Abstract
The blue-gum chalcid Leptocybe invasa Fisher & LaSalle (Hymenoptera: Eulophidae) is a gall wasp pest of Eucalyptus species, likely native to Australia. Over the past 15 years it has invaded 39 countries on all continents where eucalypts are grown. The worldwide invasion of the blue gum chalcid was attributed to a single thelytokous morphospecies formally described in 2004. Subsequently, however, males have been recorded in several countries and the sex ratio of field populations has been found to be highly variable in different areas. In order to find an explanation for such sex ratio differences, populations of L. invasa from a broad geographical area were screened for the symbionts currently known as reproductive manipulators, and both wasps and symbionts were genetically characterized using multiple genes. Molecular analyses suggested that L. invasa is in fact a complex of two cryptic species involved in the rapid and efficient spread of the wasp, the first recovered from the Mediterranean region and South America, the latter from China. All screened specimens were infected by endosymbiotic bacteria belonging to the genus Rickettsia. Two closely related Rickettsia strains were found, each infecting one of the two putative cryptic species of L. invasa and associated with different average sex ratios. Rickettsia were found to be localized in the female reproductive tissues and transovarially transmitted, suggesting a possible role of Rickettsia as the causal agent of thelytokous parthenogenesis in L. invasa. Implications for the variation of sex ratio and for the management of L. invasa are discussed.
Introduction
Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae) commonly known as blue-gum chalcid is a gall wasp of many Eucalyptus species. It was unknown until the early 2000s, when it was recorded in Italy, identified as Aprostocetus sp. [1], and in Turkey [2]. Successively it was described as L. invasa and reported as an invasive pest in the Palearctic region [3]. Due to its exclusive association with Eucalyptus, the blue-gum chalcid is believed to be native to Australia [3], where some parasitoids monophagous on L. invasa have also been recorded [4]. However, its origin remains unproven [5] because there are also a small number of Eucalyptus species native to New Guinea, Indonesia and Philippines [6,7] and there are some records of phytophagous insects that have shifted onto eucalypts and achieved pest status out of Australia [8]. Nevertheless, there is consensus that there has been a major radiation of eulophid gall inducers in Australia [9], and several species of Australian gall-inducing eulophids have become invasive within the last decade [10].
Although destructive and rapid insect invasions are widely reported [11–15], the scale and the speed of L. invasa invasion have few precedents [16,17]. Blue-gum chalcid seems to show wide ecological plasticity and indeed, in about 15 years, has spread worldwide (39 countries, spanning Europe, North Africa, Middle East, Asia, South America, Oceania and North America, S1 Table), and its invasiveness, based on the terminology in [18], reached stage V, becoming a dominant pest species [5].
Leptocybe invasa lays eggs in plant tissues causing the formation of galls on the leaf midribs and petioles and on the stem of new shoots, eventually leading to leaf-curling and premature aging of the leaves [3]. Egg overloading might cause death of juvenile shoots, while severe attacks lead to leaf fall, stunted growth and may eventually seriously weaken the tree [3]. In recently invaded countries, L. invasa can compromise the productivity of Eucalyptus plantations [17,19]. Conversely, L. invasa is not considered detrimental for Eucalyptus plantations in Australia, because the natural enemies are able to control its populations to tolerable levels [4]. Different species of eucalyptus show different susceptibility to L. invasa attacks [3] with E. grandis W. Hill, E. camaldulensis Dehnh and E. tereticornis Smith being the most susceptible [20].
The eucalyptus gall wasp was originally described as a thelytokous species [3], as field populations were represented only by females. Afterwards, males were recorded and described in some populations from Turkey [21], India [22], China [23], Thailand [24] and Taiwan [25] with a sex ratio ranging from 0.5% males in Turkish populations [21] up to 18–48% in Chinese populations [26]. Female-biased sex ratio occurs frequently in Hymenoptera [27] and, in chalcidoid lineages especially, it has been associated with infection of symbiotic intracellular bacteria able to manipulate their host’s reproduction by inducing male-killing (mortality of infected male embryos), feminization (genotypic diploid males develop as functional phenotypic females from unfertilized eggs) or more commonly thelytokous parthenogenesis (mothers produce only diploid daughters from unfertilized haploid eggs) [28–32]. In the family Eulophidae, in particular, parthenogenesis induced by bacterial endosymbionts has been documented in three different species, the Wolbachia-infected Tetrastichus coeruleus Nees [32], and the Rickettsia-infected Neochrysocharis formosa (Westwood) [29] and Pnigalio soemius (Walker) [33].
Here we studied populations of L. invasa from countries where only females have been reported (Italy, Argentina, Tunisia) ([1,34,35] S2 Table) and from countries where males are also present (Turkey, China) [21,26]. This study aims at ascertaining if: 1) cryptic species are involved in the invasive process of L. invasa; 2) the sex ratio of L. invasa is related to infection by bacterial endosymbionts. Therefore, genetic variability of L. invasa was assessed by sequencing nuclear and mitochondrial genes of different geographic populations. These populations were also screened for infection by endosymbionts, and symbiont diversity was in turn characterized by sequencing four different bacterial genes. Fluorescent in Situ Hybridization (FISH) was used to localize endosymbionts within the hosts and to test for vertical transmission.
Materials and Methods
Ethics Statement
The sampling of living material involved in our experiments included wasps, i.e. Leptocybe invasa, associated with galls on Eucalyptus sp. All sampling locations are not privately owned or protected (coordinates in Table 1). Besides neither the host plant nor the wasp species are endangered or otherwise protected, and therefore no specific permits were required for these locations/activities.
Table 1. Specimens investigated and analyses performed in the study.
