Summary
This study determined whether the membrane-permeable ventilatory stimulant, L-cysteine ethylester (L-CYSee), reversed the deleterious actions of morphine on arterial blood-gas chemistry in isoflurane-anesthetized rats. Morphine (2 mg/kg, i.v.) elicited sustained decreases in arterial blood pH, pO2 and sO2, and increases in pCO2 (all responses indicative of hypoventilation) and Alveolar-arterial gradient (indicative of ventilation-perfusion mismatch). Injections of L-CYSee (100 μmol/kg, i.v.) reversed the effects of morphine in tracheotomized rats but were minimally active in non-tracheotomized rats. L-cysteine or L-serine ethylester (100 μmol/kg, i.v.) were without effect. It is evident that L-CYSee can reverse the negative effects of morphine on arterial blood-gas chemistry and Alveolar-arterial gradient but that this positive activity is negated by increases in upper-airway resistance. Since L-cysteine and L-serine ethylester were ineffective, it is evident that cell penetrability and the sulfur moiety of L-CYSee are essential for activity. Due to its ready penetrability into the lungs, chest wall muscle and brain, the effects of L-CYSee on morphine-induced changes in arterial blood-gas chemistry are likely to involve both central and peripheral sites of action.
Keywords: morphine, L-cysteine ethylester, arterial blood-gas chemistry, halothane-anesthetized rats
1. Introduction
Systemically-administered opioids disturb arterial blood-gas chemistry in humans by direct suppression of minute ventilation (Cepeda et al., 2003; Cashman and Dolan, 2004; Taylor et al., 2005; Lötsch et al., 2006) and by negative affects on ventilation-perfusion in the lungs (Rybro et al., 1982; Chow et al., 2003; Wang et al., 2005). Systemic opioids also depress ventilation in animals by mechanisms, including central- (Campbell et al., 1995) and vagal afferent-mediated (Kaczy ska and Szereda-Przestaszewska, 2005) depression of ventilatory drive; skeletal muscle rigidity in the chest-wall and abdomen (Seamman, 1983; Niedhart et al., 1989; Bowdle, 1998); increases in pulmonary airway resistance (Willette et al., 1983); and increases in upper airway resistance via closure of the larynx (Willette et al., 1982, 1987; Bennett et al., 1997). Moreover, agonist-induced activation of central and peripheral opioid receptors blunt the hypoxic ventilatory response (see Zhang et al., 2009), and opioids such morphine and fentanyl inhibit carotid body chemoafferent activity and depress the responses of these afferents to hypoxic and hypercapnic challenges (McQueen and Rubeiro, 1980, 1981; Zimpfer et al., 1983; Kirby and McQueen, 1986; Mayer et al., 1989). Opioids including morphine also negatively affect ventilation-perfusion in the lungs of rabbits (Shafford and Schadt, 2008), pigs (Hannon and Bossone, 1991), dogs (Copland et al., 1987) and rats (Ling et al., 1985; Szikszay et al., 1986).
Although there are new therapeutics with the potential to prevent opioid-induced depression of breathing without affecting opioid-induced analgesia, none to date have been tested or proven reliably effective in human trials (Dahan et al., 2010). We have found that systemic injections of L-cysteine ethyl ester (L-CYSee) elicit dose-dependent increases in minute ventilation in rats (unpublished observations). L-CYSee is membrane-permeable (Fukui et al., 1994; Clancy et al., 2001), readily enters peripheral tissues and the brain (Servin et al., 1988), and increases intracellular pools of cysteine in these tissues (Hobbs et al., 1993; Deneke, 2000) via a membrane-associated carboxylesterase (Butterworth et al., 1993). The increased availability of cysteine directly alters the redox status of cells (Métayer et al., 2008; Winterbourn et al., 2008) and enhances glutathione production (Kimura and Kimura, 2004; Kimura, 2010), which exerts redox-dependent (reductive) effects and S-glutathiolation of proteins (Hill and Bhatnagar, 2007), and hydrogen sulfide (Kimura, 2010), which also activates redox processes and increases minute ventilation via actions in the carotid bodies (Peng et al., 2010). The enhanced biovailability of L-cysteine and L-glutathione would also promote the direct formation of the S-nitrosothiols, L-S-nitrosocysteine and L-S-nitrosoglutathione and the overall S-nitrosylation status of functional proteins in cells (Gow et al., 1991; Kharitonov et al., 1995; Keszler et al., 2010; Hu and Ho, 2011). S-nitrosothiols have diverse activities via S-nitrosylation of functional proteins (Lipton et al., 1993; Foster et al., 2003) and it is known that S-nitrosothiols within the brainstem (Lipton et al., 2001) and peripheral structures (Gaston et al., 1994, 2006; Stoyanovsky et al., 1997) exert positive effects on ventilatory function and pulmonary gas-exchange mechanisms.
It has been established that morphine alters the redox status of cells to a less reductive, more oxidative state (Polanco et al., 2009) and reduces intracellular glutathione levels (Macchia et al., 1999). Accordingly, we reasoned that the ability of L-CYSee to enhance the reductive capacity of cells both directly and via enhancement of glutathione levels may modulate the negative effects of morphine on ventilation. Moreover, L-CYSee or its free radical cation (Osburn et al., 2011) may reverse the deleterious effects of morphine by down-regulating opioid receptors (Cox et al., 1980) or by direct effects on membrane-associated proteins (Laragione et al., 2006) that regulate opioid receptor function. The initial aim of this study was to determine whether intravenous injections of the L-CYSee in isoflurane-anesthetized rats could reverse the deleterious effects of morphine on arterial blood-gas chemistry, and Alveolar-arterial O2 (A-a) gradient, an index of ventilation-perfusion status in the lung (Torda, 1981). We found that L-CYSee elicited relatively minor effects on the morphine-induced responses. In further exploring the potential reasons for the minimal effects of L-CYSee, we then performed studies in rats with a tracheotomy to test the hypothesis that L-CYSee may exacerbate morphine-induced increases in upper airway resistance. Indeed, we found that L-CYSee reversed the negative effects of morphine in rats with a tracheotomy. Taken together, it is apparent that L-CYSee can reverse the negative effects of morphine on ventilation but that its ability to increase upper airway resistance compromises gas-exchange in these rats.
