Abstract
BACKGROUND
Air-pollution levels have been trending downward progressively over the past several decades in southern California, as a result of the implementation of air quality– control policies. We assessed whether long-term reductions in pollution were associated with improvements in respiratory health among children.
METHODS
As part of the Children’s Health Study, we measured lung function annually in 2120 children from three separate cohorts corresponding to three separate calendar periods: 1994–1998, 1997–2001, and 2007–2011. Mean ages of the children within each cohort were 11 years at the beginning of the period and 15 years at the end. Linear-regression models were used to examine the relationship between declining pollution levels over time and lung-function development from 11 to 15 years of age, measured as the increases in forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) during that period (referred to as 4-year growth in FEV1 and FVC).
RESULTS
Over the 13 years spanned by the three cohorts, improvements in 4-year growth of both FEV1 and FVC were associated with declining levels of nitrogen dioxide (P<0.001 for FEV1 and FVC) and of particulate matter with an aerodynamic diameter of less than 2.5 μm (P = 0.008 for FEV1 and P<0.001 for FVC) and less than 10 μm (P<0.001 for FEV1 and FVC). These associations persisted after adjustment for several potential confounders. Significant improvements in lung-function development were observed in both boys and girls and in children with asthma and children without asthma. The proportions of children with clinically low FEV1 (defined as <80% of the predicted value) at 15 years of age declined significantly, from 7.9% to 6.3% to 3.6% across the three periods, as the air quality improved (P = 0.001).
CONCLUSIONS
We found that long-term improvements in air quality were associated with statistically and clinically significant positive effects on lung-function growth in children. (Funded by the Health Effects Institute and others.)
In previous investigations, we and others have linked exposure to ambient air pollution with lung-function impairment in children. 1–8 Reduced lung function in children has been associated with an increased risk of asthma. 9 In addition, the adverse effects of air pollution on the lungs in childhood can potentially have long-term effects: lung function lower than the predicted value for a healthy adult has been found to be associated with an increased risk of cardiovascular disease and increased mortality rate.10–12 Although progress has been made throughout the United States to reduce outdoor levels of several air pollutants, it is not known whether these reductions have been associated with improvements in children’s respiratory health.
Southern California has historically been plagued by high levels of air pollution owing to the presence of a large motor-vehicle fleet, numerous industries, the largest seaport complex in the United States, and a natural landscape that traps polluted air over the Los Angeles basin. With mounting scientific evidence of the adverse health effects of air pollution, aggressive pollution- reduction policies have been enacted. These have included strategies to control pollution from mobile and stationary sources, as well as fuel and consumer-product reformulations. As a result, air-pollution levels have been trending downward over the past several decades in southern California.
Improvements in air quality over time provide the backdrop for a “natural experiment” to examine the potential beneficial health effects. As part of the 20-year Children’s Health Study, three separate cohorts of children have had longitudinal lung-function measurements recorded over the same 4-year age range (11 to 15 years) and in the same five study communities but during different calendar periods. In this study, we examined whether changes that have occurred across these time spans in levels of nitrogen dioxide, ozone, and particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5), less than 10 μm (PM10), and between 2.5 and 10 μm (coarse particulate matter [PM10–PM2.5]) are associated with the development of lung function in children.
METHODS
PARTICIPANTS
The study sample included children recruited from three separate Children’s Health Study cohorts (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM. org). The two earlier cohorts (cohorts C and D) enrolled fourth-grade students in 1992–1993 and 1995–1996, respectively, from elementary schools in 12 southern California communities.13 The third cohort (cohort E) enrolled kindergarten and first-grade students in 2002–2003 from 13 communities, 14 9 of which overlapped with the 12 cohort C and D communities. Because of budgetary limitations, pulmonary-function testing was conducted in only 5 of the 9 overlapping communities. To facilitate direct comparisons across calendar periods, analyses were restricted to the 5 study communities (Long Beach, Mira Loma, Riverside, San Dimas, and Upland) in which pulmonary-function testing was performed in all three cohorts (Fig. S2 in the Supplementary Appendix). This sample included a total of 2120 children, including 669 in cohort C, 588 in cohort D, and 863 in cohort E.
The study protocol was approved by the institutional review board for human studies at the University of Southern California, and written informed consent was provided by a parent or legal guardian for all study participants.
