Abstract
Gestational exposure to excess T leads to intrauterine growth restriction, low birth weight, and adult metabolic/reproductive disorders in female sheep. We hypothesized that as early mediators of such disruptions, gestational T disrupts steroidal and metabolic homeostasis in both the mother and fetus by both androgenic and metabolic pathways. Maternal blood samples were measured weekly for levels of insulin, glucose, and progesterone from four groups of animals: control; gestational T (twice weekly im injections of 100 mg of T propionate from d 30 to d 90 of gestation); T plus an androgen antagonist, flutamide (15 mg/kg·d oral; T-Flutamide); and T plus the insulin sensitizer, rosiglitazone (0.11 mg/kg·d oral; T-Rosi) (n = 10–12/group). On day 90 of gestation, maternal and umbilical cord samples were collected after a 48-hour fast from a subset (n = 6/group) for the measurement of steroids, free fatty acids, amino acids, and acylcarnitines. Gestational T decreased maternal progesterone levels by 36.5% (P < .05), which was prevented by flutamide showing direct androgenic mediation. Gestational T also augmented maternal insulin levels and decreased medium chained acylcarnitines, suggesting increased mitochondrial fatty acid oxidation. These changes were prevented by rosiglitazone, suggesting alterations in maternal fuel use. Gestational T-induced increases in fetal estradiol were not prevented by either cotreatment. Gestational T disrupted associations of steroids with metabolites and progesterone with acylcarnitines, which was prevented either by androgen antagonist or insulin sensitizer cotreatment. These findings suggest a future combination of these treatments might be required to prevent alteration in maternal/fetal steroidal and metabolic milieu(s).
Gestation is a period of interaction between the mother and fetus where the mother supplies the fetus with oxygen and necessary nutrients through the placenta. Any compromise in the maternal environment will have an impact on the developmental trajectory of the fetus, potentially culminating in life-long health disorders. Existing evidence indicates that exposure of pregnant mothers to adverse conditions via nutritional deficits/excess, stress, drugs, hormonal imbalances from disease states, endocrine-disrupting chemicals, high altitude, and/or infectious agents can lead to changes in maternal milieu, altering metabolic traits of the offspring and culminating in adult metabolic diseases (1–6). The factors in the maternal milieu, however, that influence the fetal endocrine system and thus the fetal developmental trajectory remain to be delineated.
Developmentally, steroids orchestrate fetal organ differentiation and growth. Steroids can induce changes in the maternal as well as fetal compartments and perturb placental nutritional transfer. Humans can be exposed to excess steroids during critical periods of organ differentiation via altered maternal steroidogenesis, diet (phytoestrogens), or industrial endocrine disrupting chemicals with steroidogenic properties (eg, bisphenol A, phthalates, and polychlorinated biphenyls among others) (6) or disease states (7, 8). For instance, fetuses of pregnant women with polycystic ovary syndrome (PCOS) or congenital adrenal hyperplasia are exposed to higher levels of androgens [congenital adrenal hyperplasia: (9); PCOS: (10)] and shown to have small-for-gestational-age babies (11, 12), although other studies report normal-birth-weight babies (13–15). On the other hand, small or large-for-gestational-age babies have been found to increase the risk of developing PCOS (16–18). Babies born to gestational or pregestational diabetic mothers with high levels of androgens (19) exhibit macrosomia or low birth weight (LBW) (a U shape curve of birth weight) (20). A reduction in the ratio of the fetal weight to the placental weight appears to characterize diabetic pregnancies relative to nondiabetic pregnancies (21) and is coupled with a higher prevalence of obesity, diabetes, and cardiovascular disorders in adulthood (3, 20).
Animal models provide a valuable resource for understanding the impact of exposure to excess steroids (native or environmental) on the maternal and fetal endocrine and metabolic phenotype. For instance, treatment of pregnant sheep with T increased not only maternal T levels, but also fetal T and estradiol levels (22). As such, programming of adult defects in prenatal T-treated animals can be mediated via androgenic or estrogenic actions of T in these model systems. A second possibility is that programming of adult defects in offspring of gestational T-treated animals may involve a compromised maternal or fetal metabolic milieu. Evidence from studies in rhesus monkeys indicates that gestational T treatment induces maternal hyperinsulinemia (23), and their female offspring develop pancreatic morphological and functional β-cell defects as they approach menopause (24, 25). Interestingly, women with PCOS, whose characteristics most of these models recapitulate, also have elevated levels of T and insulin during their pregnancy (26). Studies with a Spanish cohort provide evidence in support of PCOS women having LBW offspring with elevated insulin levels (12), low levels of cholesterol and triglycerides (27).
A third possibility is that excess gestational T may compromise nutrient transfer to the fetus. This appears likely because gestational T treatment leads to intrauterine growth restriction (IUGR) and LBW offspring in sheep (22, 28). Changes in maternal nutrition, namely amino acids, free fatty acids (FFAs), and acylcarnitines have been found to accompany IUGR pregnancies (29–32). Consistent with nutritional compromise, gestational T excess advances placental differentiation in sheep (33), a feature they share with undernourished models [guinea pigs: (34); sheep: (35)]. Interestingly, gestational diabetes in humans is associated with elevated maternal T and altered placental growth (19). The extent to which gestational T-induced alteration in placental development compromises nutrient (eg, glucose, amino acids, and FFAs) exchange remains still to be determined. An understanding of the maternal and fetal nutritional and metabolic milieu is essential to identify mediators of IUGR. In addition, if the fetal environment parallels maternal milieu, maternal measures could serve as a surrogate for the fetal environment.
The objectives of this study are 3-fold: 1) to determine whether excess gestational T disrupts the steroidal, metabolic, and nutritional milieu of the mother and the fetus, 2) to assess whether the cotreatment with an androgen antagonist or an insulin sensitizer prevents disruptions in steroidal, metabolic, and nutritional milieu in both compartments, and 3) to examine to what extent the fetal hormonal, metabolic, and nutritional milieu parallels that of the mother.
