Abstract
This study was designed to compare the effects of linear periodization (LP) and undulating periodization (UP) on functional capacity, neuromuscular function, body composition, and cytokines in elderly sedentary women. We also aimed to identify the presence of high responders (HR), medium responders (MR), and low responders (LR) for irisin, interleukin-1 beta (IL-1β), toll-like receptor-4 (TLR-4), and brain-derived neurotrophic factor (BDNF) to resistance training (RT). Forty-nine elderly women were assigned to a control group, LP, and UP scheme. Functional capacity, body composition, maximal strength, irisin, TLR-4, BDNF, and IL-1β were evaluated. Both periodization models were effective in improving 45° leg press 1RM, chair-stand, arm curl, and time-up and go tests, with no significant differences in body composition and cytokines. Furthermore, HR, MR, and LR were identified for irisin, IL-1β, TLR-4, and BDNF, with differences between groups and moments. This study provides evidence that both periodization models were effective in improving functional capacity and neuromuscular function, with no effect on body composition and cytokines (probably as a consequence of the different responsiveness). Furthermore, for the first time, HR, MR, and LR were identified for irisin, IL1-β, TLR-4, and BDNF in response to RT.
Keywords: Resistance training, Inflammation, Biochemical markers, Responsiveness, Periodization
Introduction
Resistance training (RT) is a popular form of exercise that is recommended by national health organizations such as the American College of Sports Medicine (ACSM) and the American Heart Association (AHA). The body of knowledge from the literature examining RT supports its inclusion in daily exercise regimens of elderly populations. Moreover, RT can be performed safely to prevent the loss of lean body mass associated with sarcopenia, significantly increase muscle strength, and improve performance (Kraemer et al. 2002a, b; Westcott 2012). Additionally, RT has been shown to reduce systemic inflammation [interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α)] and is associated with lower CD14+ cell surface toll-like receptor-4 (TLR-4) expression that has a strong relationship with the aging process (Gleeson et al. 2006; Prestes et al. 2009c).
In order to maximize the benefits of RT, progressive overload (gradual increase of stress placed on the body during RT) and variation (systematic alteration of the RT program over time to allow for a training stimulus to remain optimal) are necessary (Kraemer et al. 2002a, b). One of the most commonly examined RT practices is periodization, which includes planned variation and has been shown to elicit better results as compared with nonperiodized RT, especially in the long term (more than 3 months) (Rhea and Alderman 2004).
The use of periodization in RT programs is not limited to elite athletes and has been a successful basis of exercise prescription in individuals with different training backgrounds and ages (Kraemer et al. 2002a, b). Prestes et al. (2009c) assessed the effects of linear periodization (LP) or “classic” model on cytokines and muscle strength in sedentary elderly postmenopausal women with a mean age of 63 years. The results demonstrated a significant increase in bench press, 45° leg press and standing arm curl maximal strength, while systemic cytokines, such as resistin, leptin, and interleukin-6 (IL-6), decreased after 16 weeks of training. To our knowledge, this is the only study to investigate the effects of planned variation during an RT program on muscle strength and cytokines in elderly women. Other studies have targeted only comparisons between LP, undulating periodization (UP), and reverse linear periodization (RLP) on muscle strength and body composition in sedentary (de Lima et al. 2012) and trained adult women (Prestes et al. 2009a).
In addition to the previously mentioned health-related benefits, physical exercise represents a potential auxiliary treatment to delay the onset of the neurodegenerative process. The neurophysiological hypothesis includes the release and synthesis of several neurotrophic factors that are associated with an improved cognitive function, neurogenesis, angiogenesis, and plasticity (Deslandes et al. 2009). Among blood biomarkers of neuroprotection, brain-derived neurotrophic factor (BDNF) has been widely investigated (Levinger et al. 2008; Coelho et al. 2012). However, the effects of different RT periodization models and individual responsiveness of circulating BDNF in elderly women remain to be determined.
A previous study (Coelho et al. 2012) evaluated the effect of 10-week progressive RT on muscle strength and circulating BDNF in 48 elderly women with a mean age of 70 years randomly assigned to two groups (nonfrail and frail individuals). The results demonstrated that levels of BDNF were higher in nonfrail subjects when compared with the frail group. Furthermore, the RT intervention increased the levels of BDNF and was effective in increasing muscle strength. Interestingly, individual responsiveness to RT was reported. Although, not divided into high and low responders, it was shown that some subjects significantly increased BDNF, while others did not (Coelho et al. 2012). This might explain why despite increments in muscle strength, some studies did not observe significant effects of RT on circulating BDNF (Levinger et al. 2008). This is a relevant topic, because results of studies are normally discussed at the basis of group means and not by classification of responders and nonresponders, which may lead to a misleading interpretation of results (Tajra et al. 2014; Garcia et al. 2014; Loenneke et al. 2014). Even though the presence of high and low responders should receive attention, it is not known whether such individuals can be detected for other important mediators, such as irisin, TLR-4, IL1-β, and BDNF, which play important roles in insulin resistance, inflammation, obesity (Oliveira et al. 2011; Pardo et al. 2014), and neuroplasticity of the brain (Coelho et al. 2013).
Considering the limited research comparing measures of neuromuscular adaptation, cytokines, and myokines following models of RT periodization, this study aimed to observe the effects of linear and undulating periodization in elderly sedentary women. In addition, considering that participants will have different responsiveness to one or more physiologic variables, another objective was to examine differences between participants categorized as high, medium, and low responders for cytokines and myokines in response to RT. The initial hypothesis is that both periodization models will be beneficial to improve strength and functional capacity and that subjects will be identified as low, medium, or high responders to irisin, IL1-β, TLR-4, and BDNF.