PULMONARY-FUNCTION TESTING
Trained technicians assisted the students in performing pulmonary-function maneuvers that met the standards of the American Thoracic Society, as described elsewhere.8 For each child, maximal forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were measured. For cohorts C and D, pulmonary-function testing was performed annually from 4th through 12th grade (mean ages, 10 to 18 years), with the use of rolling-seal spirometers. In cohort E, pulmonary-function testing was performed every other year, when the children were approximately 11, 13, and 15 years of age, with the use of pressure transducer–based spirometers. Of the 2120 children, 1585 (74.8%) were tested at the beginning (age 11 years) and end (age 15 years) of the follow-up period, whereas the remaining 535 children (25.2%) were tested at 11 but not at 15 years of age.
QUESTIONNAIRES
Written questionnaires regarding each child’s general health and personal exposures were completed by the parent or guardian at baseline and by the child participants annually throughout the study thereafter. The questionnaires obtained basic information about the child, including age, sex, self-identified race and ethnic background, insurance coverage, and parental education, as well as the child’s health conditions (asthma or acute respiratory illness), tobacco-smoke exposures (personal smoking, secondhand exposure, or in utero exposure), and other exposures.
AIR POLLUTANTS
Outdoor air-pollution monitoring stations in each of the study communities have been continuously measuring regional air pollutants since 1994. Data on hourly or daily concentrations of nitrogen dioxide, PM2.5, PM10, and ozone were routinely collected with Federal Reference Method or Federal Equivalent Method instrumentation. A systematic quality-assurance program was in place to review all data. Mean air-pollutant concentrations were calculated for each community over the relevant periods of exposure for each cohort (1994–1997 for cohort C, 1997–2000 for cohort D, and 2007–2010 for cohort E).
STATISTICAL ANALYSIS
The goal of the analyses was to examine the association between long-term improvements in ambient air quality and lung-function development in children from 11 to 15 years of age, measured as the increases in FEV1 and FVC during that period (hereafter referred to as 4-year growth in FEV1 and FVC). All available pulmonary-function measurements were used to estimate lung-function growth curves, including measurements at ages ranging from approximately 9 to 19 years in cohorts C and D and 10 to 16 years in cohort E. A previously developed linear-spline model,5 with knots placed at ages 12, 14, and 16 years, was used to capture the nonlinear pattern of growth during adolescence (see the Supplementary Appendix for details). The model included adjustments for sex, race, Hispanic ethnic background, height, height squared, body-mass index (BMI, the weight in kilograms divided by the square of the height in meters), BMI squared, and presence or absence of respiratory-tract illness on the day of the pulmonary-function test. The model was constructed to yield estimates of the effects of pollutants on 4-year lung-function development (from 11 to 15 years of age) and on mean attained lung function at either 11 or 15 years of age. The range from 11 to 15 years of age was targeted because it covers the overlapping age period of pulmonary-function testing across cohorts. Follow-up over this age range was conducted from 1994 through 1998 in cohort C, from 1997 through 2001 in cohort D, and from 2007 through 2011 in cohort E. The estimated health effect of each pollutant is reported as the expected difference in lung-function growth associated with a difference in exposure equal to the median of the changes in pollution in the five communities over the course of the study period (Table 1).
Table 1.
