Abstract
Background
Longitudinal migraine studies have rarely assessed headache frequency and disability variation over a year.
Methods
The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study is a cross-sectional and longitudinal Internet study designed to characterize the course of episodic migraine (EM) and chronic migraine (CM). Participants were recruited from a Web-panel using quota sampling in an attempt to obtain a sample demographically similar to the US population. Participants who passed the screener were assessed every three months with the Core (baseline, six, and 12 months) and Snapshot (months three and nine) modules, which assessed headache frequency, headache-related disability, treatments, and treatment satisfaction. The Core also assessed resource use, health-related quality of life, and other features. One-time cross-sectional modules measured family burden, barriers to medical care, and comorbidities/endophenotypes.
Results
Of 489,537 invitees, we obtained 58,418 (11.9%) usable returns including 16,789 individuals who met ICHD-3 beta migraine criteria (EM (<15 headache days/mo): n = 15,313 (91.2%); CM (≥15 headache days/mo): n = 1476 (8.8%)). At baseline, all qualified respondents (n = 16,789) completed the Screener, Core, and Barriers to Care modules. Subsequent modules showed some attrition (Comorbidities/Endophenotypes, n = 12,810; Family Burden (Proband), n = 13,064; Family Burden (Partner), n = 4022; Family Burden (Child), n = 2140; Snapshot (three months), n = 9741; Core (six months), n = 7517; Snapshot (nine months), n = 6362; Core (12 months), n = 5915). A total of 3513 respondents (21.0%) completed all modules, and 3626 (EM: n = 3303 (21.6%); CM: n = 323 (21.9%)) completed all longitudinal assessments.
Conclusions
The CaMEO Study provides cross-sectional and longitudinal data that will contribute to our understanding of the course of migraine over one year and quantify variations in headache frequency, headache-related disability, comorbidities, treatments, and familial impact.
Keywords: Migraine disorders, chronic migraine, episodic migraine, epidemiology, headache
Background
Migraine is a common and often debilitating condition, with a one-year prevalence of 11.7% to 13.2% in studies from the United States (US) (1–3). Migraine is generally divided into two groups based on headache days per month. Episodic migraine (EM) is characterized by <15 headache days per month and chronic migraine (CM) is characterized by ≥15 headache days per month. However, the definition of CM has evolved over the past 25 years (4), most recently with the 2013 release of ICHD-3 beta (5).
EM and CM have been well characterized in population studies from many regions of the world (2,6–14), and results have been summarized in meta-analyses (15,16). These studies found significant headache-related disability associated with CM and EM (2,6–8,11,15,17), although more so with CM (6,9,11,17–19). In comparison with people with EM, those with CM experience substantially greater headache impact on daily activities (11,20), higher direct medical costs (9,21,22), greater health care resource utilization (10,11), reduced health-related quality of life (11,23), and higher rates of comorbidities (9,11,19). Also, many studies have defined risk factors for migraine chronification, which have been summarized in multiple review articles (17,24–28).
Despite previous in-depth population studies, much remains to be discovered about the effects of EM and CM on individuals. Largely uninvestigated areas include 1) the natural history or longitudinal disease course of EM and CM over a single year, 2) barriers to effective diagnosis and treatment of migraine (especially CM), 3) differences between EM and CM in observed impact and burden on families, and 4) comprehensive comorbidity profiling and differential association with EM and CM. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study was designed to extend knowledge about these and other issues. The longitudinal assessments were designed to enhance understanding of the natural history of and subject-specific variations in attack frequency, disability, psychiatric comorbidities, and treatment. In addition, a series of cross-sectional modules assessed previously unexplored aspects of barriers to care, comorbidities, and family burden of migraine among those with CM and EM. In this first report from the CaMEO Study, we describe methodology and design, explore nonresponse bias, and present preliminary findings on respondent demographic and socioeconomic characteristics and headache-related disability.
Methods
Study design
Overview
The study had a screening and recruiting phase and then longitudinal assessments approximately every three months over one year. In addition, a series of cross-sectional surveys assessed health care use, family burden, and comorbidities/endophenotypes. Data collection occurred over 15 months starting in September 2012 and closing in November 2013. The study was approved by the institutional review board of the Albert Einstein College of Medicine.
Screening and recruiting
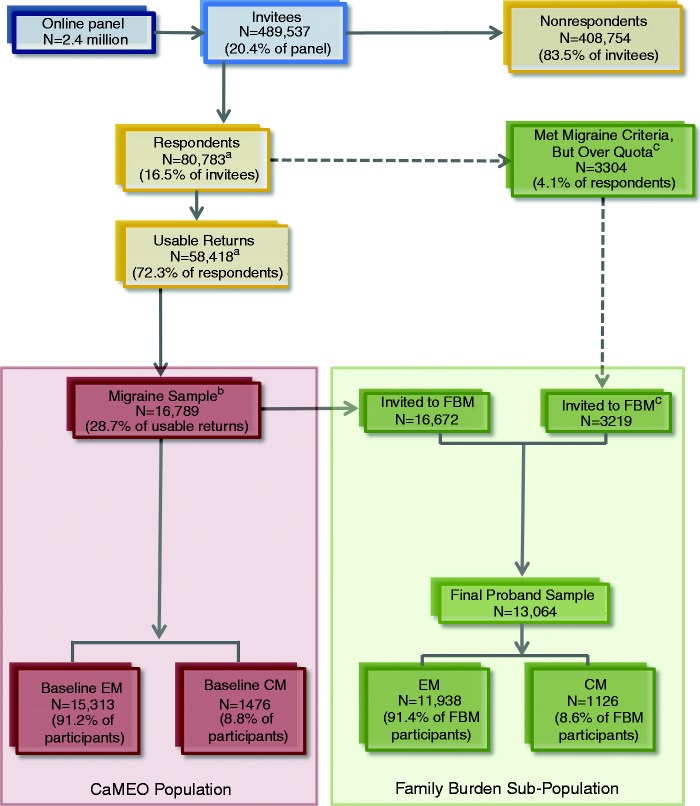
The screening and recruiting phases occurred from September 2012 through October 2012. Members of an Internet research panel (Research Now, Plano, TX), which has 2.4 million active US members, provided the sampling frame. Panel members were carefully screened and selected to be broadly representative of the US population. Quota sampling was used in an attempt to recruit a study sample that was representative of US population demography. Email invitations were sent to 489,537 panel members inviting them to complete the Screening Module (Figure 1), which was a brief survey capturing headache information and demographics (median completion time = 8 minutes; Table 1). The Study Population section describes the screener’s operating characteristics and migraine definitions used in the CaMEO Study.
Figure 1.
Participant flow diagram.
CM: chronic migraine; EM: episodic migraine; FBM: Family Burden Module; CaMEO: Chronic Migraine Epidemiology and Outcomes Study.
aN = 22,365 respondents either abandoned the survey (<20% of the survey was complete and headache status could not be identified), were over-quota, or had unusable data, which left 58,418 with usable returns.
bBaseline-sampling was quota based with the limit for the migraine sample defined as n = 17,000. Respondents who replied after quotas had been reached, but before initiation of the next sampling wave, were deemed over-quota and not included. Of the quota sample, n = 16,789 met the inclusion criteria: agreed to participate, screened positive for modified ICHD-3 beta migraine, completed initial surveys in a reasonable time (≥10 minutes), were 18 years old, were not missing headache frequency data, and reported consistent age and sex (of the 17,000 people in the migraine sample, as defined by the quotas, 211 (1.2%)) were removed during data cleaning (Table 2)). Migraine case rate was 28.7% (16,789/58,418).
cBecause of the risk of potentially low response rates for the Family Burden Module, respondents who were considered to be over-quota for CaMEO were resampled for the Family Burden Module only. Data from these over-quota respondents were not used for any other module.