Materials and Methods
Breeding, gestational treatment, and sample procurement
The Animal Care and Use Committee at the University of Michigan approved all animal procedures. Mature Suffolk ewes, 2–3 years of age, purchased from local farmers were bred and kept at the University of Michigan Sheep Research Facility (Ann Arbor, Michigan; 42°, 108 18′ N) under a natural photoperiod and provided with 0.5 kg of shelled corn and 1.0–1.5 kg of alfalfa hay per ewe per day. This provides 2.31 Mcal/kg of digestible energy that meets the nutritional requirements of pregnant ewes, as defined by the National Research Council (36). Ewes were mated with fertility proven raddled rams. The date of mating was confirmed based on paint marks appearing on the rump of ewe after being mounted by a raddled ram. They were administrated aureomycin crumbles (chlortetracycline, 250 mg per ewe per day) to reduce abortion.
Pregnant ewes (n = 44, average pregnancy weight 75.7 ± 2.2 kg) were blocked by weight and body score and randomly assigned to one of four treatment groups with treatments initiated on day 30 of gestation and ending day 90 of gestation: 1) controls (C; n = 12), administered twice weekly 2 mL corn oil, im; 2) T treated (n = 12), 100 mg testosterone propionate (Sigma-Aldrich Corp) administered twice weekly in 2 mL of corn oil, im; 3) T plus androgen antagonist, flutamide (Sigma-Aldrich Corp) treated (T-Flutamide; n = 10), flutamide administrated orally at a dose of 15 mg/kg per ewe per day; 4) T plus insulin sensitizer, rosiglitazone (Avandia; GlaxoSmithKline) treated (T-Rosi; n = 10), rosiglitazone administered orally at a dose of 0.11 mg/kg per ewe per day. Weekly blood samples were collected from all pregnant ewes starting on day 30 until day 86 of gestation (total of nine samples). All samples were collected between 7:00 and 8:00 am prior to feeding. An additional blood sample was procured on day 90 of gestation after fasting for 48 hours. All samples were collected through jugular venipunture in sodium fluoride and potassium oxalate Vacutainer tubes (BD Vacutainer). On day 90 of gestation, all dams were anesthetized with ketamine hydrochloride (Fort Dodge, IA; 3–4 mg/kg, iv) and xylazine (Anased Injection; 0.4 mg/kg per ewe, im) and subsequently maintained under general anesthesia with isoflurane (RxElite Holdings Inc). Umbilical arterial samples were collected after accessing the gravid uterus via midline and uterine wall incisions as previously described (22). Fetuses were removed for body measures and tissue procurement. After removal of fetuses, mothers were euthanized with a barbiturate overdose (15 mL, iv, Fatal Plus; Vortech Pharmaceuticals).
Fetal body measurements
The following corporal measurements were taken on day 90 for female fetuses: anogenital distance; crown-to-rump length (from the highest midpoint on the top of the head to the base of the tail); head circumference; fetal weight; and weights of adrenal, brain, heart, kidney liver, lung, ovaries, pancreas, pituitary, spleen, thymus, thyroid, and uterus. The ratio of organ weight to body weight and ratio of pituitary weight to brain weight were determined. Male fetuses were not studied.
Hormonal and metabolic measures
Plasma progesterone, LH, and FSH were measured in all weekly maternal samples and maternal and fetal samples collected at day 90 of gestation. Circulating levels of progesterone were measured using an RIA (Coat-A-Count P; Diagnostic Products Corp) validated for sheep samples (37). The sensitivity of the assay was 0.01 ± 0.003 ng/mL (n = 5 assays). Intraassay coefficients of variance (CVs), based on two quality control (QC) pools measuring 1.4 ± 0.02 ng/mL and 13.4 ± 0.2 ng/mL, were 6.9% and 5.8%, respectively. The corresponding interassay CVs were 4.5%, and 2.6%, respectively. Plasma LH and FSH concentrations were determined in duplicate using well-validated RIAs [LH: (38); FSH: (39)] and expressed in terms of NIH-LH-S12 and NIDDK-ovine FSH-1, respectively. The detection limit of the LH assay was 0.42 ± 0.04 ng/mL (n = 3 assays; mean ± SEM). The mean interassay CVs calculated based on four QC pools measuring 3.4 ± 0.1, 8.9 ± 0.2, 12.9 ± 0.3, and 23.6 ± 0.3 ng/mL were 7.1, 7.6, 8.1, and 5.1%, respectively. The intraassay CVs for the same four QC pools were 9.8%, 6.7%, 4.8%, and 5.3%, respectively. The detection limit of the FSH assay was 0.7 ± 0.1 ng/mL (n = 4). The intraassay CVs based on two FSH QC pools averaging 5.8 ± 0.1 and 15.5 ± 0.2 ng/mL were 8.5% and 6.1%, respectively. The interassay CVs for the same QC pools were 9.3% and 6.5%, respectively.
Plasma insulin and glucose were measured in all maternal and fetal samples collected at gestational day 90. Insulin concentrations were measured using a RIA kit (MP Biomedicals). The assay sensitivity was 2.0 ± 0.2 μU/mL (n = 5 assays). Mean intraassay CVs based on two QC pools measuring 29.7 ± 0.7 and 110.2 ± 2.9 μU/mL were 8.9% and 7.2%, respectively. The corresponding interassay CVs were 11.1% and 11.6%, respectively. Glucose concentrations were determined using the Glucose Oxidase Method (Pointe Scientific, Inc) as described previously (40). The intraassay CVs at 25, 75, and 150 mg/dL were 1.9%, 1.1%, and 0.9% respectively (n = 8 assays). The corresponding interassay CVs were 4.5%, 1.0%, and 1.4%, respectively.
Because evidence points to ovarian as well as adrenal contribution to the PCOS phenotype (4), which prenatal T-treated sheep recapitulate (41), plasma concentrations of androstenedione, cortisol, dehydroepiandrosterone (DHEA), deoxycorticosterone (DOC), 11-deoxycortisol, estradiol, estrone, pregnenolone, 17α-OH-pregnenolone, 17α-OH-progesterone, and T were assayed in a subset of maternal samples (those bearing female fetuses, n = 6/group) collected at day 90 and the corresponding fetal samples. These steroids were measured at the Optimized Analytical Solutions Laboratories, LLC (Durham, North Carolina) using HPLC/mass spectrometry-mass spectrometry Agilent MassHunter Workstation Data Acquisition for Triple Quad B.03.01 (B2065) and Agilent MassHunter Quantitative Analysis for QQQ (B.04.00/Build 4.0.225.0).