Estimated Differences in 4-Year Lung-Function Growth for Median Decreases in Ambient Pollutant Levels.*
| Lung-Function Measurement and Pollutant | Lung-Function Difference at 11 Years of Age | Lung-Function Difference at 15 Years of Age | Growth from 11 to 15 Years of Age | |||
|---|---|---|---|---|---|---|
| Mean (95% CI) | P Value | Mean (95% CI) | P Value | Mean (95% CI) | P Value | |
| ml | ml | ml | ||||
| FEV1 | ||||||
|
| ||||||
| Nitrogen dioxide | 119.2 (76.5 to 161.9) | <0.001 | 210.6 (156.0 to 265.2) | <0.001 | 91.4 (47.9 to 134.9) | <0.001 |
|
| ||||||
| Ozone | 15.0 (−38.5 to 68.6) | 0.58 | 8.3 (−82.9 to 99.6) | 0.86 | −6.7 (−51.0 to 37.5) | 0.77 |
|
| ||||||
| PM10 | 87.7 (50.2 to 125.2) | <0.001 | 153.2 (97.7 to 208.6) | <0.001 | 65.5 (27.2 to 103.7) | <0.001 |
|
| ||||||
| PM2.5 | 100.0 (58.9 to 141.2) | <0.001 | 165.5 (95.4 to 235.6) | <0.001 | 65.5 (17.1 to 113.8) | 0.008 |
|
| ||||||
| FVC | ||||||
|
| ||||||
| Nitrogen dioxide | 131.3 (91.0 to 171.6) | <0.001 | 300.2 (240.0 to 360.3) | <0.001 | 168.9 (127.0 to 210.7) | <0.001 |
|
| ||||||
| Ozone | 7.6 (−50.2 to 65.4) | 0.80 | 0.3 (−126.0 to 126.5) | 0.99 | −7.3 (−79.3 to 64.6) | 0.84 |
|
| ||||||
| PM10 | 93.8 (54.0 to 133.6) | <0.001 | 206.8 (124.6 to 289.1) | <0.001 | 113.0 (60.0 to 166.1) | <0.001 |
|
| ||||||
| PM2.5 | 110.1 (69.8 to 150.4) | <0.001 | 237.0 (147.2 to 326.7) | <0.001 | 126.9 (65.7 to 188.1) | <0.001 |
The estimated means of community-specific pollution effects on lung-function values at 11 years of age and at 15 years of age and growth from 11 to 15 years of age are scaled to the median of the five community-specific declines in each air pollutant — specifically, 14.1 ppb in nitrogen dioxide, 5.5 ppb in ozone (10 a.m. to 6 p.m.), 8.7 μg/m3 in particulate matter with an aerodynamic diameter of less than 10 μm (PM10), and 12.6 μg/m3 in particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5). For example, a decline of 14.1 ppb in nitrogen dioxide is associated with an increase of 91.4 ml in the growth of FEV1 from 11 to 15 years of age (the mean of the five community- specific slopes).
In addition to examining 4-year growth from 11 to 15 years of age, we analyzed the cross-sectional pulmonary-function measurements obtained for 1585 children at the end of this period (mean age, 15 years) to determine whether changes in air quality over time were associated with clinically important deficits in attained FEV1 and FVC. Using data from all three cohorts, we developed a linear prediction model for FEV1 that included adjustments for age, sex, race and ethnic background, height, height squared, BMI, BMI squared, and the presence or absence of respiratory illness. This model explained 61% of the variance in FEV1 and 69% of the variance in FVC measurements at 15 years of age (i.e., R2 = 0.61 and 0.69, respectively). For each child, we determined whether the ratio of observed to predicted FEV1 and FVC fell below each of three cutoffs for defining low lung function: 90%, 85%, and 80%. Logistic regression was used to test for temporal trends in the proportion of children with low lung function across cohorts after adjustment for community. A P value of less than 0.05 was considered to indicate statistical significance, under the assumption of a two-sided alternative hypothesis.
RESULTS
CHARACTERISTICS OF THE STUDY PARTICIPANTS
There were slightly more girls than boys (52% vs. 48%) in each of the three cohorts (Table S1 in the Supplementary Appendix). The mean age at the baseline pulmonary-function test was higher in cohort E (11.3 years) than in cohort C (10.9 years) and cohort D (10.9 years). The age-specific mean height did not differ significantly across cohorts at 11, 13, or 15 years of age. There was a significantly higher proportion of Hispanic children in cohort E than in cohorts C and D (58% vs. 31% and 33%, respectively). Cohort E also differed significantly from cohorts C and D with regard to several other factors, including exposure to passive smoke and pets (lower in cohort E) and the proportion of parents with health insurance (higher in cohort E) (Table S1 in the Supplementary Appendix).
