Abstract
A new positron emission tomography (PET) tracer, composed of 18F labeled maltohexaose (MH18F), can image bacteria in vivo with a sensitivity and specificity that is orders of magnitude better than fluorodeoxyglucose (18FDG). MH18F can detect early stage infections composed of as few as 105 E.coli colony forming units (CFUs), and can identify drug resistance in bacteria in vivo. MH18F has the potential to improve the diagnosis of bacterial infections given its unique combination of high specificity and sensitivity for bacteria.
Keywords: maltodextrin transporters, maltohexaose, radiochemistry, bacterial infections, positron emission tomography
The diagnosis of bacterial infections remains a central challenge in medicine. Infections are currently diagnosed via culturing of tissue biopsies or blood samples.[1] However these methods can only detect late stage infections that are challenging to treat. Bacterial infections therefore cause an enormous medical burden, for example, the mortality caused by bacterial infections was greater than the mortality caused from AIDs, breast cancer and prostate cancer combined.[2] Bacterial infections can be treated effectively, if diagnosed and treated at an early stage, and if the presence of drug resistance is also identified. However, this is challenging at present because the symptoms of infections look identical to a variety of other illnesses, such as cancer and inflammation.[3] Thus in this clinical environment, an imaging technology that can identify and localize bacterial infections with high sensitivity and specificity has the potential to have a significant impact on medicine.
Positron emission tomography (PET) imaging has the potential to significantly improve the diagnosis of bacterial infections due to its unparalleled sensitivity.[4] However, 18FDG is currently the only PET contrast agent available for clinical imaging of infections, and is problematic because it lacks specificity for bacteria and has high uptake in mammalian cells.[5] 18FDG therefore cannot distinguish bacterial infections from other pathologies such as cancer and inflammation, and cannot diagnose bacterial infections at an early stage.[5a, 6] Although numerous experimental PET contrast agents have been developed for imaging bacterial infections, such as radiolabeled antibiotics,[7] antimicrobial peptides,[1a] antibodies[8] or white blood cells,[9] these agents have had minimal clinical impact. Several factors have contributed to the lack of success of bacterial imaging agents, such as poor clearance due to non-specific adsorption, low target receptor expression on bacteria, or complicated radiochemical synthesis, which are challenging to perform in clinical radiochemistry labs.[10] Therefore, there is a great need for the development of new PET contrast agents that can image small numbers of bacteria with high specificity in vivo.[11]
Herein, we present a new PET tracer, composed of 18F labeled maltohexaose (MH18F) that can image bacterial infections in vivo with unprecedented sensitivity and specificity (see Scheme 1). MH18F targets the bacteria-specific maltodextrin transporter, which internalizes alpha 1,4 linked glucose oligomers (maltodextrins) as a source of glucose.[12] The maltodextrin transport system is an ideal target for imaging bacteria because of its high uptake of maltodextrins (Km of 130 μM),[13] great specificity for bacteria, and the rapid clearance of maltodextrins from un-infected tissues.[14] In addition, the maltodextrin transporter is only functional in metabolically active bacteria and MH18F uptake is therefore an indicator of bacterial viability,[14b, 15] and potentially antibiotic efficacy. Finally, MH18F should have minimal toxicity in humans because maltodextrins are a commonly used food additive.[16]
Scheme 1.
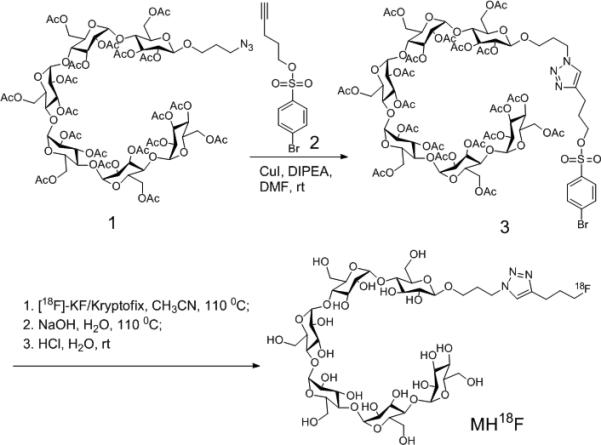
Synthesis of MH18F. MH18F is composed of 18F-fluoride conjugated to maltohexaose and was synthesized by one-step nucleophilic 18F-fluorination of brosylate-maltohexaose 3.
