Abstract
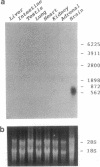
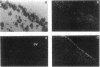
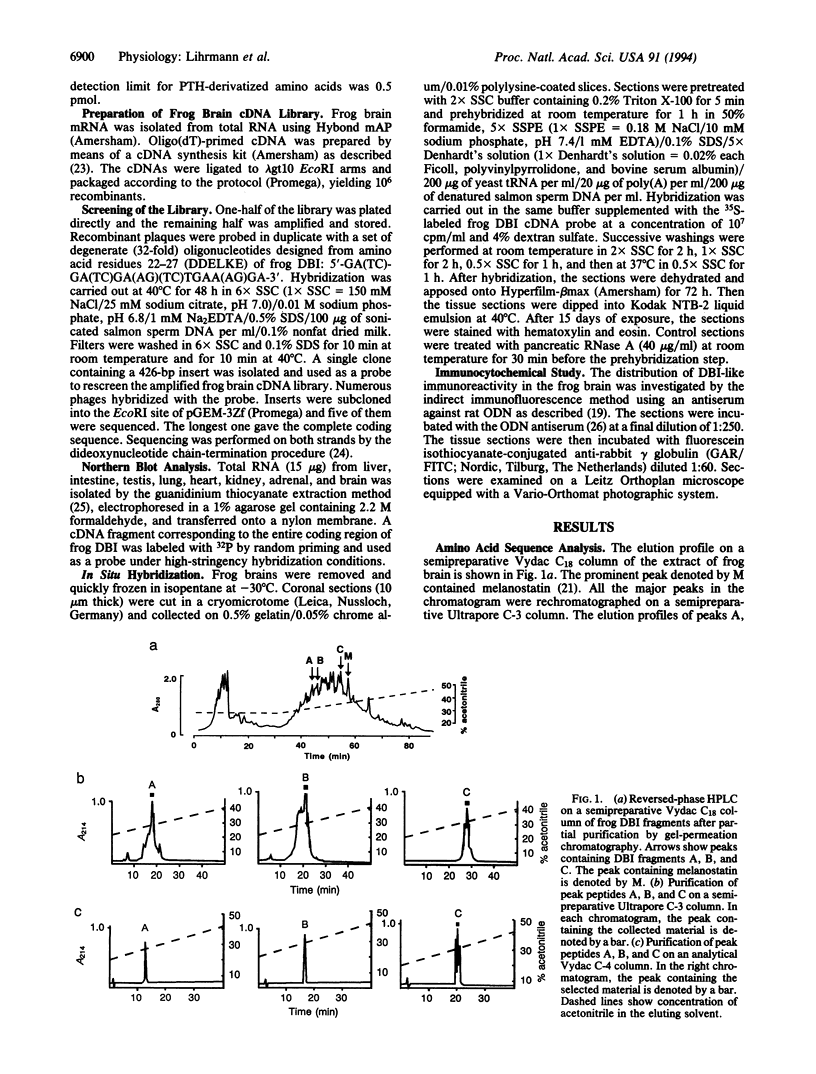
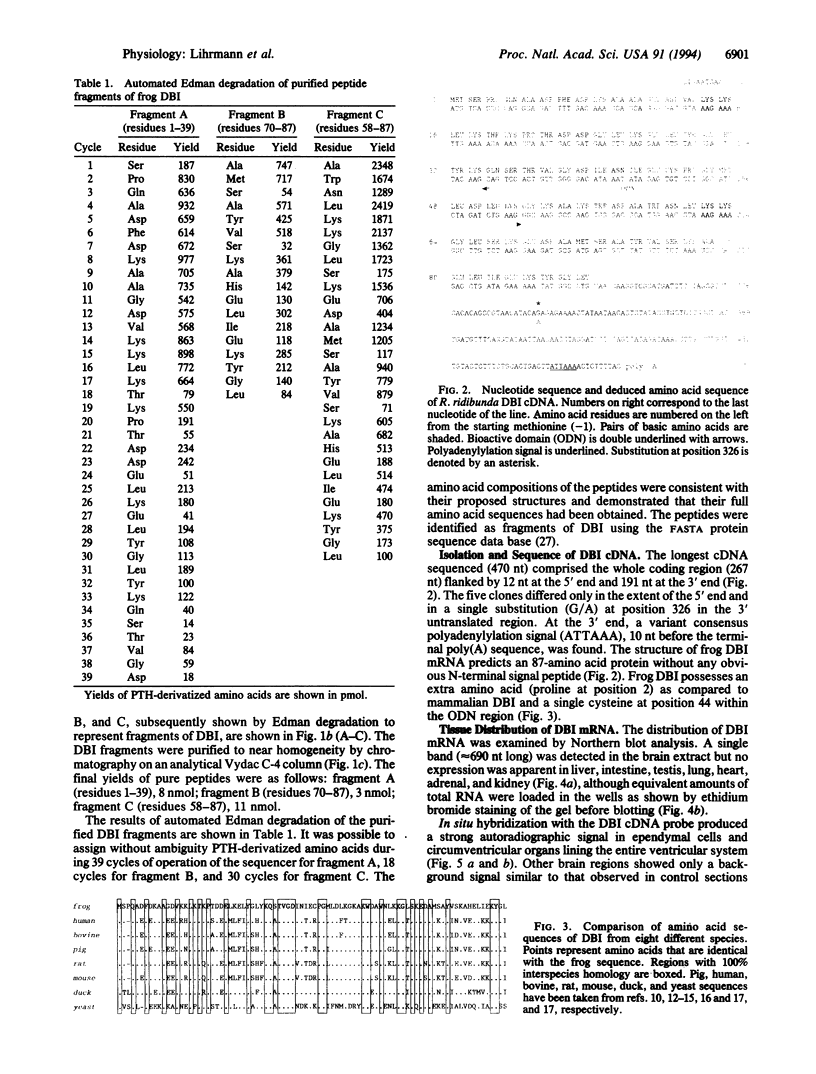
Three peptides derived from diazepam-binding inhibitor (DBI) were isolated in pure form from the brain of the frog Rana ridibunda. The primary structures of these peptides showed that they correspond to mammalian DBI-(1-39), DBI-(58-87), and DBI-(70-87). A set of degenerate primers, whose design was based on the amino acid sequence data, was used to screen a frog brain cDNA library. The cloned cDNA encodes an 87-amino acid polypeptide, which exhibits 68% similarity with porcine and bovine DBI. Frog DBI contains two paired basic amino acids (Lys-Lys) at positions 14-15 and 62-63 and a single cysteine within the biologically active region of the molecule. Northern blot analysis showed that DBI mRNA is expressed at a high level in the brain but is virtually absent in peripheral tissues. The distribution of DBI mRNA and DBI-like immunoreactivity in the frog brain was studied by in situ hybridization and immunocytochemistry. Both approaches revealed that the DBI gene is expressed in ependymal cells and circumventricular organs lining the ventricular cavity. Since amphibia diverged from mammals at least 250 million years ago, the data show that evolutionary pressure has acted to conserve the structure of DBI in the vertebrate phylum. The distribution of both DBI mRNA and DBI-like immunoreactivity indicates that DBI is selectively expressed in glial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alho H., Fremeau R. T., Jr, Tiedge H., Wilcox J., Bovolin P., Brosius J., Roberts J. L., Costa E. Diazepam binding inhibitor gene expression: location in brain and peripheral tissues of rat. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7018–7022. doi: 10.1073/pnas.85.18.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besman M. J., Yanagibashi K., Lee T. D., Kawamura M., Hall P. F., Shively J. E. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4897–4901. doi: 10.1073/pnas.86.13.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bormann J., Ferrero P., Guidotti A., Costa E. Neuropeptide modulation of GABA receptor C1- channels. Regul Pept Suppl. 1985;4:33–38. doi: 10.1016/0167-0115(85)90215-0. [DOI] [PubMed] [Google Scholar]