| Code | Locality | Longitude | Latitude | Altitude m a.s.l. | Host | Sex | PCR Rbf Rbr | Symbiont detected in DGGE | Genbank Accession Code | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insect | Rickettsia | |||||||||||||||
| 28S-D2 | COI 5’ region | COI 3’ region | ITS2 | 16S | gltA | atpA | rpmE tRNAfMet | |||||||||
| Li_IT_1 | Portici, Italy | 14°21’05” N | 40°48’55” E | 29 | E. camaldulensis | ♀ | + | Rickettsia | KP143969 | KP233972 | KP143943 | KP233904 | KP233935 | KP233920 | KP233955 | |
| Li_IT_2 | ♀ | + | Rickettsia | KP143970 | KP233973 | KP143944 | KP233905 | KP233936 | KP233921 | KP233956 | ||||||
| Li_IT_3 | ♀ | + | Rickettsia | KP143971 | KP233974 | KP143945 | KP233906 | KP233937 | KP233922 | - | ||||||
| Li_IT_4 | ♀ | + | Rickettsia | KP143972 | KP233975 | KP143946 | - | KP233938 | - | - | ||||||
| Li_IT_5 | ♀ | + | Rickettsia | KP143973 | KP233976 | KP143947 | - | KP233939 | - | - | ||||||
| Li_IT_6 | S. M. al Bagno, Italy | 17°59’49” N | 40°07’33” E | 4 | E. camaldulensis | ♀ | + | Rickettsia | - | KP233977 | - | - | - | - | - | |
| Li_IT_7 | ♀ | + | Rickettsia | - | KP233978 | - | - | - | - | - | ||||||
| Li_IT_10 | Costa Saracena Sicily, Italy | 15°07’31” N | 37°18’32” E | 22 | E. camaldulensis | ♀ | + | Rickettsia | - | KP233989 | - | KP143948 | KP233907 | KP233940 | KP233923 | - |
| Li_AR_1 | La Plata, Argentina | 57°56’20” S | 34°54’17” W | 31 | E. camaldulensis | ♀ | + | Rickettsia | KP143974 | KP233979 | KP143949 | KP233908 | KP233941 | KP233924 | KP233957 | |
| Li_AR_2* | ♀ | + | Rickettsia | KP143975 | KP233980 | KP143950 | KP233909 | KP233942 | KP233925 | KP233958 | ||||||
| Li_AR_3 | ♀ | + | Rickettsia | KP143976 | KP233981 | KP143951 | KP233910 | KP233943 | KP233926 | - | ||||||
| Li_CN_1* | Hubian, Gangzhou City, Jiangxi Province, China | 114°54’38” N | 25°53’16” E | 142 | E. globulus | ♀ | + | Rickettsia | KP143987 | KP233985 | KP143962 | KP233911 | KP233944 | KP233927 | KP233964 | |
| Li_CN_2 | ♂ | + | Rickettsia | KP143988 | KP233986 | KP143963 | KP233912 | KP233945 | KP233928 | KP233965 | ||||||
| Li_CN_3 | ♀ | + | Rickettsia | KP143989 | KP233987 | KP143964 | KP233913 | KP233946 | - | - | ||||||
| Li_CN_4 | ♀ | + | Rickettsia | KP143990 | KP233988 | KP143965 | KP233914 | KP233947 | KP233929 | - | ||||||
| Li_CN_5 | ♂ | + | Rickettsia | KP143991 | - | KP233994 | KP143966 | - | - | - | - | |||||
| Li_CN_6 | ♀ | + | - | KP143992 | KP233990 | - | KP143967 | - | - | - | - | |||||
| Li_CN_7* | ♀ | + | - | KP143993 | KP233991 | - | KP143968 | - | - | - | - | |||||
| Li_CN_8* | ♀ | + | - | KP143994 | KP233992 | - | - | - | - | - | - | |||||
| Li_CN_9* | ♀ | + | - | KP143995 | KP233993 | - | - | - | - | - | - | |||||
| Li_TK_1* | Serinyol, Antakya, Hatay Province, Turkey | 36°12’48” N | 36°22’00” E | 120 | E. camaldulensis | ♀ | + | Rickettsia | KP143977 | KP233953 | KP233966 | KP143955 | KP233918 | KP233951 | KP233933 | KP233961 |
| Li_TK_2 | ♀ | + | Rickettsia | KP143978 | KP233954 | KP233967 | KP143956 | KP233919 | KP233952 | KP233934 | KP233962 | |||||
| Li_TK_3 | ♀ | + | Rickettsia | KP143979 | - | - | KP143957 | - | - | - | - | |||||
| Li_TK_4 | ♂ | + | Rickettsia | KP143980 | - | - | KP143958 | - | - | - | KP233963 | |||||
| Li_TK_5 | ♂ | + | Rickettsia | KP143981 | - | - | KP143959 | - | - | - | - | |||||
| Li_TK_6 | ♀ | + | Rickettsia | KP143982 | - | - | KP143960 | - | - | - | - | |||||
| Li_TK_7 | ♀ | + | Rickettsia | KP143983 | - | - | KP143961 | - | - | - | - | |||||
| Li_TU_1 | Mouaden, Tunisia | 09°16’00” N | 37°09’53” E | 80 | E. camaldulensis | ♀ | + | Rickettsia | KP143984 | KP233982 | KP143952 | KP233915 | KP233948 | KP233930 | KP233959 | |
| Li_TU_2 | ♀ | + | Rickettsia | KP143985 | KP233983 | KP143953 | KP233916 | KP233949 | KP233931 | KP233960 | ||||||
| Li_TU_3 | ♀ | + | Rickettsia | KP143986 | KP233984 | KP143954 | KP233917 | KP233950 | KP233932 | - | ||||||
| Bs1 | Portici, Italy | 14°21’05” N | 40°48’55” E | 29 | Ex Bactrocera oleae on Olea europea europea | ♀ | - | - | KP233970 | KP233995 | - | - | - | - | - | |
| Am1 | Bari, Italy | 16°52’00” N | 41°07’31” E | 5 | Melilotus indicus | ♀ | - | - | KP233969 | KP233971 | KP233968 | - | - | - | - | |
Li: Leptocybe invasa; Bs: Baryscapus silvestrii; Am: Aprostocetus monacoi; +: specimen Rickettsia sp. positive in specific PCR;-: not determined.
*: destructive DNA extraction.
Insect sampling
Stands of Eucalyptus camaldulensis Dehnhardt or E. globulus Labillardière infested with L. invasa were sampled from Portici, S. Maria al Bagno and Siracusa (Italy), La Plata (Argentina) and Hubian (China). Additional material was received from Antakya (Turkey) and Mouaden (Tunisia) (Table 1). Leaves harbouring galls of different ages were removed from the trees, placed in plastic boxes and stored at room temperature until the emergence of adults. Soon after their emergence, wasps were killed in 95% ethanol and morphologically identified using the original species description of females [3] and males [21].
Molecular characterization and phylogenetic analyses of L. invasa
DNA was extracted from whole single individuals (males and females) by using both destructive and non-destructive Chelex and proteinase K based methods modified as in [36]. In this latter case, wasps were then treated and card mounted as in [37]. Three genes were sequenced: the mitochondrial cytochrome c oxydase subunit I (COI), and two ribosomal genes, the expansion segment D2 of the 28S ribosomal subunit (28S-D2) and the Internal Transcribed Spacer 2 (ITS2). COI is a very effective and universally used marker for species delimitation [38]. ITS2 has been successfully used at the species level in many Chalcidoidea taxa, e.g. Pteromalidae [39], Trichogrammatidae [40] and Eulophidae [37]. 28S-D2 has also proved diagnostic at the species level in Pteromalidae [39], Aphelinidae [41] and Eulophidae [36]. Two regions of COI were amplified, the first using the forward primer LCO1490 paired with either HCO2198 [42] or Lep-r1 [38] reverse primers, and the second pairing the forward primer C1-J-2183 [43] or Apf [44] with the reverse primer TL2-N-3014 [43]. The ribosomal gene 28S-D2 was amplified with primers D2F and D2R [39]. PCR reactions and cycling conditions for COI and 28S-D2 were set as described in [36]. For the amplification of ITS2, primers ITS2F [39] and ITS2TrichRev [40] were used in PCR reactions as in [45]. PCR products were checked on a 1.2% agarose gel stained with ethidium bromide and directly sequenced. Chromatograms were assembled, edited by eye and aligned with Bioedit 7.2.5 [46] and the sequences deposited in GenBank, with the accession numbers reported in Table 1.
All sequences with chromatograms showing ambiguous peaks were cloned. Amplicons were ethanol precipitated, ligated into the pGEM-T Easy plasmid vector (Promega), and cloned into Escherichia coli TOP10 competent cells (Invitrogen) according to the manufacturer’s instructions. Transformants were PCR-screened with universal M13 vector primers, and inserts of the expected size were sequenced. COI sequences were virtually translated into the corresponding amino acid chain to detect frame-shift mutations and stop codons, which may indicate the presence of pseudogenes [47,48].
Phylogenies for L. invasa were reconstructed using Bayesian inference (BI), utilizing MrBayes 3.1.2 [49], following the methodology reported in [37] on concatenated (ITS2–28S-D2–COI) alignment by using the GTR+G+I nucleotide model as selected by jModeltest [50]. Sharing some morphological features with L. invasa, two species of closely related genera, Baryscapus silvestrii Viggiani et Bernardo (Hymenoptera: Eulophidae) and Aprostocetus monacoi Viggiani (Hymenoptera: Eulophidae) were used as outgroups to root the tree. Sequence distances within and between populations of COI dataset were calculated as uncorrected pairwise distances (p-distances) using MEGA 4 [51]. We also looked for diagnostic single nucleotide polymorphisms (SNPs), i.e. character state in a given nucleotide position shared by all individuals from one group and different from all individuals in any other group, in COI sequences, as well as the occurrence of diagnostic non-synonymous amino acid changes.
For species delimitation, we used the Poisson tree processes model (PTP) [52], a recently developed method that been successfully applied [53]. All sequences of Tetrastichinae (5’-COI “barcoding” region) available in GenBank were included, aligned in BioEdit and run in MrBayes (GTR+G+I substitution model) for 1 million generations (rate sample = 1000, burn in value = 25%). BI tree obtained was run on the bPTP web server as in [53], for 500,000 MCMC generations. The relationships between L. invasa specimens were also investigated using Statistical Parsimony in TCS 1.21 [54] on the COI dataset, as in [55].