A synthetic strategy was devised to synthesize MH18F via nucleophilic 18-Fluorination of the maltohexaose-brosylate precursor (3) with K18F in the presence of kryptofix k222 (see Scheme 1). The reducing end of maltohexaose was selected for fluorination because the maltodextrin transporter recognizes the non-reducing end of maltodextrins and should therefore tolerate substitutions at the reducing end.[17] Azide functionalized maltohexaose 1 was synthesized from maltohexaose in 4 steps following established methods,[14b] and was conjugated with pent-4-yn-1-yl 4-bromobenzenesulfonate 2 using the Cu(I) catalyzed Huisgen cycloaddition, to afford the brosylate-maltohexaose precursor 3.[18] Radiochemical synthesis of MH18F was carried with cryptate-mediated nucleophilic substitution of the brosylate precursor 3 with potassium 18F-fluoride (K18F), followed by basic hydrolysis with NaOH and acid neutralization. A decay corrected yield of 4.2% was obtained for this synthetic procedure, starting from 18F-fluoride, with an 87 % radiochemical purity, based on radiometric HPLC (see Supplementary Figure S5).[19] The protocol for the synthesis of MH18F had a synthesis time of 100 minutes, and follows the same procedures used to make 18FDG,[20] and should therefore be achievable in clinical radiochemistry laboratories. In addition, we anticipate that the radiochemical yield of MH18F can be increased using new F-18 fluorination methodologies.[19]
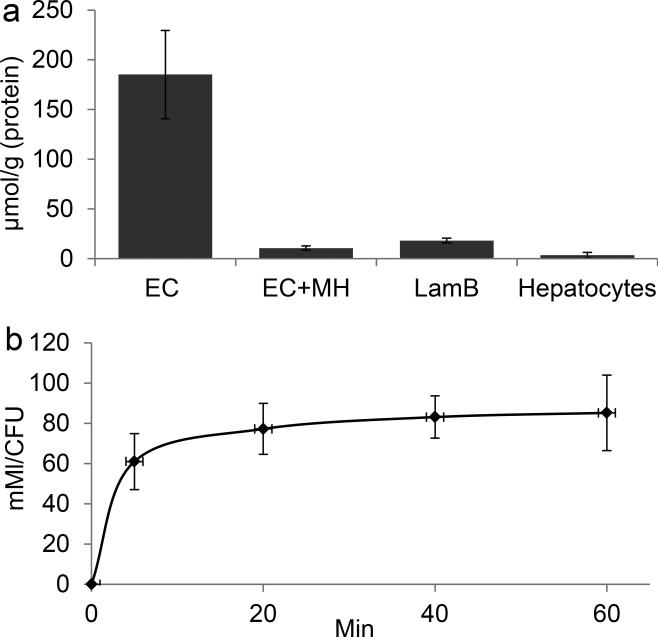
MH18F is designed to selectively target bacteria due to the presence of maltodextrin transporters in bacteria, and their absence in mammalian cells. We therefore investigated if MH19F has specificity for bacteria over mammalian cells, and if it is internalized via the maltodextrin transporter LamB, using F19-NMR. Bacteria (E.coli) and mammalian cells (hepatocytes) were incubated with a 500 μM concentration of MH19F for one hour, washed with PBS, lysed, and the cellular supernatant was analyzed using F19-NMR. Figures 1a and 1b demonstrate that MH19F has high specificity for bacteria over mammalian cells and is robustly internalized. For example, under these conditions, E.coli had accumulated 2 orders of magnitude more MH19F than hepatocytes, and reached millimolar intracellular concentrations. In addition we performed maltohexaose competition experiments and experiments with LamB mutant E.coli to determine if MH19F was being internalized via the maltodextrin transport pathway. Figure 1a demonstrates that the uptake of MH19F in E.coli could be inhibited by an excess of maltohexaose, and that there is minimal uptake of MH19F in LamB mutants, demonstrating that MH19F enters E.coli via the maltodextrin transport pathway.
Figure 1.
MH19F has high specificity for bacteria and is robustly internalized by bacteria. a, MH19F has high specificity for bacteria over hepatocytes. E. coli (EC), EC with LamB mutation (LamB) and mammalian cells were incubated with 500 μM MH19F for 1 hour in the presence or absence of 50 mM maltohexaose (MH). The intracellular MH19F concentration was determined and normalized to protein content. Bacteria robustly accumulate MH19F whereas hepatocytes have negligible uptake. The uptake of MH19F in EC is inhibited by a large excess of maltohexaose, and the uptake of MH19F in LamB mutants is significantly reduced. The results are expressed as mean micromoles per gram of protein ± s.e.m. for n = 3 per group. b, The accumulation of MH19F in EC reaches millimolar concentrations. EC were incubated with 500 μM MH19F, and the intracellular concentration of MH19F was determined at different time points, n = 3 per group.
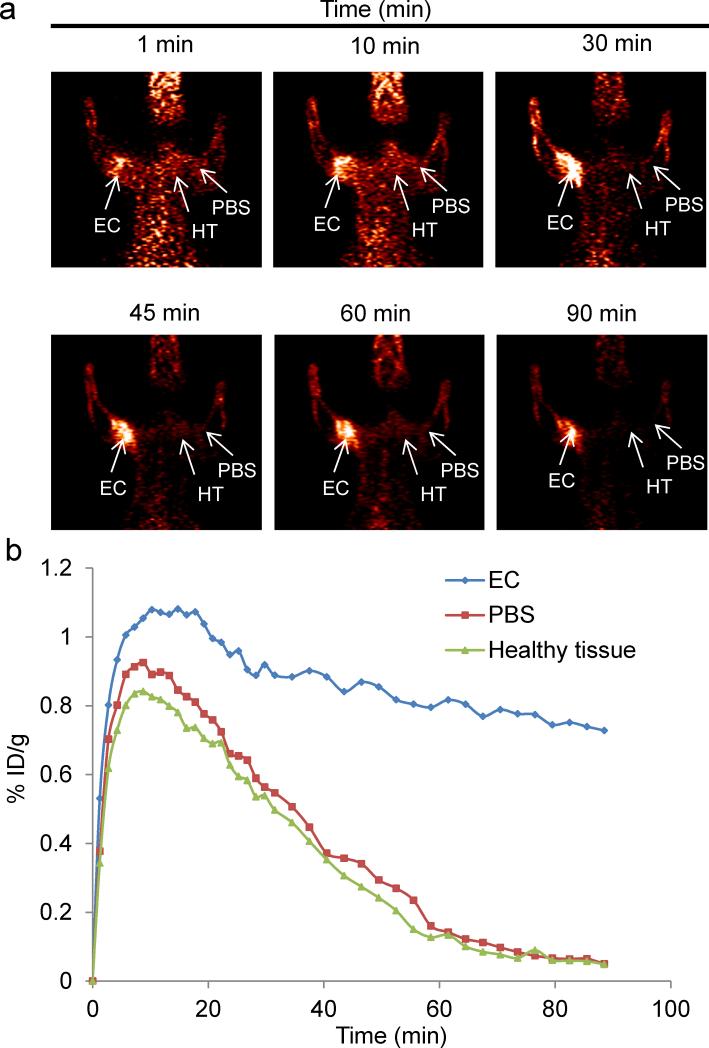
We investigated the ability of MH18F to image bacterial infections in rats. E.coli (107 CFUs) were injected into the left triceps muscle of rats, and the right triceps muscle was injected with PBS as a control. Two hours later, the rats were injected with 250 μCi of MH18F via the tail vein, and dynamic PET scans were performed using an Inveon micro PET/CT Preclinical Scanner (Siemens). Figures 2a and b demonstrates that MH18F clears well from healthy tissue but is retained in infected muscle. For example, bacterial infections were clearly visible as early as 10 min after MH18F injection and after seventy minutes had a high target-to-control contrast of 8.5, allowing bacterial infections to be easily visualized in vivo.