2. Methods
2.1. Rats and surgeries
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the Animal Care and Use Committee of the University of Virginia. Adult male Sprague-Dawley rats (n=25; Harlan, Madison, WI) were anesthetized with 2% isoflurane delivered in room air. The rats were transferred to the surgical table and anesthesia maintained by delivery of 2% isoflurane in room-air via a face mask. Rat body temperature was maintained at 37.2 ± 0.2°C via a rectal thermometer connected to a temperature-controlled heating pad. Femoral artery and vein catheters were then inserted as described previously (Lewis et al., 2006). In one set of rats, the trachea was exposed via mid-line incision and a tube inserted to by-pass the upper airway. Isoflurane was delivered via the tracheal tube from then on. In another set of rats, the trachea was exposed but not cannulated and isoflurane delivery was maintained via face-mask. After surgery, all wounds were sutured closed and the rats were maintained on 1.25% isoflurane in room-air
2.2. Blood gas measurements and determination of A-a gradient
Arterial blood samples (120 μL) were taken from rats at key time-points during the protocols. The pH, pCO2, pO2 and sO2 of these samples were measured via a blood-gas analyzer (ABL800 FLEX, Radiometer, Denmark). The calculated A-a gradient, measures the difference between alveolar and arterial blood concentrations of O2 (Torda, 1981; Story, 1996). A-a gradient = PAO2 - PaO2, where PAO2 is the partial pressure of alveolar O2, and PaO2 is the measured partial pressure of O2 in arterial blood. PAO2 = [(FiO2 x (Patm-PH2O) - (PaCO2/respiratory quotient)], where FiO2 is the fraction of O2 in inspired air; Patm is atmospheric pressure; PH2O is the partial pressure of water in inspired air; PaCO2 is the partial pressure of CO2 measured in the arterial blood, and respiratory quotient (RQ) is equal to CO2 eliminated by cells/O2 consumed by cells. In our calculations, we took FiO2 of room air to be 21% = 0.21; RQ to be 0.8; Patm to be 760 mmHg; and PH2O to be 47 mmHg (Crapo et al., 1999).
2.3. Protocols
Study 1 – L-CYSee studies. Tracheotomized rats (n=5, 317 ± 3 g) and non-tracheotomized rats (n=5, 321 ± 3 g), received an injection of morphine (2 mg/kg, i.v.) and a blood-gas sample was taken after 5, 15 and 30 min to ensure that the effects of morphine on arterial blood-gas chemistry and A-a gradient had reached plateau levels. At 35 min, the rats received an i.v. injection of vehicle (saline, pH 4.0) or L-CYSee (100 μmol/kg) and a blood-gas sample was taken 5 min later (i.e., 40 min post-morphine). The rats received a second injection of vehicle or L-CYSee (100 μmol/kg) 5 min later (i.e., 45 min post-morphine) and a blood-gas sample was taken 5 min later (i.e., 50 min post-morphine). As such, the injections of vehicle or L-CYSee were given at 35 and 45 min post-morphine and blood gas samples taken at 40 and 50 min post-morphine. Study 2 – L-cysteine study. Tracheotomized rats (n=5, 317 ± 3 g) received L-cysteine (100 μmol/kg) rather than L-CYSee in the above protocol. Study 3 –L-serine ethylester study. Tracheotomized rats (n=5, 319 ± 3 g) received L-serine ethyl ester (100 μmol/kg) rather than L-CYSee in the above protocol.
2.4. Statistics
The data are presented as mean ± SEM and were analyzed by one- or two-way ANOVA followed by Student’s modified t test with Bonferroni corrections for multiple comparisons between means (Wallenstein et al., 1980). A value of P < 0.05 denoted statistical significance.
3. Results
3.1. Effects of morphine in rats with and without tracheostomy
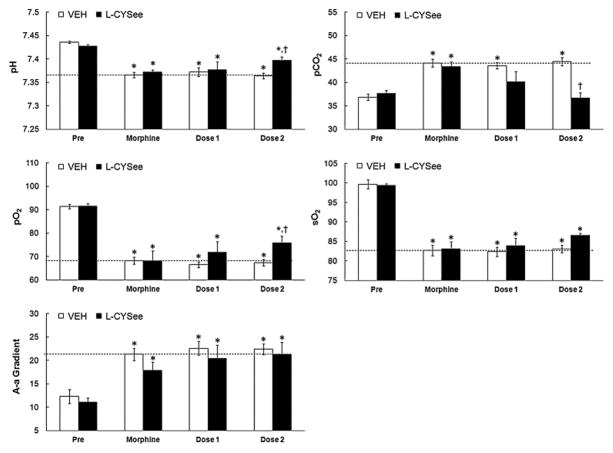
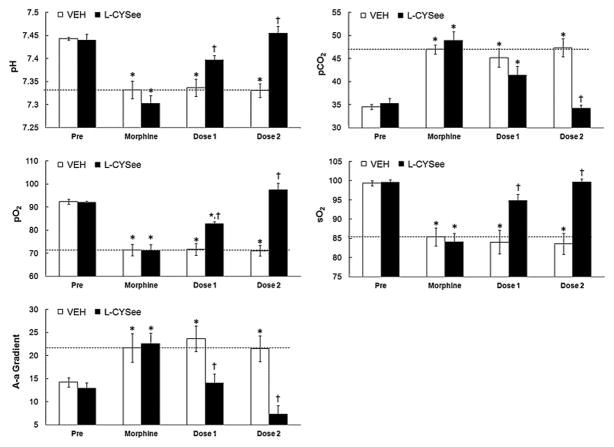
Resting parameters prior to injection of morphine were similar in the four groups of rats (Figs. 1 and 2; P >0.05, for all comparisons between the four groups). Morphine elicited sustained (i.e., present 35 min post-injection) decreases in pH, pO2 and sO2 that were accompanied by sustained increases in pCO2 and A-a gradient (Fig. 1). These responses were of similar magnitude in the four groups of rats (P >0.05, for all comparisons).
Fig. 1.
Arterial blood-gas chemistry and Alveolar-arterial (A-a) gradient values prior to administration of morphine, 30 min after injection of morphine (2 mg/kg, i.v.) and 5 min after each injection of vehicle or L-cysteine ethylester (L-CYSee, 100 μmol/kg, i.v.) in non-tracheotomized rats. The data are presented as mean ± SEM. There were 5 rats in each group.*P < significant effect of morphine. †P < 0.05, L-CYSee values versus saline values.
Fig. 2.
Arterial blood-gas chemistry and Alveolar-arterial (A-a) gradient values prior to administration of morphine, 30 min after injection of morphine (2 mg/kg, i.v.) and 5 min after each injection of vehicle or L-cysteine ethylester (L-CYSee, 100 μmol/kg, i.v.) in tracheotomized rats. The data are presented as mean ± SEM. There were 5 rats in each group.*P < significant effect of morphine. †P < 0.05, L-CYSee values versus saline values.
