes with and without a history of fracture, rather than the other way around.(6, 14, 21, 40)
Area Bone Mineral Density and Fractures
Similar to menstrual status, data for associations of areal BMD with fractures are not consistent. Duckham et al., and others found no differences in areal BMD in those with or without stress fractures, (6, 14, 21) although another study did reported a greater likelihood of oligoameneorrhea and lower areal BMD at the spine and femoral neck in athletes with fracture versus those without fracture.(40) In our study, within EA and non-athlete groups, there were no differences in BMD Z-scores in those with or without fractures. However, among AA, lumbar and whole body (but not total hip or femoral neck) BMD Z-scores were lower in those with a history of fracture, and in those with ≥2 stress fractures versus those with <2 stress fractures. The lack of association of hip BMD Z-scores with fracture may relate to weight bearing-activity partially counteracting the negative effects of a hormonally depleted state at the weight-bearing and predominantly cortical bone at the hip.
Bone Microarchitecture and Strength Estimates and Fractures
Our findings of altered bone structure and reduced strength estimates in AA are similar to our previous reports in a subpopulation of these subjects (22), as well as in anorexia nervosa and postmenopausal women.(1, 2, 22, 23). Overall, at the non-weight bearing radius, AA had greatest cortical porosity, and lowest cortical area and thickness, total volumetric BMD, stiffness and failure load. The decreased proportion of cortical bone in AA may be from enhanced endosteal resorption in the hypo-estrogenic state, as in menopause, when trabecularization of cortical bone at the endosteal border results in increased porosity.(23, 56) Our findings of negative effects of the amenorrheic state on mostly cortical but not trabecular bone (for the radius), are consistent with studies in the Kronos Early Estrogen Prevention Study in post-menopausal women, in which estrogen replacement had beneficial effects on cortical, but not trabecular microarchitecture at the radius. (23) Of interest, menarchal age was greater in AA than in EA, and after controlling for menarchal age, many differences across groups were no longer evident, particularly at the non-weight-bearing radius. This emphasizes the importance of normal menarchal timing in optimizing bone accrual. It is possible that other hormonal abnormalities associated with low energy availability and amenorrhea in athletes, such as low IGF-1 or higher cortisol levels (20), and reduced bone turnover as previously reported in AA (16), also contribute to differences in bone structural parameters (and bone density) across groups.
At the weight-bearing tibia, AA had greater total and trabecular area and cortical porosity, and lower cortical density than non-athletes. Stiffness and failure load trended lower than in EA, but were higher than in non-athletes. Greater cross-sectional area in athletes is likely from increased weight bearing activity, consistent with other studies in athletes involved in high and moderate impact sports.(48) This would lead to greater moment of inertia and resistance to bending, and lower strain for a given force; (25) and would explain the higher strength estimates in AA versus non-athletes. Increased cortical porosity in AA is likely from delayed mineralization of the expanding tibia, compounded by estrogen deficiency.
We examined microarchitecture and estimated strength differences in those with or without a history of fracture within each group. There were no microarchitecture differences between fracture and non-fracture subgroups of EA and non-athletes, suggesting that factors other than bone quality were at play. These may have included the degree of mechanical trauma, training volume, and biomechanics of gait. However, AA who fractured had lower vBMD in the outer trabecular region (meta VBMD) at both the radius and tibia. This may be from lower estrogen levels in AA leading to increased endosteal bone resorption and therefore lower density of the outer trabecular region. Trabecular thickness was lower at the radius (but not tibia) in AA who fractured, and it is possible that weight-bearing effects on the tibia are protective. One study examined quadrant specific tibial bone microarchitecture using HRpQCT in 19 athletes ages 18–45 with lower limb stress fractures and 19 controls not differentiated by menstrual status, (49) and found lower distal tibial trabecular vBMD and lower tibial cortical area in those with stress fractures, particularly in the posterior and lateral cortical regions. (49) We may have found more tibial differences had we separated the analyses according to region.