Figure 2.
In vivo PET imaging of rats infected with E.coli (107CFUs). a. Rats were infected in the left triceps muscle with 107 E.coli, injected with MH18F, and dynamic PET scans were performed for 90 minutes using a microPET/CT. Infected muscles can be easily visualized after 90 min. b. Time activity curves of decay-corrected MH18F activity in the infected rat, generated from Figure 2a. Infected muscle has an 8.5 fold increase in radioactivity over PBS injected muscle. Arrows indicate the location of infected muscle (EC), PBS injected muscle (PBS) and healthy tissue (HT).
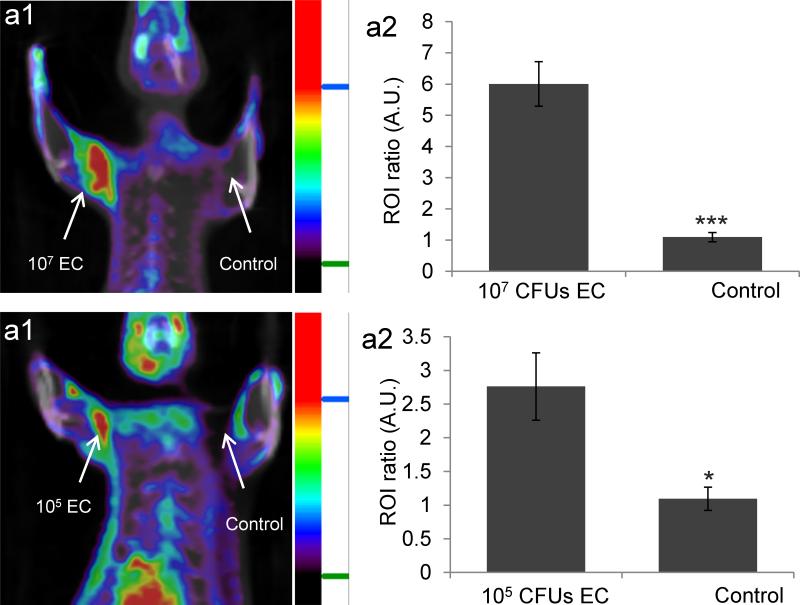
A key challenge in imaging bacteria is developing probes that have high sensitivity for bacteria.[21] Current PET tracers for imaging bacteria, such as 18FDG and radiolabeled antibiotics and antibodies, can only image 107-109 bacterial CFUs in vivo, and cannot detect infections at an early stage.[22] MH18F has the potential to detect small numbers of bacteria because of its fast transport into bacteria and its rapid clearance from un-infected tissues. We investigated the ability of MH18F to image early stage bacterial infections. E.coli (105 CFUs) were injected into the left triceps muscle of rats and imaged with MH18F as described above. Figure 3b demonstrates that MH18F is capable of detecting as few as 105 bacterial CFUs in vivo, for example, rat triceps muscles infected with 105 bacterial CFUs had a 2.7 fold increase in radioactivity over un-infected controls. Thus, MH18F's unique combination of robust transport into bacteria and clearance from healthy tissues, allows it to image bacteria with high sensitivity.
Figure 3.
MH18F can detect as few as 105 CFUs of E.coli (EC) in muscle infections. a1, MH18F can detect 107 E.coli CFUs in rats. Rats were infected with 107 E.coli and imaged with MH18F using a microPET/CT. The rat image is a representative result of four experiments, and identifies the infection site. a2, MH18F generates a 6 fold increase in radioactivity in infected muscles. b1, MH18F can detect as few as 105 E.coli in rats. Rats were infected with 105 E.coli CFUs and imaged with MH18F using a microPET/CT. The rat image is a representative result of four experiments, and identifies the infection site. b2, MH18F generates a 2.7 fold increase in radioactivity in infected muscles. Regions of interest (ROIs) including the infected muscles (target) or PBS injection areas (control) and healthy tissues (background) were identified and integrated using ASI Pro VM™ micro PET analysis software. The results in a2 and b2 are expressed as the target or control to background ratio (ROI ratio) ± s.e.m. for n = 4 per group. The ROI ratio is defined as the mean radioactivity in the target/the mean radioactivity in the background. The statistical significances in a2 and b2 were determined using a two-sample Student's t-test (*p ≤ 0.05 and ***p ≤ 0.001).