3.2. Effects of L-CYSee, L-Cysteine and L-serine ethylester in rats with and without tracheostomy
The two injections of vehicle elicited minor changes from the morphine-induced baselines in both the non-tracheotomized (Fig. 1.) and tracheotomized (Fig. 2) rats (see columns denoted “dose 1” and “dose 2”, P >0.05, for all comparisons). In non-tracheotomized rats, the first injection of L-CYSee did not affect the morphine-induced decreases in pH, pO2 and sO2 or the increases in pCO2 and A-a gradient (P >0.05, for all comparisons). The second injection of L-CYSee elicited relatively minor increases in pH and pO2, a substantial decrease in pCO2 (P < 0.05, for all comparisons to vehicle responses), but no effects on sO2 or A-a gradient (P < 0.05, for all comparisons to vehicle responses). In contrast, the injections of L-CYSee elicited a relatively dramatic reversal of the effects of morphine in tracheotomized rats (Fig. 2). The first injection of L-CYSee elicited increases in pH, pO2 and sO2 and decreases in pCO2 and A-a gradient (P >0.05, for all comparisons) whereas the second injection elicited full recovery from the effects of morphine. In contrast to L-CYSee, the injections of L-cysteine or L-serine ethylester did not modify any of the effects of morphine on arterial blood-gas chemistry or A-a gradient (Table 1).
Table 1.
Arterial blood-gas chemistry and Alveolar-arterial gradients in tracheotomized rats
| Parameter | Treatment | Sampling Periods
|
|||
|---|---|---|---|---|---|
| Pre | Morphine | Dose 1 | Dose 2 | ||
| pH | Vehicle | 7.447 ± 0.006 | 7.332 ± 0.033* | 7.337 ± 0.032* | 7.330 ± 0.031* |
| L-cysteine | 7.445 ± 0.004 | 7.328 ± 0.11* | 7.315 ± 0.008* | 7.332 ± 0.17* | |
| L-SERee | 7.440 ± 0.003 | 7.327 ± 0.018* | 7.306 ± 0.021* | 7.321 ± 0.019* | |
| pCO2 | Vehicle | 34.6 ± 0.5 | 47.0 ± 1.0* | 45.1 ± 2.0* | 47.3 ± 1.9* |
| L-cysteine | 34.4 ± 0.5 | 47.5 ± 1.5* | 46.9 ± 1.8* | 47.1 ± 1.4* | |
| L-SERee | 34.0 ± 0.7 | 47.9 ± 1.2* | 47.6 ± 1.6* | 46.3 ± 0.7* | |
| pO2 | Vehicle | 92.3 ± 1.1 | 71.4 ± 2.4* | 71.7 ± 2.6* | 71.1 ± 2.3* |
| L-cysteine | 92.1 ± 1.0 | 69.5 ± 0.8* | 69.3 ± 1.3* | 70.1 ± 0.3* | |
| L-SERee | 91.7 ± 0.06 | 70.8 ± 0.7* | 69.8 ± 2.9* | 68.7 ± 1.1* | |
| sO2 | Vehicle | 99.3 ± 0.07 | 85.4 ± 2.3* | 84.0 ± 4.1* | 83.6 ± 3.7* |
| L-cysteine | 98.8 ± 0.7 | 83.2 ± 1.7* | 84.3 ± 2.0* | 83.3 ± 1.5* | |
| L-SERee | 98.8 ± 0.7 | 83.2 ± 1.7* | 84.3 ± 2.0* | 83.3 ± 1.5* | |
| A-a | Vehicle | 14.2 ± 1.0 | 19.6 ± 3.1* | 21.6 ± 2.8* | 19.5 ± 2.8* |
| L-cysteine | 14.6 ± 1.6 | 20.8 ± 1.9* | 21.8 ± 2.2* | 20.7 ± 2.1* | |
| L-SERee | 14.5 ± 1.0 | 19.1 ± 1.5* | 20.5 ± 2.2* | 23.2 ± 1.3* | |
The data are presented as mean ± SEM. There were 5 rats in each group.
P < significant effect of morphine. A-a, Alveolar-arterial gradient. Note that neither L-cysteine nor L-serine ethylester (L-SERee) affected any of the morphine-induced responses (P >0.05 for all comparisons).
4. Discussion
The novel finding of this study is that L-CYSee elicited relatively minor effects on morphine-induced changes in arterial blood-gas chemistry and A-a gradients in rats without a tracheotomy whereas it reversed the effects of morphine in rats with a tracheotomy. Taken together, it is evident that L-CYSee is capable of antagonizing the negative effects of morphine on arterial blood-gas chemistry but that its ability to increase upper airway resistance compromises gas-exchange in morphine-treated rats. As will be discussed below, the ability of L-CYSee to enhance ventilation and elicit upper airway obstruction in non-tracheotomized rats may result in markedly negative intrathoracic pressures, which will directly gas exchange in the lungs.
4.1. Effects of morphine on arterial blood-gas chemistry and A-a gradient
Morphine elicited a sustained depression of arterial blood-gas chemistry in our isoflurane-anesthetized rats. Specifically, morphine elicited decreases in pH, pO2 and sO2 levels that were accompanied by an increase in pCO2 levels. These changes in arterial blood-gas chemistry are consistent with the known ability of morphine to suppress minute ventilation (Trescot et al., 2008; Dahan et al., 2010). The finding that baseline arterial blood-gas chemistry values and the responses elicited by morphine were similar in non-tracheotomized and tracheotomized rats suggests that the non-tracheotomized rats were adequately ventilated before injection of the opioid. Morphine also elicited a substantial increase in A-a gradient, indicative of an abnormally lower pO2 in lung blood compared to alveoli (Torda, 1981; Story, 1996). A decrease in PaO2, without a change in A-a gradient would be caused purely by hypoventilation. However, since the morphine-induced decreases in pO2 were accompanied by an increase in A-a gradient, it appears that morphine induced a ventilation-perfusion (V/Q) mismatch or shunting. As such, morphine may have directly increased pulmonary vascular resistance and/or exacerbated the hypoxic pulmonary vasoconstriction resulting from morphine-induced decrease in minute ventilation and concomitant decreases in arterial pO2. Whatever the mechanism, it appears that diminished arterial blood flow to alveoli is a major mechanism by which morphine reduced arterial pO2 in our isoflurane-anesthetized rats. These findings are consistent with evidence that morphine and other opioids increase pulmonary vascular resistance in humans (Popio et al., 1978; Mitaka et al., 1985) and animals (Schurig et al., 1978; Zola and McLeod, 1983; Copland et al., 1987; Hakim et al., 1992).