- Chartrel N., Conlon J. M., Danger J. M., Fournier A., Tonon M. C., Vaudry H. Characterization of melanotropin-release-inhibiting factor (melanostatin) from frog brain: homology with human neuropeptide Y. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3862–3866. doi: 10.1073/pnas.88.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci. 1991;49(5):325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- Ferrero P., Santi M. R., Conti-Tronconi B., Costa E., Guidotti A. Study of an octadecaneuropeptide derived from diazepam binding inhibitor (DBI): biological activity and presence in rat brain. Proc Natl Acad Sci U S A. 1986 Feb;83(3):827–831. doi: 10.1073/pnas.83.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Glaister D., Seeburg P. H., Guidotti A., Costa E. Cloning and expression of cDNA for human diazepam binding inhibitor, a natural ligand of an allosteric regulatory site of the gamma-aminobutyric acid type A receptor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7547–7551. doi: 10.1073/pnas.83.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hach M., Pedersen S. N., Börchers T., Højrup P., Knudsen J. Determination by photoaffinity labelling of the hydrophobic part of the binding site for acyl-CoA esters on acyl-CoA-binding protein from bovine liver. Biochem J. 1990 Oct 1;271(1):231–236. doi: 10.1042/bj2710231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario E., Lihrmann I., Vaudry H. Characterization of the cDNA encoding proopiomelanocortin in the frog Rana ridibunda. Biochem Biophys Res Commun. 1990 Dec 14;173(2):653–659. doi: 10.1016/s0006-291x(05)80085-3. [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Hirst M. An octadecaneuropeptide (ODN) derived from diazepam binding inhibitor increases aggressive interactions in mice. Brain Res. 1986 Sep 24;383(1-2):343–349. doi: 10.1016/0006-8993(86)90037-5. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Louiset E., Vaudry H., Cazin L. Allosteric modulation of the GABA-induced chloride current in frog melanotrophs. Ann N Y Acad Sci. 1993 May 31;680:564–566. doi: 10.1111/j.1749-6632.1993.tb19740.x. [DOI] [PubMed] [Google Scholar]
- Malagon M., Vallarino M., Tonon M. C., Vaudry H. Localization and characterization of diazepam-binding inhibitor (DBI)-like peptides in the brain and pituitary of the trout (Salmo gairdneri). Brain Res. 1992 Apr 3;576(2):208–214. doi: 10.1016/0006-8993(92)90682-y. [DOI] [PubMed] [Google Scholar]
- Malagon M., Vaudry H., Vallarino M., Gracia-Navarro F., Tonon M. C. Distribution and characterization of endozepine-like immunoreactivity in the central nervous system of the frog Rana ridibunda. Peptides. 1992 Jan-Feb;13(1):99–107. doi: 10.1016/0196-9781(92)90146-t. [DOI] [PubMed] [Google Scholar]
