Abstract
Climate change can benefit individual species, but when pest species are enhanced by warmer temperatures agricultural productivity may be placed at greater risk. We analyzed the effects of temperature anomaly on arrival date and infestation severity of potato leafhopper, Empoasca fabae Harris, a classic new world long distance migrant, and a significant pest in several agricultural crops. We compiled E. fabae arrival dates and infestation severity data at different states in USA from existing literature reviews and agricultural extension records from 1951–2012, and examined the influence of temperature anomalies at each target state or overwintering range on the date of arrival and severity of infestation. Average E. fabae arrival date at different states reveal a clear trend along the south-north axis, with earliest arrival closest to the overwintering range. E. fabae arrival has advanced by 10 days over the last 62 years. E. fabae arrived earlier in warmer years in relation to each target state level temperature anomaly (3.0 days / °C increase in temperature anomaly). Increased temperature had a significant and positive effect on the severity of infestation, and arrival date had a marginal negative effect on severity. These relationships suggest that continued warming could advance the time of E. fabae colonization and increase their impact on affected crops.
Introduction
Global surface temperature has increased by about 0.74°C in the 100 year period ending in 2005 [1] and the decade 2000–2009 was the warmest on record. There is consensus that most of the observed warming is due to human release of CO2 into the atmosphere [2]. The most recent United States Dept. of Agriculture Plant Hardiness Zone (the standard by which growers determine which plants are most likely to thrive at a location) map, which averages winter minimum temperatures from 1976–2005, has a modal increase of one full zone relative to the 1990 map that averages temperatures from 1976–1988 [3]. However, climate change has diverse effects that vary geographically, and not every species studied has accelerated its phenology in response to recent warming [4]. Increase in temperatures due to climate change may increase the risk of invasion by migrant agricultural pests [5] and long distance migration of insect pests is likely affected by climate change. Here, we relate a 62-year record of the phenology of migration by Empoasca fabae Harris, Hemiptera: Cicadellidae, also known as potato leafhopper, to the temperature anomalies associated with climate change.
Climate change affects insect population dynamics such that some species gain in prominence while others become inconspicuous. Rising temperatures directly contribute to the acceleration of phenologies in insects [6], and phenologies of several species of Lepidoptera, Aphidoidea, and Odonata [7,8] have accelerated in recent decades, though behavioral temperature regulation could buffer climate effects [9]. Earlier colonization and more rapid insect development generally increase population growth. Temperature can also affect population dynamics and geographic range of any interacting species, including host-plants, natural enemies, and parasites [5,10,11], with potentially positive or negative effects on any given species. For long distance migratory insects whose host plants are available prior to arrival, the expectation is that earlier migration will increase the severity and economic impact of infestation. Because historical records are often kept for economically significant insect pest species, they can serve as models for understanding the response of migratory species to climate change. Moreover, climate change may exacerbate agricultural problems associated with migratory pests.
Climate change may affect migratory species differently than non-migrants. Coastal and subtropical areas are expected to experience less warming than temperate and polar areas[1], and migrants avoid winter temperatures, which have risen more than summer temperatures[12]. Migration complicates the already-challenging question of how climate change alters synchrony of herbivores, host plants and natural enemies [13–15]. While the phenology of many insects has advanced with warming [7,16], the response of long-distance migratory insects and vertebrates has been less well studied than that of non-migratory insects or vertebrates [17].
E. fabae is a classic example of insect pest migration in eastern North America [18,19]. It is multivoltine with populations continuously reproducing with overlapping generations [20]. It feeds and reproduces on over 200 species of plants, including at least 26 plant families [21]. It is a key pest on a number of its numerous host plants, ranging from potato (Solanum tuberosum L.) to alfalfa (Medicago sativa L.), and to red maple trees (Acer rubrum L.) [19, 22–24]. Especially, as a key pest of alfalfa in the northcentral and northeastern United States, E. fabae results in economic yield losses of up to $66 / ha [25]. Rather than primarily affecting hosts through vectoring plant disease, E. fabae feeding initiates a cascade of biochemical and physiological changes in its host [26] that impacts agricultural producers through reduced yield or quality of plants.
Evidence suggests that E. fabae is a circular migrant [27,28]. Adults are transported by prevailing winds to the south in the fall in reproductive diapause, and overwinter in southern pine forests [29,30]. In January-February, adults move from overwintering hosts to herbaceous legumes and deciduous trees to reproduce [29]. Adults of this generation, and of subsequent generations, move northward with synoptic weather patterns [31,32] and colonize north-central and north-eastern United States, and eastern Canada [33].
Climate change may alter the migration phenology of E. fabae. Since the timing of migration may impact population ecology of E. fabae and its severity on crops, we expect climate change will impact Integrated Pest Management (IPM) programs designed to manage the pest. In this study, using historical data on E. fabae arrival time and severity data compiled from several sources, we sought to: 1) examine the trend of delayed or earlier E. fabae arrival in continental United States over a 62-year time period, 2) investigate the effect of temperature anomaly on earliest reported E. fabae arrival times, and 3) investigate the effect of arrival day and temperature anomalies on severity of E. fabae on crops. To our knowledge this research represents the first such effort examining the influence of climate change on cicadellid leafhopper migration.