Finally, when we specifically compared those AA who had sustained <2 versus ≥ 2 stress fractures, we found more pronounced differences in bone quality and strength across groups. At the radius, total cross-sectional area, total trabecular vBMD, and vBMD at both the inner and outer portions of the trabecular region were lower in the group with more fractures. This is similar to findings in postmenopausal women with a history of fragility fractures, who also had decreased vBMD in the inner and outer trabecular regions at the radius and tibia, with more pronounced changes at the radius.(51) In our study, AA with ≥ 2 stress fractures had lower stiffness and failure load at both the radius and the weight-bearing tibia, suggesting that those who do fracture do not demonstrate the beneficial effects of weight –bearing at the tibia.
Strengths and Limitations
Strengths of this study include its large sample of oligoamenorrheic athletes, thorough menstrual and training history, and BMD as well as microarchitectural assessments in groups. Limitations include its cross-sectional design, and retrospective self-report of fractures, training, and menstrual status. However, previously published work has demonstrated that self-report of fracture history (occurrence and timing) is sensitive and specific, particularly for distal forearm fractures.(29, 30)
Conclusions
Oligo-amenorrheic adolescent and young adult athletes lack much of the bone health benefits of weight-bearing exercise, such as enhancement of overall BMD and improved stiffness and failure load at weight-bearing sites. This makes them more susceptible to stress fractures than eumenorrheic athletes and non-athletes despite higher vitamin D and calcium levels. Bone microarchitectural and strength differences are more pronounced in those amenorrheic athletes who experienced multiple stress fractures, suggesting either a dose-response of amenorrhea on bone microarchitecture and strength, or individual differences in bone susceptibility to amenorrhea, leading to more bone injuries. Further work is needed to better characterize the differences in bone microarchitecture in a variety of oligoamenorrheic athletes. For sports clinicians, this study also suggests a high level of suspicion of low energy availability and menstrual dysfunction in female athletes who present with stress injuries.
Acknowledgments
We would like to thank our patients and the nurses and bionutritionists in the Massachusetts General Hospital Clinical Research Center. This work was supported by National Institutes of Health Grants 1 UL1 RR025758-01, 1UL1TR001102-01, 1 R01 HD060827-01A1 and K24 HD071843.
Footnotes
Disclosure: All authors state that they have no conflicts of interest. The results of this study do not constitute endorsement by ACSM.
Bibliography
- 1.Ackerman KE, Nazem T, Chapko D, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. The Journal of clinical endocrinology and metabolism. 2011;96(10):3123–33. doi: 10.1210/jc.2011-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerman KE, Putman M, Guereca G, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012;51(4):680–7. doi: 10.1016/j.bone.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111(4 Pt 1):844–50. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- 4.Barrack MT, Gibbs JC, De Souza MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: a prospective multisite study of exercising girls and women. The American journal of sports medicine. 2014;42(4):949–58. doi: 10.1177/0363546513520295. [DOI] [PubMed] [Google Scholar]
- 5.Bauer JS, Link TM. Advances in osteoporosis imaging. European journal of radiology. 2009;71(3):440–9. doi: 10.1016/j.ejrad.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 6.Bennell KL, Malcolm SA, Thomas SA, et al. Risk factors for stress fractures in female track-and-field athletes: a retrospective analysis. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 1995;5(4):229–35. doi: 10.1097/00042752-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bennell KL, Malcolm SA, Thomas SA, Wark JD, Brukner PD. The incidence and distribution of stress fractures in competitive track and field athletes. A twelve-month prospective study. The American journal of sports medicine. 1996;24(2):211–7. doi: 10.1177/036354659602400217. [DOI] [PubMed] [Google Scholar]
- 8.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. The Journal of clinical endocrinology and metabolism. 2005;90(12):6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 9.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2008;23(3):392–9. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 10.Brukner P, Bradshaw C, Khan KM, White S, Crossley K. Stress fractures: a review of 180 cases. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 1996;6(2):85–9. [PubMed] [Google Scholar]