- Malagon M., Vaudry H., Van Strien F., Pelletier G., Gracia-Navarro F., Tonon M. C. Ontogeny of diazepam-binding inhibitor-related peptides (endozepines) in the rat brain. Neuroscience. 1993 Dec;57(3):777–786. doi: 10.1016/0306-4522(93)90023-9. [DOI] [PubMed] [Google Scholar]
- Mandrup S., Hummel R., Ravn S., Jensen G., Andreasen P. H., Gregersen N., Knudsen J., Kristiansen K. Acyl-CoA-binding protein/diazepam-binding inhibitor gene and pseudogenes. A typical housekeeping gene family. J Mol Biol. 1992 Dec 5;228(3):1011–1022. doi: 10.1016/0022-2836(92)90888-q. [DOI] [PubMed] [Google Scholar]
- Marcus F. Preferential cleavage at aspartyl-prolyl peptide bonds in dilute acid. Int J Pept Protein Res. 1985 May;25(5):542–546. doi: 10.1111/j.1399-3011.1985.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Mocchetti I., Einstein R., Brosius J. Putative diazepam binding inhibitor peptide: cDNA clones from rat. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7221–7225. doi: 10.1073/pnas.83.19.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen I. B., Schulenberg H., Hansen H. O., Spener F., Knudsen J. A novel acyl-CoA-binding protein from bovine liver. Effect on fatty acid synthesis. Biochem J. 1987 Jan 1;241(1):189–192. doi: 10.1042/bj2410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. P., Sinha A. K., Sikela J. M., Hahn W. E. Sequence and expression of the murine diazepam binding inhibitor. Brain Res Mol Brain Res. 1989 Nov;6(2-3):101–108. doi: 10.1016/0169-328x(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Berkovich A., Krueger K. E., Costa E., Guidotti A. Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology. 1991 Sep;129(3):1481–1488. doi: 10.1210/endo-129-3-1481. [DOI] [PubMed] [Google Scholar]
- Rhéaume E., Tonon M. C., Smih F., Simard J., Désy L., Vaudry H., Pelletier G. Localization of the endogenous benzodiazepine ligand octadecaneuropeptide in the rat testis. Endocrinology. 1990 Oct;127(4):1986–1994. doi: 10.1210/endo-127-4-1986. [DOI] [PubMed] [Google Scholar]
- Rose T. M., Schultz E. R., Todaro G. J. Molecular cloning of the gene for the yeast homolog (ACB) of diazepam binding inhibitor/endozepine/acyl-CoA-binding protein. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11287–11291. doi: 10.1073/pnas.89.23.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet-Smih F., Tonon M. C., Pelletier G., Vaudry H. Characterization of endozepine-related peptides in the central nervous system and in peripheral tissues of the rat. Peptides. 