Methods
Ethics Statement
Data for this study were derived primarily from literature, and climatic data were obtained from freely available resources (National Oceanic and Atmospheric Administration (NOAA)—National Climatic Data Center). No endangered or protected species were involved in the study.
First arrival dates of E. fabae and severity of impact
The first reported arrival dates of E. fabae in various states in the breeding range across the United States were compiled from existing literature and direct reports, and the infestation severity levels were obtained from an earlier published compilation. Arrival data prior to 1998 and severity data were from obtained from several sources gathered or compiled by Maredia et al. [34]. That study combined published studies of arrival date and severity [18,31,34] with reports from a network of several cooperators in central and northeastern US states. Severity was rated on a 1–5 scale (5 denoting most severe) based on percent of (primarily alfalfa) crop above economic impact threshold (details in [34]). Because of the reduced impact of E. fabae in alfalfa in this century, recent severity data were sparse and arrival dates have been recorded less frequently as well. Arrival dates for years since 1997 were obtained from published extension newsletters or cooperator records in seven states (see S1 Table). We note that there could be systematic differences between Maredia et al. [34] and our study in the network of monitoring and reporting methods for various sources. In particular, Illinois records since 1997 were taken from the phrase “are active” in a published pest bulletin, while the earlier records were collected by G. Decker, S. J. Robertson, and E. J. Armbrust [34]. Pennsylvania and Illinois arrival dates were notably different for years since 1997, and were divided into early (<1997) and late (>1997) periods in the compiled dataset. We obtained 342 records in 19 states for the first arrival of E. fabae from 1951–2012 (S1 Dataset). In addition, we obtained 196 records from 18 states for the severity of damage by E. fabae from 1951–1997. In many of the states with high number of data records, E. fabae is particularly recognized as an economically important crop pest.
For the study period, temperature anomaly (observed difference in temperature as compared to the average temperature for the reference period 1901–2000) data were obtained from NOAA—National Climatic Data Center (http://www.ncdc.noaa.gov/cag/time-series). For each study year, temperature anomaly values were calculated for each target E. fabae arrival state (19 states), and as the averages of E. fabae overwintering states (6 states with more than half their area in overwintering zones; see [29]), during winter (January through March), spring (April through June) and both seasons (January through June). Thus, six separate temperature anomaly variables were tabulated to use as explanatory variables for statistical analyses relating temperature anomalies to E. fabae arrival date. For severity of infestation, values were obtained by averaging temperature data during summer (May—July) for each target state, and then used as a predictor variable.
Statistical Analyses
First arrival dates of E. fabae
Four data points (1.2% of total observations) on E. fabae arrival dates that were extreme outliers (Julian days 82, 86,105, 199) were removed from the data set. All statistical analyses were performed on the reduced dataset using linear mixed model (LMM) analysis with state as random factor to control for repeated measurement [35], as state was the unit of measurement for the arrival day data repeatedly collected over years. The trend of change in arrival dates over the 62-year time period was examined using LMMs based on restricted maximum likelihood (REML), with arrival date as the response variable, year as the fixed effect and state as the random effect.
Temperature influence on arrival day
The influence of temperature anomaly on arrival dates was analyzed through LMMs based on REML. For the LMM, arrival day was the response variable, temperature anomaly was a fixed effect, and states were used as a random effect to account for repeated measurement [35]. We computed multiple LMMs each with one of the six different temperature anomaly variables tabulated as the explanatory variable. We identified the best model based on both Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC) values.
The significance of fixed effects in the LMMs was determined through Wald t-test. Also, a likelihood-ratio based pseudo-R2 value [36] was calculated for each model as a measure of the proportion of total variance explained. Diagnostic plots of the models visualizing within-group residuals (standardized residuals Vs fitted values, normal Q-Q plots, histograms of residuals) and estimated random effects (normal Q-Q plots and pairs-scatter plot matrix) were used to assess model appropriateness (see [35]; pgs. 174–197).
Severity of impact
The effect of arrival date, as well as of temperature anomaly, on the severity of E. fabae infestation was analyzed using cumulative link mixed models (CLMM). CLMM was selected because it is appropriate for mixed effects models with ordinal data. CLMMs were performed on the overall data with severity of infestation as ordinal response factor, state as random effect accounting for repeated measurement, and interaction effect or individual effect of date of arrival and temperature anomaly as fixed effects. The interaction of date of arrival and temperature anomaly was tested prior to testing the individual influences of date of arrival and temperature anomaly. The significance of the fixed effects was determined through a likelihood ratio test. The proportional odds assumption for the CLMM models was verified to ensure model appropriateness (see [37] for more details).
LMMs were performed with package ‘nlme’ ([38]; v 3.1–102) and pseudo-R2 values were calculated using package ‘MuMIn’ ([39]; v 1.10.0). LMMs estimated coefficients were extracted and plotted using package ‘effects’ [40]; v 2.2–4) and CLMM were performed using package ‘ordinal’ ([41]; v 2014.12–23), all in R program [42].