Our findings that morphine increased A-a gradient agree with evidence that opioids negatively affect ventilation-perfusion in humans and animals (Ling et al., 1985; Szikszay et al., 1986; Copland et al., 1987; Hannon and Bossone, 1991; Shafford and Schadt, 2008). However, the potential ability of morphine to affect RQ (CO2 eliminated by cells/O2 consumed by cells) would have important effects on A-a gradients and the interpretation of the ability of morphine to negatively affect gas exchange in the lungs. In our calculations of A-a gradient, we took RQ to be 0.8, which assumes that morphine did not have direct effects on this parameter. Although there are reports that morphine has minimal effects on carbohydrate or lipid metabolism (Allan et al., 1983; Hauner et al., 1988), there is evidence that morphine enhances carbohydrate metabolism (Lelevich, 2011), and that morphine either increases (Nencini and Paroli, 1981) or decreases (Sablé-Amplis et al., 1975) lipid metabolism). Morphine-induced enhancement carbohydrate metabolism (Lelevich, 2011) or decrease in fat metabolism (Sablé-Amplis et al., 1975), would shift RQ to a value greater than 0.8, which would increase the calculated A-a gradient. As such, an RQ of 0.8, would underestimate the negative effects of morphine on gas exchange in the lungs. In contrast, an increase in fat metabolism (Nencini and Paroli, 1981) would shift RQ from 0.8 toward 0.66 and decrease the calculated A-a gradient. Therefore, a RQ of 0.8 would overestimate the effects of morphine on A-a gradient and gas exchange. Although or findings suggest that morphine directly (negatively) affected gas-exchange in the lungs, we cannot definitively state the exact effects of morphine on A-a gradient and gas exchange without direct measurement of RQ.
4.2. Effects of L-CYSee on the morphine-induced responses
The intravenous injection of 35S-labelled L-CYSee elicits a rapid rise (within 5 min) in 35S-L-CYSee and 35S-L-cysteine levels in the brain, lungs and chest-wall muscle of rats (Servin et al., 1988). Our key finding was that L-CYSee reversed the deleterious effects of morphine on arterial blood-gas chemistry and A-a gradient in tracheotomized rats but exerted much lesser effects in non-tracheotomized rats. It is therefore evident that the ability of L-CYSee to increase upper airway (e.g., laryngeal) resistance limits the effectiveness of L-CYSee in morphine-treated rats. Tracheostomy aids patients with marginal respiratory mechanics primarily by decreasing airway resistance (Heffner, 2001; Pierson, 2005). Accordingly, tracheostomy improves ventilatory parameters, gas exchange, pulmonary hemodynamics (Benini et al., 2002; Ferraro et al., 2004) and the mechanics of breathing (Heffner, 2001; Pierson, 2005) in patients including those on normal tidal ventilation (Namdar et al., 2010; Sofi et al., 2010; Bellani et al., 2013).
The ability of L-CYSee to improve arterial blood-gas chemistry in morphine-treated tracheotomized but not in non-tracheotomized rats is consistent with the possibility that L-CYSee increases both inspiratory effort and upper airways resistance. Non-cardiogenic pulmonary edema in children and adults occurs following various forms of upper airway obstruction (Oswalt et al., 1977; Jackson et al., 1980; Tami et al., 1986; Lang et al., 1990) and in patients with obstructive sleep apnea (Chaudhary et al., 1984). Moreover, upper airway obstruction with marked inspiratory efforts generates excessively negative intrathoracic pressures in humans (Schwartz et al., 1999) and animals (Loyd et al., 1986; Chonan et al., 1991). These abnormally negative pressures lead to an increase in transmural capillary pressure, which causes a transudation of fluid from the pulmonary capillaries into the interstitial space (Schwartz et al., 1999; da Silva et al., 2005). The lack of airflow and alveolar oxygenation during acute upper airway obstruction results in hypoxemia, which leads to a hypoxia-mediated pulmonary vasoconstriction, which also promotes pulmonary edema (Schwartz et al., 1999). The occurrence of pulmonary hemorrhage during negative pressure pulmonary edema (Muller and Miller, 1991; Schwartz et al., 1999; da Silva et al., 2005) involves stress failure of the alveolar-capillary membrane caused by the marked elevation of pulmonary capillary wall tension (Schwartz et al., 1999). Decreases in the pericapillary interstitial pressure might lead to this stress failure of the membrane (Schwartz et al., 1999). Extreme transmural pressure changes break the alveolar-capillary membrane, allowing red cell leakage into the alveoli, leading to hemorrhage (Broccard et al., 2000). Whether this L-CYSee-induced increase in upper airway resistance is due to the direct effects on the neuromuscular components of the upper airway or to actions in the brain promoting enhanced neurogenic drive, remains to be determined. Since L-CYSee reversed the effects of morphine on arterial blood-gas chemistry in tracheotomized rats, it would appear that L-CYSee does not have deleterious effects on the muscle components of the lower airway and especially those within terminal bronchioles, or negative effects on alveolar function (e.g., disturbance of surface tension which would tend to cause collapse), despite its presence in lung tissue (Butterworth et al., 1993; Hobbs et al., 1993) and bronchio-alveolar lining fluid (Lailey and Upshall, 1994) after peripheral administration. Moreover, the ability of L-CYSee to improve A-a gradient in the tracheotomized rats clearly suggests that L-CYSee can overcome the direct and/or hypoxia-induced increase in pulmonary vascular resistance. In rats without a tracheal tube, changing RQ from 0.8 to 0.66 or to 1.00 confirmed that L-CYSee would have minimal effects on A-a gradient at all RQ values. Importantly, changing RQ values from 0.8 to 0.66 or to 1.00 confirmed that L-CYSee had similar profound beneficial effects on A-a gradient at all RQ values. The lack of effect of L-CYSee on A-gradients in rats without tracheal catheters clearly suggests that L-CYSee has minimal overall effects on RQ. As such, it is tempting to assume that L-CYSee improved A-a gradient in rats with tracheal catheters by directly improving gas-exchange in the lungs, perhaps decreasing hypoxic pulmonary vasoconstriction. Although we cannot find relevant data concerning the effects of L-cysteine on RQ, there is evidence that (1) N-acetylcysteine (which increases intracellular cysteine levels) has minimal effects on lipid or carbohydrate metabolism in control rats (Novelli et al., 2009; Seiva et al., 2009), whereas it normalizes disturbances in these metabolic pathways in high-sucrose diet-induced obese rats (Novelli et al., 2009) and in rats subjected to alcohol ingestion (Seiva et al., 2009). Evidence was provided in the above studies that the therapeutic effects of N-acetylcysteine involved anti-oxidant mechanisms (Novelli et al., 2009; Seiva et al., 2009). It should be noted that morphine generates reactive oxygen species (Young et al., 2013) that are capable of altering carbohydrate and lipid metabolism (Novelli et al., 2009; Seiva et al., 2009). Accordingly, it is possible that L-CYSee overcomes the negative effects of morphine on metabolism within cells thereby tending to keep RQ at or near its normal value of 0.8.