CHANGES IN AIR QUALITY
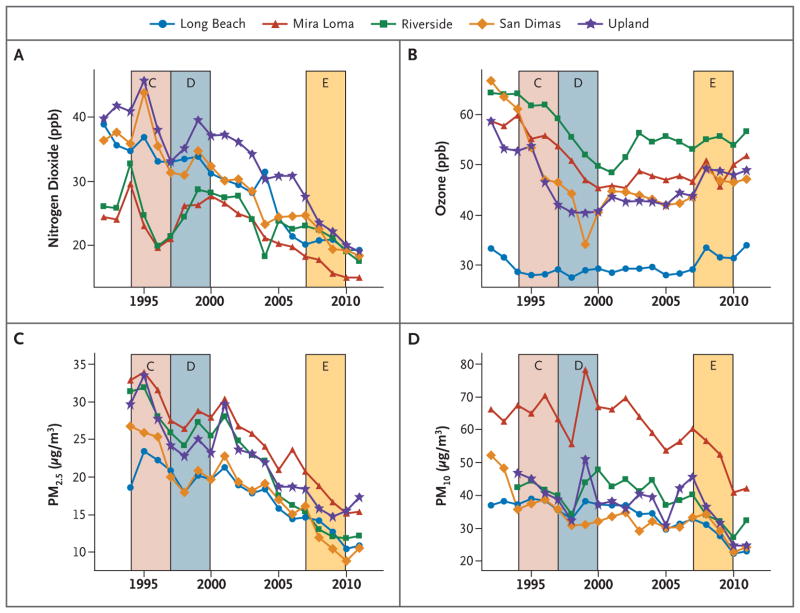
Regional air quality has improved dramatically over the course of the Children’s Health Study with respect to some pollutants (Fig. 1). The colored bands in Figure 1 represent the 4-year exposure periods for cohorts C (1994–1997), D (1997–2000), and E (2007–2010). For example, the 4-year mean PM2.5 level in the community with the highest levels of particulate matter (Mira Loma) declined from 31.5 μg per cubic meter in cohort C to 17.8 μg per cubic meter in cohort E, a 43% reduction (Table S2 in the Supplementary Appendix). All five communities had large declines in levels of PM2.5 and nitrogen dioxide. Changes in levels of PM10 and ozone over time were more modest (Fig. 1), as were changes in levels of PM10–PM2.5 (Fig. S3 in the Supplementary Appendix).
Figure 1. Levels of Four Air Pollutants from 1994 to 2011 in Five Southern California Communities.
Colored bands represent the relevant 4-year averaging period for the analysis of lung-function growth in each of the three cohorts, C, D, and E. PM2.5 denotes particulate matter with an aerodynamic diameter of less than 2.5 μm, and PM10 particulate matter with an aerodynamic diameter of less than 10 μm.
CHANGES IN LUNG-FUNCTION GROWTH
Across all three study cohorts, the mean FEV1 among girls increased from 2274 ml at 11 years of age to 3150 ml at 15 years of age, for a mean 4-year increase of 876 ml (Table S3 in the Supplementary Appendix). Among boys, the mean FEV1 was 2311 ml at 11 years of age and 3831 ml at 15 years of age, for a mean 4-year growth of 1520 ml. Similar magnitudes of growth were observed for FVC.
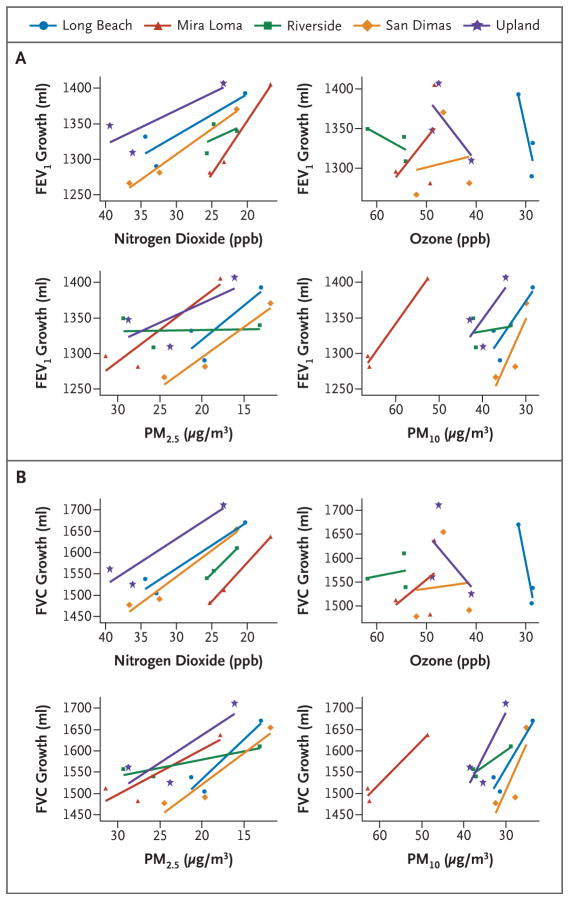
Increases in 4-year growth in both FEV1 and FVC were associated with reduced levels of nitrogen dioxide, PM10, and PM2.5 within all five study communities (Fig. 2). When the effects were averaged across communities, we found that the mean 4-year growth in FEV1 increased by 91.4 ml per decrease of 14.1 ppb in nitrogen dioxide level (P<0.001), by 65.5 ml per decrease of 8.7 μg per cubic meter in PM10 level (P<0.001), and by 65.5 ml per decrease of 12.6 μg per cubic meter in PM2.5 level (P = 0.008) (Table 1). At the beginning of follow-up (when participants were 11 years of age), significant increases in mean FEV1 values were associated with decreases in levels of nitrogen dioxide, PM10, and PM2.5 (Table 1). However, the increases in mean FEV1 at 15 years of age, after 4 years of pollution-affected growth, were even more pronounced. Analogous effects were observed for FVC. Changes in ozone (Fig. 2) and PM10–PM2.5 (Fig. S4 in the Supplementary Appendix) were not associated with differences in mean FEV1 or FVC values at 11 or 15 years of age or with 4-year growth in these values.