Results
First arrival dates of E. fabae
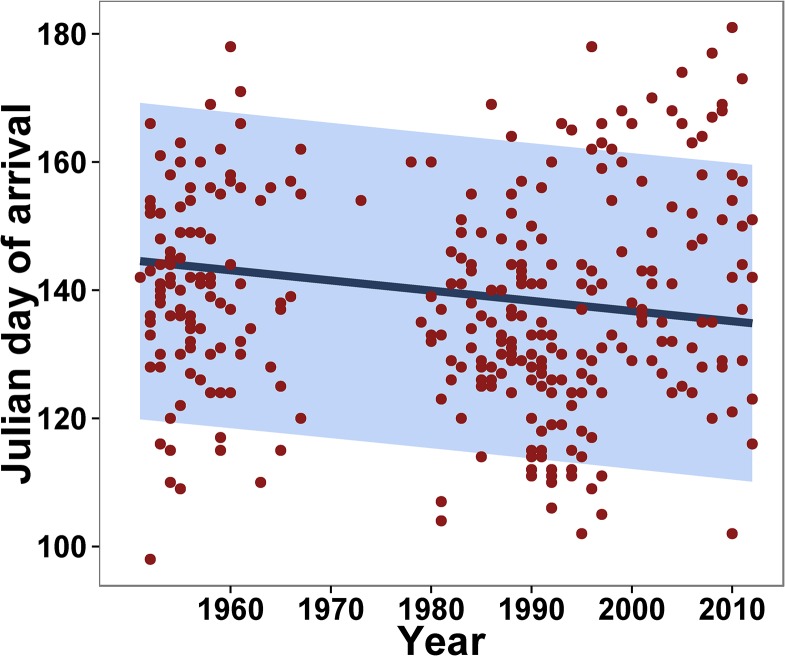
The average annual arrival date of E. fabae at different states in contiguous US, based on the compiled data, is provided in Table 1. First reported average arrival date increased along a south to north axis, corresponding to the distance from the overwintering sources in the southern part of USA (Fig 1). LMMs that examined trend of arrival demonstrated, after accounting for the state effect, a weak but significant negative association between year and arrival date (b = - 0.159, SE = 0.04, df = 316, Wald t = -3.86, P < 0.001). The model estimated that E. fabae adults arrived 0.16 days earlier with each yearly increase, or 9.7 days over 62 years (Fig 2).
Table 1. Summary of Empoasca fabae arrival and severity data across continental United States compiled for the study.
| State | Arrival data sample size | Mean arrival day ± SD | Arrival data year range | Severity data sample size | Mean severity ± SD | Severity data year range |
|---|---|---|---|---|---|---|
| Illinois | 24 | 122 ± 13.9 | 1952–1997 | 14 | 2.7 ± 1.1 | 1952–1992 |
| Illinois (>1997) | 7 | 150 ± 14.0 | 2004–2012 | - | - | - |
| Indiana | 9 | 129 ± 17.8 | 1952–1992 | 5 | 3.2 ± 1.1 | 1952–1992 |
| Iowa | 7 | 136 ± 12.6 | 1952–1992 | 3 | 4 ± 1.0 | 1953–1992 |
| Kansas | 4 | 142 ± 10.3 | 1952–1956 | 3 | 1.7 ± 0.6 | 1954–1956 |
| Kentucky | 17 | 125 ± 7.4 | 1980–1997 | 12 | 1.8 ± 1.1 | 1981–1992 |
| Maryland | 33 | 134 ± 13.3 | 1955–2012 | 18 | 3.3 ± 0.9 | 1955–1997 |
| Massachusetts | 16 | 165 ± 8.2 | 1991–2012 | - | - | - |
| Michigan | 29 | 140 ± 13.5 | 1953–2003 | 19 | 2.7 ± 1.1 | 1954–1997 |
| Minnesota | 31 | 146 ± 11.8 | 1952–2011 | 19 | 2.6 ± 1.2 | 1952–1997 |
| Missouri | 18 | 129 ± 13.0 | 1952–1995 | 14 | 2.3 ± 1.2 | 1953–1997 |
| Nebraska | 12 | 133 ± 121.3 | 1952–1989 | 9 | 2.8 ± 1.3 | 1952–1988 |
| New York | 11 | 153 ± 13.3 | 1953–1992 | 7 | 2.7 ± 1.7 | 1953–1992 |
| North Dakota | 9 | 159 ± 18.0 | 1953–1961 | 7 | 2.4 ± 1.1 | 1955–1961 |
| Ohio | 23 | 134 ± 11.4 | 1952–1997 | 14 | 2.6 ± 1.3 | 1952–1992 |
| Oklahoma | 23 | 129 ± 13.9 | 1952–1997 | 21 | 1.3 ± 0.5 | 1954–1997 |
| Pennsylvania | 6 | 141 ± 9.0 | 1955–1992 | 5 | 1.4 ± 0.5 | 1955–1992 |
| Pennsylvania (>1997) | 8 | 160 ± 10.1 | 1997–2012 | - | - | - |
| South Dakota | 7 | 155 ± 13.6 | 1952–1960 | 7 | 2.3 ± 1.0 | 1952–1960 |
| Virginia | 6 | 125 ± 14.0 | 1953–1992 | 5 | 3.4 ± 0.9 | 1953–1992 |
| Wisconsin | 42 | 133 ± 12.0 | 1951–2012 | 14 | 2.9 ± 0.9 | 1951–1992 |
The mean values provided are Julian day of arrival and severity of infestation on a scale of (1 = low to 5 = high) and States are arranged alphabetically.