Samples were subjected to a liquid/liquid extraction method followed by derivatization with dansyl chloride to enable the detection of estrone and estradiol. Each sample was analyzed using liquid chromatography/mass spectrometry-mass spectrometry with electrospray ionization. Calibration curves of steroid standard/internal standard peak area ratio vs steroid concentration were constructed and a weighted 1/x2 linear regression applied to the data. Concentrations of steroids in samples were defined based on the appropriate calibration curve. Commercially obtained female monkey plasma were used as quality controls (for cortisol, estradiol, and T: Sigma-Aldrich; and for androstenedione, DHEA, DOC, 11-deoxycortisol, estrone, pregnonelone, 17α-OH-pregnonelone, 17α-OH-progesterone: Steraloids Inc). The lower detection limits were as follows: androstenedione 0.1 ng/mL; cortisol 0.5 ng/mL; DHEA 0.5 ng/mL; 11-deoxycortisol 0.05 ng/mL; DOC 0.05 ng/mL; estradiol 5 pg/mL; estrone 5 pg/mL; pregnenolone 1 ng/mL; 17α-OH-pregnenolone 1 ng/mL; 17α-OH-progesterone 0.2 ng/mL; and T 0.02 ng/mL. The coefficients of variation for these hormone measures were as follows: androstenedione 3%, cortisol 11%, DHEA 8%, DOC 19%, 11-deoxcortisol 5%, estradiol 2%, estrone 4%, pregnenolone 16%, 17α-OH-pregnenolone 16%, 17α-OH-pregesterone 2%, and T 8%.
FFA, amino acid, and acylcarnitines measures
Circulating FFAs, amino acids, and acylcarnitines were measured in the subset of maternal samples (n = 6/group) collected at day 90 and the corresponding female fetal samples. Analysis of nonesterified FFAs was conducted using a direct method of transesterification as described previously (42). For measuring FFAs, total lipids from the fat samples were extracted using the method of solvent partition of Bligh and Dyer (43). Methyl esters were extracted with hexane and then purified by thin-layer chromatography and identified with respect to the retention flow of the standard. Fatty acid composition was determined using gas chromatography (GC). Fatty acid methyl esters were measured using an Agilent GC machine (6890 N model; Agilent Technologies) equipped with a flame ionization detector, an autosampler, and a Chemstation software for data analysis. Hydrogen was used as a carrier gas as well as flame ionization detector and nitrogen was used as a makeup gas. Intraassay CVs ranged between 0.9% and 1.1% and interassay CVs between 2.6% and 3.3%. Details on individual FFA CVs are shown in Results.
An EZ:faast GC-mass spectrometry kit from Phenomenex was used for the purification, extraction, and derivatization of amino compounds for the determination of amino acids (44). Twenty-two amino acids were measured. Prior to analysis, an internal standard (norvaline) was added to 25 μL of plasma. Amino acids in the sample were concentrated by cation exchange solid-phase extraction at acidic pH and then washed with a 2-propanol/water solution and eluted using an alkaline solution of sodium hydroxide in water mixed with 2-propanol. The eluate was derivatized by addition of propyl chloroformate, followed by mixing the solution using the vortex. This latter mixture was left for a 2-minute reaction period. The amino acid derivatives were extracted into an organic layer containing chloroform and isooctane. This layer was collected and the solvent evaporated, and the residue was reconstituted in solvent for GC-spectrometry analysis. Separation and detection was performed using a Zebron ZB-AAA column on a 6890 GC with a 5973 Mass Selective Detection system. Amino acids were quantitated based on external calibration curves generated by injection of amino acid standards derivatized in parallel with the samples. Correlation coefficient (r2) of the calibration curves of amino acids ranged from 0.9 to 0.99. The CVs of amino acids analysis ranged from 2.8% to 9.4%.
Plasma acylcarnitines were extracted using a mixture of acetonitrile and water with isotopically labeled acylcarnitine standards set B (Cambridge Isotope). The solvent was added to plasma samples at a 4:1 solvent to sample ratio, mixed, vortexed, and left sitting on ice for 5 minutes and then centrifuged at 15 000 × g for 5 minutes and extracted. The extraction step was repeated twice. The supernatants that contained acylcarnitines were transferred to an autosampler vial with insert for liquid chromatography-mass spectrometry analysis. An Agilent 1200 liquid chromatographer with Waters XBridge BEH C18 2.5-um column (2.1 × 5 cm) was used for chromatographic separation. After separation the molecules were identified using an Agilent 6410 series triple, quadruple mass spectrometer equipped with an electrospray ionization source, which was operated in positive mode. Metabolites concentrations were calculated based on ratios of unlabeled metabolites and known concentrations of isotopically labeled metabolites. Data were processed by a MassHunter workstation software, version B.04. Twenty-nine acylcarnitines (Cx:y) were measured where Cx:y is used to describe the total number of carbons and double bonds of all chains, respectively. The relative SD of duplicates of three samples for acylcarnitines ranged between 1.2% and 11.1%.
Statistical analysis
Treatment effects on corporal measurements and fetal insulin and glucose data were analyzed using a mixed model adjusted for three covariates (number of fetuses per litter, number of female fetuses, and male fetuses in the litter) and Bonferroni correction for multiple comparisons. Normality of data was tested using residual plots. Analyses of maternal plasma levels of progesterone, LH, and FSH from day 30 to day 86 of gestation involved a mixed-model procedure using treatment, time, and time by treatment interaction as a repeated variable with adjustments made for two covariates (weight of ewes and number of fetuses). A Bonferroni correction was applied for multiple comparisons. For testing treatment effects on maternal steroids on day 90 of gestation, as well as FSH, LH, glucose, and insulin, a mixed model procedure adjusted for two covariates (weight of ewes and number of fetuses) was used along with Bonferroni's correction for multiple comparisons. For fetal steroids, a Kruskal-Wallis test and Bonferroni adjustment for multiple comparisons were used. Statistical analysis was performed using SAS for Windows version 9.2 (SAS Institute). Spearman correlation methods were used to assess the association between different metabolites (eg, amino acids, FFAs, acylcarnitines, and steroids) either in maternal plasma or fetal cord blood or between the two of them. Analysis of variance using pairwise comparisons adjusted with false discovery rate method was performed to compare treatment groups against the control group. A value of P < .05 was considered significant. All data are presented as mean ± SEM.