Detection of bacterial symbionts
DGGE (Denaturing Gradient Gel Electrophoresis) analysis was used to check for the presence of bacterial symbionts in female and male specimens listed in Table 1. Bacterial 16S rRNA gene fragments were amplified by a nested PCR with the primers 341f (to which a 40-bp GC clamp was added) and 518r [56] using as template a longer fragment amplified with the primers 27F and 1513R [57] in a “touch-down” annealing protocol [58]. The following strains of arthropod reproductive manipulators were used as positive controls: Wolbachia from Encarsia formosa Gahan, Cardinium from Encarsia pergandiella Howard, Rickettsia from P. soemius, Spiroplasma from Drosophila neotestacea Grimaldi, James & Jaenike and Arsenophonus from Bemisia tabaci (Gennadius). PCR products were subjected to DGGE analysis slightly modifying the technique described in [59] (denaturing gradient 35–60% and 45–60%; 90V for 17h). In addition, to confirm DGGE results, diagnostic PCR was performed with specific primers targeting the 16S rRNA gene for Cardinium (CLOf and CLOr1 –[28]), Rickettsia (Rb-F and Rb-R—[60]), Spiroplasma (27F and TKSSsp—[61]) and Arsenophonus (27F and Ars16SR—[62]), and the ftsZ gene for Wolbachia (ftsZf1 and ftsZr1 –[63]).
Molecular characterization of Rickettsia
Multiple genes were sequenced to characterize Rickettsia at the strain level. Approximately 1000 bp of the 16S rRNA gene were amplified with Rb-F and Rb-R primers as previously described; ~600 bp of the citrate synthase (gltA) gene were obtained by a nested PCR strategy with primers CS1d and CS1273r [64] followed by CS78d paired with CS715r [64]; the subunit α of ATP synthase F1 (atpA) gene (~750 bp) was amplified with ATP syn f1 α fw and ATP syn f1 α rev primers [65]. For both genes, amplifications were carried out using a “step-up” program: 5 min of initial denaturation at 94°C, first 10 cycles step at 94°C for 40 sec, 37°C for 2 min and 72°C for 90 sec and the second 35 cycles step with the same denaturation and elongation parameters and annealing temperature set at 48°C for 1 min. The amplification was completed by holding for 5 min at 72°C. Finally, the intergenic spacer rpmE-tRNAfMet, known to be useful for characterizing strains of Rickettsia [66], was sequenced using rpmEF and rpmER primers [67]. All sequences were assembled, edited and aligned with Bioedit 7.2.5 [46], and deposited in GenBank, with the accession numbers reported in Table 1.
Rickettsia phylogeny and host-endosymbiont relationship
Sequences of the 16S rRNA, gltA and atpA genes were aligned with homologous sequences available in GenBank. The related species Orientia tsutsugamushi (Hayashi) was included as outgroup in the 16S rRNA and atpA genes analyses [68], whereas a Wolbachia strain was used as outgroup in the gltA gene analysis. For Rickettsia 16s rRNA, gltA and atpA genes, phylogenetic reconstructions using Bayesian inference was performed using MrBayes 3.1.2 [49], following the methodology reported in [37]. Intergenic spacer region rpmE-tRNAfMet sequences of L. invasa were compared to each other and checked against GenBank for highest similar matches.
To test for congruence between the host and endosymbiont trees, trees of L. invasa populations and of their own Rickettsia symbionts were compared. For this analysis, ML trees were built including P. soemius and its own Rickettsia sequences as outgroups, as the only eulophid taxon currently available in GenBank for which all five insect and symbiont genes here used are available.
Fluorescence microscopy
Localization of Rickettsia within the host’s reproductive tissues was carried out, for the Italian population, using fluorescence in situ hybridization (FISH) with the Rickettsia-specific probe RickPn-Cy3 [33] and the universal probe EUB338-Cy5 [69]. Ovaries and mature eggs were extracted from adult females in a drop of PBS buffer under a stereomicroscope. Ovaries and eggs were subjected to the whole-mount FISH method reported by [33] except for the duration of egg dechorionation (10 min in 50% commercial bleach in PBS solution). Stained samples were observed both under a ZEISS Axiophot 2 epifluorescence microscope and under the Leica confocal TCS SP5 microscope. Images obtained on multiple focal planes were stacked with software Leica application Suite, Advances fluorescence 2.4.1. The specificity of the observed signals was verified with the following control experiments: no-probe control and RNase-digested control. Nuclei of the host cells were counterstained with DAPI (0.4 μg/ml) in mounting medium.
Results
Morphological characterization of Leptocybe invasa
All female and male specimens from different countries were identified as L. invasa, and did not show any appreciable difference in their morphology. Voucher material (listed in Table 1) is deposited at the Institute for Sustainable Plant Protection (IPSP, Portici, Italy).
Molecular characterization and phylogenetic analyses of L. invasa
The ribosomal gene 28S-D2 sequences were obtained for 27 specimens (Table 1). Sequences ranged from 488 to 578 nt, and, after trimming, the final alignment consisted of 482 bp. The ribosomal gene 28S-D2 sequences of Italian, Tunisian, Argentinean and Turkish samples were identical to each other and differed by a single nucleotide from Chinese sequences (Table 2). Similarly, ITS2 sequences from Italian, Tunisian, Argentinean and Turkish samples were identical, whereas the Chinese sequences had a single missing nucleotide (427 bp versus 428 bp) (Table 2).
Table 2. Synthetic representation of the L. invasa and of its Rickettsia symbiont characterization.
Two regions of COI (1455 nucleotides in total) were sequenced from each of 19 specimens (Table 1). The same COI haplotype was recovered from the Italian, Argentinean and Tunisian populations, whereas one and two unique haplotypes were recovered from the Chinese and Turkish populations, respectively (Table 2). Clones of COI amplicons, that showed ambiguous peaks in the chromatograms of sequences from the Turkish population, mostly resulted from pseudogenes, except for two clones that were then used in subsequent analyses. BI phylogenetic analyses of the concatenated ITS2, 28S-D2 and COI (S1 Fig) showed two well-supported clades. The first clade includes L. invasa from China, and the second clade includes Italian, Argentinean and Tunisian populations (hereafter called lineage A) and the Turkish population.
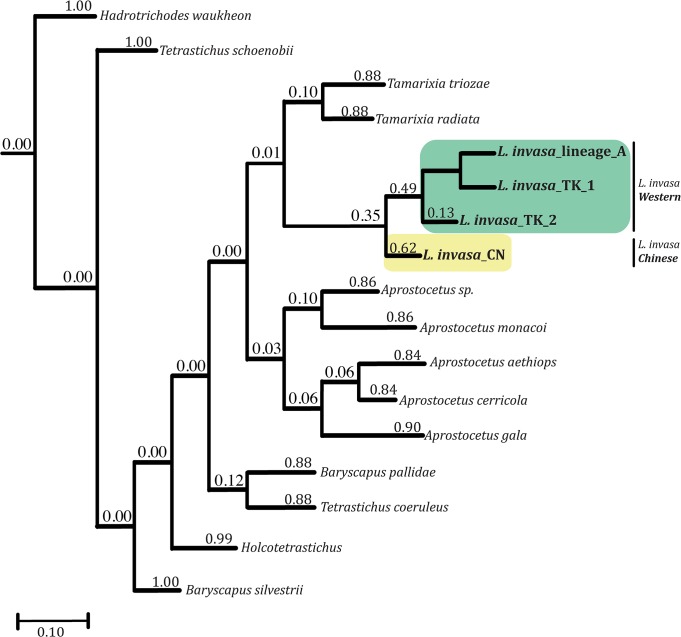
The bPTP analysis indicates a moderate support for the existence of two cryptic species in the blue gum chalcid complex, delimiting L. invasa from China as a putative species (hereafter called Chinese lineage) and the monophyletic clade including lineage A and Turkish specimens as a second putative species (hereafter called Western lineage) (Fig 1). Based on this analysis, there is 65% probability that the four COI haplotypes do not represent a single species, 49% probability that the 3 haplotypes “lineage A”, “TK1” and “TK2” represent a single species (which is consistent with the slightly high uncorrected intra-specific distances in the Turkish population, see below) and 62% probability that the Chinese population is a separate species. Statistical parsimony on the COI dataset yielded two separate networks, corresponding to the Western and Chinese lineages. The connection limit necessary to obtain a single network was 48 steps.