Fig 1. Average arrival date of Empoasca fabae at different states across continental United States during 1951–2012, and the overwintering range (stripe shaded region; adapted from Taylor and Shields [29]).
Map was generated with spatial data on administrative state boundaries available freely through United States Census Bureau (https://www.census.gov/geo/maps-data/data/tiger-line.html).
Fig 2. Trends in arrival date (Julian day) of Empoasca fabae in United States during 1951–2012 estimated through LMM.
The straight line represents the slope and the extent of shaded region represents lower and upper 95% confidence intervals as estimated by the LMM, and the points represent raw data.
Temperature influence on arrival day
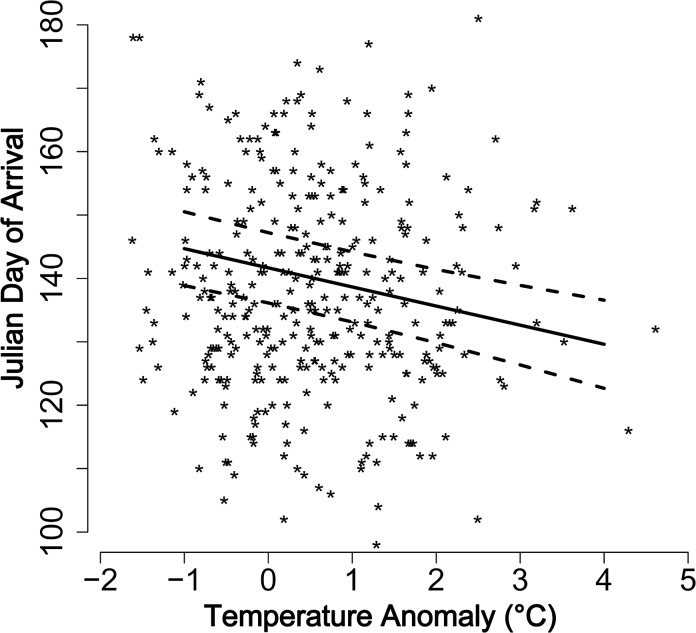
LMM with temperature anomaly calculated for the target states across both winter and summer (January through June), was selected based on AIC and BIC values (see Table 2). Arrival date (in Julian days) of E. fabae was significantly influenced negatively by temperature anomaly at each individual target states during January—June (141.68 (±2.8)– 3.01 (±0.63) temperature anomaly, Wald-t = -4.80, d. f. = 316, P < 0.001). E. fabae arrived three days earlier in relation to 1°C increase in temperature anomaly. The anomaly accounted for a third of the variation in arrival day (pseudo-R2 = 0.37; Fig 3).
Table 2. Summary of LMM results analyzing the influence of temperature anomaly on the overall earliest arrival day of Empoasca fabae in the United States during 1951–2012.
| Temperature anomaly data range | Data months | Estimate | SE | pval | Pseudo—R2 | AIC | BIC | logLik |
|---|---|---|---|---|---|---|---|---|
| Individual target states | January—June | -3.01 | 0.63 | <0.001 | 0.37 | 2687.3 | 2702.6 | -1339.6 |
| Individual target states | January—April | -1.68 | 0.39 | <0.001 | 0.37 | 2692.3 | 2707.6 | -1342.2 |
| Overwintering states | January—June | -2.82 | 0.87 | <0.001 | 0.35 | 2698.3 | 2713.6 | -1345.2 |
| Overwintering states | January—April | -1.38 | 0.51 | <0.001 | 0.34 | 2702.4 | 2717.7 | -1347.2 |
| Individual target states | April—June | -1.61 | 0.65 | 0.0136 | 0.34 | 2703.2 | 2718.5 | -1347.6 |
| Overwintering states | April—June | -1.63 | 0.90 | 0.07 | 0.33 | 2705.4 | 2720.7 | -1348.7 |
For each LMM, temperature anomaly was calculated differently as averages based on distributional range and seasons (see methods for details on overwintering and target states). Models are arranged in increasing values of AIC and BIC (Akaike information and Bayesian information criteria respectively).
Fig 3. Arrival date (Julian day) of Empoasca fabae in relation to temperature anomaly in United States during 1951–2012, estimated through LMM.
The straight lines represent the slope and dotted lines represent the lower and upper 95% confidence intervals as estimated by the LMM, and the points represents raw data.