Table 1.
Screening, core, and snapshot module construct areas and validated instruments.
| Domain | Instrument/brief description | Module |
||
|---|---|---|---|---|
| Screening (baseline) | Core (baseline, six and 12 months) | Snapshot (three and nine months) | ||
| Headache day frequency | • Number of headache days in past three months • Three-item; rated for past 90 days, 60 days, and 30 days | X | X | X |
| Headache treatments | • Headache treatments in past 30 days • Acute and preventive Rx and OTC medication usage, frequency of usage, overuse | X | X | |
| Headache-resource use | • Past six-month health care-resource use • Health care professional and hospital visits, frequency for headache and for other health reasons | X | ||
| Activity in school, work/paid employment, household work or chores, and nonwork | • Migraine Disability Assessment (MIDAS)a • Five-item, lost time and productivity in past three months (number of days missed) | X | X | |
| Daily performance | • Migraine-Specific Quality of Life Questionnaire (MSQ)a • Fourteen-item, six-point frequency scale, on headache-related behavioral and emotional/lifestyle impairment over past four weeks | X | ||
| Headache-related burden in work, school, family/social life, plans, commitments, and emotion or cognition | • Migraine Interictal Burden Scale (MIBS-4)a • Four-item, five-point frequency scale; rated for past four weeks | X | ||
| Treatment satisfaction over past four weeks (or last time headache was treated) | • Migraine-Treatment Optimization Questionnaire (M-TOQ)a • Five-item, “yes” or “no” questionnaire | X | X | |
| Presence of depression over last two weeks | • Patient Health Questionnaire, nine-item depression screener (PHQ-9)a • Nine-item, four-point frequency scale; depression is coded as a dichotomous variable using the DSM-IV and PHQ-9 clinical algorithm | X | ||
| Presence/severity of generalized anxiety disorder over last two weeks | • Generalized Anxiety Disorder, seven-item screener (GAD-7)a • Seven-item, four-point frequency scale | X | ||
| Severity of seven ICHD-3 beta migraine-defining features plus visual aura | • Migraine Symptom Severity (MSS) Score • Eight-item, four-point frequency scale; one “yes” or “no” question | X | ||
| Presence/severity of major events in previous 12 months | • Major Life Events Scale (MLE)b • Six-item “yes” or “no” questionnaire with five-point severity scale | X | ||
DSM-IV: Diagnostic and Statistical Manual for Mental Disorders, 4th ed.; ICHD-3 beta: International Classification of Headache Disorders, 3rd edn (beta version) (5); NRS: numerical rating schedule; OTC: over the counter; PHQ: Patient Health Questionnaire; Rx: prescription.
Validated instrument.
Adapted from Horowitz M, Schaefer C, Hiroto D, et al. Life event questionnaires for measuring presumptive stress. Psychosom Med 1977; 39: 413–431 (29).
Longitudinal modules
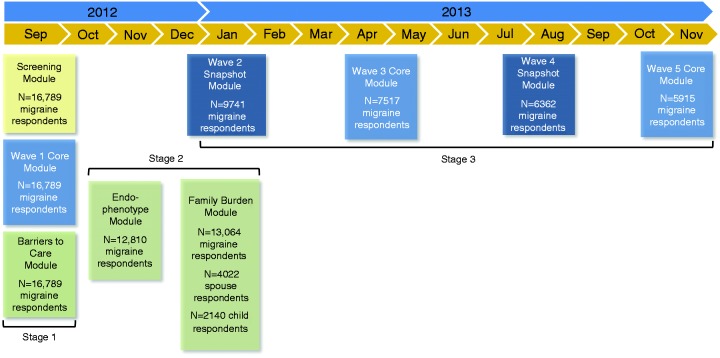
Qualified participants received emails at approximately three-month intervals (three, six, nine, and 12 months after baseline was sent) inviting them to complete multi-component Internet surveys (Figure 2). The longitudinal assessments were of two kinds: 1) the Core Module and 2) the Snapshot Module. The Core was offered three times (baseline, six, and 12 months) and the Snapshot was offered twice (three and nine months). The Core Module (median completion time = 10 minutes) consisted of 10 construct areas, most of which used validated instruments measuring headache frequency, depression, anxiety, interictal burden, headache-related disability, health-related quality of life, major life events, treatment/treatment satisfaction, and health care resource utilization (Table 1). The Snapshot Module (median completion time = 6 minutes) was an abbreviated version of the Core and assessed headache day frequency, headache-related disability, and any changes in headache treatments or treatment satisfaction in the past three months (Table 1). The components common to the Core and Snapshot modules will be used to assess longitudinal variation in headache frequency and disability (Figure 2).
Figure 2.
Study design.
N = number of returns for that module only, and does not represent a running total of participation in previous modules.
Module completion dates are as follows. Stage 1: Screening, Wave 1 Core, and Barriers to Care, September 17–October 30, 2012. Stage 2: Comorbidities/Endophenotypes, October 10–December 17, 2012; at the conclusion of wave 4 and before start of wave 5, nonrespondents to the Comorbidities/Endophenotypes Module were resampled (August 19–October 3, 2013). Family Burden-Proband, November 14, 2012–January 28, 2013. Family Burden-Partner, November 30, 2012–October 30, 2013. Family Burden-Child, January 11–October 30, 2013; at conclusion of wave 3 and before wave 4, nonrespondents to the Family Burden Survey were resampled (Proband, April 22–September 4, 2013; Partner, May 7–October 30, 2013; Child: May 3–October 30, 2013). Stage 3: Wave 2 Snapshot, December 21, 2012–February 19, 2013. Wave 3 Core, March 20–May 15, 2013. Wave 4 Snapshot, June 20–August 19, 2013. Wave 5 Core, September 19–November 19, 2013.
For Wave 1 Core, no cases could attrit; the total returns screening positive for migraine were n = 17,000 out of n = 58,629 (these cases plus the n = 10,044 who were over-quota and n = 12,110 who abandoned produce the total returns of n = 80,783); of these, n = 211 (1.2%) were removed during cleaning, resulting in a final sample of n = 16,789 qualified respondents. All five waves of longitudinal assessments were completed by n = 3626 of n = 16,789 (21.6%) respondents; four of five waves by n = 2364 (14.1%), three of five waves by n = 2415 (14.4%), two of five waves by n = 3109 (18.5%), and only one wave (i.e. baseline) by n = 5275 (31.4%).
Cross-sectional modules
In addition to the longitudinal components, several cross-sectional modules were administered one time each (Figure 2). These modules assessed barriers to care, comorbidities/endophenotypes, and family burden.
The order of module administration was embedded in the longitudinal design (Figure 2) and may be described as having three stages. In stage 1, baseline assessments consisted of the Screener, first Core Module, and Barriers to Care Module in quick succession from September 2012 to October 2012. This was followed by stage 2, during which the Comorbidities/Endophenotypes Module and Family Burden Module were administered from October 2012 through February 2013. Stage 3 collected the remaining four waves of longitudinal data, with the final surveys emailed in September 2013 and closed in November 2013. Receipt and initiation of modules were staggered based on subject-specific completion; thus, while Figure 2 would suggest stages 2 and 3 overlapped, at the respondent level there was no overlap, though an individual could be completing a stage 2 module at the time that another was initiating a stage 3 module.
Assessments
Questionnaire development
The CaMEO Study modules included validated instruments and surveys as well as original items developed based on the results of focus groups, expert opinion, and pretesting among target respondents.