4.3. Effects of L-cysteine and L-serine ethylester on the morphine-induced responses
As with 35S-L-CYSee, the injection of 35S-L-cysteine elicited a rapid rise in 35S-L-cysteine levels in the lungs but unlike 35S-L-CYSee, 35S-L-cysteine did not accumulate in the chest-wall muscle or brain (Servin et al., 1988). The lack of tissue penetration in these vital organs may explain why L-cysteine did not reverse the deleterious effects of morphine in tracheotomized rats. Moreover, the lack of effect of L-serine ethylester suggests that the sulfur moiety is vital to the actions of L-CYSee and the ethyl ester moiety of L-CYSee in itself did not confer activity other than allowing for plasma membrane and/or intracellular delivery. As such, it appears that the ability of L-CYSee to reverse the actions of morphine depend upon its delivery of L-cysteine into the plasma membranes and/or cytoplasm of cells within the periphery (e.g., lungs and ventilatory muscles) and/or the brain including those protected by a blood-brain-barrier (e.g., brainstem), and those including the area postrema, that are not (Johnson and Gross, 1993). The possibility that L-CYSee accumulates and exerts effects within other key structures such as the primary glomus cells and chemosensory nerves in the carotid bodies remains to be determined.
4.4. Potential mechanisms by which L-CYSee reverses the morphine-induced responses
It is unlikely that L-CYSee interacts (e.g., chelates) with morphine in vivo since L-cysteine and morphine do not interact when added together (Nagamatsu et al., 1982). However, in vitro studies have demonstrated that whereas 1 mM concentrations of cysteine, glutathione and dithiothreitol do not affect opioid receptor binding, high concentrations of these thiols (20 mM) induce a rapid loss of opiate receptor binding (Cox et al., 1980). Based on the assumption that our rats (≈0.3 kg) have about 20 ml of circulating blood (Ringler and Dabich, 1979), the first injection of L-CYSee (100 μmol/kg) would at the instant of injection, result in a blood level of 1.5 mM (i.e., each rat received ≈ 30 μmol of L-CYSee resulting in levels of 30 μmol/20 ml = 1.5 mM). Assuming no degradation or tissue distribution of L-CYSee, the second injection would have elevated plasma levels to 3.0 mM. Taken with the lack of effects of L-cysteine, it is unlikely that L-CYSee, at the total dose used in this study, reversed the effects of morphine via direct actions on membrane proteins (Laragione et al., 2006) and especially opiate receptors (Cox et al., 1980). Considering the known sites of action of morphine (see Introduction), it is feasible that L-CYSee acted in the carotid bodies to reverse the negative effects of morphine on primary glomus cells/chemoafferents and the responses of the carotid body to hypoxia and hypercapnia. Consistent with a putative increase in laryngeal muscle activity, L-CYSee may increase the activity of respiratory muscles within the chest and diaphragm via direct actions or via actions within the brain. Finally, the beneficial effects of L-CYSee on A-a gradient in the tracheotomized rats raises the possibility that L-CYSee diminished the direct and/or hypoxia-driven increase in pulmonary vascular resistance.
Morphine alters the redox status of neuroblastoma x glioma hybrid cells to a less reductive, more oxidative state in an opioid receptor antagonist-sensitive manner (Polanco et al., 2009) and decreases the levels of reduced glutathione in cultured epithelial cells (Macchia et al., 1999). As such, L-CYSee may overcome the actions of morphine by enhancing intracellular levels of cysteine (Butterworth et al., 1993; Hobbs et al., 1993; Deneke, 2000), glutathione (Kimura and Kimura, 2004; Kimura, 2010), and hydrogen sulfide (Kimura, 2010). Cysteine (Deneke, 2000; Métayer et al., 2008; Winterbourn et al., 2008), glutathione (Hill and Bhatnagar, 2007) and hydrogen sulfide (Peng et al., 2010) exert redox-dependent effects in cells and hydrogen sulfide promotes minute ventilation via actions in the carotid bodies (Peng et al., 2010). Finally, the generation of intracellular S-nitrosothiols such as L-S-nitrosoglutathione and an increase overall S-nitrosylation status of proteins in cells (Gow et al., 1991; Kharitonov et al., 1995; Keszler et al., 2010; Hu and Ho, 2011) may contribute to the ventilatory excitant effects of L-CYSee in morphine-treated rats. This especially is because (1) microinjections of S-nitrosothiols into the nucleus tractus solitarii of conscious rats cause pronounced increases minute ventilation (Lipton et al., 2001), (2) S-nitrosothiols promote skeletal muscle activity via activation of ryanodine receptors (Stoyanovsky et al., 1997), and (3) S-nitrosothiols exert positive effects on ventilatory function and pulmonary gas-exchange mechanisms (Gaston et al., 1994, 2006; Lipton et al., 2001). The overriding question is whether L-CYSee and down-stream products directly reverse the processes by which morphine suppresses ventilatory function or actively overrides these processes via mechanisms that are independent of the morphine-sensitive processes (e.g., direct increase in carotid body activity).
4.5. Summary
The study shows that L-CYSee reverses the effects of morphine on arterial blood-gas chemistry and A-a gradient in isoflurane-anesthetized rats but that this positive effect is negated by its ability to close the upper airway. Whether the effects of L-CYSee on upper airway tone points to a role of L-cysteine/down-stream products in the physiological/pathophysiological regulation of upper airway smooth muscle remains to be determined. It is feasible that L-CYSee may be used to overcome morphine-induced depression of ventilatory function in intubated patients undergoing surgery or in the post-operative setting (Taylor et al., 2005). We are currently determining whether analogues of L-CYSee such as D-CYSee retain the positive but not the negative aspects of L-CYSee.
Highlights.
L-cysteine ethylester (L-CYSee) reversed the negative actions of morphine on arterial blood-gas (ABG) chemistry in tracheotomized rats.
L-CYSee exerted minimal effects on the negative actions of morphine in non-tracheotomized rats.
L-cysteine or L-serine ethylester (L-SERee) did not affect the negative actions of morphine on ABG chemistry in tracheotomized rats.
L-CYSee reverses the effects of morphine on ABG chemistry but this positive activity is negated by increases in upper-airway resistance.
Since L-cysteine and L-SERee were ineffective, it is evident that cell penetrability and the sulfur moiety of L-CYSee are essential for activity.