Results
Fetal female body measurements
Fetal female body measurements, the ratio of organ weight to body weight, and pituitary weight to brain weight of female fetuses at day 90 of gestation were similar among all groups (Supplemental Table 1). Gestational T treatment increased the anogenital distance in female fetuses relative to corresponding controls (T: 7.6 ± 0.1 vs C: 0.8 ± 0.1 cm, P < .0001). Cotreatment with the androgen antagonist prevented the masculinizing effects of T (T-Flutamide: 2.6 ± 0.6; T vs T-Flutamide, P < .01), whereas cotreatment with the insulin sensitizer had no effect (T-Rosi: 7.4 ± 0.2; C vs T-Rosi; P < .0001). No other anthropomorphic features were affected by the treatments.
Impact of prenatal T and interventions on maternal and fetal hormonal milieu
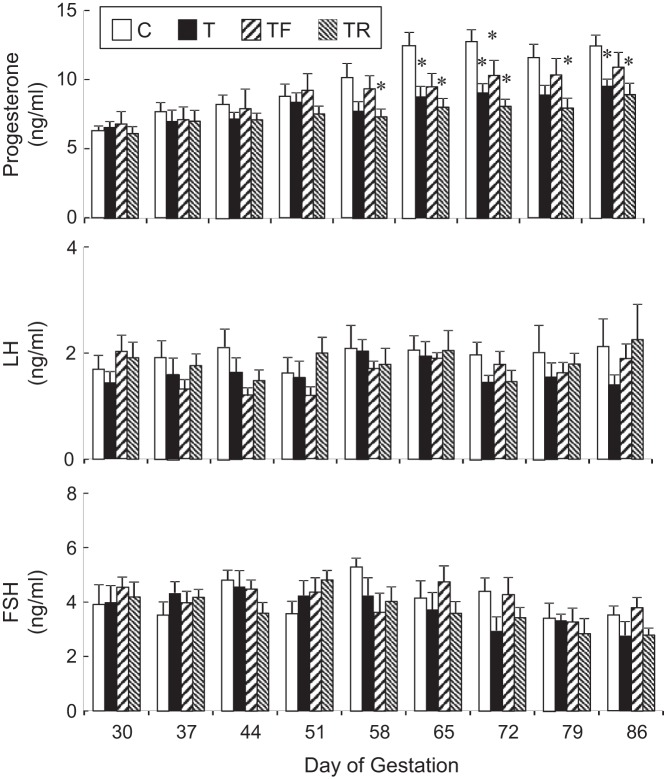
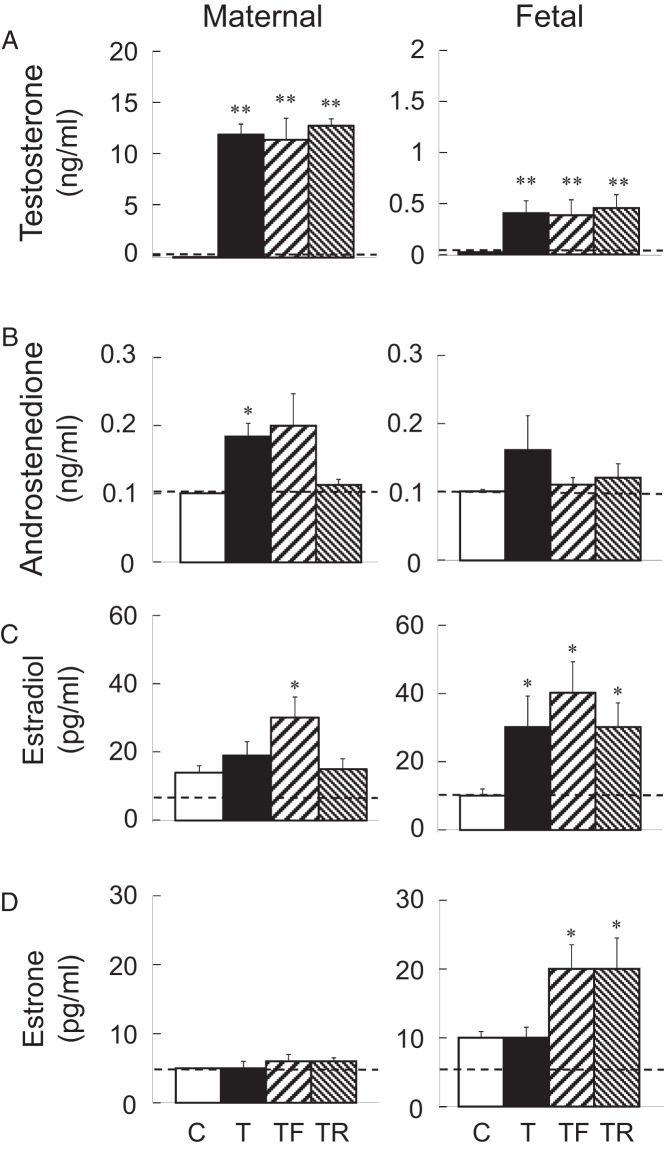
Mean maternal concentrations of progesterone, LH, and FSH from day 30 to day 86 of gestation, are shown in Figure 1. Gestational T treatment reduced plasma progesterone levels on days 65, 72, and 86 of gestation (P < .05). Plasma progesterone levels were also lower in the T-Rosi group relative to controls on gestational days 58, 65, 72, 79, and 86. Plasma progesterone levels in the T-Flutamide group only differed from control on day 72 of gestation. Maternal LH and FSH concentrations were similar across treatment groups at all the time points studied with no significant differences observed. Mean maternal and fetal testosterone, androstenedione, estradiol, and estrone concentrations on day 90 of gestation are shown in Figure 2. Gestational T treatment increased maternal T concentrations relative to the C group (P < .01). T levels in the intervention groups (T-Flutamide, T-Rosi) were similar to that achieved in the T-treated group (Figure 2A). Testosterone also increased maternal androstenedione relative to the C group (P < .05) (Figure 2B). This increase was prevented in the T-Rosi but not the T-Flutamide group. T did not alter maternal estradiol or estrone levels (Figure 2, C and D). An increase in estradiol was evident in T-Flutamide but not T-Rosi mothers. T also increased T concentrations in the fetal milieu relative to controls (P < .01). Concentrations of T achieved in the two intervention groups (T-Flutamide, T-Rosi) were similar to that achieved in the gestational T-treated group (Figure 2A). T increased fetal estradiol levels in the gestational T-treated group, and this was not prevented in the two intervention groups (Figure 2C). There was no impact of gestational T on fetal estrone levels (Figure 2D). Surprisingly, an increase in estrone levels relative to controls was evident in both the intervention groups (T-Flutamide and T-Rosi) (Figure 2C).