Fig 1. Species delimitation of Leptocybe invasa specimens based on bPTP analysis on the COI dataset.
Clades highlighted with coloured boxes and names in bold after a | symbol correspond to recognised putative species of L. invasa. Posterior delimitation probabilities values are reported above branches.
The distance between Chinese and Turkish samples was 3.7%, that between Chinese lineage and lineage A was 3.1% (Table A in S1 File), and that between Chinese and Western lineages was 3.6% (Table B in S1 File). The two Turkish haplotypes resulted in an intraspecific distance of 1.5% (Table C in S1 File). Analysis of diagnostic sites for COI sequences showed that the Chinese lineage is distinguishable by 43 SNPs (S3 Table). Three diagnostic non-synonymous amino acid changes were found in the Chinese lineage: valine instead of isoleucine, methionine instead of isoleucine and serine instead of threonine.
Detection of bacterial symbionts
DGGE analysis of PCR-amplified 16S rRNA gene showed that the only endosymbiont infecting all tested L. invasa adults (26 females and 4 males) was Rickettsia (data not shown). Congruent results were obtained by PCR screening, which revealed that 100% of individuals were infected only by Rickettsia.
Molecular characterization of Rickettsia
Rickettsia 16S rRNA gene sequences (ranged from 744 to 837 bp) were obtained from 16 specimens (Table 1). No intra-lineage variation was found except within the Turkish population, where two sequences were recovered: one in specimen Li-TK1, shared with lineage A, and another found exclusively in specimen Li-TK2 (different by two nt). 16S rRNA gene of the Chinese lineage differed from all the others by two nt (Table 2). Sequences of the Rickettsia gltA gene (499 bp), obtained from 18 wasps (Table 1), were invariant, except for in the Chinese lineage, which differed by a single nucleotide. Sequences of the atpA gene (681 bp), obtained from 15 wasps (Table 1), were identical in Rickettsia found in all L. invasa specimens. rpmE-tRNAfMet sequences (381 bp) were obtained from 11 wasps (Table 1). Rickettsia in individuals of the Western lineage showed the same sequence, which differed from the sequence found in Rickettsia in the individuals of the Chinese lineage by three nucleotides. The Chinese and all other samples sequences had a match of 97 and 98% identity with R. felis rpmE-tRNAfMet sequences, respectively.
Rickettsia phylogeny and host-endosymbiont relationship
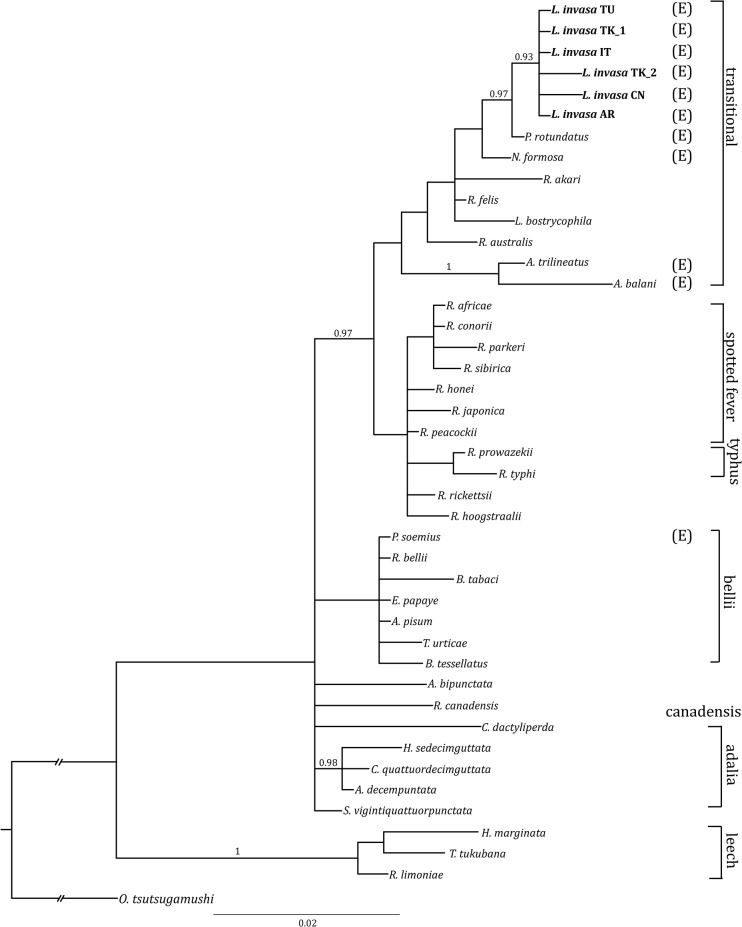
BI phylogenetic analyses of 16S rRNA, gltA and atpA genes showed that Rickettsia symbionts of L. invasa form a lineage in the Rickettsia transitional group (Fig 2 and S2 Fig). The host and symbiont trees have an identical topology, suggesting coevolution (S3 Fig).
Fig 2. Bayesian majority-rule consensus tree based on the 16S rRNA dataset.
BI tree shows the phylogenetic placement of the symbiont from L. invasa within the genus Rickettsia. The evolutionary model selected by MrModeltest2 was HKY+G. The host is provided whenever the symbiont is not identified at the species level. Posterior probabilities are reported above branches. Scale bar indicates the number of substitutions per site.
Fluorescence microscopy
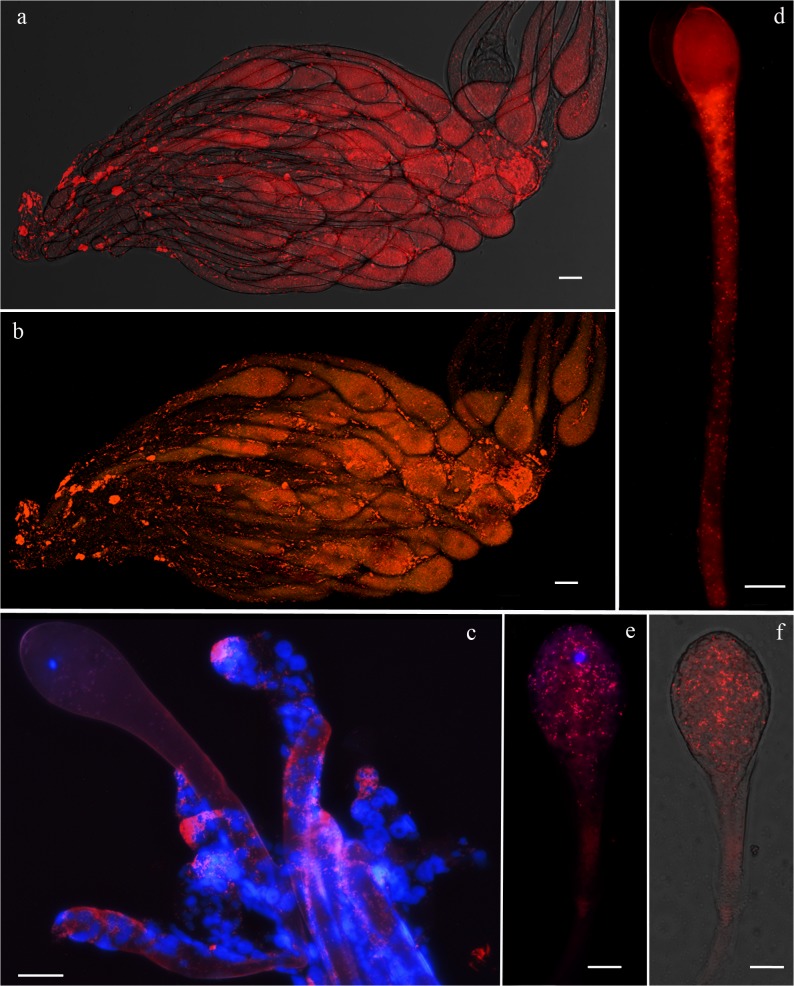
Using FISH analysis, we found that Rickettsia occur in the reproductive tissues of L. invasa females. Inside the ovary, dense clusters of bacteria were observed in the ovarioles (Fig 3A) and in the germarium (Fig 3C). Simultaneous probing with the Rickettsia specific probe and the universal bacterial probe (S4A and S4B Fig) did not reveal the occurrence of different bacteria out of Rickettsia (Fig 3B) showing a full overlay of two probes. Within the developed oocytes, bacteria were densely distributed both near the nucleus (Fig 3D and 3E) and in the peduncle (Fig 3F). Negative controls (no-probe and RNase-digested controls) did not display any signal, confirming the specificity of the detected signals.