Figure 2. Mean 4-Year Lung-Function Growth versus the Mean Levels of Four Pollutants.
The mean growth in forced expiratory volume in 1 second (FEV1) (Panel A) and the mean growth in forced vital capacity (FVC) (Panel B) from 11 to 15 years of age are plotted against the corresponding levels of nitrogen dioxide, ozone, PM2.5, and PM10 for each community and cohort.
The estimated pollution-related effects on 4-year FEV1 and FVC growth remained significant in sensitivity analyses (Table S4 in the Supplementary Appendix). For example, the associations between improved lung-function development and reduced nitrogen dioxide levels in Table 1 (shown as “base model” in Table S4 in the Supplementary Appendix) remained significant and of similar magnitude when additional adjustment was made for in utero or passive tobacco-smoke exposure, personal smoking, health insurance, parental education, asthma at baseline, or several indoor exposures, including cats, dogs, and mold or mildew. There were significant effects on lung-function growth in both boys and girls, although the magnitude of the air-pollution effect was significantly larger in boys than in girls with respect to both FEV1 (P = 0.04) and FVC (P = 0.001). There were also significant pollution effects on lung-function growth in Hispanic white and non-Hispanic white children and in children with asthma and children without asthma. Although the magnitude of the nitrogen dioxide effect on 4-year growth in FEV1 and FVC was nearly twice as large in children with asthma, as compared with children without asthma, the difference was not significant for either lung-function measure. Pollution-effect estimates were of a magnitude similar to those in the base model when the sample was restricted to children with complete 4-year follow-up data. Sensitivity analyses of the other pollutants yielded results similar to those for nitrogen dioxide (data not shown). We found no significant association between growth in height and change in pollution during the study period (Table S5 in the Supplementary Appendix), which indicates that our findings on lung-function growth are probably not the result of a secular trend in general development.
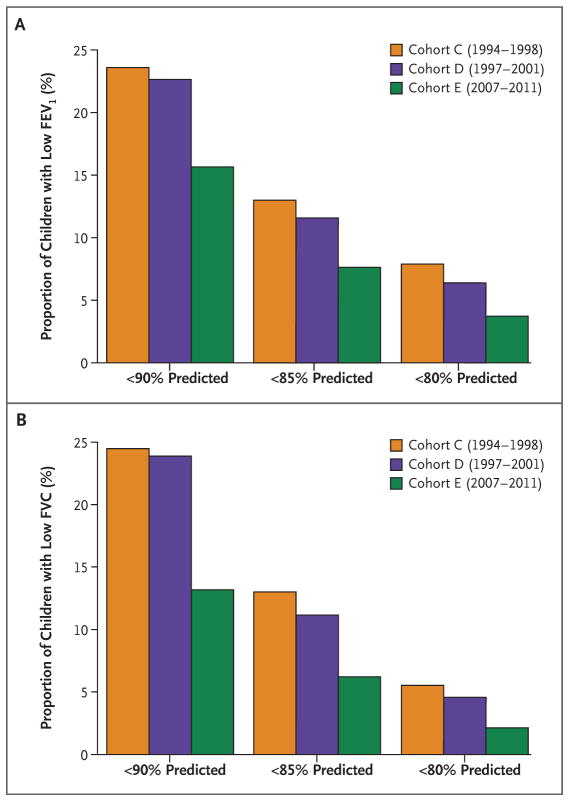
The large improvements in lung function associated with reduced air pollution (Table 1) suggest that the mean attained FEV1 and FVC values at 15 years of age were substantially larger in cohort E (the cohort with the lowest level of exposure, as seen in Fig. 1) than in cohorts C and D. Further analysis of lung function at 15 years of age also revealed significant differences across cohorts in the proportion of children with low lung function (Fig. 3). For example, at a cutoff of 80% for the ratio of observed to predicted values, the proportion of children with low FEV1 was 7.9% in cohort C, 6.3% in cohort D, and only 3.6% in cohort E (P<0.005). Similar significant trends were observed for FVC.