Figure 1.
Maternal progesterone (top), LH (middle), and FSH (bottom) plasma levels (mean ± SEM) from days 30 to 86 of gestation at weekly intervals (C, n = 12; T, n = 12, T-Flutamide, n = 10; T-Rosi, n = 10). Values with asterisk differ significantly (P < .05) from the control group. C, controls; T, treated with T; TF, treated with T plus flutamide; TR, treated with T plus rosiglitazone.
Figure 2.
Maternal (left panels) and fetal (right panels) samples. T (top; A), androstenedione (upper middle; B), estradiol (lower middle; C), and estrone (bottom; D) plasma levels (mean ± SEM) on day 90 of gestation are shown. For maternal samples, a subset of the entire group was randomly selected for steroid measures (n = 6/group). The corresponding fetal subset (range per group: n = 6–9) samples were assayed. Values with asterisks differ significantly from controls. **, P < .01; *, P < .05). C, controls; T, treated with T; TF, treated with T plus flutamide; TR, treated with T plus rosiglitazone.
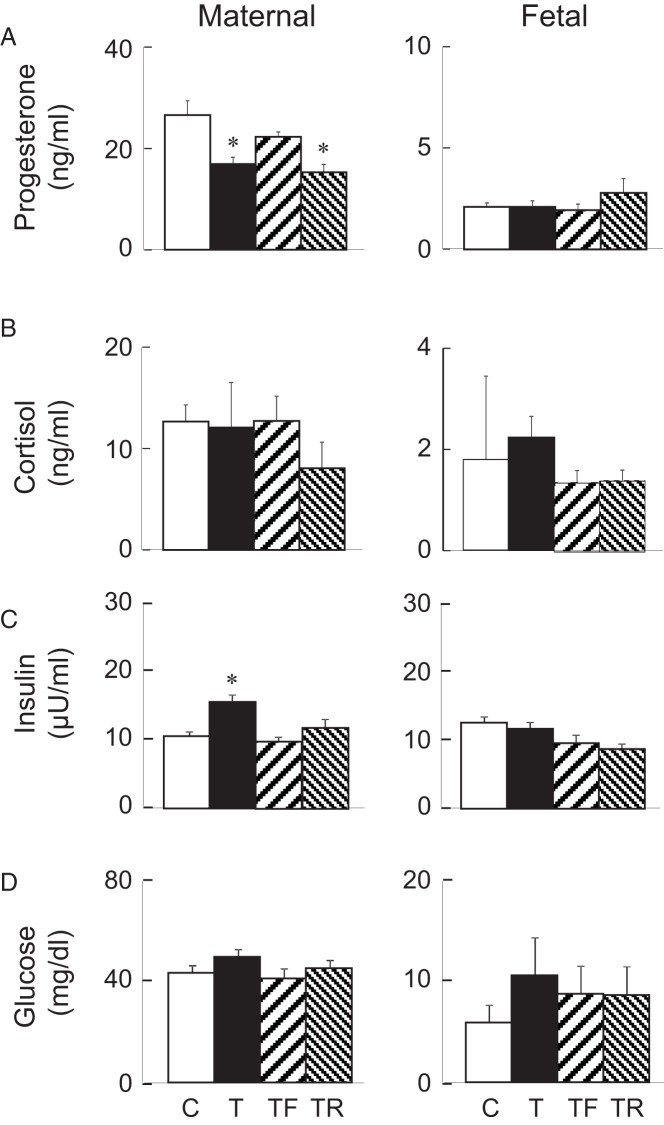
T reduced maternal progesterone concentrations relative to the C group on fetal day 90. Progesterone concentrations in the T-Flutamide group were similar to those of controls, consistent with earlier time points discussed above but reduced in the T-Rosi intervention group (Figure 3A). Fetal progesterone levels were similar across all groups. Maternal and fetal cortisol levels did not differ across treatment groups (Figure 2B). Other steroids measured (DOC, 11-deoxycortisol, DHEA, pregnenolone, and 17α-OH-pregnenolone) were near the detection limit of the assay in both the mother and the fetus (not shown). Gestational T increased maternal insulin in gestational T-treated group relative to the C group (Figure 3C). Both T-Flutamide and T-Rosi interventions prevented maternal hyperinsulinemia. Fetal insulin levels were similar among all groups. Glucose levels were similar in the maternal and fetal compartments across all treatment groups (Figure 3D).
Figure 3.
Maternal (left panels) and fetal (right panels) samples. Progesterone (top; A), cortisol (upper middle; B), insulin (lower middle; C), and glucose (bottom; D) plasma levels (mean ± SEM) on day 90 of gestation are shown. All maternal (range per group: n = 10–12) and fetal (range per group: n = 7–12) samples were included in these measures. Values with asterisks differ significantly from controls. *, P < .05. C, controls; T, treated with T; TF, treated with T plus flutamide; TR, treated with T plus rosiglitazone.
Impact of prenatal T and interventions on maternal and fetal amino acids, acylcarnitines, and FFAs
Mean circulating levels of maternal amino acids (n = 23), acylcarnitines (n = 28), and FFAs (n = 10) are shown on Supplemental Tables 2–4. Those metabolites that had the highest circulating levels plus those showing significant differences among treatment groups (five metabolites per category) are included in Table 1. Maternal circulating levels of amino acids in the gestational T-treated group as well as the two cotreatment groups (T-Flutamide, T-Rosi) did not differ from the control group. Gestational T treatment decreased circulating levels of acylcarnitines relative to controls (C12-OH: P < .01, C14-OH: P < .05), reflective of intermediates of fatty acid oxidations. This decrease was prevented by insulin sensitizer (T-Rosi group) but not androgen antagonist (T-Flutamide group) cotreatment. Gestational T treatment did not alter the maternal circulating FFAs levels. α-Linolenic acid was elevated in the T-Flutamide compared with the C group (P < .05).
Table 1.