Fig 3. FISH on L. invasa ovarioles and eggs.
Distribution of Rickettsia in the ovary (A and B), germaria (C) and the eggs (D, E, and F) of Leptocybe invasa. In the ovary Rickettsia bacteria (bright red spots) are inside the developing oocytes (A) and are densely clustered in the germaria (C). Merged image of ovarioles simultaneously stained with the Rickettsia specific probe RickPn-Cy3 and the universal bacterial probe EUB338-Cy5, showing Rickettsia cells in orange (B). Within the egg, bacteria are distributed in the head (E and F) and in the peduncle (D). DAPI-stained nuclei are blue. Bars, 20 μm.
Discussion
Specimens of L. invasa from different countries here examined all correspond to a single morphospecies. However, the integration of phylogenetic analysis, bPTP species delimitation method, statistical parsimony, COI distances and diagnostic SNPs, genetic differences at the strain level of Rickettsia symbionts, and sex ratio differences supports separation of the Chinese and Western lineages, which can be therefore treated as putative species.
Indeed, two genetically distinct lineages of L. invasa were identified on the basis of molecular, phylogenetic and species delimitation analyses. One lineage included populations from the Mediterranean region and South America, the other was present in China. The two lineages showed differences in all sequenced genes. Whereas ITS2 resulted to be more conserved than in other chalcidoid taxa, variation in COI and 28S-D2 is compatible with species level divergence reported previously. Indeed, 28S-D2 is often invariant between closely related species that are instead well differentiated on the base of other genes (usually COI) and biological traits [70,45]. In one case, even Palearctic and Nearctic species of the genus Pnigalio (Eulophidae) shared the same 28S-D2 sequence [45]. The single 28S-D2 polymorphism in L. invasa is consistent with what already observed in Eulophidae, where a high variation in COI can correspond to just a single diagnostic polymorphism in 28S-D2 [36]. As for COI, the divergence (<4%) is similar to that between two species pairs of the genus Encarsia (Aphelinidae) (Gebiola et al. in prep). The lack of COI genetic variation in specimens from the same locality (with the exception of the Turkish population) could be explained either by founder effects (the reduced genetic variation that occurs when a population is established starting from a few or a single specimen) [71], or by endosymbiont infection. Endosymbionts, acting as reproductive manipulators, are considered responsible for the very low mitochondrial genetic diversity in infected populations [32, 72], indeed they can induce selective sweeps that indirectly impact the existing polymorphism of mtDNA [73,74].
Among the known reproductive manipulators, Rickettsia was the only one found to infect L. invasa. Phylogenetic reconstructions based on three different genes (16S rRNA, gltA and atpA) placed this symbiont within the Rickettsia transitional group, in a clade that includes the symbionts of the eulophid wasps Pediobius rotundatus (Fonscolombe) and N. formosa [68]. Congruently, the rpmE-tRNAfMet gene sequence showed the highest similarity with R. felis, a species also belonging to the transitional group [68,75]. In particular, Rickettsia gene sequence analysis revealed the occurrence of two closely related bacterial strains, one associated with the putative Western species and one with the putative Chinese species. The 16S rDNA, gltA, atpA and rpmE-tRNAfMet sequences of the symbionts of the Western species samples showed no differences with the exception of 16S rRNA gene sequence of the Turkish specimen Li-TK2, differing by two nt. Instead, the Rickettsia strain associated with the Chinese species differed from the Western species in three out of four genes (16S rRNA, gltA and rpmE-tRNAfMet).
The strict association between the two Rickettsia strains and the two L. invasa lineages is also evident by the striking congruence between symbiont and host phylogenetic trees (S3 Fig), which suggests strict vertical transmission of the symbiont. Congruent topologies of host and symbiont molecular phylogenies are consistent with coevolution, but could be caused by other factors as well, such as resource tracking, where the symbiont evolves in response to a host trait with a phylogenetic signal [76].
Populations of L. invasa are known to have different sex ratios over their geographic distribution. Males have never been recorded from Italy ([1], see also S2 Table), Tunisia [34] and Argentina [35], whereas males are rare in Turkey (sex ratio 0–0.5%) [21]. In these countries, it is clear that L. invasa reproduces by thelytokous parthenogenesis. In contrast, a high frequency of males has been recorded in populations from China (males ranging from 18–48% in some populations) [17,26] and in populations from South–East Asia (Taiwan, India, Thailand) [22,23,24,25]. Interestingly, the genetic differentiation within L. invasa shown here mirrors the geographic variation in sex ratio. However, despite the occurrence of males, there is evidence suggesting that some populations from South-East Asia also reproduce by thelytokous parthenogenesis (although biparental populations cannot be excluded). Indeed, populations with only occasional males occur in China as well [77]. Furthermore, virgin females of the Chinese population studied in this work (Wang, unpublished observations) and of a Thai population [24] are able to produce both females and males, with male progeny apparently being not functional [24].
Why do such differences in the sex ratio exist between thelytokous populations of the Western and Chinese putative species? Results discussed above and the evidence that Rickettsia symbionts of L. invasa occur at high density within the ovaries and the eggs and is transmitted vertically to the progeny with very high efficiency support the hypothesis that Rickettsia can induces thelytokous parthenogenesis in L. invasa. Removing Rickettsia symbionts by feeding females antibiotics should restore the production of male progeny [33,78], but, despite numerous attempts, we were not able to rear and thus cure infected L. invasa. Furthermore, the interaction between host and symbiont genotype plays an important role in the phenotypic effect and transmission efficiency of reproductive manipulators [79]. Low transmission efficiency of Rickettsia could make the sex ratio of thelytokous individuals more susceptible to the effect of environmental factors. Efficiency of symbiont transmission through the host germline, but also penetrance of the reproductive phenotype, and infection prevalence in the host population, may be correlated with bacterial density [80]. Bacterial density is in turn regulated by genetic factors of the host and the symbiont itself and is strongly influenced by environmental factors, like temperature, antibiotics, and host age [81,82]. The general variation in bacterial density in response to temperatures indicates that there can be large spatial, temporal, and seasonal differences in endosymbiont densities and functions in natural populations. High temperatures, by reducing the symbiont density within the reproductive tissues of the host, can induce the production of male progeny by infected parthenogenetic females and variable sex ratios in field populations [83,84]. In the case of L. invasa, laboratory experiments with a Chinese population have shown that at constant temperatures the frequency of males in the progeny of thelytokous females increases from 2% at 20–23°C to 7% around 30°C [77]. Therefore, it is possible that the association between Rickettsia strain and its Chinese host is weaker (in terms of bacterial density and/or transmission efficiency) than that occurring with its Western host, and consequently more susceptible to the effect of high and/or low temperatures. Another plausible explanation for the different sex ratios of the Chinese species may be a more recent origin of the symbiotic association with Rickettsia than in the Western species. Thus, the production of frequent male offspring by infected Chinese females may be due to a maladaptive side effect of incomplete coevolution between host and symbiont, as recorded in another host—parasitoid system [85]. Besides, a possible influence of the host plant cannot be excluded, as the Chinese specimens were collected on E. globulus instead of E. camaldulensis. It has been demonstrated in other systems that plants on which phytophagous insects feed may influence the host-symbiont relationship [86,87]. Lastly, unless a direct involvement of Rickettsia in the thelytokous reproduction [33,78] or in the oogenesis [88] of L. invasa is conclusively demonstrated (by obtaining males or no eggs respectively from cured females), we cannot rule out the possibility that thelytoky could be genetically determined.