Severity of impact
CLMM revealed a non-significant interaction term (Z value 1.70, P = 0.090) of the interaction of arrival day and temperature anomaly on infestation severity of E. fabae. CLMM with only arrival day as predictor variable was marginally significant based on a likelihood ratio test (χ2 = - 3.73, d.f. = 1, P = 0.053). Also, results showed a mild yet positive association between temperature anomaly and severity levels with 0.54 (±0.17) times greater overall odds for higher infestation with increasing state temperature anomaly which was significant in a likelihood ratio test (χ2 = 11.14, d.f. = 1, P < 0.001). In particular, the odds ratio for higher severity levels increased with temperature anomaly with 1.5 (± 0.30) times greater odds between severity scales 3 and 4, and 3.7 (±0.45) times greater odds between severity levels 4 and 5.
Discussion
Climate change may aggravate migratory insect pest impacts in agriculture. We found a trend of earlier arrival over a 62-year time period ending in 2012 (9.7 days), with a significant negative relationship between mean annual temperature anomaly and arrival of E. fabae in its summer range. Results also revealed a significant positive effect of temperature on E. fabae severity of infestation, and a marginally significant negative effect of arrival date on potato leafhopper severity of infestation. While future impacts are very challenging to anticipate due to interactions among many factors influencing insect population dynamics, these results suggest an increasingly earlier time of colonization by E. fabae with continued warming, and that severity of infestation may also increase with warming.
The relationship between temperature and arrival date, -3.0 days °C-1 over all states, is within the range observed in other insects studied. The best studied systems to date are the Aphidoidea (-10 to -14 days °C-1 [43]; 0.611±0.11 days year-1 [44]) and Lepidoptera (~7 days °C-1 [45]; -2 to -10 days °C-1 [46]) with a few other cases including Apis mellifera L. (-5.5 days °C-1), Leptinotarsa decemlineata (Say) (-5.8 days °C-1), Bactrocera oleae (Rossi) (-5.2 days °C-1 [47]), Odonata (-3.4 days °C-1 [48]), and several species including Cicadidae and Orthoptera (-2.7 days °C-1 [16]). Predicting future arrival dates in E. fabae is challenging because of imprecise knowledge of the location of source populations and proximate causes of movement and settling.
The overwintering range of E. fabae extends north of the Gulf States [29,49], but it is not known which overwintering populations contribute most to the migration into northern states. Temperature effects on migration are diverse along the overwintering range, based on the best current understanding of E. fabae overwintering phenology. After overwintering on evergreens in a non-reproductive state, a largely female population shifts to leguminous hosts and deciduous trees in early spring [30]. It is unknown the degree to which indirect cues, such as photoperiod, vs. direct cues, such as population density or availability or quality of hosts, stimulate migration [50]. Increased temperature probably allows earlier migration through accelerated development of legumes and spring leafhopper generations. In addition, earlier stress or senescence and harvest of spring host plants may lead to migration. Thus, E. fabae migration may not be limited solely by readiness of spring migrants.
Temperature can operate on several processes necessary for E. fabae colonization of spring and summer ranges. It could advance the timing of favorable synoptic weather systems to carry migrants north. Literature on changes in the timing of synoptic weather systems and climate change is sparse. Kossin [51] found that the start of the north Atlantic hurricane season has been advancing, but these synoptic storm systems form later in the summer. Hondula and Davis [52] observed a decrease in the frequency of low-pressure transition days with increasing temperature in the Midwest, suggesting fewer southerly winds available for migration. Higher temperatures could shift the northern limit of the overwintering range and shorten migratory distances, allowing smaller scale and shorter weather patterns to suffice for migration. Finally, increased temperatures could advance the timing of favorable conditions (including host plant development) for colonists of northern states. E. fabae was detected on yellow sticky cards following two favorable weather systems for migration before they were widely detected using sweep nets later in the spring [53], suggesting migratory events sometimes precede host colonization.
Annual mean global and national temperatures have increased most consistently and clearly since 1980. Yet, there does not appear to be an increase in the advance of reported occurrence since 1980 (see Fig 2). In our study, the advancement of arrival time over the 62-year time period ending 2012 (~10 days) was greater than that predicted by temperature (3 days earlier arrival / °C increase in anomaly), but visually much of the advancement in arrival (as seen in Fig 2) precedes the greatest warming. The inconsistency between the response of E. fabae phenology to temperature and the observed trends over time is similar to an existing report for other insect species [16]. In that study the pattern was attributed to confounding factors from declining populations, or development and urbanization of habitats over the same time period. In our example a decline of E. fabae pest status, due to resistant alfalfa varieties [54] and incidental suppression in potato by systemic neonicotinoid insecticides targeted at Colorado potato beetle [55], has reduced search effort and the ability to detect early spring colonizers. It is also possible that factors other than temperature limit migration or colonization and have opposed or limited any effect during the last decade.
Ultimately changes in phenology due to warming are ecologically significant if they affect population size and interactions with other species. Historical records of infestation severity offer an opportunity to test both the ecological impact of climate effects on phenology, as well as phenological effects on abundance. For example, Cocu et al. [43] found a strong effect of mild winters on early migration in the aphid, Myzus persicae (Sulz.), which was associated with highly damaging outbreaks. We found a direct influence of temperature anomaly on infestation severity, and a marginally significant negative relationship between arrival date and severity. An earlier analysis using subsets (three states and two regions) of the same severity data did not find the predicted correlation between first occurrence date and infestation severity [34]. However, we agree with Maredia et al. [34] that many factors besides arrival date contribute to severity. Our results indicate that temperature during the growing season has a greater impact on severity than arrival time. Precipitation [56] and agricultural practices [57,58] also likely have large effects.