Figure 3. Proportions of Children with Low Lung Function in Each Cohort.
The proportions of children with lung function below 90%, 85%, or 80% of the predicted value at 15 years of age in cohorts C, D, and E are shown for FEV1 (Panel A) and FVC (Panel B).
DISCUSSION
This study shows an association between secular improvements in air quality in southern California and measurable improvements in lung-function development in children. Improved lung function was most strongly associated with lower levels of particulate pollution (PM2.5 and PM10) and nitrogen dioxide. These associations were observed in boys and girls, Hispanic white and non-Hispanic white children, and children with asthma and children without asthma, which suggests that all children have the potential to benefit from improvements in air quality.
In addition to improvements in lung-function development from 11 to 15 years of age, we also found a strong association between a reduction in air pollution and a reduction in the proportion of children with clinically low FEV1 and FVC at 15 years of age. In general, the age range of 11 to 15 years captures a period during which lungs are developing rapidly in both boys and girls. Lung-function development continues in girls until their late teens and in boys until their early 20s, but at a much-reduced rate as compared with the rate during the earlier adolescent period.15,16 It is therefore likely that the improved function we observed in the children who were less exposed to the pollutants will persist into their adulthood. A higher level of lung function in early adulthood may decrease the risk of respiratory conditions.17 However, the greatest benefit of improvements in lung-function development may occur later in life, because it has been shown that greater lung function in adulthood can contribute to lower risks of premature death and other adverse health outcomes.18–24 Consistent with the growth effects we have observed in children, there is evidence that reduced exposure to pollution in adulthood can slow the decline in lung function25 and increase life expectancy.26,27
In southern California, motor vehicles are a primary source of PM2.5, PM10, and nitrogen dioxide, through direct tailpipe emissions as well as downwind physical and photochemical reactions of vehicular emissions.28,29 Gasoline-powered and diesel-powered engines contribute to high levels of these pollutants, and improved emission standards for both types of vehicles have contributed to the observed declines in air pollutants. Control strategies implemented in the 1970s and 1980s focused primarily on reducing the levels of ozone, a pollutant with a long history of demonstrated acute health effects.30 Although levels of ozone continued to decline in the 1990s and 2000s, the changes were smaller than for nitrogen dioxide and particulate matter, and we did not observe ozone-related effects on lung-function growth. This finding is consistent with our previous report that decreased lung-function growth was related to increased exposure to nitrogen dioxide and particulate matter but not to ozone.6 Only a few other studies have addressed the long-term effects of ozone on lung function in children, and the results have been inconsistent. 31 Because of high correlations among reductions of PM2.5, PM10, and nitrogen dioxide (Table S6 in the Supplementary Appendix), we could not assess the independent associations between lung function and each of these pollutants. Many other studies have also been unable to identify the health effects of specific pollutants that are constituents of a multipollutant mixture.3,32 However, the results of our investigation make it clear that broad-based efforts to improve general air quality are associated with substantial and measurable public health benefits.
A main directive of the 1970 U.S. Clean Air Act was to establish “…ambient air quality standards … allowing an adequate margin of safety … requisite to protect the public health ….” A basic tenet of the act is that changes in airborne pollutant levels can lead to improved public health and that the scientific evidence needed to determine the appropriate levels for those standards can be identified. Our observation of improvements in air quality and subsequent improvements in longitudinal respiratory health outcomes may provide objective evidence in support of that basic tenet.
The data necessary to conduct this study were collected over a period of nearly two decades. Strengths of the study include the use of consistent protocols for collecting health, covariate, and air-quality data over the entire study period. Although the extended follow-up period can be viewed as a strength, it also presented several challenges. A change in spirometers during the course of the study was necessary to replace aging equipment and raises the issue of instrumental comparability. To address this, we conducted an additional analysis to show that our findings are robust to the use of different spirometers (Table S7 in the Supplementary Appendix).