Acknowledgments
This work was supported by grants from Galleon Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan EH, Green IC, Titheradge MA. The stimulation of glycogenolysis and gluconeogenesis in isolated hepatocytes by opioid peptides. Biochem J. 1983;216:507–510. doi: 10.1042/bj2160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani G, Deab SA, Pradella A, Mauri T, Citerio G, Foti G, Pesenti A. Effect of percutaneous tracheostomy on gas exchange in hypoxemic and non-hypoxemic mechanically ventilated patients. Respir Care. 2013;58:482–486. doi: 10.4187/respcare.01889. [DOI] [PubMed] [Google Scholar]
- Benini A, Rossi N, Maisano P, Marcolin R, Patroniti N, Pesenti A, Foti G. Translaryngeal tracheostomy in acute respiratory distress syndrome patients. Intensive Care Med. 2002;28:726–730. doi: 10.1007/s00134-002-1246-1. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Abrams JT, Van Riper DF, Horrow JC. Difficult or impossible ventilation after sufenidil-induced anaesthesia is caused primarily by vocal cord closure. Anesthesiology. 1997;87:1070–1074. doi: 10.1097/00000542-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Bowdle TA. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19:189–193. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- Broccard AF, Liaudet L, Aubert JD, Schnyder P, Schaller MD. Negative pressure post-tracheal extubation alveolar hemorrhage. Anesth Analg. 2001;92:273–275. doi: 10.1097/00000539-200101000-00055. [DOI] [PubMed] [Google Scholar]
- Butterworth M, Upshall DG, Cohen GM. A novel role for carboxylesterase in the elevation of cellular cysteine by esters of cysteine. Biochem Pharmacol. 1993;46:1131–1137. doi: 10.1016/0006-2952(93)90460-e. [DOI] [PubMed] [Google Scholar]
- Campbell C, Weinger MB, Quinn M. Alterations in diaphragm EMG activity during opiate-induced respiratory depression. Respir Physiol. 1995;100:107–117. doi: 10.1016/0034-5687(94)00119-k. [DOI] [PubMed] [Google Scholar]
- Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Brit J Anaesth. 2004;93:212–223. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74:102–112. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- Chaudhary BA, Nadimi M, Chaudhary TK, Speir WA. Pulmonary edema due to obstructive sleep apnea. South Med J. 1984;77:499–501. doi: 10.1097/00007611-198404000-00022. [DOI] [PubMed] [Google Scholar]
- Chonan T, Hida W, Kikuchi Y, Shindoh C, Taguchi O, Miki H, Takishima T. Effects of elastic loading and exercise on pulmonary gas exchange in dogs. Tohoku J Exp Med. 1991;164:157–167. doi: 10.1620/tjem.164.157. [DOI] [PubMed] [Google Scholar]
- Chow MY, Goh MH, Boey SK, Thirugnanam A, Ip-Yam PC. The effects of remifentanil and thoracic epidural on oxygenation and pulmonary shunt fraction during one-lung ventilation. J Cardiothorac Vasc Anesth. 2003;17:69–72. doi: 10.1053/jcan.2003.12. [DOI] [PubMed] [Google Scholar]
- Clancy R, Cederbaum AI, Stoyanovsky DA. Preparation and properties of S-nitroso-L-cysteine ethyl ester, an intracellular nitrosating agent. J Med Chem. 2001;44:2035–2038. doi: 10.1021/jm000463f. [DOI] [PubMed] [Google Scholar]
- Copland VS, Haskins SC, Patz JD. Oxymorphone: cardiovascular, pulmonary, and behavioral effects in dogs. Am J Vet Res. 1987;48:1626–1630. [PubMed] [Google Scholar]
- Cox BM, Leslie FM, Dunlap CE., 3rd The use of ascorbate as a probe of opioid receptor structure: evidence for two independent mechanisms of receptor destruction by ascorbate. J Recept Res. 1980;1:329–354. doi: 10.3109/10799898009044104. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160:1525–1531. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- Da Silva PS, Monteiro Neto H, Andrade MM, Neves CV. Negative-pressure pulmonary edema: a rare complication of upper airway obstruction in children. Pediatr Emerg Care. 2005;21:751–754. doi: 10.1097/01.pec.0000186430.92388.a6. [DOI] [PubMed] [Google Scholar]
- Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151–180. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- Ferraro F, Capasso A, Troise E, Lanza S, Azan G, Rispoli F, Anello CB. Assessment of ventilation during the performance of elective endoscopic-guided percutaneous tracheostomy: clinical evaluation of a new method. Chest. 2004;126:159–164. doi: 10.1378/chest.126.1.159. [DOI] [PubMed] [Google Scholar]
- Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- Fukui K, Kaneda M, Takahashi E, Washio M, Doi K. Protective effects of sulfhydryl compounds on HOCl-induced intracellular Ca2+ increase in single rat ventricular myocytes. J Mol Cell Cardiol. 1994;26:455–461. doi: 10.1006/jmcc.1994.1056. [DOI] [PubMed] [Google Scholar]
- Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med. 2006;173:1186–1193. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149:538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J Biol Chem. 1991;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- Hakim TS, Grunstein MM, Michel RP. Opiate action in the pulmonary circulation. Pulm Pharmacol. 1992;5:159–165. doi: 10.1016/0952-0600(92)90036-g. [DOI] [PubMed] [Google Scholar]
- Hannon JP, Bossone CA. Cardiovascular and pulmonary effects of morphine in conscious pigs. Am J Physiol. 1991;261:R1286–R1293. doi: 10.1152/ajpregu.1991.261.5.R1286. [DOI] [PubMed] [Google Scholar]
- Hauner H, Glatting G, Ditschuneit HH, Pfeiffer EF. Endogenous opiates do not influence glucose and lipid metabolism in rat adipocytes. Exp Clin Endocrinol. 1988;91:350–354. doi: 10.1055/s-0029-1210768. [DOI] [PubMed] [Google Scholar]
- Heffner JE. The role of tracheotomy in weaning. Chest. 2001;120(6 suppl):477S–481S. doi: 10.1378/chest.120.6_suppl.477s. [DOI] [PubMed] [Google Scholar]
- Hill BG, Bhatnagar A. Role of glutathiolation in preservation, restoration and regulation of protein function. IUBMB Life. 2007;59:21–26. doi: 10.1080/15216540701196944. [DOI] [PubMed] [Google Scholar]
- Hobbs MJ, Butterworth M, Cohen GM, Upshall DG. Structure-activity relationships of cysteine esters and their effects on thiol levels in rat lung in vitro. Biochem Pharmacol. 1993;45:1605–1612. doi: 10.1016/0006-2952(93)90301-c. [DOI] [PubMed] [Google Scholar]