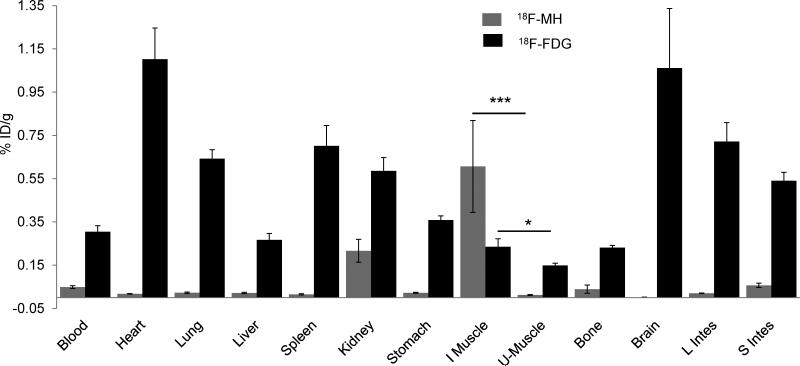
18FDG is currently the only PET radiopharmaceutical available for imaging bacterial infections; however, 18FDG has significant limitations due to its high uptake in mammalian cells.[23] To determine the translational potential of MH18F, a biodistribution study was performed with MH18F and 18FDG to compare their specificity for bacteria and non-specific adsorption in healthy tissues. Rats were infected with 109 colony forming units (CFUs) of E.coli and intravenously injected with either MH18F or 18FDG. After one hour post administration, the various organs were harvested and their radioactivty was measured. Figure 4 demonstrates that MH18F is specific for bacteria and has excellent clearance from healthy tissues. For example, MH18F generated a 30 fold difference in accumulation between infected muscles versus healthy muscles, and in contrast, 18FDG generated only a 1.5 fold difference. The improved biodistribution pattern of MH18F over 18FDG is due to the exclusive expression of maltohexaose transporters in bacteria in contrast to the high expression of glucose transporters in mammalian cells.[12c, 24] This allowed MH18F to clear from all of the major organs including heart, lung, brain, liver, bone and muscle, whereas 18FDG had significant accumulation within these tissues. For example, in infected rats, the ratio of accumulation of MH18F in infected muscle versus liver was 5, whereas for 18FDG, this ratio was only 0.3, and for other reported PET contrast agents the infected muscle to liver ratio is also generally less than 1.[1a, 7-8] The excellent clearance of MH18F allowed it to target bacteria much better than 18FDG, and MH18F therefore has the potential to image bacterial infections in a variety of anatomical areas.
Figure 4.
MH18F is more effective than 18FDG at imaging bacterial infections. A biodistribution study was performed with either MH18F or 18FDG in rats infected with 109 E.coli CFUs. MH18F is efficiently cleared from un-infected tissues, whereas 18FDG has significant accumulation within the major organs. The results are expressed as % injected dose/gram tissue ± s.e.m. for n = 4 per group. Statistical significance was determined using a two-sample Student's t-test (*p ≤ 0.05 and ***p ≤ 0.001).
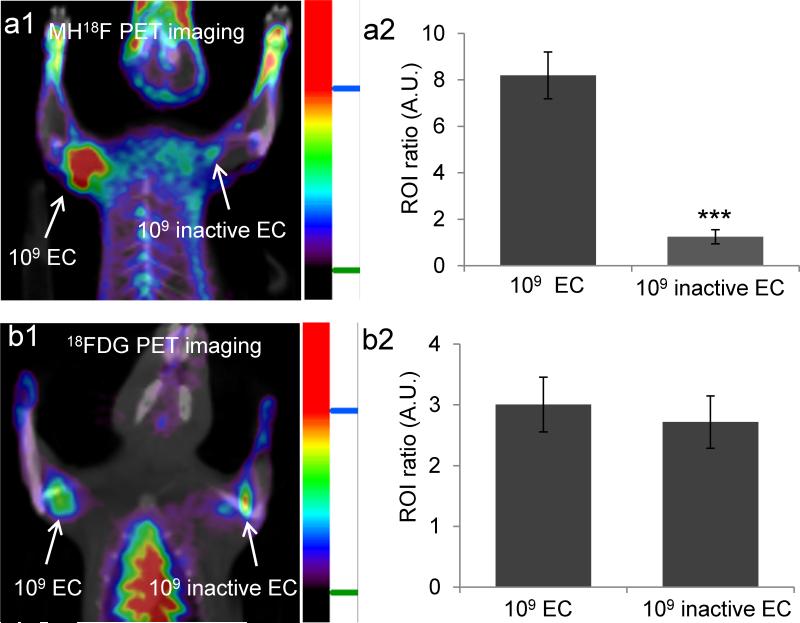
At present, there is no direct method available to monitor the efficacy of antibiotic treatment, and doctors therefore have to rely on non-specific and imprecise clinical indicators to guide antibiotic therapy.[25] MH18F has the potential to image bacterial drug resistance because it targets ATP binding cassette (ABC) transporters,[26] which require ATP for internalizing their substrates, connecting MH18F's uptake with cellular metabolism and bacterial viability. We therefore investigated if MH18F could distinguish between live versus dead bacteria and identify resistance to therapy. We first performed PET imaging with MH18F and 18FDG, and compared their ability to monitor bacterial metabolic activity in vivo. Rats were injected with 109 CFUs of live E.coli in their left triceps and 109 CFUs of metabolically inactive E.coli (sodium azide treated) in their right triceps. Two hours later, the rats were injected with 250 μCi of either MH18F or 18FDG via the tail vein, and imaged using an Inveon micro PET/CT Scanner. Figure 5a shows that MH18F can distinguish between live versus metabolically inactive bacteria, for example metabolically active E.coli had a 7 fold increase in relative radioactivity over sodium azide treated metabolically inactive bacteria, demonstrating that MH18F is being actively transported by bacteria in vivo. In contrast, Figure 5b shows that 18FDG could not distinguish between live versus dead bacteria, due to its high uptake by inflammatory cells.