The change from annual testing in cohorts C and D to testing every other year in cohort E, as a result of budgetary constraints, may raise concern about dropout of participants in cohort E. In general, bias can occur in a cohort study if dropout depends simultaneously on both outcome and exposure. In our study, however, participant attrition during the follow-up period was not jointly associated with baseline lung function and several measures of exposure, including cohort membership and cohort-specific mean levels of nitrogen dioxide and particulate matter, the pollutants that showed significant associations with lung-function growth. In addition, the magnitude and significance of our observed growth effects were similar among participants with complete follow-up (Table S4 in the Supplementary Appendix), making it unlikely that selective dropout is responsible for our observed associations.
The shift in ethnic background across cohorts to a more Hispanic population, synchronous with general trends occurring more broadly in southern California,33,34 raises potential concerns about confounding by factors specific to ethnic background. Also, because this is an observational study, it is possible that one or more additional factors associated with both lung-function growth and change in air quality over time could confound our pollution analyses. However, we conducted many sensitivity analyses and found that none of these factors appreciably affected our estimates or inferences. Furthermore, because the mean growth in height from 11 to 15 years of age did not vary over the study period, one might conclude that the change in growth is specific to the lung, with improvement in air quality serving as an important contributing factor.
Another limitation of our study is the lack of a pure “control” community — that is, a community in which there was no change in pollution during the study period. However, we studied five different communities with differing magnitudes of improvement in air quality, which collectively serve as five replicate experiments of our within-community temporal-trend experiment. We conducted an additional analysis that showed that the expected gain in lung function over time within any one community was aligned with the magnitude of improvement in air quality within that community (Fig. S5 in the Supplementary Appendix). The trends in these effects suggest that if we had had a pure control community, we would have seen little change in lung-function growth. This analysis also suggests that even modest improvements in air quality can lead to improved health, although with only five communities included in the study, we caution that we do not have adequate data to make definitive conclusions about the exposure–response relationship.
We have shown that improved air quality in southern California is associated with statistically and clinically significant improvements in childhood lung-function growth. The pollutants we found to be associated with lung-function growth — nitrogen dioxide, PM2.5, and PM10 — are products of primary fuel combustion and are likely to be at increased levels in most urban environments. These pollutants were among those effectively reduced through targeted policy strategies. If we make the not-unreasonable assumption of causality, the magnitude of the effects we observed and the importance of lung function over the course of the human lifetime justify the efforts that have been made to improve air quality.
Supplementary Material
Acknowledgments
Supported in part by contracts with the Health Effects Institute (4910-RFA11-1/12-4) and the California Air Resources Board (A033-186) and by grants from the National Institute of Environmental Health Sciences (ES011627, ES07048, and ES022719) and the Hastings Foundation.
Dr. McConnell reports holding a research contract from funds from an air-quality-violations settlement between the South Coast Air Quality Management District, a California state regulatory agency, and BP.
We thank the participating students and their families, the school staff and administrators, the regional and state air monitoring agencies, and the members of the health testing field team.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We dedicate this article to Dr. John M. Peters, who conceived the original Children’s Health Study design, directed the study over most of its time, and recruited and inspired the colleagues who worked with him to investigate the effects of air pollution on children’s health.