Methods
Participants
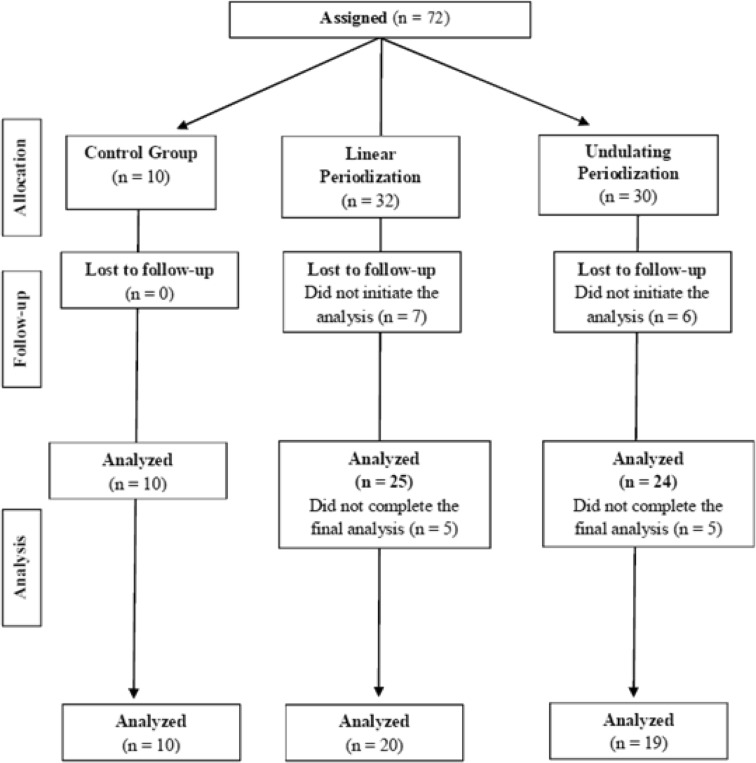
Initially, 72 sedentary elderly women were recruited and assigned into three groups: (a) a group that completed 16 weeks of RT with LP (n = 32), (b) a group that completed 16 weeks of RT with UP (n = 30) and a control group (n = 10). A total of 49 subjects completed all phases of the study and were included in the final analysis: LP (n = 20), UP (n = 19), and control group (n = 10) (see Fig. 1). Each participant completed a thorough physical examination, including a medical history, resting and exercise electrocardiogram, blood pressure assessment, anthropometric, and orthopaedic evaluation prior to participation in the experimental protocols. The inclusion criteria were as follows: female, sedentary (American College of Sports Medicine 2009), body mass index (BMI) ≤30.0 kg/m2, and age ≥60 years. Participants with physical disabilities, diagnosis of diabetes, cardiovascular diseases, hypertension (systolic blood pressure >140 mmHg and diastolic blood pressure >90 mmHg), smoking, drug/alcohol abuse, and recent (last 2 years) use of hormonal replacement were excluded from the trial. Informed consent was obtained from all individual participants included in the study. The protocol was approved by the Catholic University of Brasilia Research Ethics Committee for Human Use (Protocol #235/2010).
Fig. 1.
Flow diagram participants
Anthropometrics and body composition
Anthropometric status was investigated by the following measures: height (to the nearest 0.1 cm) and body mass (to nearest 0.1 kg), and these were used to calculate BMI (body mass/height2). All circumferences were obtained in triplicate using a nonelastic tape measure and averaged to determine the final reported circumference. Waist, neck, and hip circumferences were measured. Waist circumference was measured with individual standing up in the midway point between last floating rib and iliac crest; neck circumference was measured with participant stand erect and your head positioned in horizontal Frankfurt plane. The upper edge of the measuring tape was placed just below the laryngeal prominence and applied perpendicularly to the long axis of the neck. Hip circumference was measured with the individual standing up using the maximum circumference of the hip. Percentage body fat (PBF) and fat-free mass were determined by DEXA (General Electric-GE model 8548 BX1L, Lunar DPX type, software Encore 2005; Rommelsdorf, Germany). The tests included a complete body scan of the volunteers, in the supine position, with the apparatus always regulated and operated by a technically trained professional. The legs were secured by nonelastic straps at the knees and ankles, and the arms were aligned along the trunk with the palms facing the thighs. All metal objects were removed from the subject before the scan.
Functional tests
Functional fitness was measured by the 6-min walk test, 30-s chair-stand, arm curl, and flexibility according to the procedures of Jones and Rikli (2002). The 6-min walk test was administered according to the guidelines of the American Thoracic Society (2002). For the chair stand up test, the subject started in a seated position and was instructed to complete the highest number of squats possible during 30 s. To complete the arm curl test, the subject who sat on a chair was instructed to complete the greatest number of repetitions possible during 30 s. The timed-up and go test consisted of rising from a chair and walking as fast as possible to a cone 3 m away, circling around the cone, and returning to sit on the chair. Flexibility was tested using a standard sit-and-reach box by asking the subject to sit on the floor and bend at the trunk with the upper limbs projected forward, elbows extended up to the limit of its joint amplitude.
Strength assessments
One-repetition maximum (1 RM) tests for bench press, 45° leg press, and standing arm curl were used to determine the maximal strength. The 1 RM tests were completed on the same day with a minimum of 10 min of rest between the tests in the following order: bench press, 45° leg press, and arm curl. After a general warm-up (10 min of low intensity treadmill running), subjects completed eight repetitions with an estimated 50 % of 1 RM (according to the loads estimated in the adaptation period). After 1 min of rest, three repetitions with an estimated 70 % of 1 RM were performed. Three minutes later, subsequent trials were performed for 1 RM with progressively heavier weights until the 1 RM was determined within three attempts, using 3- to 5-min rest periods between trials. The range of motion and exercise technique was standardized according to the descriptions of Brown and Weir (2001). To guarantee stabilization of 1 RM prior to the beginning of training, the pretraining 1 RM was determined on two separate days with 3 days between them. A higher interclass correlation was found between the first and the second trial (bench press r = 0.95, 45° leg press r = 0.98, and arm curl r = 0.98).
Resistance training
Participants completed 2 weeks of RT familiarization before initiating the UP and LP RT programs, consisting of one exercise for each main muscle group and two sets of 15 submaximal repetitions. The periodization scheme was based in a previous study from our laboratory (Prestes et al. 2009b; Prestes et al. 2009c). After the familiarization period, participants initiated the LP and UP RT programs with two weekly sessions throughout the 16-week treatment period. Training machines were from Righetto Fitness Equipment (Sao Paulo, Brazil). A qualified experienced RT professional carefully supervised all training sessions. The order of exercises during RT sessions was as follows: bench press, 45° leg press, seated low row, leg extension, leg curl, triceps pulley extension, adduction and abduction machines, standing arm curl, and seated calf raise. For all listed exercises, three sets leading to concentric failure were performed, and the number of repetitions and rest intervals between sets and exercises were followed according to the intensity prescribed for the training session. The mean duration to complete one repetition was 3–4 s and training sessions lasted approximately 40–50 min. Although diet was not controlled, the participants were encouraged to maintain similar food ingestion during the intervention period.
In the LP program, the four initial weeks consisted of three sets of 12–14 RM, and from the fifth to the eighth week, three sets of 10–12 RM were performed. Weeks 9–12 consisted of 8–10 RM and 13–16 weeks three sets of 6–8 RM.