Modules
The Barriers to Care Module (median completion time = 20 minutes) assessed the nature, patterns, and reasons for medical consultation; self-reported medical diagnosis; and treatment availability and utilization in those respondents who were diagnosed, to better define the existing barriers to effective care for EM and CM (Table 3). Together, stage 1 Screening, Core, and Barriers to Care modules took a median of 38 minutes to complete.
Table 3.
Other modules.
| Module | Domain/brief description |
|---|---|
| Barriers to care | • Headache information: knowledge about headache and medical care |
| • Consulting behaviors: motivation for consulting or not consulting a doctor/prescribing HCP (current, lapsed, or never consulted); reasons for changes in consulting patterns | |
| • Interactions with doctor:a among consulters only, tests to diagnose, symptoms discussed, and satisfaction | |
| • Awareness: awareness and use of headache diary, knowledge of headache triggers and types of treatments | |
| • Non-prescribing HCP:a consultation patterns among non-prescribing HCPs | |
| • Segmentation, knowledge, and attitudes: attitudes about HCPs, headache, and headache treatments; ability to function with headache | |
| • Experience and expectations: experiences and expectations for treatment efficacy (acute/preventive); important factors in choosing a treatment; interest in treatment options | |
| Comorbidities/Endophenotypes | • Migraine features: usual onset, duration, and treatment; presence of allodynia (12-item Allodynia Symptom Checklist (ASC-12)); five-point frequency allodynia screener); migraine triggers; prodrome features |
| • Allergies and respiratory disorders: presences of different types of allergies, asthma (European Community Respiratory Health Survey-II: Asthma), immunologic disorders, and nonallergic rhinitis | |
| • Chronic pain: total pain index and pain questions from SF-36 (2002 version, two items); presence of temporomandibular disorder, fibromyalgia (Fibromyalgia Rapid Screening Tool), chronic fatigue syndrome, pain disorders (excluding irritable bowel syndrome and headache, including osteoarthritis and joint hypermobility) | |
| • Sleep: presence of insomnia (sleep scale from MOS), sleep apnea (Berlin Sleep Apnea Questionnaire), restless leg syndrome, or other sleep problems | |
| • Autonomic disorders: presence of postural orthostatic tachycardia syndrome, orthostatic hypotension, syncope, vertigo, autonomic disorders, cardiac disorders, and stroke | |
| • Psychiatric disorders: presence of post-traumatic stress disorder (PCL-C and PC-PTSD screeners), generalized anxiety disorder, and other psychiatric disorders | |
| • Internal disorders: presence of gastroesophageal reflux disease (GERD-Q), irritable bowel syndrome (IBS Module 1), and overactive bladder | |
| Family burdenb | • Family structure: relationship status, children within family |
| • Overall burden: burden assessments, absenteeism/presenteeism for routine daily activities | |
| • Family activities: absenteeism/presenteeism for family activities, number of missed important family events, life events, and vacations | |
| • Interactions with partner and children: impact of headache on communication, planning, and supporting partner and/or children | |
| • Financial impact: impact of headache on career, future life planning, and finances | |
| • Life without headache: expectations of life without headache |
GERD-Q: Gastroesophageal Reflux Disease Questionnaire; HCP: health care professional; IBS-1: Irritable Bowel Syndrome Module 1, validated, uses Rome III criteria; MOS: Medical Outcomes Study; PC-PTSD: Primary Care Post-Traumatic Stress Disorder; PCL-C: Post-Traumatic Stress Disorder Checklist-Civilian Version.
Doctor was defined for the respondent as an HCP who is licensed to prescribe medications and included a medical doctor (MD/DO), nurse practitioner, physician assistant, or a dentist (DDS). A complimentary or alternative HCP (non-prescribing) was defined as a chiropractor, psychologist, massage therapist, acupuncturist, physical therapist, naturopath, natural health consultant, or any other type of alternative HCP.
Domains based on Proband Module, but Partner and Child modules had a similar structure.
In stage 2, the Comorbidities/Endophenotypes Module (median completion time = 19 minutes) collected data on a broad set of indicators (e.g. migraine features, allergies and respiratory disorders, chronic pain, chronic fatigue, sleep problems, autonomic disorders, psychiatric disorders, and internal disorders; Table 3), in an attempt to discover new empirically supported subclasses of migraine and increase taxonomic classification accuracy within the migraine spectrum.
The Family Burden Module assessed headache-related burden from the perspective of the migraineur (proband), their partners (i.e. spouses/significant others), and their children. The Proband Family Burden Module assessed the proband’s perspective on the impact of their headaches on family well-being, interpersonal relationships, and family and social interactions and activities as well as the impact of migraine on family finances, career, and health-related quality of life (Table 3). Partners and children were identified by the proband; participating partners and children reported their perspectives on the effect of the proband’s migraines on family life.
Study population
Migraine respondents
The migraine Screening Module (see Table 1), based on the American Migraine Study (AMS)/the American Migraine Prevalence and Prevention (AMPP) Study diagnostic module (7,8), was used for migraine classification. This screener has a sensitivity of 100% and specificity of 82% for migraine diagnosis (30), and sensitivity of 91% and specificity of 80% for CM diagnosis (31). The screener was based on lifetime recall of migraine symptoms associated with the respondents’ most severe headache (e.g. unilateral, pulsating, moderate/severe pain intensity, routine activity exacerbation of headache pain, nausea, phonophobia, photophobia), and was used to make modified ICHD-3 beta migraine classifications (5). Although the AMS/AMPP diagnostic module was based on ICHD-2 migraine criteria, no significant changes occurred between ICHD-2 and ICHD-3 beta that are related to the criteria used in this study. The AMS/AMPP diagnostic module classification criteria were considered modifications of ICHD-3 beta migraine criteria because two criteria were not confirmed: ≥5 lifetime migraine events (criterion A) and duration of attack untreated from four to 72 hours (criterion B).
In addition, this module assessed baseline headache frequency with a single open-response item on three-month recall of headache days, which was averaged to obtain a one-month headache day rate to classify respondents as having EM or CM using modified Silberstein-Lipton criteria (headache frequency of ≥15 (CM) or <15 (EM) days per month over the preceding three months) (32,33). The modified Silberstein-Lipton CM criteria employed in the CaMEO Study align to ICHD-3 beta criteria for CM, except for the requirement that ≥8 of the headache days per month be migraine (criterion C from the ICHD-3 beta CM criteria). Criterion C was not employed because it is difficult to assess in a large, self-report data-collection paradigm and requires the use of a diary and physician interview to accurately assess.
Only reliable respondents were included in the final sample (defined as those who completed baseline surveys in a reasonable time to read and respond to items (≥10 minutes), and reported consistent age and sex). Respondents eligible for inclusion were reliable active panel members, aged ≥18 years, met migraine criteria, and had experienced ≥1 headache within the past 12 months.
Family members
For the proband, partners were defined as “currently in a relationship with a spouse, partner, or significant other.” Partners were eligible to participate if they had cohabitated with the proband for ≥2 months. Eligible child respondents included adolescent/adult children, grandchildren, and stepchildren aged 13 to 29 years, cohabitating with the proband for ≥2 months. Because of the risk of potentially low partner and child Family Burden Module response rates, baseline respondents who were considered to be over-quota, but otherwise qualified for the CaMEO Study, were resampled for the Family Burden Module (Figure 1). Data from these over-quota respondents were not used for any other module.
Statistical methods
Sample size determinants
Because we planned to compare people with CM and EM in multiple ways, no formal sample size calculations were performed. We targeted a sample of ≥315 individuals with CM who would complete all study assessments over the course of a year. We assumed that prevalence of migraine in this US panel would be 12%, and that of those people with migraine 7% would have CM. In order to achieve a sample of n = 315 individuals with CM completing all modules and assessments, after accounting for anticipated response and attrition rates, a total of 489,537 panelists were invited to complete the screening survey.