Circulating Maternal Metabolites (Amino Acids, Acylcarnitines, and FFAs) (Nanomoles per Milliliter) With the Highest Concentrations in Plasma and Those That Differed Significantly Among Treatment Groups
| Metabolites | C | T | T-Flutamide | T-Rosi | CV, % | P Adj |
|---|---|---|---|---|---|---|
| Amino acids | ||||||
| Glycine | 461.3 ± 28.6 | 498.2 ± 78.6 | 415.9 ± 47.6 | 453.7 ± 45.2 | 8.0 | NS |
| Valine | 175.3 ± 14.2 | 166.9 ± 8.2 | 187.8 ± 16.4 | 189.4 ± 11.9 | 4.5 | NS |
| Leucine | 152.3 ± 9.7 | 125.5 ± 7.9 | 151.3 ± 14.4 | 150.5 ± 12.74 | 5.7 | NS |
| Glutamic acid | 151.1 ± 10.5 | 124.9 ± 5.7 | 166.0 ± 8.9 | 169.1 ± 18.4 | 9.1 | NS |
| Alanine | 145.8 ± 21.3 | 144.3 ± 9.7 | 137.5 ± 17.4 | 141.5 ± 19.6 | 5.4 | NS |
| Acylcarnitines | ||||||
| L-carnitine | 11.559 ± 2.066 | 14.067 ± 1.655 | 16.844 ± 4.243 | 10.763 ± 1.305 | 8.6 | NS |
| Decenoyl (C10:1) | 1.756 ± 0.311 | 3.602 ± 0.815 | 2.267 ± 0.668 | 2.844 ± 0.630 | 1.8 | NS |
| Propionyl-(C3) | 0.592 ± 0.025 | 0.741 ± 0.060 | 0.595 ± 0.081 | 0.861 ± 0.104 | 1.7 | NS |
| 3-Hydroxydodecanoyl- (C12-OH) | 0.009 ± 0.001a | 0.004 ± 0.001b | 0.004 ± 0.001b | 0.007 ± 0.001a | 9.3 | <.01 |
| 3-Hydroxytetradecanoyl- (C14-OH) | 0.025 ± 0.005a | 0.010 ± 0.001b | 0.008 ± 0.001b | 0.018 ± 0.002a | 5.3 | <.05 |
| FFAs | ||||||
| Stearic acid (18:0) | 41.64 ± 4.11 | 40.99 ± 5.92 | 37.46 ± 3.52 | 35.51 ± 2.03 | 0.11 | NS |
| Oleic acid (18:1 [n-9]) | 31.16 ± 6.28 | 40.94 ± 4.39 | 41.70 ± 7.32 | 32.47 ± 2.36 | 0.37 | NS |
| Palmitic acid (16:0) | 15.75 ± 1.74 | 17.88 ± 2.19 | 19.43 ± 2.55 | 15.74 ± 1.09 | 1.09 | NS |
| Linoleic acid (18:2) | 2.69 ± 0.59 | 4.69 ± 1.09 | 5.67 ± 0.78 | 6.07 ± 1.83 | 0.57 | NS |
| α-Linolenic acid (18:3 [n-3]) | 0.10 ± 0.030a | 0.55 ± 0.17a | 0.92 ± 0.19b | 0.43 ± 0.12a | 0.43 | <.05 |
Abbreviations: Adj, adjusted; C, controls; CV, coefficient of variation; NS, not significant; T, treated with T; T-Flutamide, treated with T plus flutamide; T-Rosi, treated with T plus rosiglitazone. Values with different superscript letters (a, b) differ significantly (P < .05). NS values do not differ significantly (P > .05). All P values are shown as an adjusted P value (P adj). For a more complete list, see supplemental Tables 3–5.
Fetal levels of amino acids (n = 23), acylcarnitines (n = 28), and FFAs (n = 8) are shown in Supplemental Tables 5–7. Those metabolites that had the highest circulating levels plus those showing significant differences among treatment groups (five metabolites per category) are included in Table 2. Similar to observations in maternal circulation, amino acid levels did not differ among the four treatment groups. Fetal acylcarnitines did not differ among groups with one exception; fetal C3 levels were reduced (P < .05) in the T-Rosi relative to the control group. FFAs, myristic acid, α-linolenic acid, palmitoleic acid, and γ-linolenic acid that were detected in maternal circulation (Supplemental Table 4) were not present in the umbilical cord blood, whereas vaccenic acid was detected only in cord but not maternal blood.
Table 2.
Circulating Fetal Metabolites (Amino Acids, Acylcarnitines, and FFAs) (Nanomoles per Milliliter) With the Highest Concentrations in Plasma and Those That Differed Significantly Among Treatment Groups
| Metabolites | C | T | T-Flutamide | T-Rosi | CV, % | P Adj |
|---|---|---|---|---|---|---|
| Amino acids | ||||||
| Serine | 998.1 ± 65.8 | 834.8 ± 32.7 | 939.3 ± 101.9 | 1097.1 ± 80.9 | 9.0 | NS |
| Glycine | 587.4 ± 47.6 | 524.2 ± 25.3 | 523.0 ± 45.5 | 667.5 ± 65.0 | 8.0 | NS |
| Threonine | 389.7 ± 27.9 | 490.2 ± 35.7 | 410.0 ± 60.3 | 356.1 ± 40.1 | 7.0 | NS |
| Glutamine | 333.0 ± 39.7 | 289.7 ± 36.8 | 344.9 ± 37.3 | 273.4 ± 51.1 | 8.6 | NS |
| Valine | 293.0 ± 32.9 | 327.5 ± 25.8 | 316.9 ± 36.1 | 270.4 ± 17.6 | 4.5 | NS |
| Acylcarnitines | ||||||
| L-carnitine | 34.352 ± 6.124 | 34.663 ± 5.738 | 31.626 ± 3.818 | 41.486 ± 9.113 | 8.6 | NS |
| Decenoyl (C10:1) | 1.858 ± 0.505 | 3.397 ± 0.742 | 0.861 ± 0.227 | 2.057 ± 0.469 | 1.8 | NS |
| Propionyl-(C3) | 0.961 ± 0.093a | 0.905 ± 0.103a | 0.881 ± 0.169a | 1.850 ± 0.186b | 1.7 | <.05 |
| Butyryl-(C4) | 0.759 ± 0.038 | 0.835 ± 0424 | 0.822 ± 0.178 | 1.389 ± 0.236 | 1.3 | NS |
| Isovaleryl-(C5) | 0.199 ± 0.021 | 0.177 ± 0.017 | 0.169 ± 0.031 | 0.314 ± 0.055 | 1.2 | NS |
| FFAs | ||||||
| Stearic acid (18:0) | 3.78 ± 0.46 | 3.56 ± 0.27 | 3.42 ± 0.19 | 3.63 ± 0.18 | 0.11 | NS |
| Palmitic acid (16:0) | 2.99 ± 0.29 | 2.95 ± 0.14 | 3.51 ± 0.16 | 4.033 ± 0.38 | 1.09 | NS |
| Oleic acid (18:1 [n-9]) | 1.44 ± 0.34 | 2.37 ± 0.11 | 2.55 ± 0.18 | 2.57 ± 0.36 | 0.37 | NS |
| Linoleic acid (18:2) | 0.39 ± 0.16 | 0.14 ± 0.02 | 0.17 ± 0.01 | 0.15 ± 0.016 | 0.57 | NS |
| Vaccenic acid (18:1 [n-7]) | 0.37 ± 0.02 | 0.39 ± 0.02 | 0.37 ± 0.01 | 0.37 ± 0.05 | 1.24 | NS |
Abbreviations: Adj, adjusted; C, controls; CV, coefficient of variation; NS, not significant; T, treated with T; T-Flutamide, treated with T plus flutamide; T-Rosi, treated with T plus rosiglitazone. Values with different superscript letters (a, b) differ significantly (P < .05. NS values do not differ significantly (P > .05). All P values are shown as an adjusted P value (P adj). For a more complete list, see Supplemental Tables 6–8.