Figure 5.
MH18F can distinguish between live versus dead bacteria and can discriminate infections from inflammation. a1, MH18F can distinguish between live versus dead bacteria in vivo. Rats were infected with 109 live and dead E.coli and imaged with MH18F using a microPET/CT. The rat image is a representative result of four experiments, and demonstrates that MH18F does not accumulate in dead bacteria. a2, E.coli infected tissues had a 7 fold increase in radioactivity over muscles treated with dead bacteria. b1, 18FDG cannot distinguish between live and dead E.coli infected tissues. Rats were infected with 109 live and dead E.coli CFUs and imaged with 18FDG using a microPET/CT. The image is a representative result of four experiments, and demonstrates that 18FDG cannot discriminate live bacteria from dead bacteria. b2, 18FDG accumulates in both live and dead bacteria infected tissues. ROIs including the infected muscles (target) and healthy tissues (background) from a1 and b1were identified and integrated using ASI Pro VM™ micro PET analysis software. The results in a2 and b2 are expressed as ROI ratio ± s.e.m. for n = 4 per group. The ROI ratio is defined as the mean radioactivity in the target/the mean radioactivity in the background. The statistical significance in a2 was determined using a two-sample Student's t-test (***p ≤ 0.001).
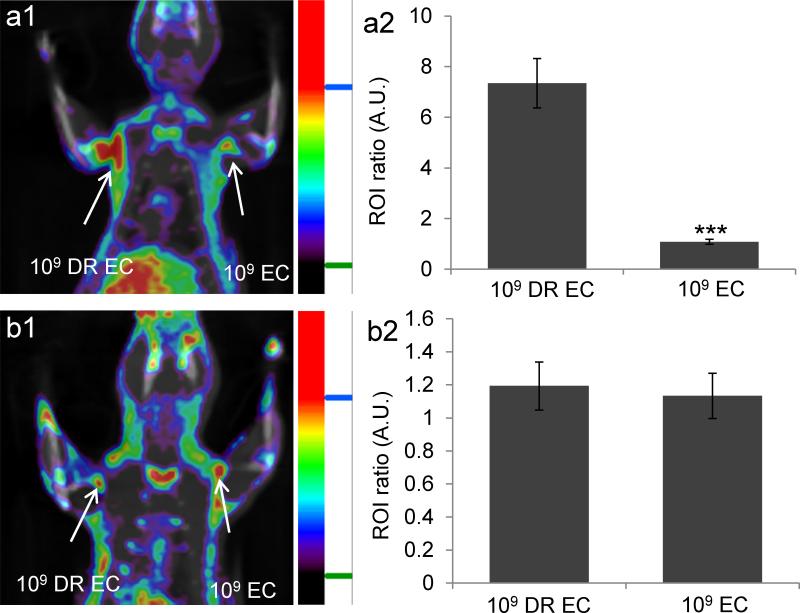
Based on these results we investigated if MH18F could identify bacterial drug resistance in vivo and measure antibiotic efficacy. Rats were infected with ampicillin-resistant E.coli (109 CFUs) and wild-type E.coli (109 CFUs), treated with ampicillin and imaged with MH18F. Figure 6a demonstrates that MH18F can measure the efficacy of antibiotics in vivo and rapidly identify drug resistance. For example, ampicillin-resistant E.coli generated an 8.2 fold increase in PET signal intensity over susceptible E.coli, due to their increased survival under antibiotic treatment. In addition, we investigated if MH18F could monitor the treatment of ampicillin resistant bacteria with ciprofloxacin, and be used as a real time methodology to assess antibiotic efficacy. Figure 6b demonstrates that in rats treated with ciprofloxacin, both ampicillin-resistant E.coli (109 CFUs) and wild-type E.coli (109 CFUs) infected tissues have very low accumulation of MH18F, indicating that MH18F can quantify the effects of antibiotics, and can be used to guide the selection of antibiotics.
Figure 6.
MH18F can measure drug resistance and monitor the therapeutic effect of antibiotics in vivo. a1, MH18F can identify drug resistance in bacteria in vivo. Rats were infected with 109 CFUs of ampicillin-resistant E.coli (DR EC) and wild-type E.coli (EC), treated with ampicillin and imaged with MH18F using a microPET/CT. The rat image is a representative result of four experiments, and demonstrates that MH18F only accumulates in DR EC infected muscles. a2, DR EC generated a 10 fold increase in radioactivity over EC. b1, MH18F can monitor the therapeutic effect of antibiotics. Rats were infected with DR EC and EC, treated with ciprofloxacin and imaged with MH18F using a microPET/CT. The rat image is a representative result of four experiments, and demonstrates that both DR EC and EC infected muscles have weak accumulation of MH18F. a2, Both infected tissues have weak radioactivity. ROIs including the infected muscles (target) and healthy tissues (background) from a1 and b1 were identified and integrated using ASI Pro VM™ micro PET analysis software. The results in a2 and b2 are expressed as ROI ratio ± s.e.m. for n = 4 per group. The ROI ratio is defined as the mean radioactivity in the target/the mean radioactivity in the background. The statistical significance in a2 was determined using a two-sample Student's t-test (***p ≤ 0.001).