Nonresponse bias
Nonresponse bias was explored through a variety of methods and contrasts; details of these methods and results are provided in the online Supplementary Appendix.
Statistical analyses
Descriptive and inferential statistics were used to compare the EM and CM samples on baseline survey demographics and outcomes. Continuous and count outcomes were described using means and standard deviations; inferential contrasts for continuous outcomes were based on analysis of variance (ANOVA) mean differences and 95% confidence intervals (CI), while rate ratios (RR; 95% CI) derived from negative binomial regression models were employed for count outcomes. Binary and multinomial outcomes were described using the number and percentage endorsing. Inferential contrasts for binary outcomes were based on odds ratios (OR; 95% CI) obtained from logistic regression models. For ordered multinomial category outcomes, cumulative ORs (95% CI) were obtained from cumulative logistic regression models. All statistical tests were two-tailed (significance level, 0.05) and were not adjusted for multiple comparisons. Imputation methods for missing values were not conducted. All analyses were conducted using SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
CaMEO sample and baseline response rates
Of 489,537 invitees, 80,783 (16.5%) responded to the screening survey (58,418 (11.9%) with usable returns; Figure 1). Overall, 3.4% (16,789/489,537) of invitees and 28.7% (16,789/58,418) of respondents with usable data met the inclusion criteria. Of the 16,789 respondents who qualified for inclusion, 15,313 (91.2%) were classified as EM and 1476 (8.8%) as CM at baseline (Stage 1: Screening; Figure 2). Module completion rates over the duration of the study showed respondent attrition (Table 4). A total of 3513 (21.0%) respondents completed all modules, and 3626 (21.6%) (CM: n = 323 (22.0%); EM: n = 3303 (21.6%)) completed all longitudinal assessments (five waves of response composed of three Core and two Snapshot modules).
Table 4.
Distribution of respondents with episodic (EM) and chronic migraine (CM) by study module.
| Time of emailing | Module(s) | EM respondents, n (%) | CM respondents, n (%) | Total respondents,a n |
|---|---|---|---|---|
| Month 0 (Baseline) | Screening, Core (wave 1), and Barriers to Care | 15,313 (91.2) | 1476 (8.8) | 16,789c |
| Month 1 (resampled in month 11)e | Comorbidities/endophenotypes | 11,699 (91.3) | 1111 (8.7) | 12,810 |
| Month 2 (resampled in month 7)e | Family Burden Module (Proband)b | 11,938 (91.4) | 1126 (8.6) | 13,064 |
| Month 3 | Snapshot (wave 2) | 9022 (92.6) | 719 (7.4) | 9741c |
| Month 3 (resampled in month 8)e | Family Burden Module (Partner) | 3642 (90.6) | 380 (9.4) | 4022 |
| Month 4 (resampled in month 8)e | Family Burden Module (Child) | 1536 (88.6)d | 197 (11.4)d | 2140d |
| Month 6 | Core (wave 3) | 6990 (93.0) | 527 (7.0) | 7517c |
| Month 9 | Snapshot (wave 4) | 5956 (93.6) | 406 (6.4) | 6362 |
| Month 12 | Core (wave 5) | 5502 (93.0) | 413 (7.0) | 5915c |
Total number of respondents who completed each of these modules.
Family Burden Module was supplemented with additional respondents who were over quota but otherwise qualified per the CaMEO Study criteria (see Figure 2).
Includes those who completed each wave (Snapshot/Core Module). A subset of this population completed all 5 waves (Core/Snapshot; n = 3626).
The total number of children responding to the Family Burden Module (Child version) was 2140, representing 1733 unique probands. Rates of EM and CM respondents were calculated based on the number of unique probands (n = 1733), since some had multiple children respond to the survey.
A fraction of the sample did not complete either the Family Burden or Comorbidities/endophenotypes modules during stage 2, and were resampled during stage 3 to minimize nonresponse.
Stages 1 and 2: Cross-sectional module response rates
Barriers to Care, Comorbidities/Endophenotypes, Family Burden modules
All qualified respondents (n = 16,789) completed the Barriers to Care module (EM: n = 15,313 (91.2%); CM: n = 1476 (8.8%)). The Comorbidities/Endophenotypes survey was sent to 16,763 (99.8%) respondents, of whom 15,289 (91.2%) were EM and 1474 (8.8%) were CM at baseline. Of the 16,763 invitations, a total of 12,810 respondents returned completed surveys with valid data (EM: n = 11,699 (91.3%); CM: n = 1111 (8.7%); Table 4). The Proband Family Burden Module was sent to 19,891 respondents, 16,672 (83.8%) of whom came from CaMEO baseline and 3219 (16.2%) of whom came from the baseline over-quota sample. Of the 19,891 respondents invited to participate in the Proband Family Burden Module, 18,131 (91.2%) were EM and 1760 (8.8%) were CM at baseline, and 13,064 respondents returned completed surveys with valid data (EM: n = 11,938 (91.4%); CM: n = 1126 (8.6%); Figure 1). The Partner Family Burden Module was sent to 7141 individuals identified by the proband, and 4886 partners returned completed surveys; 4022 of these returns had valid data (EM proband: n = 3642 (90.6%); CM proband: n = 380 (9.4%); Table 3). The Child Family Burden Module was sent to 2762 children identified by the proband, and 2404 children returned completed surveys; 2140 of these returns had valid data (EM proband: n = 1536 (88.6%); CM proband: n = 197 (11.4%); rates calculated based on the number of unique probands (n = 1733) because some probands had multiple children respond to the survey).
Stage 3: Longitudinal module response rates
Core and Snapshot modules
Longitudinal return and attrition rates were calculated (Table 2). Over the five longitudinal data collection periods, respondents had to participate at baseline, but were free to respond or not respond at any wave thereafter; 21.6% (3626/16,789) of respondents responded in all five waves, 31.4% (5275/16,789) responded only at baseline (wave 1), and the remaining 47.0% had some variation of participation in two to four waves (two waves, n = 3109 (18.5%); three waves, n = 2415 (14.4%); four waves, n = 2364 (14.1%); Table 5). Response and attrition rates were not different between EM and CM groups.
Table 2.
Longitudinal return and attrition rates.
| Wave | Outgob n (%) | Returnedb n (%) | Attritedb n (%) | Removed during cleaningc n (%) (wave specific) | Final cleanedc n (%) (wave specific) | Final cleanedb n (%) |
|---|---|---|---|---|---|---|
| 1a | 17,000d | 211 (1.2) | 16,789 (98.8) | 16,789 (98.8) | ||
| 2 | 16,681 (99.4) | 10,023 (59.7) | 6766 (40.3) | 282 (2.8) | 9741 (97.2) | 9741 (58.0) |
| 3 | 16,373 (97.5) | 7840 (46.7) | 8949 (53.3) | 323 (4.1) | 7517 (95.9) | 7517 (44.8) |
| 4e | 14,715 (87.7) | 6584 (39.2) | 10,205 (60.8) | 222 (3.4) | 6362 (96.6) | 6362 (37.9) |
| 5 | 16,316 (97.2) | 6128 (36.5) | 10,661 (63.5) | 213 (3.5) | 5915 (96.5) | 5915 (35.2) |
For wave 1 no cases could attrit, the total returns screening positive for migraine were N = 17,000 out of N = 58,629 (these cases plus the N = 10,044 who were over-quota and N = 12,110 who abandoned produce the total returns of N = 80,783), of these, 211 (1.2%) were removed during cleaning resulting in a final sample of N = 16,789 respondents.
Percentages in these cells denominated by baseline total (n = 16,789).
Percentages in these cells denominated by wave-specific returns.