The likely existence of more than one species behind the morphospecies L. invasa could have important implications in terms of pest management. For example, parasitoids introduced in several countries [19] could have a different degree of specificity towards the two cryptic species, being able to parasitize only one of them or performing sub-optimally on different host species. It is therefore very important to determine whether the host range of the main parasitoid species described as monophagous (Selitrichodes kryceri Kim & La Salle, Selitrichodes neseri Kelly & La Salle, and Quadrastichus mendeli Kim & La Salle) [4,89] includes both cryptic species of L. invasa. Moreover, an influence on parasitoid performance might be caused not just by different hosts but also by the presence of diverse bacteria, as recently showed in other systems where different species of endosymbionts, or even slightly different strains, have an impact on the specificity of host—parasitoid interactions [90–93].
Concluding Remarks
We showed that the world wide distribution of L. invasa very likely involves at least two species showing distinct sex-ratios, that Rickettsia may be the causal agent of thelytokous reproduction, that two different symbiont strains are associated with the two putative host species, and that the host evolutionary history is recapitulated in the relationship of their microbial symbionts. Based on these results, a better understanding of the interaction between the host and symbiont is critical to explain biological differences. Furthermore, a deeper knowledge and characterization of the different populations of L. invasa from around the world is an essential challenge that should be addressed because of its consequences on pest management. As L. invasa is now widespread and biological control seems to be the best solution of its management, it is important to reassess the efficiency of the parasitoids currently used on both cryptic gall wasps, in order to avoid failures of biological control programs.
Supporting Information
The evolutionary model selected by MrModeltest2 was GTR+G. Scale bar indicates the number of substitutions per site.
(TIF)
(a) Bayesian phylogeny based on atpA sequences (Evol. model: GTR+I+G); (b) Bayesian phylogeny based on gltA sequences (Evol. model: TVM+G). The host is provided whenever the symbiont is not identified at the species level. Posterior probabilities are reported above branches. Scale bar indicates the number of substitutions per site.
(TIF)
Bootstrap values (>75%) are reported above branches. Scale bar indicates the number of substitutions per site.
(TIF)
Rickettsia bacteria, stained with Rickettsia specific probe RickPn-Cy3, appear like bright red spots on the ooplasm background (A), while appear like bright green spots on the ooplasm background (B) when stained with universal bacterial probe EUB388-Cy5. Bars, 20 µm.
(TIF)
(DOCX)
*Presence of males.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors wish to thank Khaled Abbes and Mikdat Doğanlar for the samplings in Tunisia and Turkey, Danilo Ercolini for his advices on DGGE, Jiajie Zhang and Alexandros Stamatakis for their elucidations on bPTP, Anna Giulia Nappo for her precious technical help and Molly Hunter for reviewing the manuscript. Lastly we thank Tamsin Majerus and an anonymous reviewer whose comments helped improving the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files, except for sequences that are available from the GenBank database http://www.ncbi.nlm.nih.gov/genbank/ (from accession numbers KP143943 to KP143995 and from KP233904 to KP233995).
Funding Statement
This research activity was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca with the project titled: Insects and globalization: sustainable control of exotic species in agro-forestry ecosystems (GEISCA) PRIN, id: 2010CXXHJE; and partly by the 7th FP Marie Curie Actions People International Research Staff Exchange Scheme (IRSES) (IPRABIO, grant 269196).
References
- 1. Viggiani G, Laudonia S, Bernardo U. The increase of insect pests in Eucalyptus. Inf Agr. 2001;58: 86–87. [Google Scholar]
- 2. Aytar F. Natural biology, distribution and control method of Leptocybe invasa Fisher & La Salle (Hym., Eulophidae), eucalyptus gall wasps in Turkey. Journal of DOA. 2003;9: 47–66. [Google Scholar]
- 3. Mendel Z, Protasov A, Fisher N. Taxonomy and biology of Leptocybe invasa gen. and sp. n. (Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus . Aust J Entomol. 2004;43: 101–113. [Google Scholar]
- 4. Kim I-K, Mendel Z, Protasov A, Blumberg D, La Salle J. Taxonomy, biology and efficacy of two Australian parasitoids of the eucalyptus gall wasp, Leptocybe invasa Fisher and La Salle (Hymenoptera: Eulophidae: Tetrastichinae). Zootaxa. 2008;1910: 1–20. [Google Scholar]
- 5.EPPO 2013 PQR database. Paris, France: European and Mediterranean Plant Protection Organization. Available: http://www.eppo.int/DATABASES/pqr/pqr.htm.
- 6. Hill KD, Johnson L. Systematic studies in the eucalypts. 10. New tropical and subtropical eucalypts from Australia and New Guinea (Eucalyptus, Myrtaceae). Telopea. 2000;8: 503–539. [Google Scholar]
- 7. Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S. Agroforestree Database: a tree reference and selection guide version 4.0; 2009. World Agroforestry Centre, Kenya: Available: http://www.worldagroforestry.org/resources/databases/agroforestree [Google Scholar]
- 8. Paine TD, Steinbauer MJ, Lawson SA. Native and exotic pests of Eucalyptus: a worldwide perspective. Annu Rev Entomol. 2011;56: 181–201. 10.1146/annurev-ento-120709-144817 [DOI] [PubMed] [Google Scholar]
- 9. Austin AD, Yeates DK, Cassis G, Fletcher MJ, La Salle J, Lawrence JF, et al. Insects 'Down Under'—Diversity, endemism and evolution of the Australian insect fauna: examples from select orders. Australian J Entomol. 2004;43: 216–234. [Google Scholar]
- 10. La Salle J, Arakelian G, Garrison RW, Gates MW. A new species of invasive gall wasp (Hymenoptera: Eulophidae: Tetrastichinae) on blue gum (Eucalyptus globulus) in California. Zootaxa. 2009;2121: 35–43. [Google Scholar]
- 11. Johnson DM, Liebhold AM, Tobin PC, Bjoernstad O. Allee effects and pulsed invasion by the gypsy moth. Nature (Lond.). 2006;444: 361–363. [DOI] [PubMed] [Google Scholar]
- 12. Rubinoff D, Holland BS, Shibata A, Messing RH, Wright MG. Rapid invasion despite lack of genetic variation in the erythrina gall wasp (Quadrastichus erythrinae Kim). Pacific Science. 2010;64: 23–31. [Google Scholar]
- 13. Desneux N, Luna MG, Guillemaud T, Urbaneja A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci. 2011;84: 403–408. [Google Scholar]
- 14. Gilioli G, Pasquali S, Tramontini S, Riolo F. Modelling local and long-distance dispersal of invasive chestnut gall wasp in Europe. Ecol Model. 2013;263: 281–290. [Google Scholar]
- 15.Lopez VM, Rugman-Jones PF, Coleman TW, Hoddle MS, Stouthamer R. Population genetics of goldspotted oak borer, Agrilus auroguttatus Schaeffer (Coleoptera: Buprestidae): investigating the origin of an invasive pest of native oaks in California. Biol Invasions. 2014;: 10.1007/s10530-014-0672-7 [DOI]
- 16. Wylie FR, Speight MR. Insect pests in tropical forestry CABI; 2012. [Google Scholar]
- 17. Zheng XL, Li J, Yang ZD, Xian ZH, Wei JG. A review of invasive biology, prevalence and management of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae). Afr Entomol. 2014;22: 68–79. [Google Scholar]