Results of correlation analyses (r > 0.800) among circulating levels of metabolites (amino acids, acylcarnitines, and FFAs) in the maternal circulation are shown in Supplemental Table 8. In the maternal circulation, strong positive correlations were found between glycine, valine, leucine, isoleucine, and proline and other metabolites in C females with most of these associations being lost in gestational T-treated females (see Supplemental Table 8 for more details). Positive associations of threonine with leucine, serine with methionine, asparagine with alanine, glycine, valine, and methionine as well as phenylalanine found in C females were lost in the gestational T treated group. The loss of association of asparagine with phenylalanine and serine with methionine was prevented in both the T-Flutamide and T-Rosi groups, whereas asparagine with methionine was prevented only in the T-Rosi group. In maternal circulation, a positive correlation between acylcarnitine species within a difference of two to four carbons was present in the C group. Many of these associations lost in the gestational T-treated group were mainly in acylcarnitines C2, C4, and C14. Many of the positive associations seen in controls remained in the T-Flutamide or T-Rosi group, with only a few retained in both T-Flutamide and T-Rosi groups.
Results of correlation analyses (r > 0.800) among circulating levels of metabolites (amino acids, acylcarnitines, and FFAs) within the fetus are shown in Supplemental Table 9. In the fetal circulation, alanine, leucine, isoleucine, and proline were positively correlated among themselves and/or glutamine and tyrosine in C fetuses. Three of these associations, namely leucine-tyrosine, isoleucine-glutamine, and proline-alanine, were lost in gestational T-treated and T-Flutamide fetuses but not in the T-Rosi (see Supplemental Table 9 for details). Positive correlation of C8, C8:1, C14, 14:1, C16, or C18 with other acylcarnitines (C14, C14:1, C14:2, C14-OH, C16, C18, C18:1, C18:2-OH) were found in fetal circulation of the C group with all being lost in the gestational T-treated group. Many of these changes paralleled what was seen in the maternal circulation. Insulin sensitizer intervention (T-Rosi group) prevented the loss of associations among fetal acylcarnitines except between C14:1 and C14:2. Androgen antagonist intervention (T-Flutamide group) prevented the loss of the following three associations C8 with C18:2-OH, C14 with C10, and C14:1 with C14:2.
Significant associations between maternal and fetal metabolites from the correlation analyses are shown in Table 3. For instance maternal sarcosine and tryptophan, negatively correlated with fetal acylcarnitines C10:1 and C18:2-OH, respectively. Maternal acylcarnitines C14 and C16 correlated positively with fetal valine, leucine, and α-aminoisobutyric acid. Most of the associations found between maternal and fetal metabolites were lost in the gestational T-treated group barring association between tryptophan with acylcarnitine C8:1 and acylcarnitine C14 with C14:2. The loss of maternal-fetal associations of glycine with palmitic acid, tryptophan with C18:2-OH, acylcarnitine C14 with leucine, acylcarnitine C16 with α-aminoisobutyric acid, acylcarnitine C5 with methionine was prevented in the T-Rosi group. The association between maternal C16-OH with fetal isoleucine and C14:2 with α-aminoisobutyric acid lost in the gestational T-treated group was retained the T-Flutamide group.
Table 3.
Correlation of Maternal and Fetal Metabolites
| Maternal | Fetal | C | T | T-Flutamide | T-Rosi | |
|---|---|---|---|---|---|---|
| FFA (maternal) | Arachidic acid (20:0) | Arachidonic acid (20:4) | 0.928a | 0.314 | 0.030 | 0.030 |
| Amino acids (maternal) | Glycine | Palmitic acid (16:0) | 0.829a | 0.086 | 0.086 | −0.880a |
| Sarcosine | Decenoyl-(C10:1) | −0.890a | −0.543 | −0.77b | −0.200 | |
| Ornithine | Oleic acid (18:1 [n-9]) | −0.600 | −0.890a | −0.200 | −0.770b | |
| Tryptophan | Octenoyl-(C8:1) | −0.928a | 0.971a | 0.152 | −0.920a | |
| Tryptophan | 3-Hydroxyoctadecadienyl- (C18:2-OH) | −0.820a | 0.029 | −0.370 | −0.840a | |
| Acylcarnitines (maternal) | Tetradecanoyl-(C14) | Valine | 0.943a | 0.486 | −0.480 | 0.771b |
| Tetradecanoyl-(C14) | Leucine | 0.943a | 0.600 | −0.200 | 0.886a | |
| Hexadecanoyl-(C16) | α-Aminoisobutyric acid | 0.829a | 0.086 | −0.480 | 1.000a | |
| 3-Hydroxyhexadecanoyl- (C16-OH) | Isoleucine | 0.986a | 0.500 | 0.812a | 0.638 | |
| Isovaleryl-(C5) | Methionine | −0.943a | −0.147 | 0.086 | −0.830a | |
| Tetradecadienoyl-(C14:2) | α-Aminoisobutyric acid | −0.829a | 0.029 | −0.810a | −0.230 | |
| Isovaleryl-(C5) | Arachidoyl-(C20:2) | −0.899a | 0.179 | −0.690b | 0.154 | |
| Tetradecanoyl-(C14) | Tetradecadienyl-(C14:2) | −0.870a | −0.841a | 0.200 | 0.829a | |
| Octadecadienyl-(C18:2) | Glutaryl-(C5-DC) | −0.971a | −0.338 | 0.171 | −0.760b | |
| Arachidoyl-(C20) | Butyryl-(C4) | −0.899a | −0.116 | −0.090 | 0.029 |
Abbreviations: C, controls; T, treated with T; T-Flutamide, treated with T plus flutamide; T-Rosi, treated with T plus rosiglitazone.