- 11.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41(4):505–15. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(5):983–93. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbon R, Sambrook PN, Deakin V, et al. Bone density of elite female athletes with stress fractures. The Medical journal of Australia. 1990;153(7):373–6. doi: 10.5694/j.1326-5377.1990.tb125491.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen YT, Tenforde AS, Fredericson M. Update on stress fractures in female athletes: epidemiology, treatment, and prevention. Current reviews in musculoskeletal medicine. 2013;6(2):173–81. doi: 10.1007/s12178-013-9167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christo K, Prabhakaran R, Lamparello B, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008;121(6):1127–36. doi: 10.1542/peds.2007-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compston JE. Sex steroids and bone. Physiological reviews. 2001;81(1):419–47. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- 18.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2004;19(12):1976–81. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 19.De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. British journal of sports medicine. 2014;48(4):289. doi: 10.1136/bjsports-2013-093218. [DOI] [PubMed] [Google Scholar]
- 20.De Souza MJ, West SL, Jamal SA, Hawker GA, Gundberg CM, Williams NI. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone. 2008;43(1):140–8. doi: 10.1016/j.bone.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ open. 2012;2(6) doi: 10.1136/bmjopen-2012-001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faje AT, Karim L, Taylor A, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. The Journal of clinical endocrinology and metabolism. 2013;98(5):1923–9. doi: 10.1210/jc.2012-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farr JN, Khosla S, Miyabara Y, Miller VM, Kearns AE. Effects of estrogen with micronized progesterone on cortical and trabecular bone mass and microstructure in recently postmenopausal women. The Journal of clinical endocrinology and metabolism. 2013;98(2):E249–57. doi: 10.1210/jc.2012-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Topics in magnetic resonance imaging: TMRI. 2006;17(5):309–25. doi: 10.1097/RMR.0b013e3180421c8c. [DOI] [PubMed] [Google Scholar]
- 25.Garrett WE, Kirkendall DT. Exercise and Sport Science. Philadelphia: Lippincott Williams Wilkins; 2000. p. 227. [Google Scholar]
- 26.Gilsanz V, Chalfant J, Kalkwarf H, et al. Age at onset of puberty predicts bone mass in young adulthood. The Journal of pediatrics. 2011;158(1):100–5. 5 e1–2. doi: 10.1016/j.jpeds.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2. Stanford, Calif: Stanford University Press; 1959. p. xvi.p. 256. [Google Scholar]
- 28.Harel Z, Gold M, Cromer B, et al. Bone mineral density in postmenarchal adolescent girls in the United States: associated biopsychosocial variables and bone turnover markers. The Journal of adolescent health: official publication of the Society for Adolescent Medicine. 2007;40(1):44–53. doi: 10.1016/j.jadohealth.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Honkanen K, Honkanen R, Heikkinen L, Kroger H, Saarikoski S. Validity of self-reports of fractures in perimenopausal women. American journal of epidemiology. 1999;150(5):511–6. doi: 10.1093/oxfordjournals.aje.a010040. [DOI] [PubMed] [Google Scholar]
- 30.Ismail AA, O’Neill TW, Cockerill W, et al. Validity of self-report of fractures: results from a prospective study in men and women across Europe. EPOS Study Group. European Prospective Osteoporosis Study Group. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000;11(3):248–54. doi: 10.1007/s001980050288. [DOI] [PubMed] [Google Scholar]
- 31.Kelsey JL, Bachrach LK, Procter-Gray E, et al. Risk factors for stress fracture among young female cross-country runners. Medicine and science in sports and exercise. 2007;39(9):1457–63. doi: 10.1249/mss.0b013e318074e54b. [DOI] [PubMed] [Google Scholar]
- 32.Kirmani S, Christen D, van Lenthe GH, et al. Bone structure at the distal radius during adolescent growth. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2009;24(6):1033–42. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta orthopaedica Scandinavica Supplementum. 1983;202:1–109. [PubMed] [Google Scholar]