The degree to which preparedness to migrate, suitable weather systems, or readiness to support migratory populations after colonization pose the greatest limits to migratory species [59,60] is a continuing debate to which climate-induced population changes should contribute greatly. In turn, knowledge of the limiting factors on migratory species is needed to make any predictions about the effects of changing climate and how to manage economically important migrants. The historical records often available for agricultural pests are a valuable resource for predicting and testing climate effects. Continued and focused sampling effort is needed to build on and take advantage of those records.
Supporting Information
(DOCX)
Temperature anomaly (°C) data for overwintering range and target states for winter, spring, and both winter and spring are also provided.
(XLSX)
Acknowledgments
We thank Conor O’Leary for his assistance in collecting temperature data. Ruth Hazzard, Michael Gray, Aaron Hager, helped collate individual state arrival date information and JD Carlson provided manuscripts and background on Michigan arrival and synoptic weather systems. Comments from three anonymous reviewers improved the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by PSC-CUNY award 43- 65677, https://www.rfcuny.org/rfwebsite/research/content.aspx?catID=1190 (MB), and by Hatch Act Formula Grant MD-ENTM-1016, http://www.csrees.usda.gov/business/awards/formula/hatch.html (WL). Funding for Open Access provided by the UMD Libraries Open Access Publishing Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pachauri RK, Reisinger A. Climate Change 2007 Synthesis Report: Summary for Policymakers Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, Geneva, Switzerland: IPCC Secretariat; 2007. [Google Scholar]
- 2. Weart SR. The idea of anthropogenic global climate change in the 20th century. Wiley Interdiscip Rev Clim Change. 2010;1: 67–81. 10.1002/wcc.6 [DOI] [Google Scholar]
- 3. Daly C, Widrlechner MP, Halbleib MD, Smith JI, Gibson WP. Development of a new USDA plant hardiness zone map for the United States. J Appl Meteorol Climatol. 2012;51: 242–264. 10.1175/2010JAMC2536.1 [DOI] [Google Scholar]
- 4. Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421: 37–42. 10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- 5. Porter JH, Parry ML, Carter TR. The potential effects of climatic change on agricultural insect pests. Agric For Meteorol. 1991;57: 221–240. 10.1016/0168-1923(91)90088-8 [DOI] [Google Scholar]
- 6. Taylor F. Ecology and evolution of physiological time in insects. Am Nat. 1981;117: 1–23. [Google Scholar]
- 7. Menéndez R. How are insects responding to global warming? Tijdschr Voor Entomol. 2007;150: 355–365. [Google Scholar]
- 8. Ovaskainen O, Skorokhodova S, Yakovleva M, Sukhov A, Kutenkov A, Kutenkova N, et al. Community-level phenological response to climate change. Proc Natl Acad Sci. 2013;110: 13434–13439. 10.1073/pnas.1305533110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heinrich B. Why have some animals evolved to regulate a high body temperature? Am Nat. 1977;111: 623–640. [Google Scholar]
- 10. Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421: 57–60. 10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- 11. Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. The distributions of a wide range of taxonomic groups are expanding polewards. Glob Change Biol. 2006;12: 450–455. 10.1111/j.1365-2486.2006.01116.x [DOI] [Google Scholar]
- 12. Karl TR, Melillo JM, Peterson T C, editors. Global Climate Change Impacts in the United States New York: Cambridge University Press; 2009. [Google Scholar]
- 13. Cannon RJC. The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Glob Change Biol. 1998;4: 785–796. 10.1046/j.1365-2486.1998.00190.x [DOI] [Google Scholar]
- 14. Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, et al. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol. 2002;8: 1–16. 10.1046/j.1365-2486.2002.00451.x [DOI] [Google Scholar]
- 15. Parmesan C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob Change Biol. 2007;13: 1860–1872. 10.1111/j.1365-2486.2007.01404.x [DOI] [Google Scholar]
- 16. Ellwood ER, Diez JM, Ibáñez I, Primack RB, Kobori H, Higuchi H, et al. Disentangling the paradox of insect phenology: are temporal trends reflecting the response to warming? Oecologia. 2012;168: 1161–1171. 10.1007/s00442-011-2160-4 [DOI] [PubMed] [Google Scholar]
- 17. Robinson R, Crick H, Learmonth J, Maclean I, Thomas C, Bairlein F, et al. Travelling through a warming world: climate change and migratory species. Endanger Species Res. 2009;7: 87–99. 10.3354/esr00095 [DOI] [Google Scholar]
- 18. Medler JT. Migration of the potato leafhopper-a report on a cooperative study. J Econ Entomol. 1957;50: 493–497 pp. [Google Scholar]
- 19. Chasen EM, Dietrich C, Backus EA, Cullen EM. Potato leafhopper (Hemiptera: Cicadellidae) ecology and integrated pest management focused on alfalfa. J Integr Pest Manag. 2014;5: 1–8. 10.1603/IPM13014 [DOI] [Google Scholar]
- 20. Hogg DB, Hoffman GD. Potato leafhopper population dynamics In: Armbrust EJ, Lamp WO, editors. History and perspectives of potato leafhopper (Homoptera: Cicadellidae) research. Annapolis, MD, USA: Entomological Society of America Misc. publications; 1989. pp. 26–34. [Google Scholar]
- 21. Lamp WO, Nielsen GR, Danielson SD. Patterns among host plants of potato leafhopper, Empoasca fabae (Homoptera: Cicadellidae). J Kans Entomol Soc. 1994;67: 354–368. [Google Scholar]
- 22.Noetzel DM, Cutkomp LK, Harein PK. Estimated annual losses due to insects in Minnesota 1981–1983. St. Paul, Minnesota, USA.: University of Minnesota, Institute of Agriculture, Agricultural Extension Service.; 1985. Report No.: AG-BU-2415.