The overall training loads were equal between LP and UP. However, in the UP group, training loads varied in a daily basis. A different intensity for each training session was adopted according to the following sequence during the 16 weeks: 12–14 RM, 10–12 RM, 8–10 RM, and 6–8 RM. The rest interval between sets and exercises was as follows: 12–14 RM: 60 s; 10–12 RM: 80 s; 8–10 RM: 100 s; and 6–8 RM: 120 s. Both testing and training sessions took place between 2 p.m. and 3 p.m. after lunch and under a controlled standardized temperature.
Subgrouping for high, medium, and low responders
High responders (HR) for irisin and BDNF were defined as a ∆Irisin and ∆BDNF increment >80th percentile after training (>9.20 and 14.12 % for BDNF and irisin, respectively), medium responder (MR) between 50th and 80th percentile (2.80–9.20 % and −1.60–14.12 % for BDNF and irisin, respectively) and low responders (LR) as <50th percentile (<2.80 and <−1.60 % for BDNF and irisin, respectively). High responders for IL1-β and TLR-4 were defined as ∆IL1-β and ∆TLR-4 decrement <25th percentile (−20.37 and −17.70 % for IL1-β and TLR-4, respectively) after training, medium responders between 25th and 80th percentile (−20.37–6.74 % and −17.70–13.40 % for IL1-β and TLR-4, respectively) and low responders as >80th percentile (>6.74 and >13.40 % for IL1-β and TLR-4, respectively). For the other systemic markers, the individual responsiveness was not determined. In this case, some subjects presented undetectable measures.
Biochemical assays
Participants reported to the laboratory between 08:00–10:00 a.m., after an overnight fast, and after blood collection from the antecubital vein, samples were centrifuged at room temperature at 2.500 rpm for 10 min. All subjects were encouraged to avoid smoking, alcohol, and caffeine consumption, as well as unusual physical activity. The serum was stored at 80 °C for further analysis. Serum was analyzed for BDNF, IL-1β, IL-1ra, IL-10, and IL-15 (R&D System Inc., Minneapolis, MN, USA), irisin, and TLR-4 (MyBioSource Inc., San Diego, CA, USA). Systemic blood markers were assessed by using commercially available enzyme-linked immunosorbent assay (ELISA) kits. All samples were determined in duplicate to guarantee the precision of the results. The minimal detectable doses for dosages were as follows: 184.38 pg/mL BDNF, 0.92 pg/mL IL-1β, 2.31 pg/mL IL-1ra, 5.50 pg/mL IL-10, 5.23 pg/mL IL-15, 27.85 ng/mL irisin, and 0.20 ng/mL TLR-4.
Statistical analyses
Considering a power of 80 %, an alpha error of 0.05, and assuming a standard deviation of 53 kg, the sample size necessary to detect a mean increase of 45 kg on 45° leg press was calculated to be 13 individuals in each experimental group. For the analyses of normality and homogeneity, the Shapiro-Wilk and Levene tests were used, respectively. To examine the functional fitness tests, anthropometric, body composition, maximal strength, and cytokine response to RT, an ANOVA [3 × 2, groups × time (pre- and post-16 weeks)] was used. When differences were indicated between groups, a Hochberg Post Hoc test was applied. In the case of non-normality, Kruskal-Wallis, Wilcoxon, and Mann-Whitney analysis were applied. Bonferroni adjustment was used for all analyses. The assumption of non-normality was detected for BDNF, IL-1β, IL-1ra, IL-10, and IL-15.
For effect size calculation, the following formulas were applied (Rhea 2004).
where ME = the mean of the experimental group, MC = the mean of the control group, and SDC = the standard deviation of the control group (Rhea 2004). For determination of the magnitude of effect sizes, we considered the following values for untrained individuals (Rhea 2004). Trivial (<0.50), small (0.50–1.25), moderate (1.25–1.9), and large (>2.0).
The intention-to-treat principle was applied to the analysis of the outcomes for all participants based on their assigned treatment. In addition, the per-protocol analysis was conducted without participants who did not attend or complete the final evaluations. There were no differences between per protocol and intention-to-treat analysis. An alpha level of ≤0.05 was considered significant, and P values were two-tailed. Data are reported as mean ± standard deviation (SD). All analyses were conducted with SPSS version 18.0 (SPSS Inc., Chicago). The sample power was calculated by the software G*Power 3.1.6 (Faul et al. 2007).
Results
Functional capacity
The UP group (F(2.59) = 4.43, P = 0.016) performed higher chair stand repetitions as compared with the control group. Both LP (F(2.59) = 26.86, P = 0.001) and UP (F(2.59) = 26.86, P = 0.001) groups presented higher arm curl repetitions as compared with the control group CG before training. Additionally, LP and UP groups displayed an increase in chair stand (P = 0.001) and arm curl repetitions (P = 0.001) following 16 weeks of RT. The LP and UP groups presented an increase in the repetitions for chair stand (F(2.56) = 24.48, P = 0.001) and arm curl (F(2.56) = 24.48, P = 0.001) as compared with the control group, while only UP diminished the time to complete the time-up and go test as compared with the control group (F(2.56) = 3.72, P = 0.03) (Table 1).
Table 1.