References
- 1.Urman R, McConnell R, Islam T, et al. Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax. 2014;69:540–7. doi: 10.1136/thoraxjnl-2012-203159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mölter A, Agius RM, de Vocht F, et al. Long-term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect. 2013;121:1232–8. doi: 10.1289/ehp.1205961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehring U, Gruzieva O, Agius RM, et al. Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect. 2013;121:1357–64. doi: 10.1289/ehp.1306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Götschi T, Sunyer J, Chinn S, et al. Air pollution and lung function in the European Community Respiratory Health Survey. Int J Epidemiol. 2008;37:1349–58. doi: 10.1093/ije/dyn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauderman WJ, Vora H, McConnell R, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–7. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 6.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 7.Gauderman WJ, Gilliland GF, Vora H, et al. Association between air pollution and lung function growth in southern California children: results from a second cohort. Am J Respir Crit Care Med. 2002;166:76–84. doi: 10.1164/rccm.2111021. [DOI] [PubMed] [Google Scholar]
- 8.Peters JM, Avol E, Gauderman WJ, et al. A study of twelve southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159:768–75. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 9.Islam T, Gauderman WJ, Berhane K, et al. Relationship between air pollution, lung function and asthma in adolescents. Thorax. 2007;62:957–63. doi: 10.1136/thx.2007.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgiopoulou VV, Kalogeropoulos AP, Psaty BM, et al. Lung function and risk for heart failure among older adults: the Health ABC Study. Am J Med. 2011;124:334–41. doi: 10.1016/j.amjmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–9. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 12.Ryan G, Knuiman MW, Divitini ML, James A, Musk AW, Bartholomew HC. Decline in lung function and mortality: the Busselton Health Study. J Epidemiol Community Health. 1999;53:230–4. doi: 10.1136/jech.53.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters JM, Avol E, Navidi W, et al. A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159:760–7. doi: 10.1164/ajrccm.159.3.9804143. [DOI] [PubMed] [Google Scholar]
- 14.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–72. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burrows B, Cline MG, Knudson RJ, Taussig LM, Lebowitz MD. A descriptive analysis of the growth and decline of the FVC and FEV1. Chest. 1983;83:717–24. doi: 10.1378/chest.83.5.717. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Dockery DW, Wypij D, et al. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis. 1993;148:1502–8. doi: 10.1164/ajrccm/148.6_Pt_1.1502. [DOI] [PubMed] [Google Scholar]
- 17.Mckean MC, Leech M, Lambert PC, Hewitt C, Myint S, Silverman M. A model of viral wheeze in nonasthmatic adults: symptoms and physiology. Eur Respir J. 2001;18:23–32. doi: 10.1183/09031936.01.00073101. [DOI] [PubMed] [Google Scholar]
- 18.Ashley F, Kannel WB, Sorlie PD, Masson R. Pulmonary function: relation to aging, cigarette habit, and mortality. Ann Intern Med. 1975;82:739–45. doi: 10.7326/0003-4819-82-6-739. [DOI] [PubMed] [Google Scholar]
- 19.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;294:1071–5. doi: 10.1056/NEJM197605132942001. [DOI] [PubMed] [Google Scholar]
- 20.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–5. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannel WB, Hubert H, Lew EA. Vital capacity as a predictor of cardiovascular disease: the Framingham study. Am Heart J. 1983;105:311–5. doi: 10.1016/0002-8703(83)90532-x. [DOI] [PubMed] [Google Scholar]
- 22.Knuiman MW, James AL, Divitini ML, Ryan G, Bartholomew HC, Musk AW. Lung function, respiratory symptoms, and mortality: results from the Busselton Health Study. Ann Epidemiol. 1999;9:297–306. doi: 10.1016/s1047-2797(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder EB, Welch VL, Couper D, et al. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158:1171–81. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 24.Schünemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–64. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 25.Lepeule J, Litonjua AA, Coull B, et al. Long-term effects of traffic particles on lung function decline in the elderly. Am J Respir Crit Care Med. 2014;190:542–8. doi: 10.1164/rccm.201402-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope CA, III, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–86. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–72. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarder SB, Fine PM, Sioutas C. Seasonal and spatial variability of the size-resolved chemical composition of particulate matter (PM10) in the Los Angeles basin. J Geophys Res-Atmos. 2005;110:1–14. [Google Scholar]
- 29.McDonald BC, Dallmann TR, Martin EW, Harley RA. Long-term trends in nitrogen oxide emissions from motor vehicles at national, state, and air basin scales. J Geophys Res-Atmos. 2012;117:1–11. [Google Scholar]
- 30.Bascom R, Bromberg P, Costa D, et al. Health effects of outdoor air pollution. Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 31.Tager IB. Air pollution and lung function growth: is it ozone? Am J Respir Crit Care Med. 1999;160:387–9. doi: 10.1164/ajrccm.160.2.ed09-99. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Poon R, Chen L, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668–74. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Profiles of general demographic characteristics. U.S. Census Bureau; 2000. ( http://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml) [Google Scholar]
- 34.Department of Commerce Economics and Statistics Administration, U.S. Census Bureau. California: 2010 summary population and housing characteristics 2010 census of population and housing. U.S. Census Bureau; 2012. Issued December 2012 CPH-1-6. ( http://www.census.gov/prod/cen2010/cph-1-6.pdf) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.