In conclusion, in this report we present a bacterial targeted PET tracer, termed MH18F, which can image bacteria in vivo with sensitivity and specificity that is orders of magnitude better than previously reported PET tracers. MH18F can also identify drug resistance and can therefore potentially assist physicians in prescribing antibiotics. Finally, MH18F can be synthesized in one radiochemical step from clinically available K18F, and therefore has the potential to rapidly enter into clinical trials.
Supplementary Material
Acknowledgments
This project has been funded in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. NIH R01 1R01HL096796-02 (N.M.), NIH U01 268201000043C-0-0-1, NIH R211R21AI098799-01 (N.M.), NIH 7UM1AI068636-07 and NIH R01 AI088023-03.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
Contributor Information
Xinghai Ning, Department of Bioengineering, UC Berkeley, 284 Hearst Memorial Mining Building, UC Berkeley, Berkeley, CA 94720 (USA).
Wonewoo Seo, Department of Radiology and Imaging Sciences, Emory University (USA).
Seungjun Lee, Department of Bioengineering, UC Berkeley, 284 Hearst Memorial Mining Building, UC Berkeley, Berkeley, CA 94720 (USA).
Kiyoko Takemiya, Division of Cardiology, Department of Medicine, Emory University School of Medicine (USA).
Mohammad Rafi, Department of Bioengineering, UC Berkeley, 284 Hearst Memorial Mining Building, UC Berkeley, Berkeley, CA 94720 (USA).
Xuli Feng, Department of Bioengineering, UC Berkeley, 284 Hearst Memorial Mining Building, UC Berkeley, Berkeley, CA 94720 (USA).
Daiana Weiss, Division of Cardiology, Department of Medicine, Emory University School of Medicine (USA).
Xiaojian Wang, Department of Bioengineering, UC Berkeley, 284 Hearst Memorial Mining Building, UC Berkeley, Berkeley, CA 94720 (USA).
Larry Williams, Department of Radiology and Imaging Sciences, Emory University (USA).
Vernon M. Camp, Department of Radiology and Imaging Sciences, Emory University (USA)
Malveaux Eugene, Department of Radiology and Imaging Sciences, Emory University (USA).
W. Robert Taylor, Division of Cardiology, Department of Medicine and the Department of Biomedical Engineering, Emory University School of Medicine and the Atlanta VA Medical Center (USA).
Mark Goodman, Department of Radiology and Imaging Sciences, Emory University (USA).
Niren Murthy, Department of Bioengineering, UC Berkeley, 284 Hearst Memorial Mining Building, UC Berkeley, Berkeley, CA 94720 (USA).
References
- 1.a Lupetti A, Welling MM, Pauwels EK, Nibbering PH. Lancet Infect. Dis. 2003;3:223–229. doi: 10.1016/s1473-3099(03)00579-6. [DOI] [PubMed] [Google Scholar]; b van Doorn LJ, Henskens Y, Nouhan N, Verschuuren A, Vreede R, Herbink P, Ponjee G, van Krimpen K, Blankenburg R, Scherpenisse J, Quint W. J. Clin. Microbiol. 2000;38:13–17. doi: 10.1128/jcm.38.1.13-17.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Lipsky BA, Itani K, Norden C. G. Linezolid Diabetic Foot Infections Study. Clin. Infect. Dis. 2004;38:17–24. doi: 10.1086/380449. [DOI] [PubMed] [Google Scholar]; b Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr., Musher DM, Niederman MS, Torres A, Whitney CG. A. Infectious Diseases Society of, S. American Thoracic. Clin. Infect. Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Gillissen A, Paparoupa M. Clin. Respir. J. 2014 doi: 10.1111/crj.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sfanos KS, Isaacs WB, De Marzo AM. Am. J. Clin. Exp. Urol. 2013;1:3–11. [PMC free article] [PubMed] [Google Scholar]
- 4.a Phelps ME. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ametamey SM, Honer M, Schubiger PA. Chem. Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 5.a Glaudemans AW, Signore A. Eur. J. Nucl. Med. Mol. Imag. 2010;37:1986–1991. doi: 10.1007/s00259-010-1587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Erba PA, Bandera F, Sollini M, Tascini C. J. Am. Coll. Cardiol. 2012;60:1435–1436. doi: 10.1016/j.jacc.2012.05.039. author reply 1437. [DOI] [PubMed] [Google Scholar]; c Keidar Z, Nitecki S. Semin. Nucl. Med. 2013;43:396–402. doi: 10.1053/j.semnuclmed.2013.04.004. [DOI] [PubMed] [Google Scholar]; d del Rosal T, Goycochea WA, Mendez-Echevarria A, Garcia-Fernandez de Villalta M, Baquero-Artigao F, Coronado M, Marin MD, Albajara L. Eur. J. Pediatr. 2013;172:1111–1115. doi: 10.1007/s00431-013-1983-x. [DOI] [PubMed] [Google Scholar]