- 23. Lamp WO. Reduced Empoasca fabae (Homoptera: Cicadellidae) density in oat–alfalfa intercrop systems. Environ Entomol. 1991;20: 118–126. [Google Scholar]
- 24. Bentz J-A, Townsend AM. Feeding injury, oviposition, and nymphal survivorship of the potato leafhopper on red maple and Freeman maple clones. Environ Entomol. 1999;28: 456–460. [Google Scholar]
- 25. Lamp WO, Nielsen GR, Dively GP. Insect pest-induced losses in alfalfa: patterns in Maryland and implications for management. J Econ Entomol. 1991;84: 610–618. [Google Scholar]
- 26. Backus EA, Serrano MS, Ranger CM. Mechanisms of hopperburn: an overview of insect taxonomy, behavior, and physiology. Annu Rev Entomol. 2005;50: 125–151. 10.1146/annurev.ento.49.061802.123310 [DOI] [PubMed] [Google Scholar]
- 27. Taylor PS, Shields EJ, Tauber MJ, Tauber CA. Induction of reproductive diapause in Empoasca fabae (Homoptera: Cicadellidae) and its implications regarding southward migration. Environ Entomol. 1995;24: 1086–1095. [Google Scholar]
- 28. Shields EJ, Testa AM. Fall migratory flight initiation of the potato leafhopper, Empoasca fabae (Homoptera: Cicadellidae): observations in the lower atmosphere using remote piloted vehicles. Agric For Meteorol. 1999;97: 317–330. 10.1016/S0168-1923(99)00075-1 [DOI] [Google Scholar]
- 29. Taylor PS, Shields EJ. Phenology of Empoasca fabae (Harris) (Homoptera: Cicadellidae) in its overwintering area and proposed seasonal phenology. Environ Entomol. 1995;24: 1096–1108. [Google Scholar]
- 30. Taylor PS, Shields EJ. Development of migrant source populations of the potato leafhopper (Homoptera: Cicadellidae). Environ Entomol. 1995;24: 1115–1121. [Google Scholar]
- 31. Pienkowski RL, Medler JT. Synoptic weather conditions associated with long-range movement of the potato leafhopper, Empoasca fabae, into Wisconsin. Ann Entomol Soc Am. 1964;57: 588–591. [Google Scholar]
- 32. Taylor RAJ, Reling D. Preferred wind direction of long-distance leafhopper (Empoasca fabae) migrants and its rrelevance to the return migration of small insects. J Anim Ecol. 1986;55: 1103–1114. 10.2307/4436 [DOI] [Google Scholar]
- 33.DeLong DM. Biological studies on the leafhopper Empoasca fabae as a bean pest [Internet]. United States Department of Agriculture, Economic Research Service; 1938 p. 60 pp,. Report No.: 618. Available: https://ideas.repec.org/p/ags/uerstb/166147.html
- 34. Maredia KM, Whalon ME, Gage SH, Kaeb MJ. Observations of first occurrence and severity of potato leafhopper, Empoasca fabae (Harris), (Homoptera: Cicadellidae) in the north central and eastern United States. Gt Lakes Entomol. 1998;31: 73–84. [Google Scholar]
- 35. Pinheiro JC, Bates DM. Mixed effects models in S and S-Plus. New York: Springer; 2000. [Google Scholar]
- 36. Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78: 691–692. 10.1093/biomet/78.3.691 [DOI] [Google Scholar]
- 37.Christensen RHB. A tutorial on fitting cumulative link mixed models with clmm2 from the ordinal package [Internet]. 2014. Available: http://cran.r-project.org/web/packages/ordinal/vignettes/clmm2_tutorial.pdf
- 38.Pinheiro J, Bates DM, Debroy S, Sarkar D, EISPACK authors, R-core. nlme: Linear and nonlinear mixed effects models [Internet]. 2013. Available: http://cran.r-project.org/web/packages/nlme/index.html
- 39.Bartoń K. MuMIn: Multi-model inference [Internet]. 2013. Available: http://cran.r-project.org/web/packages/MuMIn/index.html
- 40. Fox J. Effect displays in R for generalised linear models. J Stat Softw. 2003;8: 1–27. [Google Scholar]
- 41.Christensen RHB. ordinal: Regression models for ordinal data [Internet]. 2014. Available: http://www.cran.r-project.org/web/packages/ordinal/index.html
- 42. R Development Core Team. R: A language and environment for statistical computing The R foundation for statistical computing, Vienna, Austria: [Internet]. 2014. Available: http://www.R-project.org/ [Google Scholar]