- 18. Colautti RI, MacIsaac HJ. A neutral terminology to define “invasive” species. Divers Distrib. 2004;10: 135–141. [Google Scholar]
- 19. Lawson S, Griffiths M, Nahrung H, Noack A, Wingfield M, Wilcken C, et al. Biological control of eucalypt pests overseas and in Australia 1–40. Report Australian Centre for International Agricultural Research (ACIAR), Canberra; 2012. [Google Scholar]
- 20. Thu PQ, Dell B, Burgess TI. Susceptibility of 18 eucalypt species to the gall wasp Leptocybe invasa in the nursery and young plantations in Vietnam. Science Asia. 2009; 35:113–117. [Google Scholar]
- 21. Doğanlar O. Occurrence of Leptocybe invasa Fisher & La Salle, 2004 (Hymenoptera: Chalcidoidea) on Eucalyptus camaldulensis in Turkey, with a description of the male sex. Zool Middle East. 2005;35: 112–114. [Google Scholar]
- 22. Gupta A, Poorani J. Taxonomic studies on a collection of Chalcidoidea (Hymenoptera) from India with new distribution records. JoTT. 2009;1: 300–304. [Google Scholar]
- 23. Chen H-Y, Yao J-M, Xu Z-F. First description of the male of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae) from China. J Environ Entomol. 2009;31: 285–287. [Google Scholar]
- 24. Sangtongpraow B, Charernsom K, Siripatanadilok S. Longevity, fecundity and development time of Eucalyptus gall wasp, Leptocybe invasa Fisher and La Salle (Hymenoptera: Eulophidae) in Kanchanaburi province, Thailand. Thai J Agric Science. 2011;44: 155–163. [Google Scholar]
- 25. Tung GS, La Salle J. Pest alert-a newly discovered invasion of gall-forming wasps, Leptocybe invasa (Fisher & La Salle), on eucalyptus trees in Taiwan. Formosan Entomol. 2010;30: 241–244. [Google Scholar]
- 26. Liang Y, Jiyue LI, Xiujun W, Zuozhen LI. Observation on the male ratio of Leptocybe invasa adult. Forest Pest and Disease. 2010;5: 21–22. [Google Scholar]
- 27. Heimpel GE, de Boer JG. Sex determination in the hymenoptera. Annu Rev Entomol. 2008;53: 209–230. [DOI] [PubMed] [Google Scholar]
- 28. Weeks AR, Velten R, Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc R Soc Lond B. 2003;270: 1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adachi-Hagimori T, Miura K, Stouthamer R. A new cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera. Proc R Soc B. 2008;275: 2667–2673. 10.1098/rspb.2008.0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giorgini M, Monti MM, Caprio E, Stouthamer R, Hunter MS. Feminization and the collapse of haplodiploidy in an asexual parasitoid wasp harbouring the bacterial symbiont Cardinium . Heredity. 2009;102: 365–371. 10.1038/hdy.2008.135 [DOI] [PubMed] [Google Scholar]
- 31. Reumer BM, van Alphen JJM, Kraaijeveld K. Ecology, Wolbachia infection frequency and mode of reproduction in the parasitoid wasp Tetrastichus coeruleus (Hymenoptera: Eulophidae). Mol Ecol. 2010;19: 1733–1744. 10.1111/j.1365-294X.2010.04599.x [DOI] [PubMed] [Google Scholar]
- 32. Reumer BM, van Alphen JJM, Kraaijeveld K. Population genetics of Wolbachia-infected, parthenogenetic and uninfected, sexual populations of Tetrastichus coeruleus (Hymenoptera: Eulophidae). Mol Ecol. 2013;22: 4433–4444. 10.1111/mec.12397 [DOI] [PubMed] [Google Scholar]
- 33. Giorgini M, Bernardo U, Monti MM, Nappo AG, Gebiola M. Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl Environ Microb. 2010;76: 2589–2599. 10.1128/AEM.03154-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhahri S, Ben Jamaa M, Lo Verde G. First record of Leptocybe invasa and Ophelimus maskelli eucalyptus gall wasps in Tunisia. Tunisian J Plant Protec. 2010;5: 229–234. [Google Scholar]
- 35.Aquino DA, Botto E, Loiacono MS, Pathauer P. "Avispa de la agalla del eucalipto,” Leptocybe invasa Fischer & La Salle (Hymenoptera: Eulophidae: Tetrastichinae), en Argentina. RIA / Trabajos en prensa. 2011;1–6.
- 36. Gebiola M, Bernardo U, Monti MM, Navone P, Viggiani G. Pnigalio agraules (Walker) and Pnigalio mediterraneus Ferrière and Delucchi (Hymenoptera: Eulophidae): two closely related valid species. J Nat Hist. 2009;43: 2465–2480. [Google Scholar]
- 37. Gebiola M, Gómez-Zurita J, Monti MM, Navone P, Bernardo U. Integration of molecular, ecological, morphological and endosymbiont data for species delimitation within the Pnigalio soemius complex (Hymenoptera: Eulophidae). Mol Ecol. 2012;21: 1190–1208. 10.1111/j.1365-294X.2011.05428.x [DOI] [PubMed] [Google Scholar]
- 38. Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator . Proc Natl Acad Sci Usa. 2004;101: 14812–14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell BC, Steffen-Campbell JD, Werren JH. Phylogeny of the Nasonia species complex (Hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28S rDNA sequences. Insect Mol Biol. 1993;2: 225–237. [DOI] [PubMed] [Google Scholar]
- 40. Stouthamer R, Hu J, van Kan FJ, Platner GR, Pinto JD. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma . BioControl. 1999;43: 421–440. [Google Scholar]
- 41. Babcock CS, Heraty JM. Molecular Markers Distinguishing Encarsia formosa and Encarsia luteola (Hymenoptera: Aphelinidae). Ann Entomol Soc Am. 2000;93: 738–744. [Google Scholar]
- 42. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech. 1994;3: 294–299. [PubMed] [Google Scholar]
- 43. Simon C, Frati F, Beckenbach AT, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87: 651–701. [Google Scholar]
- 44. Bernardo U, Monti MM, Nappo AG, Gebiola M, Russo A, Pedata PA, et al. Species status of two populations of Pnigalio soemius (Hymenoptera: Eulophidae) reared from two different hosts: An integrative approach. Biol Control. 2008;46: 293–303. [Google Scholar]
- 45. Gebiola M, Bernardo U, Burks R. A reevaluation of the generic limits of Pnigalio Schrank (Hymenoptera: Eulophidae) based on molecular and morphological evidence. Zootaxa. 2010;2484: 35–44. [Google Scholar]
- 46. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41: 95–98. [Google Scholar]
- 47. Zhang D, Hewitt G. Nuclear integrations: challenges for mitochondrial DNA markers. Tree. 1996;11: 247–251. [DOI] [PubMed] [Google Scholar]
- 48. Bensasson D, Zhang DX, Hartl DL, Hewitt GM. Mitochondrial pseudogenes: evolution's misplaced witnesses. Trends Ecol Evol. 2001;16: 314–321. [DOI] [PubMed] [Google Scholar]
- 49. Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 50. Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14: 817–818. [DOI] [PubMed] [Google Scholar]
- 51. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 52. Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29: 2869–2876. 10.1093/bioinformatics/btt499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cottontail VM, Kalko EKV, Cottontail I, Wellinghausen N, Tschapka M, Perkins SL, et al. High local diversity of Trypanosoma in a common bat species, and implications for the biogeography and taxonomy of the T. cruzi clade. PLoS ONE. 2014;9:e108603 10.1371/journal.pone.0108603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9: 1657–1659. [DOI] [PubMed] [Google Scholar]
- 55. Hart M, Sunday J. Things fall apart: biological species form unconnected parsimony networks. Biol Lett. 2007;3: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ercolini D. PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. Journal Microbiol Meth. 2004;56: 297–314. [DOI] [PubMed] [Google Scholar]