- Hu TM, Ho SC. Similarity and dissimilarity of thiols as anti-nitrosative agents in the nitric oxide-superoxide system. Biochem Biophys Res Commun. 2011;404:785–789. doi: 10.1016/j.bbrc.2010.12.059. [DOI] [PubMed] [Google Scholar]
- Jackson FN, Rowland V, Corssen G. Laryngospasm-induced pulmonary edema. Chest. 1980;78:819–821. doi: 10.1378/chest.78.6.819. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Kaczyńska K, Szereda-Przestaszewska M. Involvement of vagal opioid receptors in respiratory effects of morphine in anaesthetized rats. J Physiol Pharmacol. 2005;56:195–203. [PubMed] [Google Scholar]
- Keszler A, Zhang Y, Hogg N. Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: how are S-nitrosothiols formed? Free Radic Biol Med. 2010;48:55–64. doi: 10.1016/j.freeradbiomed.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J Biol Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- Kirby GC, McQueen DS. Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. Brit J Pharmacol. 1986;88:889–898. doi: 10.1111/j.1476-5381.1986.tb16263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lailey AF, Upshall DG. Thiol levels in rat bronchio-alveolar lavage fluid after administration of cysteine esters. Hum Exp Toxicol. 1994;13:776–780. doi: 10.1177/096032719401301106. [DOI] [PubMed] [Google Scholar]
- Lang SA, Duncan PG, Shephard DA, Ha HC. Pulmonary oedema associated with airway obstruction. Can J Anaesth. 1990;37:210–218. doi: 10.1007/BF03005472. [DOI] [PubMed] [Google Scholar]
- Laragione T, Gianazza E, Tonelli R, Bigini P, Mennini T, Casoni F, Massignan T, Bonetto V, Ghezzi P. Regulation of redox-sensitive exofacial protein thiols in CHO cells. Biol Chem. 2006;387:1371–1376. doi: 10.1515/BC.2006.172. [DOI] [PubMed] [Google Scholar]
- Lelevich SV. Comparative characteristics of glucose metabolism in the liver of rats under acute alcohol and morphine intoxication. Biomed Khim. 2011;57:615–623. doi: 10.18097/pbmc20115706615. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Hashmi-Hill MP, Owen JR, Sandock K, Robertson TP, Bates JN. The vasodilator potency of the endothelium-derived relaxing factor, L-S-nitrosocysteine, is impaired in Spontaneously Hypertensive rats. Vasc Pharmacol. 2006;44:476–490. doi: 10.1016/j.vph.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Ling GS, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J Pharmacol Exp Ther. 1985;232:149–155. [PubMed] [Google Scholar]
- Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Loyd JE, Nolop KB, Parker RE, Roselli RJ, Brigham KL. Effects of inspiratory resistance loading on lung fluid balance in awake sheep. J Appl Physiol. 1986;60:198–203. doi: 10.1152/jappl.1986.60.1.198. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Dudziak R, Freynhagen R, Marschner J, Geisslinger G. Fatal respiratory depression after multiple intravenous morphine injections. Clin Pharmacokinet. 2006;45:1051–1060. doi: 10.2165/00003088-200645110-00001. [DOI] [PubMed] [Google Scholar]
- Macchia I, Palamara AT, Bué C, Savini P, Ciriolo M, Gaziano R, di Francesco P. Increased replication of Sendai virus in morphine-treated epithelial cells: evidence for the involvement of the intracellular levels of glutathione. Int J Immunopharmacol. 1999;21:185–193. doi: 10.1016/s0192-0561(98)00080-0. [DOI] [PubMed] [Google Scholar]
- Mayer N, Zimpfer M, Raberger G, Beck A. Fentanyl inhibits the canine carotid chemoreceptor reflex. Anesth Analg. 1989;69:756–762. [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. Inhibitory actions of methionine-enkephalin and morphine on the cat carotid chemoreceptors. Brit J Pharmacol. 1980;71:297–305. doi: 10.1111/j.1476-5381.1980.tb10939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. Effects of beta-endorphin, vasoactive intestinal polypeptide and cholecystokinin octapeptide on cat carotid chemoreceptor activity. QJ Exp Physiol. 1981;66:273–284. doi: 10.1113/expphysiol.1981.sp002556. [DOI] [PubMed] [Google Scholar]
- Métayer S, Seiliez I, Collin A, Duchêne S, Mercier Y, Geraert PA, Tesseraud S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J Nutr Biochem. 2008;19:207–215. doi: 10.1016/j.jnutbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Mitaka C, Sakanishi N, Tsunoda Y, Mishima Y. Comparison of hemodynamic effects of morphine, butorphanol, buprenorphine and pentazocine on ICU patients. Bull Tokyo Med Dent Univ. 1985;32:31–39. [PubMed] [Google Scholar]
- Muller NL, Miller RA. Diffuse pulmonary hemorrhage. Radiol Clin North Am. 1991;29:965–971. [PubMed] [Google Scholar]
- Nagamatsu K, Kido Y, Terao T, Ishida T, Toki S. Protective effect of sulfhydryl compounds on acute toxicity of morphinone. Life Sci. 1982;30:1121–1127. doi: 10.1016/0024-3205(82)90533-1. [DOI] [PubMed] [Google Scholar]
- Namdar T, Stollwerck PL, Stang FH, Klotz KF, Lange T, Mailänder P, Siemers F. Early postoperative alterations of ventilation parameters after tracheostomy in major burn injuries. Ger Med Sci. 2010;8 doi: 10.3205/000099. Doc10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencini P, Paroli E. The lipolytic activity of met-enkephalin, leu-enkephalin, morphine, methadone and naloxone in human adipose tissue. Pharmacol Res Commun. 1981;13:535–540. doi: 10.1016/s0031-6989(81)80023-9. [DOI] [PubMed] [Google Scholar]
- Niedhart P, Burgener MC, Schweiger J, Suter PM. Chest wall rigidity during fentanyl and midazolam-fentanyl induction: ventilatory and hemodynamic effects. Acta Anaesthesiol Scand. 1989;33:1–5. doi: 10.1111/j.1399-6576.1989.tb02849.x. [DOI] [PubMed] [Google Scholar]
- Novelli EL, Santos PP, Assalin HB, Souza G, Rocha K, Ebaid GX, Seiva FR, Mani F, Fernandes AA. N-acetylcysteine in high-sucrose diet-induced obesity: energy expenditure and metabolic shifting for cardiac health. Pharmacol Res. 2009;59:74–79. doi: 10.1016/j.phrs.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Osburn S, Steill JD, Oomens J, O’Hair RA, van Stipdonk M, Ryzhov V. Structure and reactivity of the cysteine methyl ester radical cation. Chemistry. 2011;17:873–879. doi: 10.1002/chem.201002042. [DOI] [PubMed] [Google Scholar]