Functional fitness capacity for experimental and control groups
| Variable | Control | LP | UP |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 95 % confidence interval for mean | 95 % confidence interval for mean | 95 % confidence interval for mean | |
| Before training | |||
| Chair stand, reps | 17.30 ± 2.71 (15.36–19.23) | 19.75 ± 4.12 (18.01–21.48) | 21.76 ± 4.55 (18.87–23.64) † |
| Arm curl, reps | 15.40 ± 1.83 (14.08–16.71) | 22.70 ± 3.74 (21.12–24.29) † | 24.68 ± 3.55 (23.21–26.14) † |
| TUG, s | 7.10 ± 0.58 (6.56–7.64) | 6.68 ± 1.01 (6.25–7.11) | 6.47 ± 0.88 (6.10–6.83) |
| 6-MWT, m | 504.00 ± 48.40 (469.37–538.62) | 521.56 ± 79.76 (487.88–555.24) | 536.78 ± 54.80 (514.15–559.40) |
| Flexibility, cm | 23.08 ± 7.01 (18.06–28.10) | 23.70 ± 8.71 (20.02–27.38) | 22.44 ± 6.89 (19.60–25.29) |
| After training | |||
| Chair stand, reps | 17.33 ± 2.87 (15.12–19.54) | 24.50 ± 4.55 (21.91–26.18) †* | 24.40 ± 3.18 (22.90–25.89) †* |
| Arm curl, reps | 15.44 ± 1.94 (13.95–16.93) | 28.75 ± 5.90 (25.98–31.51) †* | 29.15 ± 4.59 (27.00–31.29) †* |
| Tug, s | 7.11 ± 0.80 (6.48–7.73) | 6.15 ± 1.21 (5.58–6.72) | 5.90 ± 0.82 (5.51–6.29) † |
| 6-MWT, m | 501.66 ± 50.74 (462.66–540.67) | 546.13 ± 67.58 (514.50–577.76) | 536.02 ± 64.19 (505.98–566.06) |
| Flexibility, cm | 21.87 ± 6.23 (17.08–26.66) | 25.68 ± 8.23 (21.82–29.53) | 23.87 ± 6.04 (21.04–26.69) |
| Prepost effect size | |||
| Chair stand, reps | 0.01 (trivial) | 0.93 (small) | 0.61 (small) |
| Arm curl, reps | 0.02 (trivial) | 1.30 (moderate) | 1.02 (small) |
| Tug, s | 0.01 (trivial) | 0.46 (trivial) | 0.44 (trivial) |
| 6-MWT, m | 0.05 (trivial) | 0.26 (trivial) | 0.66 (trivial) |
| Flexibility, cm | 0.17 (trivial) | 0.16 (trivial) | 0.22 (small) |
| Magnitude of changes between groups | |||
| LP vs. UP | LP vs. control | UP vs. control | |
| Chair stand, reps | 0.03 (trivial) | 2.49 (large) | 2.46 (large) |
| Arm curl, reps | −0.08 (trivial) | 6.86 (large) | 7.06 (large) |
| Tug, s | 0.30 (trivial) | 1.2 (small) | 1.51(moderate) |
| 6-MWT, m | 0.15 (trivial) | 0.87 (small) | 0.67 (small) |
| Flexibility, cm | 0.29 (trivial) | 0.61(small) | 0.32 (trivial) |
Data are expressed by means and SD
LP linear periodization training, UP undulating periodization training, TUG time-up and go test, 6-MWT 6-min walking test, reps repetitions
†Difference between LP or UP vs. control at the same time point (<0.05)
*Difference between pretraining vs. posttraining (<0.05)
Anthropometrics and body composition
There were no significant differences between groups (P > 0.05) and pretest versus posttest measures (P > 0.05) for the anthropometric and body composition variables (Tables 2 and 3).
Table 2.
Anthropometry for experimental and control groups
| Variable | Control | LP | UP |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 95 % confidence interval for mean | 95 % confidence interval for mean | 95 % confidence interval for mean | |
| Before training | |||
| Age, years | 66.90 ± 7.56 | 69.20 ± 6.05 | 65.52 ± 4.72 |
| Weight, kg | 58.94 ± 6.66 (54.17–63.70) | 62.69 ± 12.03 (57.72–67.65) | 66.71 ± 9.50 (62.60–70.80) |
| Height, m | 1.52 ± 0.05 (1.48–1.56) | 1.52 ± 0.06 (1.49–1.54) | 1.52 ± 0.05 (1.49–1.54) |
| BMI, kg/m2 | 25.11 ± 3.43 (22.65–27.57) | 27.06 ± 3.78 (27.16–30.44) | 28.80 ± 3.78 (27.16–30.44) |
| Neck, cm | 32.72 ± 1.99 (31.29–34.14) | 33.50 ± 3.18 (32.19–34.81) | 34.91 ± 2.62 (33.77–36.04) |
| Waist, cm | 77.44 ± 10.13 (70.18–84.69) | 84.99 ± 12.29 (29.92–90.07) | 85.98 ± 9.73 (81.77–90.19) |
| Hip, cm | 93.32 ± 4.21 (90.30–96.33) | 98.65 ± 10.83 (94.18–103.12) | 101.78 ± 8.12 (98.26–105.29) |
| Waist-hip ratio, cm | 0.82 ± 0.07 (0.77–0.88) | 0.86 ± 0.07 (0.83–0.90) | 0.84 ± 0.06 (0.81–0.87) |
| After training | |||
| Weight, kg | 58.80 ± 7.05 (53.37–64.22) | 60.79 ± 12.52 (54.35–67.23) | 69.33 ± 11.09 (62.42–74.24) |
| Height, m | 1.53 ± 0.05 (1.49–1.57) | 1.51 ± 0.07 (1.47–1.54) | 1.52 ± 0.04 (1.50–1.55) |
| BMI, kg/m2 | 24.77 ± 3.45 (22.11–27.42) | 26.56 ± 5.47 (23.75–29.37) | 29.31 ± 4.61 (26.85–31.77) |
| Neck, cm | 32.41 ± 1.84 ( 30.99–33.82) | 32.42 ± 3.27 (30.73–34.10) | 34.28 ± 2.12 (33.15–35.41) |
| Waist, cm | 76.21 ± 9.92 (68.57–83.84) | 80.61 ± 11.63 (74.64–86.59) | 85.61 ± 11.49 (79.49–91.74) |
| Hip, cm | 93.11 ± 4.41 (89.72–96.50) | 93.26 ± 18.79 (83.60–102.92) | 101.82 ± 10.07 (96.45–107.91) |
| Waist-hip ratio, cm | 0.81 ± 0.07 (0.75–0.87) | 0.92 ± 0.39 (0.72–1.12) | 0.83 ± 0.07 (0.80–0.87) |
Data are expressed by means and SD
LP linear periodization training, UP undulating periodization training, BMI body mass index
Table 3.