- 43. Cocu N, Harrington R, Rounsevell MDA, Worner SP, Hullé M, the EXAMINE project participants. Geographical location, climate and land use influences on the phenology and numbers of the aphid, Myzus persicae, in Europe. J Biogeogr. 2005;32: 615–632. 10.1111/j.1365-2699.2005.01190.x [DOI] [Google Scholar]
- 44. Bell JR, Alderson L, Izera D, Kruger T, Parker S, Pickup J, et al. Long-term phenological trends, species accumulation rates, aphid traits and climate: five decades of change in migrating aphids. J Anim Ecol. 2015;84: 21–34. 10.1111/1365-2656.12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forister ML, Shapiro AM. Climatic trends and advancing spring flight of butterflies in lowland California. Glob Change Biol. 2003;9: 1130–1135. 10.1046/j.1365-2486.2003.00643.x [DOI] [Google Scholar]
- 46. Roy DB, Sparks TH. Phenology of British butterflies and climate change. Glob Change Biol. 2000;6: 407–416. 10.1046/j.1365-2486.2000.00322.x [DOI] [Google Scholar]
- 47. Gordo O, Sanz JJ. Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia. 2005;146: 484–495. 10.1007/s00442-005-0240-z [DOI] [PubMed] [Google Scholar]
- 48. Hassall C, Thompson DJ, French GC, Harvey IF. Historical changes in the phenology of British Odonata are related to climate. Glob Change Biol. 2007;13: 933–941. 10.1111/j.1365-2486.2007.01318.x [DOI] [Google Scholar]
- 49. Sidumo AJ, Shields EJ, Lembo A. Estimating the potato leafhopper Empoasca fabae (Homoptera: Cicadellidae) overwintering range and spring premigrant development by using geographic information system. J Econ Entomol. 2005;98: 757–764. 10.1603/0022-0493-98.3.757 [DOI] [PubMed] [Google Scholar]
- 50. Medler JT. Long-range displacement of Homoptera in the central United States. Proc XI Int Congr Entomol. 1962;3: 30–35. [Google Scholar]
- 51. Kossin JP. Is the North Atlantic hurricane season getting longer? Geophys Res Lett. 2008;35: L23705 10.1029/2008GL036012 [DOI] [Google Scholar]
- 52. Hondula DM, Davis RE. Decline in wintertime air-mass transition frequencies in the USA. Clim Res. 2011;46: 121–136. [Google Scholar]
- 53. Carlson JD, Whalon ME, Landis DA, Gage SH. Springtime weather patterns coincident with long-distance migration of potato leafhopper into Michigan. Agric For Meteorol. 1992;59: 183–206. 10.1016/0168-1923(92)90092-I [DOI] [Google Scholar]
- 54. Sulc RM, Mccormick JS, Hammond RB, Miller DJ. Population responses of potato leafhopper (Hemiptera: Cicadellidae) to insecticide in glandular-haired and non-glandular-haired alfalfa cultivars. J Econ Entomol. 2014;107: 2077–2087. 10.1603/EC14205 [DOI] [PubMed] [Google Scholar]
- 55. Huseth AS, Groves RL, Chapman SA, Alyokhin A, Kuhar TP, Macrae IV, et al. Managing colorado potato beetle insecticide resistance: new tools and strategies for the next decade of pest control in potato. J Integr Pest Manag. 2014;5: A1–A8. 10.1603/IPM14009 [DOI] [Google Scholar]
- 56. Schroeder PC, Brandenburg RL, Nelson CJ. Interaction between moisture stress and potato leafhopper (Homoptera: Cicadellidae) damage in alfalfa. J Econ Entomol. 1988;81: 927–933. 10.1093/jee/81.3.927 [DOI] [Google Scholar]
- 57. Pienkowski RL, Medler JT. Effects of alfalfa cuttings on the potato leafhopper, Empoasca fabae . J Econ Entomol. 1962;55: 973–978. [Google Scholar]
- 58. Cuperus GW, Watrin CG, Radcliffe EB. Influence of postharvest alfalfa stubble on potato leafhopper, Empoasca fabae (Harris) (Homoptera: Cicadellidae). J Kans Entomol Soc. 1986;59: 246–252. [Google Scholar]
- 59. Sillett TS, Holmes RT. Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol. 2002;71: 296–308. 10.1046/j.1365-2656.2002.00599.x [DOI] [Google Scholar]
- 60. Brower LP, Taylor OR, Williams EH, Slayback DA, Zubieta RR, Ramírez MI. Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv Divers. 2012;5: 95–100. 10.1111/j.1752-4598.2011.00142.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Temperature anomaly (°C) data for overwintering range and target states for winter, spring, and both winter and spring are also provided.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.