See Figure 2 for how we arrive at the n = 17,000 migraine cases enrolled in the sample.
The drop in outgo at wave 4 is reviewed in detail in the Discussion under the section on “Benefits/risks of conducting large scale epidemiologic studies using national online sampling panels.”
Table 5.
Longitudinal response patterns.a
| Number of waves participating, n (%) | All CaMEO respondents (N = 16,789) | Baseline episodic migraine CaMEO respondents (N = 15,313) | Baseline chronic migraine CaMEO respondents (N = 1476) |
|---|---|---|---|
| 1 | 5275 (31.4) | 4801 (31.4) | 474 (32.1) |
| 2 | 3109 (18.5) | 2839 (18.5) | 270 (18.3) |
| 3 | 2415 (14.4) | 2209 (14.4) | 206 (14.0) |
| 4 | 2364 (14.1) | 2161 (14.1) | 203 (13.8) |
| 5 | 3626 (21.6) | 3303 (21.6) | 323 (21.9) |
Over the five waves of longitudinal data collection, respondents had to participate at baseline, but were free to respond or not respond at any wave thereafter. Response patterns for one wave and five waves can occur only a single way, but a variety of response patterns could be observed for two to four waves of participation.
Respondent demographic and comorbidity contrasts
Demographic, socioeconomic, and comorbidity differences were noted between EM and CM respondents (Table 6). Compared with the EM group, those with CM were more likely to be female (73.8% vs 81.1%; OR (95% CI) = 1.52 (1.33–1.75)), white (83.3% vs 87.5%; OR (95% CI) = 1.41 (1.20–1.66)), and obese (34.6% vs 41.6%; OR (95% CI) = 1.34 (1.21–1.50)), and had more family members (mean 2.9 vs 3.1 members; RR (95% CI) = 1.04 (1.01–1.08)). They were equally likely to be married or in a civil union (49.4% vs 47.6%; OR (95% CI) = 0.93 (0.84–1.04)). Respondents with CM completed fewer years of education (EM, 45.9% vs CM, 34.9% had ≥bachelor’s degree; OR (95% CI) = 0.63 (0.56–0.71)), were less likely to be employed full or part time (66.0% vs 56.4%; OR (95% CI) = 0.66 (0.60–0.74)), and were more likely to have lower annual individual incomes (cumulative OR (95% CI) = 0.65 (0.59–0.72)) and household incomes (cumulative OR (95% CI) = 0.63 (0.57–0.69)). Compared with those with EM, the CM group experienced higher Migraine Disability Assessment (MIDAS) scores (mean, 13.1 (Grade III, moderate) vs 60.5 (Grade IV, severe); RR (95% CI) = 4.63 (4.31–4.98)), higher rates of depression (30.0% vs 56.6%; OR (95% CI) = 3.05 (2.74–3.40)), and greater rates of anxiety (28.1% vs 48.4%; OR (95% CI) = 2.40 (2.16–2.67); Table 6).
Table 6.
Demographic, socioeconomic, and headache characteristics of participants with episodic and chronic migraine.
| Variables | Episodic migraine (N = 15,313) | Chronic migraine (N = 1476) | Contrast | Chronic vs episodic |
|
|---|---|---|---|---|---|
| Point estimate (95% CI)a | p value | ||||
| Demographics | |||||
| Age (years), mean (SD) | 40.6 (14.5) | 41.0 (13.8) | Mean difference | 0.39 (−0.38 to 1.16) | 0.32 |
| Female, n (%) | 11,298 (73.8) | 1197 (81.1) | Odds ratio | 1.52 (1.33–1.75) | < 0.001 |
| Race, n (%) White | 12,752 (83.3) | 1292 (87.5) | Odds ratio | 1.41 (1.20–1.66) | <0.001 |
| Ethnicity, n (%) Hispanic | 2039 (13.3) | 191 (12.9) | Odds ratio | 0.97 (0.83–1.13) | 0.69 |
| BMI, n (%) Obese | 5305 (34.6) | 614 (41.6) | Odds ratio | 1.34 (1.21–1.50) | <0.001 |
| Socioeconomics | |||||
| Number of household members, mean (SD) | 2.9 (1.4) | 3.1 (1.6) | Rate ratio | 1.04 (1.01–1.08) | 0.003 |
| Marital status, n (%) | |||||
| Married/civil union | 7561 (49.4) | 703 (47.6) | Odds ratio | 0.93 (0.84–1.04) | 0.20 |
| Highest level of education, n (%) | |||||
| ≥Bachelor’s degree | 7032 (45.9) | 515 (34.9) | Odds ratio | 0.63 (0.56–0.71) | <0.001 |
| Current employment, n (%) | |||||
| Full- or part-time | 10,112 (66.0) | 832 (56.4) | Odds ratio | 0.66 (0.60–0.74) | <0.001 |
| Annual individual income, n (%) | |||||
| <$25,000 | 6426 (42.1) | 761 (51.7) | Cumulative | Reference | <0.001 |
| $25,000–$49,999 | 4032 (26.4) | 400 (27.2) | odds ratio | 0.65 (0.59–0.72) | |
| $50,000–$99,999 | 3769 (24.7) | 243 (16.5) | |||
| ≥$100,000 | 1022 (6.7) | 67 (4.6) | |||
| Annual household income, n (%) | |||||
| <$25,000 | 2571 (16.9) | 365 (25.0) | Cumulative | Reference | <0.001 |
| $25,000–$49,999 | 3424 (22.5) | 372 (25.4) | odds ratio | 0.63 (0.57–0.69) | |
| $50,000–$99,999 | 5945 (39.1) | 513 (35.1) | |||
| ≥$100,000 | 3262 (21.5) | 212 (14.5) | |||
| Headache/medical characteristics | |||||
| MIDAS score, mean (SD) | 13.1 (22.2) | 60.5 (70.4) | Rate ratio | 4.63 (4.31–4.98) | <0.001 |
| Headache frequency, mean (SD) | 3.2 (3.2) | 20.8 (4.9) | Rate ratio | 6.49 (6.21–6.78) | <0.001 |
| PHQ-9 Depression, n (%) | 4589 (30.0) | 836 (56.6) | Odds ratio | 3.05 (2.74–3.40) | <0.001 |
| Generalized anxiety disorder, n (%) | 4307 (28.1) | 715 (48.4) | Odds ratio | 2.40 (2.16–2.67) | <0.001 |
BMI: body mass index; CM: chronic migraine; EM: episodic migraine; MIDAS: Migraine Disability Assessment; PHQ-9: Nine-item Patient Health Questionnaire; CI: confidence interval.
Reference values were “male”, “other race”, “not Hispanic”, “not obese”, “not married/civil union”, “<bachelor’s degree” and “not employed full or part time.” Mean difference obtained from analysis of variance model, rate ratio obtained from negative binomial regression model, odds ratios obtained from logistic regression model, and cumulative odds ratios obtained from cumulative logistic regression model.
Discussion
The CaMEO Study is a large Internet survey of the US population designed to identify individuals with EM and CM and chart their longitudinal course with assessments every three months over one year. In addition, the CaMEO Study assessed barriers to care, comorbidities/endophenotypes, and family burden of EM and CM. A large number of studies examining EM, CM, or both (e.g. systematic reviews by Natoli et al. (16) and Stovner et al. (34,35)) summarize the migraine epidemiology literature. Table 7 summarizes only the US studies because they are most relevant to this US-based Internet-based survey. Previous large population-based studies designed to identify and characterize US migraineurs with EM or CM (including prevalence estimates) used postal mail surveys or telephone interviews (Table 7); only the International Burden of Migraine Study (IBMS), which included the US, used Internet surveys. As with the CaMEO Study, IBMS was not designed to be population based; however, IBMS was a cross-sectional international burden of illness study, and therefore did not have longitudinal assessments.