Body composition for experimental and control groups
| Variable | Control | LP | UP |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 95 % confidence interval for mean | 95 % confidence interval for mean | 95 % confidence interval for mean | |
| Before Training | |||
| Body fat, % | 37.76 ± 6.03 (39.27–42.71) | 40.62 ± 6.39 (37.98–43.26) | 42.65 ± 6.83 (39.83–45.47) |
| Fat free mass,% | 62.24 ± 6.03 (57.91–66.56) | 59.37 ± 6.39 (56.73–62.01) | 57.42 ± 6.82 (54.60–60.23) |
| Body fat, kg | 21.38 ± 5.65 (17.34–25.42) | 25.46 ± 7.78 (22.24–28.67) | 27.65 ± 7.76 (24.44–30.85) |
| Fat free mass, kg | 34.22 ± 2.52 (32.42–36.03) | 34.90 ± 4.53 (33.03–36.77) | 36.02 ± 3.44 (34.60–37.45) |
| After Training | |||
| Body fat, % | 37.87 ± 6.39 (32.96–42.79) | 40.01 ± 5.47 (37.29–42.74) | 42.11 ± 7.77 (38.57–45.66) |
| Fat-free mass, % | 62.12 ± 6.39 (57.20–67.03) | 59.98 ± 5.47 (57.26–62.70) | 57.88 ± 7.77 (54.33–61.42) |
| Body fat, kg | 21.47 ± 5.98 (16.87–26.07) | 23.67 ± 7.29 (20.05 -27.30) | 27.76 ± 8.68 (23.81–3.71) |
| Fat-free mass, kg | 34.09 ± 2.64 (32.06–36.12) | 34.35 ± 5.83 (31.44–37.25) | 36.62 ± 3.25 (35.14–38.11) |
Data are expressed by means and SD
LP linear periodization training, UP undulating periodization training
Maximal strength
There were significant differences between groups (F(2.117) = 27.06, P = 0.001) and pretest versus posttest measures (F(1.117) = 8.31, P = 0.005) for the 45° leg press. The UP (F(2.58) = 8.71, P = 0.001) and LP (F(2.58) = 8.71, P = 0.001) presented a higher 45° leg press strength as compared with the control group before training. Additionally, the LP (F(2.58) = 18.53, P = 0.001) and UP (F(2.58) = 18.53, P = 0.001) groups increased leg press muscle strength by a higher amount as compared with the control group after 16 weeks of training. Moreover, LP (P = 0.001) and UP (P = 0.001) groups increased leg press strength as compared with pretraining (Table 4).
Table 4.
Maximal strength variables for experimental and control groups
| Variable | Control | LP | UP |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 95 % confidence interval for mean | 95 % confidence interval for mean | 95 % confidence interval for mean | |
| Before training | |||
| 45° Leg press, kg | 55.80 ± 14.96 (90.95–113.70) | 107.60 ± 52.85 (86.19–129.00) † | 115.68 ± 29.61 (104.45–127.90) † |
| Bench press, kg | 24.17 ± 5.01 (20.58–27.75) | 23.20 ± 5.48 (20.97–25.50) | 25.02 ± 4.93 (22.99–27.06) |
| Arm curl, kg | 16.91 ± 2.71 (14.96–18.85) | 15.27 ± 2.94 (14.06–16.49) | 17.11 ± 2.37 (14.96–18.85) |
| After training | |||
| 45° Leg press, kg | 54.44 ± 15.20 (120.76–149.30) | 158.42 ± 52.62 (133.05–183.78) †* | 150.35 ± 47.01 (128.34–172.35) †* |
| Bench press, kg | 23.77 ± 5.09 (19.80–27.64) | 26.31 ± 5.06 (23.87–28.75) | 26.38 ± 5.16 (23.96–28.30) |
| Arm curl, kg | 17.00 ± 2.92 (16.80–14.80–19.19) | 17.46 ± 3.32 (15.81–19.12) | 18.05 ± 2.71 (16.74–19.36) |
| Pre-Post Effect size | |||
| 45° Leg press, kg | 0.09 (trivial) | 0.96 (small) | 1.17 (small) |
| Bench press, kg | 0.07 (trivial) | 0.56 (small) | 0.27 (trivial) |
| Arm curl, kg | 0.03 (trivial) | 0.74 (small) | 0.39 (trivial) |
| Magnitude of changes between groups | |||
| LP vs. UP | LP vs. control | UP vs. control | |
| 45° Leg press, kg | 0.17 (small) | 6.84 (large) | 6.30 (large) |
| Bench press, kg | −0.01 (small) | 0.49 (trivial) | 0.51 (small) |
| Arm curl, kg | −0.21(small) | 0.15 (small) | 0.35 (trivial) |
Data are expressed by means and SD
LP linear periodization training, UP undulating periodization training
†Difference between LP or UP vs. control at the same time point (<0.05)
*Difference between pretraining vs. posttraining (<0.05)
Cytokines
There were significant group differences (F(2.89) = 5.41, P = 0.006), but not for pretest versus posttest measures (F(1.89) = 0.05, P = 0.81) in irisin. The LP group demonstrated higher levels of irisin as compared with the control group before training (F(2.47) = 4.70, P = 0.014) (Table 5).
Table 5.
Cytokines of experimental and control groups
| Variable | Control | LP | UP |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 95 % confidence interval for mean | 95 % confidence interval for mean | 95 % confidence interval for mean | |
| Before training | |||
| Irisin, ng/mL | 169.62 ± 36.55 (143.47–195.77) | 230.00 ± 55.88 (203.19–257.06) † | 202.10 ± 52.30 (176.89–227.31) |
| TLR-4, ng/mL | 9.54 ± 5.45 (5.74–13.54) | 7.80 ± 4.61 (5.34–10.26) | 6.69 ± 4.40 (4.57–8.81) |
| BDNF‡, pg/mL | 1433.18 ± 361.42 | 1530.13 ± 159.24 | 1540.57 ± 107.46 |
| IL-1β‡, pg/mL | 31.06 ± 38.79 | 6.83 ± 16.27 | 15.73 ± 27.85 |
| IL-1ra‡, pg/mL | 110.85 ± 272.20 | 149.32 ± 276.56 | 1411.18 ± 1365.79 |
| IL-10‡, pg/mL | 74.94 ± 49.48 | 1664.89 ± 1439.63 | 2538.22 ± 1937.41 |
| IL-15‡, pg/mL | 12.03 ± 11.12 | 230.16 ± 369.67 | 280.74 ± 280.23 |
| After training | |||
| Irisin, ng/mL | 173.64 ± 36.35 (145.70–201.59) | 217.49 ± 53.55 (191.68–243.31) | 202.23 ± 76.94 (165.14–239.32) |
| TLR4, ng/mL | 9.46 ± 5.75 (5.04–13.88) | 7.21 ± 5.50 (4.33–10.09) | 6.24 ± 4.59 (4.02–8.45) |
| BDNF‡, pg/mL | 1473.20 ± 359.08 | 1528.72 ± 148.73 | 1689.28 ± 234.54 |
| IL-1 β‡, pg/mL | 43.78 ± 45.14 | 6.55 ± 15.58 | 15.21 ± 27.65 |
| IL-1ra‡, pg/mL | 123.65 ± 288.08 | 156.44 ± 202.87 | 1015.80 ± 1311.87 |
| IL-10‡, pg/mL | 102.17 ± 21.21 | 1139.33 ± 1342.02 | 2112.67 ± 1600.71 |
| IL-15‡, pg/mL | 14.30 ± 12.43 | 175.46 ± 267.04 | 267.23 ± 265.91 |
Data are expressed by means and SD
LP linear periodization training, UP undulating periodization training, TLR-4 tool-like receptor-4, BDNF brain-derived neurotrophic factor, IL-1β interleukin-1 beta, IL-1ra interleukin-1 receptor antagonist, IL-10 interleukin-10, IL-15 interleukin-15
†Difference between LP vs. control at the same time point (<0.05)
‡These variables are not normally distributed
There were no significant group (F(2.90) = 2.82, P = 0.065) and pretest versus posttest measures (F(1.90) = 0.08, P = 0.77) differences for TLR-4. There were no significant group differences before training for BDNF (H(2): 0.47, P = 0.78), IL-1β (H(2): 5.4, P = 0.06), IL-10 (H(2): 3.8, P = 0.16), and IL-15 (H(2): 4.41, P = 0.11), while IL-1ra (H(2): 6.32, P = 0.035) displayed a difference. However, following Bonferoni adjustment, the difference became null.