- Oswalt CE, Gates GA, Holmstrom FMG. Pulmonary edema as a complication of acute airway obstruction. JAMA. 1977;238:1833–1835. [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson DJ. Tracheostomy and weaning. Respir Care. 2005;50:526–533. [PubMed] [Google Scholar]
- Polanco MJ, Alguacil LF, Albella B, Segovia JC, González-Martín C. Yohimbine prevents the effect of morphine on the redox status of neuroblastomaxglioma NG108–15 cells. Toxicol Lett. 2009;189:115–120. doi: 10.1016/j.toxlet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Popio KA, Jackson DH, Ross AM, Schreiner BF, Yu PN. Hemodynamic and respiratory effects of morphine and butorphanol. Clin Pharmacol Ther. 1978;23:281–287. doi: 10.1002/cpt1978233281. [DOI] [PubMed] [Google Scholar]
- Ringler DH, Dabich L. Hematology and clinical biochemistry. In: Baker HJ, Lindsey JR, Weisbroth SH, editors. The Laboratory Rat, Volume I, Biology and Diseases. Academic Press, American College Laboratory Animal Medicine Series; New York: 1979. pp. 105–121. [Google Scholar]
- Rybro L, Schurizek BA, Petersen TK, Wernberg M. Postoperative analgesia and lung function: a comparison of intramuscular with epidural morphine. Acta Anaesthesiol Scand. 1982;26:514–518. doi: 10.1111/j.1399-6576.1982.tb01810.x. [DOI] [PubMed] [Google Scholar]
- Sablé-Amplis R, Agid R, Abadie D. Some effects of morphine on lipid metabolism in normal, tolerant and abstinent rats. Life Sci. 1975;16:1477–1482. doi: 10.1016/0024-3205(75)90045-4. [DOI] [PubMed] [Google Scholar]
- Schurig JE, Cavanagh RL, Buyniski JP. Effect of butorphanol and morphine on pulmonary mechanics, arterial blood pressure and venous plasma histamine in the anesthetized dog. Arch Int Pharmacodyn Ther. 1978;233:296–304. [PubMed] [Google Scholar]
- Servin AL, Goulinet S, Renault H. Pharmacokinetics of cysteine ethyl ester in rat. Xenobiotica. 1988;18:839–847. doi: 10.3109/00498258809041722. [DOI] [PubMed] [Google Scholar]
- Schwartz DR, Maroo A, Malhotra A, Kesselman H. Negative pressure pulmonary hemorrhage. Chest. 1999;115:1194–1197. doi: 10.1378/chest.115.4.1194. [DOI] [PubMed] [Google Scholar]
- Seiva FR, Amauchi JF, Rocha KK, Ebaid GX, Souza G, Fernandes AA, Cataneo AC, Novelli EL. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43:649–656. doi: 10.1016/j.alcohol.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Shafford HL, Schadt JC. Respiratory and cardiovascular effects of buprenorphine in conscious rabbits. Vet Anaesth Analg. 2008;35:326–332. doi: 10.1111/j.1467-2995.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Sofi K, Wani T. Effect of tracheostomy on pulmonary mechanics: an observational study. Saudi J Anaesth. 2010;4:2–5. doi: 10.4103/1658-354X.62606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story DA. Alveolar oxygen partial pressure, alveolar carbon dioxide partial pressure, and the alveolar gas equation. Anesthesiology. 1996;84:1011. doi: 10.1097/00000542-199604000-00036. [DOI] [PubMed] [Google Scholar]
- Stoyanovsky D, Murphy T, Anno PR, Kim YM, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21:19–29. doi: 10.1016/s0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- Szikszay M, Snyder FR, London ED. Interactions between verapamil and morphine on physiological parameters in rats. J Pharmacol Exp Ther. 1986;238:192–197. [PubMed] [Google Scholar]
- Tami TA, Chu F, Wildes TO, Kaplan M. Pulmonary edema and acute upper airway obstruction. Laryngoscope. 1986;96:506–509. doi: 10.1288/00005537-198605000-00007. [DOI] [PubMed] [Google Scholar]
- Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190:752–756. doi: 10.1016/j.amjsurg.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Torda TA. Alveolar-arterial oxygen tension difference: a critical look. Anaesth Intensive Care. 1981;9:326–330. doi: 10.1177/0310057X8100900403. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11 (2 Suppl):S133–S153. [PubMed] [Google Scholar]
- Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H, Drummer O, Goodman C, Cherry G, Cunnington D, Kronborg I. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–1356. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- Willette RN, Krieger AJ, Sapru HN. Opioids increase laryngeal resistance and motoneuron activity in the recurrent laryngeal nerve. Eur J Pharmacol. 1982;80:57–63. doi: 10.1016/0014-2999(82)90177-7. [DOI] [PubMed] [Google Scholar]
- Willette RN, Barcas PP, Krieger AJ, Sapru HN. Pulmonary resistance and compliance changes evoked by pulmonary opiate receptor stimulation. Eur J Pharmacol. 1983;91:181–188. doi: 10.1016/0014-2999(83)90463-6. [DOI] [PubMed] [Google Scholar]
- Willette RN, Evans DY, Doorley BM. The in situ isolated larynx for evaluating peripheral opiate receptor antagonists. J Pharmacol Methods. 1987;17:15–25. doi: 10.1016/0160-5402(87)90033-7. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Young AP, Gruber RB, Discala JF, May WJ, McLaughlin D, Palmer LA, Lewis SJ. Co-activation of μ- and δ-opioid receptors elicits tolerance to morphine-induced ventilatory depression via generation of peroxynitrite. Respir Physiol Neurobiol. 2013;186:255–264. doi: 10.1016/j.resp.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Opioid μ-receptors in medullary raphe region affect the hypoxic ventilation in anesthetized rats. Respir Physiol Neurobiol. 2009;168:281–288. doi: 10.1016/j.resp.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimpfer M, Beck A, Mayer N, Raberger G, Steinbereithner K. Effects of morphine on the control of the cardiovascular system by the carotid-sinus-reflex and by the carotid chemoreflex. Anaesthesist. 1983;32:60–66. [PubMed] [Google Scholar]
- Zola EM, McLeod DC. Comparative effects and analgesic efficacy of the agonist-antagonist opioids. Drug Intell Clin Pharm. 1983;17:411–417. doi: 10.1177/106002808301700601. [DOI] [PubMed] [Google Scholar]