1992 Nov-Dec;13(6):1219–1225. doi: 10.1016/0196-9781(92)90032-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Gentry L. E., Marquardt H., Todaro G. J. Isolation and characterization of a putative endogenous benzodiazepineoid (endozepine) from bovine and human brain. J Biol Chem. 1986 Sep 15;261(26):11968–11973. [PubMed] [Google Scholar]
- Slobodyansky E., Berkovich A., Bovolin P., Wambebe C. The endogenous allosteric modulation of GABAA receptor subtypes: a role for the neuronal posttranslational processing products of rat brain DBI. Adv Biochem Psychopharmacol. 1990;46:51–60. [PubMed] [Google Scholar]
- Slobodyansky E., Guidotti A., Wambebe C., Berkovich A., Costa E. Isolation and characterization of a rat brain triakontatetraneuropeptide, a posttranslational product of diazepam binding inhibitor: specific action at the Ro 5-4864 recognition site. J Neurochem. 1989 Oct;53(4):1276–1284. doi: 10.1111/j.1471-4159.1989.tb07425.x. [DOI] [PubMed] [Google Scholar]
- Steyaert H., Tonon M. C., Tong Y., Smihrouet F., Testart J., Pelletier G., Vaudry H. Distribution and characterization of endogenous benzodiazepine receptor ligand (endozepine)-like peptides in the rat gastrointestinal tract. Endocrinology. 1991 Oct;129(4):2101–2109. doi: 10.1210/endo-129-4-2101. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Rose T. M., Shoyab M. Human DBI (endozepine): relationship to a homologous membrane associated protein (MA-DBI). Neuropharmacology. 1991 Dec;30(12B):1373–1380. doi: 10.1016/s0028-3908(11)80004-3. [DOI] [PubMed] [Google Scholar]
- Tonon M. C., Adjeroud S., Lamacz M., Louiset E., Danger J. M., Desrues L., Cazin L., Nicolas P., Vaudry H. Central-type benzodiazepines and the octadecaneuropeptide modulate the effects of GABA on the release of alpha-melanocyte-stimulating hormone from frog neurointermediate lobe in vitro. Neuroscience. 1989;31(2):485–493. doi: 10.1016/0306-4522(89)90391-6. [DOI] [PubMed] [Google Scholar]
- Tonon M. C., Désy L., Nicolas P., Vaudry H., Pelletier G. Immunocytochemical localization of the endogenous benzodiazepine ligand octadecaneuropeptide (ODN) in the rat brain. Neuropeptides. 1990 Jan;15(1):17–24. doi: 10.1016/0143-4179(90)90155-r. [DOI] [PubMed] [Google Scholar]
- Webb N. R., Rose T. M., Malik N., Marquardt H., Shoyab M., Todaro G. J., Lee D. C. Bovine and human cDNA sequences encoding a putative benzodiazepine receptor ligand. DNA. 1987 Feb;6(1):71–79. doi: 10.1089/dna.1987.6.71. [DOI] [PubMed] [Google Scholar]