Responders and nonresponders for cytokines
There were significant differences between groups for irisin (H(2): 31.00, P = 0.001), IL1-β (H(2): 29.57, P = 0.001), TLR-4 (H(2): 27.73, P = 0.001), and BDNF (H(2): 22.50, P = 0.001). The high responders for irisin demonstrated a higher percent increase as compared with medium (U = 0.00, P = 0.001) and low responders (U = 0.00, P = 0.001) following 16 weeks of RT. The high and medium responders increased the serum concentrations of irisin after training (P = 0.016 and P = 0.010, respectively), while the low responders decreased the serum concentrations of irisin after training (P = 0.001) (Table 6).
Table 6.
Responders and nonresponders for cytokines and myokines
| Responders for ∆Irisin | Before training Mean ± SD |
After training Mean ± SD |
∆% |
|---|---|---|---|
| High (n = 7) | 170.71 ± 64.87 | 251.60 ± 36.59 | 47.38†* |
| Medium (n = 12) | 219.20 ± 52.35 | 233.79 ± 59.86 | 6.65* |
| Low (n = 19) | 226.30 ± 60.67 | 176.63 ± 68.93 | −21.94* |
| Responders for ∆IL-1β | |||
| High (n = 9) | 3.66 ± 2.31 | 2.65 ±1.81 | −27.59†* |
| Medium (n = 18) | 14.87 ± 27.64 | 14.06 ± 26.25 | −5.44* |
| Low (n = 9) | 10.72 ± 22.62 | 11.83 ± 24.37 | 10.35* |
| Responders for ∆TLR-4 | |||
| High (n = 9) | 7.85 ± 4.78 | 5.33 ± 4.01 | −32,10†* |
| Medium (n = 19) | 6.21 ± 3.12 | 6.04 ± 2.84 | −2,73 |
| Low (n = 7) | 9.04 ± 6.75 | 11.11 ± 8.36 | 22,89* |
| Responders for ∆BDNF | |||
| High (n = 8) | 1344.47 ± 352.77 | 1629.27 ± 112.09 | 22,09†* |
| Medium (n = 12) | 1591.25 ± 221.62 | 1660.08 ± 227.83 | 4,32* |
| Low (n = 18) | 1627.10 ± 223.18 | 1565.95 ± 230.92 | −3,75* |
†Difference between high vs. medium and low (<0.05)
*Difference between pretraining vs. posttraining (<0.05)
For IL-1β, the high responders demonstrated a higher percent decrease as compared with medium (U = 0.00, P = 0.001) and low responders (U = 0.00, P = 0.001) groups. The high and medium responders displayed a significant decrease in IL-1β serum concentrations after training (P = 0.004 for both), while the low responders increased the serum concentrations of IL-1β after training (P = 0.008) (Table 6).
The TLR-4 decreased by a higher amount in the high responders as compared with than medium (U = 0.00, P = 0.001) and low responders (U = 0.00, P = 0.001). Moreover, high responders significantly decreased the serum concentrations of TLR-4 after training (P = 0.004), while there were no differences for the medium responders (P = 0.72). The low responders presented an increase in TLR-4 serum concentrations (P = 0.016) (Table 6).
For BDNF, the high responders demonstrated a higher percent increase as compared with medium (U = 0.00, P = 0.001) and low responders (U = 0.00, P = 0.001). The high and medium responders increased the serum concentrations of BDNF after training (P = 0.008 and P = 0.001, respectively), while the low responders decreased the serum concentrations of BDNF after training (P = 0.009) (Table 6).
Discussion
The purpose of this investigation was to determine neuromuscular and cytokine differences between periodization type (linear versus undulating) in elderly women. In addition, a secondary aim was to utilize plasma biomarkers to categorize individuals who can be considered high responders, medium responders, and low responders to a resistance-training program. Our results revealed that both LP and UP were effective in increasing lower limb muscle strength and functional capacity (chair stand and arm curl tests), with no significant effects on body composition and cytokines. Moreover, the UP group improved in the timed-up and go test, while the LP group exhibited no significant increase in this parameter following 16 weeks of RT. To the best of knowledge, this was the first study to categorize subjects into HR, MR, and LR, and as expected, we were able to identify individuals utilizing irisin, IL1-β, TLR-4, and BDNF, confirming our initial hypothesis.
The improvements in chair stand and timed-up and go tests induced by both RT protocols are important indicators of functional ability in elderly subjects. The chair stand provides a reasonably valid indication of lower body strength (Jones and Rikli 2002), and the timed-up and go test is a simple screening test that is a sensitive and predictive measure of fall risk among older adults (Shumway-Cook et al. 2000).
There was no significant alteration in anthropometrics and body composition. These results corroborate a previous study from Prestes et al. (2009b) who compared the effects of daily UP and LP in 40 experienced RT men and found no significant changes in body composition after 12 weeks of training. Although the repetition maximum zones were similar when compared with this study, the methodology and increments for LP and UP were different. In addition, contrary to Prestes et al. (2009b), this study used a more sensitive method to evaluate changes in body composition. These results may be explained by the lack of a more rigid dietetic control and the absence of combined aerobic training. Furthermore, de Lima et al. (2012) concluded that LP and UP performed with a high number of repetitions may be considered an appropriate intervention for untrained young women, leading to better improvements in body composition. However, results depend on the periodization model, microcycle duration, subject characteristics, training background, volume, and intensities selected. Because of this, further studies controlling for confounding variables are needed to clarify the benefit of different periodization models in elderly women.