Table 7.
Characteristics of other large-scale epidemiologic migraine studies that assessed chronic migraine and episodic migraine in the United States.
| Study | Study initiation/ country | Data collection method/study design | Respondent sample size, n/migraine diagnosis criteria/migraine sample size, n | Data focus |
|---|---|---|---|---|
| AMS (8) | 1989 US | Mailed survey Population-based, cross-sectional study | 20,468 respondents screened using 1988 IHS diagnostic criteriab Final sample of 2479 migraineurs | Prevalence estimates Headache-related symptoms Headache frequency Headache-related disability |
| Northeast Baltimore County Survey (13, 14) | 1993 US | Telephone interview survey Geographic-population- based cross-sectional study | 13,343 respondents screened using 1988 IHS diagnostic criteria Final sample included 1750 migraineurs | Prevalence estimates Sociodemographics Headache diagnosis Headache-related symptoms Headache frequency Headache-related disability Headache severity |
| AMS II (7) | 1999 US | Mailed survey Population-based, cross-sectional study | 29,727 respondents screened using 1988 IHS diagnostic criteriab Final sample of 3738 migraineurs | Prevalence estimates Sociodemographics Headache-related symptoms Headache frequency Headache-related disability Severe headache frequency |
| AMPP (6) | 2004 US | Mailed survey Population-based, longitudinal cohort study | 162,756 respondents screened using ICHD-2 diagnostic criteria Final sample of 19,189 migraineurs | Prevalence estimates Sociodemographics Headache diagnosis Headache-related symptoms Headache frequency (EM and CM) Headache-related disability Headache severity Health care resource use Medication use |
| IBMS (10) | 2009 Internationala | Web-based survey Quota-sampled, cross-sectional study | 10,650 respondents screened using ICHD-2 diagnostic criteria Final sample of 8726 migraineurs | Burden of illness Sociodemographics Headache diagnosis Headache-related symptoms Headache frequency (EM and CM) Headache-related disability Headache severity Comorbidities Headache-related quality of life Health care resource utilization |
| CaMEO | 2012 US | Web-based survey Quota-sampled,c Internet–population- based cohort study with longitudinal (three-month intervals) and cross-sectional surveys | 58,418 respondents screened using modified ICHD-3 beta diagnostic criteria Final sample of 16,789 migraineurs | Burden of illness Sociodemographics Headache-related symptoms Headache frequency Headache-related disability Headache-related quality of life Health care resource utilization Treatment satisfaction Barriers to care Comorbidities Family burden |
AMS: American Migraine Study; AMPP: American Migraine Prevalence and Prevention Study; CaMEO: Chronic Migraine Epidemiology and Outcomes Study; CM: chronic migraine; EM: episodic migraine; IBMS: International Burden of Migraine Study; ICHD-2: International Classification of Headache Disorders, 2nd edn.
Australia, Canada, France, Germany, Italy, Spain, United Kingdom, Taiwan, United States (US).
Met the study case definition for migraine, which was based on (but not strictly the same as) 1988 International Headache Society (IHS) diagnostic criteria. Data collection was not stratified by episodic or chronic forms of migraine, because this distinction was not made until 2004, with the release of ICHD-2.
Previous studies characterized migraine prevalence in the US. CaMEO was not designed to assess overall migraine prevalence and used quota sampling to provide a population construct in an attempt to represent the US population demography.
The CaMEO Study replicated many well-known findings regarding EM and CM. In CaMEO, chronic migraineurs were 52% more likely to be female, 41% more likely to be white, 34% more likely to be obese, 205% more likely to have depression, and 140% more likely to have anxiety than episodic migraineurs (Table 6). Results from CaMEO and AMPP Studies and IBMS were similar in that those with CM were primarily female and significantly more likely than those with EM to be obese, white, and have psychiatric comorbidities (9,36). Within IBMS, the percentage of females was not significantly different between the EM and CM groups, but the proportion of females was still higher in the CM group versus the EM group (like CaMEO); significantly different ages were noted in IBMS (unlike CaMEO), but the mean actual age was almost identical between the two studies, with chronic migraineurs being slightly older than episodic migraineurs (9,10). In a single-center study (19) of consecutive outpatients with EM and CM (with probable medication overuse (revised ICHD-2R) (37)), the CM group had a greater proportion of females and was older. Study design and population differences, including international versus US participation, may result in inconsistent demographic data between studies.
The CaMEO Study found that the rate of disability days per month (using MIDAS) was 3.63 times greater for those with CM than for those with EM. This is consistent with previous studies (6,9,18,38). Thus, chronic migraineurs are less able to perform work and leisure activities than those with EM, in line with previous findings of a direct correlation between attack frequency and headache-related disability for people with migraine (38).
In the CaMEO Study, socioeconomic status was reduced in those with CM compared with those with EM, as chronic migraineurs were found to have less education, less full- or part-time employment, and were more likely to have lower annual income. This finding is generally consistent with previous studies (17,19,36,39); however, IBMS and the Taiwanese clinic study found similar annual household income in EM and CM groups (9,10,39). This discrepancy with the CaMEO Study may be caused by potentially different income sources internationally (e.g. work, government-issued disability payments). A single-center outpatient study (19) found the CM group had less education, and was more likely to be married than those with EM.
Assessment of potential bias
All surveys are prone to selection bias, which can include under-coverage, voluntary response bias, and nonresponse bias. Under-coverage occurs when some members of the population are inadequately represented in the sample. The CaMEO Study addressed this by balancing the target sample on key demographics and by adjusting for demographic characteristics in the analyses. Voluntary response bias occurs when respondents are self-selected volunteers and may represent those with the strongest opinions or most severe disease. Although not directly assessed in the CaMEO Study, we suspect that this effect may work in both directions; more severe migraine cases may be both unable to respond and more interested in responding, which may negate this potential source of bias. Beyond the rates of CM, the AMPP Study, US IBMS, AMS, and CaMEO Study profiles are similar (2,6–8,10), which suggests that biases in CaMEO are not very different from these earlier studies.
The CaMEO Study was not designed to be a prevalence study. We acknowledged at inception that use of online panel sampling would limit generalizability in a way that mailed surveys do not, thus precluding explorations of prevalence. But prevalence has been well addressed (6,15,16) and was not of central interest here. Though we used probability sampling of quotas from a nationally distributed online panel to obtain the CaMEO migraine sample, response rates were low. Only 16.5% of invitees responded, 11.9% of whom provided useable data.
Analyses of nonresponse are imperative to understand the implications of survey results, as characteristics of nonrespondents may differ from those of respondents (40). Nonresponse bias occurs when individuals chosen for the sample are unwilling or unable to participate in the survey, causing respondents to differ in meaningful ways from nonrespondents. To assess this potential source of bias in the CaMEO Study, we sent a follow-up survey to nonrespondents to obtain demographics and disease severity (see the online Supplementary Appendix). Comparison of respondent and nonrespondent demographic data found that large sample sizes exaggerated the significance of small differences (online Supplementary Table 1). Respondents to the Nonrespondent Survey and the initial survey are similar; however, the low rate of participation in the Nonrespondent Survey leaves open the possibility of nonresponse bias.