- 57. Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dar S, Kuenen J, Muyzer G. Nested PCR-denaturing gradient gel electrophoresis approach to determine the diversity of sulfate-reducing bacteria in complex microbial communities. Appl Environ Microbiol. 2005;71: 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microb. 1993;59: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microb. 2006;72: 3646–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fukatsu T, Tsuchida T, Nikoh N, Koga R. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl Environ Microb. 2001;67: 1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum . Mol Ecol. 2002;11: 2123–2135. [DOI] [PubMed] [Google Scholar]
- 63. Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc Lond B. 1995;261: 55–63. [DOI] [PubMed] [Google Scholar]
- 64. Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the Rickettsiae. Int J Syst Bacteriol. 1997;47: 252–261. [DOI] [PubMed] [Google Scholar]
- 65. Vitorino L, Chelo IM, Bacellar F, Ze-Ze L. Rickettsiae phylogeny: a multigenic approach. Microbiology. 2007;153: 160–168. [DOI] [PubMed] [Google Scholar]
- 66. Merhej V, Raoult D. Rickettsial evolution in the light of comparative genomics. Biol Rev. 2010;86: 379–405. 10.1111/j.1469-185X.2010.00151.x [DOI] [PubMed] [Google Scholar]
- 67. Fournier PE, Zhu Y, Ogata H, Raoult D. Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. J Clin Microbiol. 2004;42: 5757–5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. BCM Biology. 2009;7: 6 http://www.biomedcentral.com/1741-7007/7/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coombs MT, Costa HS, De Barro P, Rosell RC. Pre-Imaginal Egg Maturation and Bacteriocyte Inclusion in Bemisia aff. gigantea (Hemiptera: Aleyrodidae). Ann Entomol Soc Am. 2007;100: 736–744. [Google Scholar]
- 70. Heraty JM, Woolley JB, Hopper KR, Hawks DL, Kim JW, et al. Molecular phylogenetics and reproductive incompatibility in a complex of cryptic species of aphid parasitoids. Mol Phylogenet Evol. 2007;45: 480–493. [DOI] [PubMed] [Google Scholar]
- 71. Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol. 2008;17: 431–449. [DOI] [PubMed] [Google Scholar]
- 72. Johnstone RA, Hurst GD. Maternally inherited male‐killing microorganisms may confound interpretation of mitochondrial DNA variability. Biol J Linn Soc. 1996;58: 453–470. [Google Scholar]
- 73. Engelstädter J, Hurst GDD. The ecology and evolution of microbes that manipulate host reproduction. Ann Rev Ecol Evol & Syst. 2009;40: 127–149. 10.1093/molbev/mss070 [DOI] [PubMed] [Google Scholar]
- 74. Gueguen G, Vavre F, Gnankine O, Peterschmitt M, Charif D, Chiel E, et al. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol. 2010;19: 4365–4378. 10.1111/j.1365-294X.2010.04775.x [DOI] [PubMed] [Google Scholar]
- 75. Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, et al. Plasmids and Rickettsial Evolution: Insight from Rickettsia felis . PLoS ONE. 2007;2(3): e266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McFrederick QS, Taylor DR. Evolutionary history of nematodes associated with sweat bees. Mol Phylogenet Evol. 2013;66: 847–856. 10.1016/j.ympev.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 77.Zhu F-L, Ren S-X, Qiu B-L, Wu J-H. Effect of temperature on life table parameters of Leptocybe invasa (Hymenoptera: Eulophidae). Austral Entomol. 2014;: 10.1111/aen.12094 [DOI]
- 78. Hagimori T, Abe Y, Date S, Miura K. The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Curr Microbiol. 2006;52: 97–101. [DOI] [PubMed] [Google Scholar]
- 79. Veneti Z, Zabalou S, Papafotiou G, Paraskevopoulos C, Pattas S, Livadaras I, et al. Loss of reproductive parasitism following transfer of male- killing Wolbachia to Drosophila melanogaster and Drosophila simulans . Heredity. 2012;109: 306–312. 10.1038/hdy.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Unckless R, Boelio L, Herren J, Jaenike J. Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proc R Soc Lond B. 2009;276: 2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jaenike J. Spontaneous emergence of a new Wolbachia phenotype. Evolution. 2007;61: 2244–2252. [DOI] [PubMed] [Google Scholar]
- 82. Bordenstein SR, Bordenstein SR. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS ONE. 2011;6: e29106 10.1371/journal.pone.0029106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Giorgini M. Induction of males in thelytokous populations of Encarsia meritoria and Encarsia protransvena: a systematic tool. BioControl. 2001;46: 427–438. [Google Scholar]
- 84.Huigens ME, Stouthamer R. Parthenogenesis associated with Wolbachia. In: Bourtzis K, Miller TA (eds) Insect symbiosis, vol 2., CRCBoca Raton, FL, pp 247–266. 2003.
- 85. Reumer BM, van Alphen JJM, Kraaijeveld K. Occasional males in parthenogenetic populations of Asobara japonica (Hymenoptera: Braconidae): low Wolbachia titer or incomplete coadaptation? Heredity. 2012;108: 341–346. 10.1038/hdy.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ferrari J, Scarborough CL, Godfray HCJ. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia. 2007;153: 323–329. [DOI] [PubMed] [Google Scholar]
- 87. Biere A, Tack AJM. Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Funct Ecol. 2013;27: 646–660. [Google Scholar]
- 88. Perotti MA, Clarke HK, Turner BD, Braig HR. Rickettsia as obligate and mycetomic bacteria. Faseb J. 2006;20: E1646–1656. [DOI] [PubMed] [Google Scholar]
- 89. Kelly J, La Salle J, Harney M, Dittrich-Schroder G, Hurley BP. Selitrichodes neseri n. sp, a new parasitoid of the eucalyptus gall wasp Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae). Zootaxa. 2012;3333: 50–57. [Google Scholar]
- 90. Hansen AK, Jeong G, Paine TD, Stouthamer R. Frequency of secondary symbiont infection in an invasive psyllid relates to parasitism pressure on a geographic scale in California. Appl Environ Microbiol. 2007;73: 7531–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vorburger C. The evolutionary ecology of symbiont-conferred resistance to parasitoids in aphids. Insect Sci. 2014;21: 251–264. 10.1111/1744-7917.12067 [DOI] [PubMed] [Google Scholar]
- 92. Rouchet R, Vorburger C. Experimental evolution of parasitoid infectivity on symbiont-protected hosts leads to the emergence of genotype specificity. Evolution. 2014;68: 1607–1616. 10.1111/evo.12377 [DOI] [PubMed] [Google Scholar]
- 93. Asplen MK, Bano N, Brady CM, Desneux N, Hopper KR, Malouines C, et al. Specialisation of bacterial endosymbionts that protect aphids from parasitoids. Ecol Entomol. 2014;39: 736–739. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The evolutionary model selected by MrModeltest2 was GTR+G. Scale bar indicates the number of substitutions per site.
(TIF)
(a) Bayesian phylogeny based on atpA sequences (Evol. model: GTR+I+G); (b) Bayesian phylogeny based on gltA sequences (Evol. model: TVM+G). The host is provided whenever the symbiont is not identified at the species level. Posterior probabilities are reported above branches. Scale bar indicates the number of substitutions per site.
(TIF)
Bootstrap values (>75%) are reported above branches. Scale bar indicates the number of substitutions per site.
(TIF)
Rickettsia bacteria, stained with Rickettsia specific probe RickPn-Cy3, appear like bright red spots on the ooplasm background (A), while appear like bright green spots on the ooplasm background (B) when stained with universal bacterial probe EUB388-Cy5. Bars, 20 µm.
(TIF)
(DOCX)
*Presence of males.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files, except for sequences that are available from the GenBank database http://www.ncbi.nlm.nih.gov/genbank/ (from accession numbers KP143943 to KP143995 and from KP233904 to KP233995).