Regarding muscle strength, the studies comparing linear and undulating perodization exhibited conflictant results. Rhea et al. (2002) reported that UP induced a higher percentage increase in bench press and leg press muscle strength (28.78 and 55.78 %, respectively) than LP (14.37 and 25.61 %, respectively) in young trained individuals. However, De Lima et al. (2012) showed that in sedentary young women, both LP and UP improved absolute muscle strength without significant differences between groups. However, a greater effect size was found for the LP in leg press (2.99 versus 1.73), bench press (1.77 and 0.95), and biceps curl (1.30 and 1.19). In our study, both periodization models significantly improved leg press muscle strength. Although a small improvement was observed with regard to the upper body exercises, no statistically significant differences were found. Effect size was of greater magnitude for UP (1.17) than LP (0.96) in the leg press, while LP had greater effect size in the bench press (0.56 and 0.27) and arm curl (0.74 and 0.39) as compared with UP. The difference between studies may be related with periodization methodology and the subjects analyzed. Rhea et al. (2002) used trained individuals and maximum repetition zone between 4 and 8 maximal repetitions. De lima et al. (2012) used sedentary young women and maximum repetition zone between 15 and 30 maximal repetitions, and our study used older women and maximum repetition zone between 6 and 14 maximal repetitions. It is important to note that this difference between training approached makes the comparison between studies difficult.
A previous study using the same LP scheme and older women (Prestes et al. 2009c) revealed a significant increase in bench press, 45° leg press, and standing arm curl strength after 16 weeks of RT. When comparing the delta increment and effect size (ES), the study of Prestes et al. (2009c) demonstrated a 22, 29, and 9 % increment with an ES of 1.25 (moderate), 8.96 (large), and 4.42 (large) for bench press, 45° leg press, and standing arm curl, respectively. However, this study demonstrated a 13, 47, and 14 % increment with an ES of 0.56 (small), 0.96 (small), and 0.74 (small) for the same exercises. One can hypothesize that these differences would be attributed to a higher variability within subjects in this study as compared with the study of Prestes et al. (2009c).
This was the first study to compare the effects of LP and UP on cytokines in elderly women. The results revealed that regardless of the periodization model, RT was not effective in changing irisin, TLR-4, BDNF, IL1-β, IL1-ra, IL-10, and IL-15 when all subjects were analyzed together. The results were somewhat different from the initial expectation, but according to Machado and Willardson (2010), trained individuals tend to exhibit a disproportionate increase in serum creatine kinase (CK) activity after an RT bout (consisting of three sets of 10 RM for the chest press, cable pulldown, biceps curl, triceps extension, leg extension, and prone leg curl with either 1- or 3-min rest intervals between sets). These individuals have been defined as HR and LR. This is a relevant topic because results of most RT studies are typically reported and discussed in terms of group means, and not by the classification of responders and nonresponders.
In addition, Machado and Willardson (2010) reported that an accepted definition of HR and LR currently does not exist in the literature, and that the mechanisms responsible for the disproportionate response in HR are not clearly understood. In fact, motivated by this finding, our research group reported that elderly individuals classified as HR experienced greater CK and interleukin-6 responses to an acute eccentric resistance exercise session (consisting of seven sets of 10 repetitions at 110 % of 10RM, with 3-min rest intervals between sets) (Tajra et al. 2014).
To note, adverse responses to exercise can be found for strength (Garcia et al. 2014), hemodynamic variables (Loenneke et al. 2014), plasma fasting insulin, plasma triglycerides, and plasma high density-lipoprotein variables (Bouchard et al. 2012). Because of this, this phenomenon of ‘high responders’ and ‘low responders’ may provide a helpful insight into the mechanisms responsible for training adaptations and methods of training prescription (Mann et al. 2014).
Irisin release is induced by exercise in mice and humans (Bostrom et al. 2012; Pardo et al. 2014). While an increase in irisin level elevates energy expenditure in mice, it is accompanied by no changes in movement or food intake. The increase in energy expenditure induced by irisin has been associated with improvements in clinical aspects of obesity and metabolic disorders (Bostrom et al. 2012). Based on the previously reported literature, we speculated that HR for irisin in the current investigation would have a greater decrement in body fat compared to MR and LR groups, but found no differences between groups (data not shown). Future studies that are specifically directed toward this research question are needed to clarify this hypothesis.
Interleukin 1 beta (IL1-β) is a protein released from blood monocytes and adipose tissue in response to infectious or inflammatory stimuli (Cannon et al. 1989). High responders were verified for this protein and for TLR-4; however, it is premature to speculate that these subjects are more susceptible to infections. Nevertheless, future studies should investigate if being an HR for BDNF would delay the onset of neurodegenerative processes as compared with LR.
It should be noted that not all subjects who are classified as nonresponders for one blood parameter will necessarily be nonresponders for other markers (Mann et al. 2014). For example, the same individual that was an HR for irisin in the present study was also an LR for BDNF. These are important findings because the identification of subjects at risk for an adverse condition (ex. Alzheimer) can be potentially made with greater precision. Additionally, at risk individuals may be offered guidance for reducing disease consequences or increasing benefits through exercise by changing the prescription according to their individual responsiveness to training. The present study has some limitations that should be considered, such as genetic variability of the population analyzed, lack of more precise control of nongenetic determinants of training responses (ex. sleep and stress and nutritional status), and overload control (volume load and volume index) between periodizations. In addition, participants were only separated for responsiveness, independently of periodization training mode. So, the individual responsiveness for one inflammatory marker might be related to the mode of periodization applied. Therefore, further evidence supporting the role of a standardized training intervention, as supported by Mann et al. (2014), will provide helpful insights into mechanisms of training adaptation and methods of training prescription. Although baseline values might contribute to differences in responsiveness to exercise, a high interindividual variability is consistently reported by training studies, even within homogenous groups of previously untrained subjects and after fully compliant and supervised training (Hecksteden et al. 2015).
Conclusions
This study suggests that both periodization models were effective in improving functional capacity and lower limb strength, with no significant effects on body composition and cytokines, when the whole group was analyzed, reinforcing the clinical importance of RT for elderly women. Furthermore, for the first time, high responders and low responders were identified for irisin, IL-1β, TLR-4, and BDNF. This different responsiveness may avoid misleading interpretation of results in research and also in the future, may help to individualize training prescription to optimize results.