This study has several limitations. All data were collected via self-report, and no supporting documentation or medical or pharmacy records were collected for verification, as this would be impractical given the large study size. As noted above, despite quota sampling, baseline demographic characteristics differed between the CaMEO Study respondents and nonrespondents (online Supplementary Tables 1 and 2). Consistent with other epidemiologic studies (6,9,36), the large sample sizes in CaMEO may have made minor demographic differences become significant. The migraine-positive case rate in the overall Nonrespondent Survey was generally similar to that of the respondents, suggesting disease state did not present a sampling bias. However, data for only 2.0% of nonrespondents (8225/408,754) were available for these analyses, and thus may not be representative of the entire set of nonrespondents. Other analyses of nonrespondents have found that nonresponse was associated with low socioeconomic status, but not health status (40). Because the variable of interest in our study is health status (i.e. migraine), it is possible that any differences that may have been caused by nonresponse may not have a great effect (40).
Benefits/risks of conducting large-scale epidemiologic studies in national online sampling panels
Internet research panels provide the advantage of enabling access to large numbers of willing survey participants who can be sampled and stratified according to pre-identified demographic and disease/symptom-based criteria. In CaMEO, over the course of a year, we succeeded in screening and following 16,789 migraineurs, replicated many of the findings of prior studies like the AMPP Study during the data processing and verification phase, and succeeded in obtaining our target sample proportions. We succeeded in guiding a total of 3626 migraineurs through five complete waves of longitudinal assessments and 3513 migraineurs with complete data through all nine study modules. However, Internet data capture, by virtue of its electronic and nearly automatic nature, is not faultless and the risk remains that some groups of interest may be over- or under-sampled because of issues of Internet access (41–44) and a lack of rigor in the management of Internet panel members.
Internet sampling panels often have limits on the number of studies in which members can participate. These limits are set by the panelist and are designed to minimize panelist burden and optimize participation. Our sampling protocol required panelist limits to be overridden so that baseline participants would receive invitations to all modules and every phase of the quarterly longitudinal assessments. This occurred in nearly all cases, except in wave 4 of the longitudinal assessment and a few cases spread across other modules.
In wave 4 panelist limits were not overridden for 1657 active participants. As a result, these 1657 participants never received invitations for wave 4; the number of these cases that would have responded in wave 4 had they been invited is unclear. To estimate the impact of the omission of these cases, we focused on a decomposition of the 34% of cases (556/1657) who had not attrited by wave 3. Our best estimate of those we would have lost has a lower and upper estimate based on responsiveness patterns among these 34% of cases. Our lower bound is based on those who responded sequentially in all waves 1 to 3, but not in wave 5. Our upper bound is based on those who responded in every wave but wave 4, the wave to which they were not invited. Our lower bound total sample is 184 subjects, who represent 2.8% of the 6362 wave 4 respondents. These 184 cases were composed of 173 persons with EM (2.9% of wave 4 EM respondents) and 11 individuals with CM (2.7% of wave 4 CM respondents). Our upper bound total sample was n = 372, constituting 5.8% of the 6362 wave 4 respondents. These 372 cases were composed of 335 people with EM (5.6% of the wave 4 EM respondents) and 37 people with CM (9% of wave 4 CM respondents). For our primary group of interest, there were an estimated 11 to 37 additional CM cases that could have been obtained at wave 4 but were not, and our expected CM sample for wave 4 in the absence of this limit filter error would move from the observed 406 CM cases to 417 or 443 CM cases, depending on the estimate.
There were also 199 cases who were eligible for at least one component of the Family Burden Module (either proband or partner), 15 cases who should have been invited to the Comorbidities/Endophenotypes Module, and four cases who should have been invited to the wave 2 Snapshot Module who were not invited because of this restriction limit error.
Despite limitations, the strengths of the CaMEO Study include a large nationwide sample (n = 16,789) and repeated assessments at three-month intervals. This provides a novel longitudinal perspective on the course and consequences of migraine. Cross-sectional modules were designed to fill a gap in knowledge about migraine and will provide novel data on previously unexamined aspects of migraine, including headache-related family burden, barriers to and patterns of migraine care, endophenotypes, and comorbidities among those with CM and EM.
Conclusions
Baseline results from the CaMEO Study confirmed previous findings that CM is associated with increased headache-related disability, psychiatric comorbidities, and greater financial and occupational burden than is EM. As more data are analyzed, the CaMEO Study will continue to provide a naturalistic understanding of the longitudinal course and consequences of EM and CM; quantify variations in headache frequency, headache-related disability, comorbidities, medication use, and effect of migraine on the family unit; and contribute a wealth of information to the limited amount of data on CM. The insights gleaned from the CaMEO Study into the characteristics of EM and CM will help to optimize future treatment paradigms to improve outcomes for the many individuals affected by migraine.
Clinical implications
Limited data are available to characterize chronic migraine.
The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study was designed with a particular emphasis on understanding the differences between chronic and episodic migraine, and aims to provide a naturalistic understanding of the longitudinal course and consequences of migraine (every three months over 12 months) and quantify variations in headache frequency, headache-related disability, comorbidities, medication use, and familial impact, as a complement to earlier studies designed to assess prevalence and burden of illness.
Supplementary Material
Funding
This study and analysis were sponsored by Allergan, Inc. (Irvine, CA). The study sponsor was involved in the study design, data collection, data analysis, data interpretation and the writing of the article. AA, MR, VM, DS, KF, DB, and RL were involved in development of the Web-based questionnaire. AA, MR, DS, KF, and RL were involved in development of the statistical analyses plan. DS and KF were responsible for conducting all the statistical analyses in this manuscript. All authors had full access to all of the data. The corresponding author had final responsibility for submission of this paper. All authors met the International Committee of Medical Journal Editors (ICMJE) authorship criteria. Neither honoraria nor payments were made for authorship.
Writing and editorial assistance were provided to the authors by Amanda M. Kelly, MPhil, MSHN, and Kristine W. Schuler, MS, of Complete Healthcare Communications Inc. (Chadds Ford, PA) and funded by Allergan, Inc. (Irvine, CA).
Conflicts of interest
Richard B. Lipton, MD, receives research support from the National Institutes of Health (NIH) (PO1 AG03949 (Program Director, Project and Core Leader), RO1AG025119 (Investigator), RO1AG022374-06A2 (Investigator), RO1AG034119 (Investigator), RO1AG12101 (Investigator), K23AG030857 (Mentor), K23NS05140901A1 (Mentor), and K23NS47256 (Mentor)), the National Headache Foundation, and the Migraine Research Fund; serves on the editorial board of Neurology; has reviewed for the National Institute on Aging (NIA) and National Institute of Neurological Disorders and Stroke (NINDS); holds stock options in eNeura Therapeutics; serves as consultant, advisory board member, or has received honoraria from Allergan, American Headache Society, Autonomic Technologies, Boehringer-Ingelheim Pharmaceuticals, Boston Scientific, Bristol Myers Squibb, Cognimed, Colucid, Eli Lilly, Endo Pharmaceuticals, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, NuPathe, Pfizer and Vedanta.
Dawn C. Buse, PhD, has received grant support and honoraria from Allergan Inc./MAP Pharmaceuticals, Novartis, NuPathe, and Zogenix. She is an employee of Montefiore Medical Center, which has received research support funded by Allergan Inc., Colucid, Endo Pharmaceuticals, GlaxoSmithKline, MAP Pharmaceuticals, Merck & Co. Inc., NuPathe, Novartis, Ortho-McNeil, and Zogenix, via grants to the National Headache Foundation. Aubrey Manack Adams, PhD, is an Allergan Inc. employee and receives stock options. Daniel Serrano, PhD, is an employee of Endpoint Outcomes. This work was completed while serving as a consultant for Vedanta Research, and he has received support funded by Allergan Inc., Colucid, Endo Pharmaceuticals, GlaxoSmithKline, MAP Pharmaceuticals, Merck & Co. Inc., NuPathe, Novartis, and Ortho-McNeil, via grants to the National Headache Foundation. Michael L. Reed, PhD, Valerie Marske, and Kristina M. Fanning, PhD, are employees of Vedanta Research, which has received support funded by Allergan Inc., Colucid, Endo Pharmaceuticals, GlaxoSmithKline, MAP Pharmaceuticals, Merck & Co. Inc., NuPathe, Novartis, and Ortho-McNeil, via grants to the National Headache Foundation.