- 34.Lloyd T, Chinchilli VM, Johnson-Rollings N, Kieselhorst K, Eggli DF, Marcus R. Adult female hip bone density reflects teenage sports-exercise patterns but not teenage calcium intake. Pediatrics. 2000;106(1 Pt 1):40–4. doi: 10.1542/peds.106.1.40. [DOI] [PubMed] [Google Scholar]
- 35.Lyons RA, Delahunty AM, Kraus D, et al. Children’s fractures: a population based study. Injury prevention: journal of the International Society for Child and Adolescent Injury Prevention. 1999;5(2):129–32. doi: 10.1136/ip.5.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma NS, Gordon CM. Pediatric osteoporosis: where are we now? The Journal of pediatrics. 2012;161(6):983–90. doi: 10.1016/j.jpeds.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 37.Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008;42(6):1203–13. doi: 10.1016/j.bone.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Bmj. 1996;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro M, Hecker AT, Bouxsein ML, Myers ER. Failure load of thoracic vertebrae correlates with lumbar bone mineral density measured by DXA. Calcified tissue international. 1995;56(3):206–9. doi: 10.1007/BF00298611. [DOI] [PubMed] [Google Scholar]
- 40.Myburgh KH, Hutchins J, Fataar AB, Hough SF, Noakes TD. Low bone density is an etiologic factor for stress fractures in athletes. Annals of internal medicine. 1990;113(10):754–9. doi: 10.7326/0003-4819-113-10-754. [DOI] [PubMed] [Google Scholar]
- 41.Nattiv A, Kennedy G, Barrack MT, et al. Correlation of MRI grading of bone stress injuries with clinical risk factors and return to play: a 5-year prospective study in collegiate track and field athletes. The American journal of sports medicine. 2013;41(8):1930–41. doi: 10.1177/0363546513490645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine position stand. The female athlete triad. Medicine and science in sports and exercise. 2007;39(10):1867–82. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 43.Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(4):882–90. doi: 10.1359/jbmr.091020. [DOI] [PubMed] [Google Scholar]
- 44.Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842–8. doi: 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- 45.Rizzoli R, Bonjour JP, Ferrari SL. Osteoporosis, genetics and hormones. Journal of molecular endocrinology. 2001;26(2):79–94. doi: 10.1677/jme.0.0260079. [DOI] [PubMed] [Google Scholar]
- 46.Roberts BJ, Thrall E, Muller JA, Bouxsein ML. Comparison of hip fracture risk prediction by femoral aBMD to experimentally measured factor of risk. Bone. 2010;46(3):742–6. doi: 10.1016/j.bone.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Salamone LM, Glynn NW, Black DM, et al. Determinants of premenopausal bone mineral density: the interplay of genetic and lifestyle factors. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1996;11(10):1557–65. doi: 10.1002/jbmr.5650111024. [DOI] [PubMed] [Google Scholar]
- 48.Schipilow JD, Macdonald HM, Liphardt AM, Kan M, Boyd SK. Bone microarchitecture, estimated bone strength, and the muscle-bone interaction in elite athletes: an HR-pQCT study. Bone. 2013;56(2):281–9. doi: 10.1016/j.bone.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Schnackenburg KE, Macdonald HM, Ferber R, Wiley JP, Boyd SK. Bone quality and muscle strength in female athletes with lower limb stress fractures. Medicine and science in sports and exercise. 2011;43(11):2110–9. doi: 10.1249/MSS.0b013e31821f8634. [DOI] [PubMed] [Google Scholar]
- 50.Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: Hormonal determinants and disorders of peak bone mass in children. The Journal of clinical endocrinology and metabolism. 2000;85(11):3951–63. doi: 10.1210/jcem.85.11.6994. [DOI] [PubMed] [Google Scholar]
- 51.Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(12):2572–81. doi: 10.1002/jbmr.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. The Journal of clinical endocrinology and metabolism. 1992;75(4):1060–5. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 53.Vilayphiou N, Boutroy S, Sornay-Rendu E, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in postmenopausal women. Bone. 2010;46(4):1030–7. doi: 10.1016/j.bone.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(7):1521–6. doi: 10.1002/jbmr.46. [DOI] [PubMed] [Google Scholar]
- 55.Wolman RL, Clark P, McNally E, Harries M, Reeve J. Menstrual state and exercise as determinants of spinal trabecular bone density in female athletes. Bmj. 1990;301(6751):516–8. doi: 10.1136/bmj.301.6751.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375(9727):1729–36. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]