Acknowledgments
Funding
The authors acknowledge the financial support from Conselho Nacional de Pesquisa (483549/2011-8).
Conflict of interest
The authors declare no conflict of interest in the present study.
References
- American College of Sports Medicine position stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Häkkinen K, Jenkins NT, Karavirta L, Kraus WE, Leon AS, Rao DC, Sarzynski MA, Skinner JS, Slentz CA, Rankinen T. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Weir J. ASEP procedures recommentation I: Accurate assessment of muscular strength and power. J Exercise Physiol Online. 2001;4(3):1–21. [Google Scholar]
- Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ. Increased interleukin 1 beta in human skeletal muscle after exercise. Am J Physiol. 1989;257(2 Pt 2):R451–5. doi: 10.1152/ajpregu.1989.257.2.R451. [DOI] [PubMed] [Google Scholar]
- Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56(1):10–5. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Coelho FM, Pereira DS, Lustosa LP, Silva JP, Dias JM, Dias RC, Queiroz BZ, Teixeira AL, Teixeira MM, Pereira LS. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr. 2012;54(3):415–20. doi: 10.1016/j.archger.2011.05.014. [DOI] [PubMed] [Google Scholar]
- De Lima C, Boullosa DA, Frollini AB, Donatto FF, Leite RD, Gonelli PR, Montebello MI, Prestes J, Cesar MC. Linear and daily undulating resistance training periodizations have differential beneficial effects in young sedentary women. Int J Sports Med. 2012;33(9):723–7. doi: 10.1055/s-0032-1306324. [DOI] [PubMed] [Google Scholar]
- Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, Pompeu FA, Coutinho ES, Laks J. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59(4):191–8. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Garcia P, Nascimento DD, Tibana RA, Barboza MM, Willardson JM, Prestes J. Comparison between the multiple-set plus 2 weeks of tri-set and traditional multiple-set method on strength and body composition in trained women: a pilot study. Clin Physiol Funct Imaging. 2014 doi: 10.1111/cpf.12192. [DOI] [PubMed] [Google Scholar]
- Gleeson M, McFarlin B, Flynn M. Exercise and Toll-like receptors. Exerc Immunol Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- Hecksteden A, kraushaar J, Scharhag-Rosenberger F, Theisen D, Senn S, Meyer Individual response to exercise training – a statistical perspective. J Appl Physiol. 2015 doi: 10.1152/japplphysiol.00714.2014. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE (2002) Measuring functional fitness of older adults. The Journal on Active Aging 24-30
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34(2):364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Ratamess NA, French DN. Resistance training for health and performance. Curr Sports Med Rep. 2002;1(3):165–71. doi: 10.1249/00149619-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Levinger I, Goodman C, Matthews V, Hare DL, Jerums G, Garnham A, Selig S. BDNF, metabolic risk factors, and resistance training in middle-aged individuals. Med Sci Sports Exerc. 2008;40(3):535–41. doi: 10.1249/MSS.0b013e31815dd057. [DOI] [PubMed] [Google Scholar]
- Loenneke JP, Fahs CA, Abe T, Rossow LM, Ozaki H, Pujol TJ, Bemben MG. Hypertension risk: exercise is medicine* for most but not all. Clin Physiol Funct Imaging. 2014;34(1):77–81. doi: 10.1111/cpf.12059. [DOI] [PubMed] [Google Scholar]
- Machado M, Willardson JM (2010) Short recovery augments magnitude of muscle damage in high responders. Med Sci Sports Exerc 42(7):1370–4 [DOI] [PubMed]
- Mann TN, Lamberts RP, Lambert MI. High responders and low responders: factors associated with individual variation in response to standardized training. Sports Med. 2014;44(8):1113–24. doi: 10.1007/s40279-014-0197-3. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, Bagarolli RA, Guadagnini D, Carvalheira JB, Saad MJ. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60(3):784–96. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pardo M, Crujeiras AB, Amil M, Aguera Z, Jiménez-Murcia S, Baños R, Botella C, de la Torre R, Estivill X, Fagundo AB, Fernández-Real JM, Fernández-García JC, Fruhbeck G, Gómez-Ambrosi J, Rodríguez R, Tinahones FJ, Fernández-Aranda F, Casanueva FF. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int J Endocrinol. 2014;2014:857270. doi: 10.1155/2014/857270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestes J, De Lima C, Frollini AB, Donatto FF, Conte M. Comparison of linear and reverse linear periodization effects on maximal strength and body composition. J Strength Cond Res. 2009;23(1):266–74a. doi: 10.1519/JSC.0b013e3181874bf3. [DOI] [PubMed] [Google Scholar]
- Prestes J, Frollini AB, de Lima C, Donatto FF, Foschini D, de Cássia Marqueti R, Figueira A, Jr, Fleck SJ. Comparison between linear and daily undulating periodized resistance training to increase strength. J Strength Cond Res. 2009;23(9):2437–42b. doi: 10.1519/JSC.0b013e3181c03548. [DOI] [PubMed] [Google Scholar]
- Prestes J, Shiguemoto G, Botero JP, Frollini A, Dias R, Leite R, Pereira G, Magosso R, Baldissera V, Cavaglieri C, Perez S. Effects of resistance training on resistin, leptin, cytokines, and muscle force in elderly post-menopausal women. J Sports Sci. 2009;27(14):1607–15c. doi: 10.1080/02640410903352923. [DOI] [PubMed] [Google Scholar]
- Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res. 2004;18(4):918–20. doi: 10.1519/14403.1. [DOI] [PubMed] [Google Scholar]
- Rhea MR, Alderman BL. A meta-analysis of periodized versus nonperiodized strength and power training programs. Res Q Exerc Sport. 2004;75(4):413–22. doi: 10.1080/02701367.2004.10609174. [DOI] [PubMed] [Google Scholar]
- Rhea MR, Ball SB, Phillips WT, and Burkett LN (2002) A comparison of linear and daily undulating periodization with equated volume and intensity for strength. J Strength Cond Res 16:250–55. [PubMed]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- Tajra V, Tibana RA, Vieira DC, de Farias DL, Teixeira TG, Funghetto SS, Silva AO, de Sousa NM, Willardson J, Karnikowski MG, Prestes J. Identification of high responders for interleukin-6 and creatine kinase following acute eccentric resistance exercise in elderly obese women. J Sci Med Sport. 2014;17(6):662–6. doi: 10.1016/j.jsams.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep. 2012;11(4):209–16. doi: 10.1249/JSR.0b013e31825dabb8. [DOI] [PubMed] [Google Scholar]