- 6.a Corstens FHM, van der Meer JWM. The Lancet. 1999;354:765–770. doi: 10.1016/S0140-6736(99)06070-5. [DOI] [PubMed] [Google Scholar]; b Gemmel F, Dumarey N, Welling M. Semin. Nucl. Med. 2009;39:11–26. doi: 10.1053/j.semnuclmed.2008.08.005. [DOI] [PubMed] [Google Scholar]; c van Oosten M, Schäfer T, Gazendam JAC, Ohlsen K, Tsompanidou E, de Goffau MC, Harmsen HJM, Crane LMA, Lim E, Francis KP, Cheung L, Olive M, Ntziachristos V, van Dijl JM, van Dam GM. Nat. Commun. 2013:4. doi: 10.1038/ncomms3584. [DOI] [PubMed] [Google Scholar]
- 7.Langer O, Muller U, Brunner M, Zeitlinger M, Joukhadar C, Mitterhauser M, Wadsak W, Kletter K, Muller M. Eur. J. Nucl. Med. Mol. Imag. 2004;31:S447–S447. [Google Scholar]
- 8.a Rusckowski M, Gupta S, Liu G, Dou S, Hnatowich DJ. J. Nucl. Med. 2004;45:1201–1208. [PubMed] [Google Scholar]; b Rennen HJ, Makarewicz J, Oyen WJ, Laverman P, Corstens FH, Boerman OC. Nucl. Med. Biol. 2001;28:401–408. doi: 10.1016/s0969-8051(01)00208-6. [DOI] [PubMed] [Google Scholar]
- 9.a Bhattacharya A, Kochhar R, Sharma S, Ray P, Kalra N, Khandelwal N, Mittal BR. J. Nucl. Med. 2014;55:1267–1272. doi: 10.2967/jnumed.114.137232. [DOI] [PubMed] [Google Scholar]; b Jones HA, Peters AM, Clark JC. Nucl. Med. Commun. 2001;22:601–602. doi: 10.1097/00006231-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 10.a Petrik M, Haas H, Schrettl M, Helbok A, Blatzer M, Decristoforo C. Nucl. Med. Biol. 2012;39:361–369. doi: 10.1016/j.nucmedbio.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Langer O, Brunner M, Zeitlinger M, Ziegler S, Müller U, Dobrozemsky G, Lackner E, Joukhadar C, Mitterhauser M, Wadsak W, Minar E, Dudczak R, Kletter K, Müller M. Eur. J. Nucl. Med. Mol. Imag. 2005;32:143–150. doi: 10.1007/s00259-004-1646-2. [DOI] [PubMed] [Google Scholar]; c Petruzzi N, Shanthly N, Thakur M. Semin. Nucl. Med. 2009;39:115–123. doi: 10.1053/j.semnuclmed.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lu C, Jiang Q, Tan C, Tang J, Zhang J. Molecules. 2012;17:8518–8532. doi: 10.3390/molecules17078518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Signore A, Mather SJ, Piaggio G, Malviya G, Dierckx RA. Chem. Rev. 2010;110:3112–3145. doi: 10.1021/cr900351r. [DOI] [PubMed] [Google Scholar]
- 12.a Shuman HA. Ann. Microbiol. (Paris) 1982;133A:153–159. [PubMed] [Google Scholar]; b Klebba PE. Res. Microbiol. 2002;153:417–424. doi: 10.1016/s0923-2508(02)01340-2. [DOI] [PubMed] [Google Scholar]; c Charbit A. Front. Biosci. 2003;8:s265–274. doi: 10.2741/1046. [DOI] [PubMed] [Google Scholar]; d Gopal S, Berg D, Hagen N, Schriefer EM, Stoll R, Goebel W, Kreft J. PLoS One. 2010;5:e10349. doi: 10.1371/journal.pone.0010349. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Boos W, Shuman H. Microbiol. Mol. Biol. Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a Dippel R, Boos W. J Bacteriol. 2005;187:8322–8331. doi: 10.1128/JB.187.24.8322-8331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Freundlieb S, Ehmann U, Boos W. J Biol Chem. 1988;263:314–320. [PubMed] [Google Scholar]
- 14.a Reuss R, Ludwig J, Shirakashi R, Ehrhart F, Zimmermann H, Schneider S, Weber MM, Zimmermann U, Schneider H, Sukhorukov VL. J. Membr. Biol. 2004;200:67–81. doi: 10.1007/s00232-004-0694-7. [DOI] [PubMed] [Google Scholar]; b Ning X, Lee S, Wang Z, Kim D, Stubblefield B, Gilbert E, Murthy N. Nat. Mater. 2011;10:602–607. doi: 10.1038/nmat3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharff AJ, Rodseth LE, Spurlino JC, Quiocho FA. Biochemistry. 1992;31:10657–10663. doi: 10.1021/bi00159a003. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd R, Robertson A, Ofman D. Food Hydrocolloid. 2000;14:281–286. [Google Scholar]
- 17.a Dippel R, Boos W. J. Bacteriol. 2005;187:8322–8331. doi: 10.1128/JB.187.24.8322-8331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Freundlieb S, Ehmann U, Boos W. J. Biol. Chem. 1988;263:314–320. [PubMed] [Google Scholar]