Clinicaltrials.gov identifier: NCT01648530
References
- 1.Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia 2010; 30: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. [DOI] [PubMed] [Google Scholar]
- 3.Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2013; 53: 1278–1299. [DOI] [PubMed] [Google Scholar]
- 4.Manack A, Turkel C, Silberstein S. The evolution of chronic migraine: Classification and nomenclature. Headache 2009; 49: 1206–1213. [DOI] [PubMed] [Google Scholar]
- 5.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 6.Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: Results from the American Migraine Prevalence and Prevention Study. Headache 2012; 52: 1456–1470. [DOI] [PubMed] [Google Scholar]
- 7.Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache 2001; 41: 646–657. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Lipton RB, Celentano DD, et al. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992; 267: 64–69. [PubMed] [Google Scholar]
- 9.Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: Results from the International Burden of Migraine Study (IBMS). Cephalalgia 2011; 31: 301–315. [DOI] [PubMed] [Google Scholar]
- 10.Payne KA, Varon SF, Kawata AK, et al. The International Burden of Migraine Study (IBMS): Study design, methodology, and baseline cohort characteristics. Cephalalgia 2011; 31: 1116–1130. [DOI] [PubMed] [Google Scholar]
- 11.Wang SJ, Wang PJ, Fuh JL, et al. Comparisons of disability, quality of life, and resource use between chronic and episodic migraineurs: A clinic-based study in Taiwan. Cephalalgia 2013; 33: 171–181. [DOI] [PubMed] [Google Scholar]
- 12.Katsarava Z, Manack A, Yoon MS, et al. Chronic migraine: Classification and comparisons. Cephalalgia 2011; 31: 520–529. [DOI] [PubMed] [Google Scholar]
- 13.Scher AI, Stewart WF, Liberman J, et al. Prevalence of frequent headache in a population sample. Headache 1998; 38: 497–506. [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Lipton RB, Liberman J. Variation in migraine prevalence by race. Neurology 1996; 47: 52–59. [DOI] [PubMed] [Google Scholar]
- 15.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007; 27: 193–210. [DOI] [PubMed] [Google Scholar]
- 16.Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: A systematic review. Cephalalgia 2010; 30: 599–609. [DOI] [PubMed] [Google Scholar]
- 17.Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep 2012; 16: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigal ME, Serrano D, Reed M, et al. Chronic migraine in the population: Burden, diagnosis, and satisfaction with treatment. Neurology 2008; 71: 559–566. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache 2007; 47: 65–72. [DOI] [PubMed] [Google Scholar]
- 20.Buse DC, Manack AN, Serrano D, et al. Headache impact of chronic and episodic migraine: Results from the American Migraine Prevalence and Prevention study. Headache 2012; 52: 3–17. [DOI] [PubMed] [Google Scholar]
- 21.Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: Results from the International Burden of Migraine Study (IBMS). Headache 2011; 51: 1058–1077. [DOI] [PubMed] [Google Scholar]
- 22.Bloudek LM, Stokes M, Buse DC, et al. Cost of healthcare for patients with migraine in five European countries: Results from the International Burden of Migraine Study (IBMS). J Headache Pain 2012; 13: 361–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meletiche DM, Lofland JH, Young WB. Quality-of-life differences between patients with episodic and transformed migraine. Headache 2001; 41: 573–578. [DOI] [PubMed] [Google Scholar]
- 24.Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology 2008; 71: 1821–1828. [DOI] [PubMed] [Google Scholar]
- 25.Bigal ME, Lipton RB. Clinical course in migraine: Conceptualizing migraine transformation. Neurology 2008; 71: 848–855. [DOI] [PubMed] [Google Scholar]
- 26.Manack A, Buse DC, Serrano D, et al. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology 2011; 76: 711–718. [DOI] [PubMed] [Google Scholar]
- 27.Manack AN, Buse DC, Lipton RB. Chronic migraine: Epidemiology and disease burden. Curr Pain Headache Rep 2011; 15: 70–78. [DOI] [PubMed] [Google Scholar]
- 28.Bigal ME, Lipton RB. What predicts the change from episodic to chronic migraine? Curr Opin Neurol 2009; 22: 269–276. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz M, Schaefer C, Hiroto D, et al. Life event questionnaires for measuring presumptive stress. Psychosom Med 1977; 39: 413–431. [DOI] [PubMed] [Google Scholar]
- 30.Lipton RB, Diamond S, Reed M, et al. Migraine diagnosis and treatment: Results from the American Migraine Study II. Headache 2001; 41: 638–645. [DOI] [PubMed] [Google Scholar]
- 31.Liebenstein M, Bigal M, Sheftell F, et al. Validation of the Chronic Daily Headache Questionnaire (CDH-Q), abstract F25. Presented at: 49th Annual Scientific Meeting of the American Headache Society, Chicago, IL, June 7–11, 2007.
- 32.Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near-daily headaches: Field trial of revised IHS criteria. Neurology 1996; 47: 871–875. [DOI] [PubMed] [Google Scholar]
- 33.Silberstein SD, Lipton RB, Solomon S, et al. Classification of daily and near-daily headaches in the headache clinic. Proposed revisions to the International Headache Society criteria. In: Olesen J. (ed). Frontiers in headache research, New York: Raven Press Ltd, 1994, pp. 117–126. [Google Scholar]
- 34.Stovner LJ, Andree C. Eurolight Steering Committee. Impact of headache in Europe: A review for the Eurolight project. J Headache Pain 2008; 9: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stovner LJ, Zwart JA, Hagen K, et al. Epidemiology of headache in Europe. Eur J Neurol 2006; 13: 333–345. [DOI] [PubMed] [Google Scholar]
- 36.Buse DC, Manack A, Serrano D, et al. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 2010; 81: 428–432. [DOI] [PubMed] [Google Scholar]
- 37.Olesen J, Bousser MG, Diener HC, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006; 26: 742–746. [DOI] [PubMed] [Google Scholar]
- 38.Bigal ME, Rapoport AM, Lipton RB, et al. Assessment of migraine disability using the migraine disability assessment (MIDAS) questionnaire: A comparison of chronic migraine with episodic migraine. Headache 2003; 43: 336–342. [DOI] [PubMed] [Google Scholar]
- 39.Wang SJ, Wang PJ, Fuh JL, et al. Comparisons of disability, quality of life, and resource use between chronic and episodic migraineurs: A clinic-based study in Taiwan. Cephalalgia 2013; 33: 171–181. [DOI] [PubMed] [Google Scholar]
- 40.Ekholm O, Gundgaard J, Rasmussen NK, et al. The effect of health, socio-economic position, and mode of data collection on non-response in health interview surveys. Scand J Public Health 2010; 38: 699–706. [DOI] [PubMed] [Google Scholar]
- 41.Wyatt JC. When to use web-based surveys. J Am Med Inform Assoc 2000; 7: 426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi NG, Dinitto DM. The digital divide among low-income homebound older adults: Internet use patterns, eHealth literacy, and attitudes toward computer/Internet use. J Med Internet Res 2013; 15: e93–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rainie L. Internet, broadband, and cell phone statistics. http://www.pewinternet.org/files/old-media//Files/Reports/2010/PIP_December09_update.pdf (accessed 26 August 2014).
- 44.Zickuhr K and Smith A. Digital differences. http://pewinternet.org/reports/2012/Digital-differences.aspx (accessed 26 August 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.