- 18.a Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]; b Kolb HC, Finn MG, Sharpless KB. Angew. Chem. 2001;113:2056–2075. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.a O'Hagan D, Schaffrath C, Cobb SL, Hamilton JT, Murphy CD. Nature. 2002;416:279. doi: 10.1038/416279a. [DOI] [PubMed] [Google Scholar]; b Kim DW, Choe YS, Chi DY. Nucl. Med. Biol. 2003;30:345–350. doi: 10.1016/s0969-8051(03)00017-9. [DOI] [PubMed] [Google Scholar]; c Kim DW, Chi DY. Angew. Chem. 2004;116:489–491. [Google Scholar]; Angew. Chem. Int. Ed. 2004;43:483–485. doi: 10.1002/anie.200352760. [DOI] [PubMed] [Google Scholar]; d Kim DW, Ahn DS, Oh YH, Lee S, Kil HS, Oh SJ, Lee SJ, Kim JS, Ryu JS, Moon DH, Chi DY. J. Am. Chem. Soc. 2006;128:16394–16397. doi: 10.1021/ja0646895. [DOI] [PubMed] [Google Scholar]; e Glaser M, Arstad E. Bioconjugate Chem. 2007;18:989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]; f Vaidyanathan G, Zalutsky MR. Nucl. Med. Biol. 1992;19:275–281. [Google Scholar]; g Iwata R, Pascali C, Bogni A, Horvath G, Kovacs Z, Yanai K, Ido T. Appl. Radiat. Isot. 2000;52:87–92. doi: 10.1016/s0969-8043(99)00117-7. [DOI] [PubMed] [Google Scholar]; h Prante O, Einsiedel J, Haubner R, Gmeiner P, Wester HJ, Kuwert T, Maschauer S. Bioconjugate Chem. 2007;18:254–262. doi: 10.1021/bc060340v. [DOI] [PubMed] [Google Scholar]; i Ting R, Adam MJ, Ruth TJ, Perrin DM. J. Am. Chem. Soc. 2005;127:13094–13095. doi: 10.1021/ja053293a. [DOI] [PubMed] [Google Scholar]; j Lee E, Kamlet AS, Powers DC, Neumann CN, Boursalian GB, Furuya T, Choi DC, Hooker JM, Ritter T. Science. 2011;334:639–642. doi: 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Lee E, Hooker JM, Ritter T. J. Am. Chem. Soc. 2012;134:17456–17458. doi: 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Huiban M, Tredwell M, Mizuta S, Wan Z, Zhang X, Collier TL, Gouverneur V, Passchier J. Nat. Chem. 2013;5:941–944. doi: 10.1038/nchem.1756. [DOI] [PubMed] [Google Scholar]
- 20.a Surasi DS, Bhambhvani P, Baldwin JA, Almodovar SE, O'Malley JP. J. Nucl. Med. Technol. 2014;42:5–13. doi: 10.2967/jnmt.113.132621. [DOI] [PubMed] [Google Scholar]; b Nakao R, Ito T, Yamaguchi M, Suzuki K. Nucl. Med. Biol. 2008;35:239–244. doi: 10.1016/j.nucmedbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.a Bettegowda C, Foss CA, Cheong I, Wang Y, Diaz L, Agrawal N, Fox J, Dick J, Dang LH, Zhou S, Kinzler KW, Vogelstein B, Pomper MG. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1145–1150. doi: 10.1073/pnas.0408861102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Smith BA, Akers WJ, Leevy WM, Lampkins AJ, Xiao S, Wolter W, Suckow MA, Achilefu S, Smith BD. J. Am. Chem. Soc. 2009;132:67–69. doi: 10.1021/ja908467y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a Pellegrino D, Bonab AA, Dragotakes SC, Pitman JT, Mariani G, Carter EA. J. Nucl. Med. 2005;46:1522–1530. [PubMed] [Google Scholar]; b Fischman AJ, Livni E, Babich J, Alpert NM, Liu YY, Thom E, Cleeland R, Prosser BL, Callahan RJ, Correia JA. Antimicrob. Agents Chemother. 1992;36:2286–2292. doi: 10.1128/aac.36.10.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a Varagnolo L, Stokkel MPM, Mazzi U, Pauwels EKJ. Nucl. Med. Biol. 2000;27:103–112. doi: 10.1016/s0969-8051(99)00109-2. [DOI] [PubMed] [Google Scholar]; b Hanasono MM, Kunda LD, Segall GM, Ku GH, Terris DJ. Laryngoscope. 1999;109:880–885. doi: 10.1097/00005537-199906000-00007. [DOI] [PubMed] [Google Scholar]; c Lind P, Igerc I, Beyer T, Reinprecht P, Hausegger K. Eur. J. Nucl. Med. Mol. Imag. 2004;31:S125–S134. doi: 10.1007/s00259-004-1535-8. [DOI] [PubMed] [Google Scholar]; d Lubezky N, Metser U, Geva R, Nakache R, Shmueli E, Klausner JM, Even-Sapir E, Figer A, Ben-Haim M. J. Gastrointest. Surg. 2007;11:472–478. doi: 10.1007/s11605-006-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a Strauss LG, Koczan D, Klippel S, Pan L, Willis S, Sachpekidis C, Dimitrakopoulou-Strauss A. Am. J. Nucl. Med. Mol. Imaging. 2013;3:417–424. [PMC free article] [PubMed] [Google Scholar]; b Deron P, Vangestel C, Goethals I, De Potter A, Peeters M, Vermeersch H, Van de Wiele C. Nuklearmedizin. 2011;50:15–21. doi: 10.3413/nukmed-0324-10-06. [DOI] [PubMed] [Google Scholar]; c Wandersman C. Ann. Microbiol. (Paris) 1982;133A:161–163. [PubMed] [Google Scholar]
- 25.Roberts JA, Norris R, Paterson DL, Martin JH. Br. J. Clin. Pharmacol. 2012;73:27–36. doi: 10.1111/j.1365-2125.2011.04080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morbach S, Tebbe S, Schneider E. J. Biol. Chem. 1993;268:18617–18621. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.