A significant correlation between both metabolites within treatment group.
A tendency (from P > .05 to P < .08) in the correlation between both metabolites within treatment group.
Results of correlation analysis relating circulating levels of maternal steroids with maternal metabolites (amino acids, acylcarnitines, and FFAs) within the mother and within the fetus are summarized in Supplemental Tables 10 and 11, respectively, and between the mother and the fetus in Table 4. In the maternal circulation, there was a strong negative correlation between progesterone and acylcarnitines (C2, C4, C6, C8:1, C10, and C20:2) in the C group, an effect that was lost in the gestational T-treated group and not prevented by either interventions (Supplemental Table 10). A similar direction of changes was observed in the fetal circulation, with a negative correlation existing between progesterone and long chained acylcarnitines (C18:1, C18:2, and C20:4) in the control group that was lost again in all three treatment groups (Supplemental Table 11). Fetal estradiol also negatively correlated with most acylcarnitines (C3, C18:1, C18:2, C20:1, and C20:2). The association between estradiol and C20:1 and C20:2 was lost in the gestational T-treated group and this loss was prevented in the T-Flutamide group. The significant correlations between maternal steroids and fetal metabolites are shown in Table 4. A predominant negative correlation was found between estradiol and medium to long-chained acylcarnitines in the gestational T-treated group.
Table 4.
Correlation of Maternal Steroids With Fetal Metabolites
| Maternal | Fetal | C | T | T-Flutamide | T-Rosi | |
|---|---|---|---|---|---|---|
| FFAs (fetal) | P4 | Stearic acid (18:0) | −0.300 | 0.420a | −0.140 | −0.140 |
| Amino acids (fetal) | Estradiol | Phenylalanine | 0.350 | 0.940a | −0.030 | −0.290 |
| Estradiol | Tryptophan | 0.350 | 0.94a | 0.88a | −0.290 | |
| P4 | Aspargine | 1.000a | −0.370 | −0.030 | 0.420 | |
| Acylcarnitines (fetal) | Estradiol | Glutaryl-(C5-DC) | −0.181 | 0.559a | 0.539 | 0.304 |
| Estradiol | Octenoyl-(C8:1) | −0.726 | 0.250a | −0.226 | −0.761 | |
| Estradiol | Decenoyl-(C10:1) | −0.363 | −0.791a | −0.759b | 0.577 | |
| Estradiol | Tetradecenoyl-(C14:1) | −0.354 | −0.632a | −0.638 | −0.577 | |
| Estradiol | 3-Hydroxytetradecanoyl-(C14-OH) | −0.707 | −0.791a | −0.678 | −0.577 | |
| Estradiol | Hexadecanoyl-(C16) | −0.354 | −0.949a | −0.334 | −0.577 | |
| Estradiol | Octadecanoyl-(C18) | −0.354 | −0.949a | −0.516 | −0.577 | |
| Estradiol | Octadecenoyl-(C18:1) | 0.354 | −0.949a | −0.395 | −0.577 | |
| Estradiol | 3-Hydroxyoctadecadienoyl-(C18:2-OH) | −0.726 | −0.730a | −0.820 | −0.577 | |
| Estradiol | Arachidoyl-(C20) | −0.181 | −0.892a | −0.678 | −0.592 | |
| P4 | Propionyl-(C3) | 0.900a | −0.080 | −0.650 | −0.080 | |
| Testosterone | Dodecanoyl-(C12) | 0.700 | 0.540a | −0.820a | −0.080 |
Abbreviations: C, controls; P4, progesterone; T, treated with T; T-Flutamide, treated with T plus flutamide; T-Rosi, treated with T plus rosiglitazone.
A significant correlation between both metabolites within a treatment group.
A tendency (from P > .05 to P < .08) in the correlation between both metabolites within a treatment group.
Discussion
The findings from this study provide evidence of the following: 1) gestational T treatment decreases circulating levels of progesterone and two of the minor species of medium chain acylcarnitines generated by the first step in trifunctional protein metabolizing the desaturated fatty acids and increases insulin in the maternal milieu with the progestogenic compromise prevented by cotreatment with the androgen antagonist, and acylcarnitine and insulin compromise prevented by cotreatment with the insulin sensitizer; 2) gestational T treatment perturbs the positive relationship between maternal progesterone and maternal acylcarnitines, with androgen antagonist cotreatment preventing this effect; 3) neither cotreatment prevents gestational T treatment-induced increase in fetal estradiol; and 4) gestational T treatment perturbs the association between maternal and fetal metabolites, predominantly that of acylcarnitines with insulin sensitizer cotreatment preventing 50% of this loss of association. Taken together, these findings indicate that gestational T treatment has a differential impact on both the maternal steroidal and metabolic milieu with an impact on progesterone likely mediated through androgenic action, whereas the increase in insulin and decrease in acylcarnitines affected by metabolic programming. In contrast, increased fetal estrogen levels appear not to be mediated via androgenic or metabolic programming but more likely via estrogenic action.
The perturbation of maternal acylcarnitines and insulin homeostasis by gestational T treatment is similar to metabolic changes reported for pregnant women with gestational diabetes (45). Although IUGR pregnancies are characterized by a reduction in acylcarnitines, the lack of effect of gestational T treatment on amino acids, FFAs, and majority of acylcarnitines (except two) in day 90 fetuses does not necessarily reflect the array of metabolic alterations that may take place later on, leading up to the IUGR phenotype evident at day 140 of gestation in this animal model.
Impact of prenatal T and interventions on maternal and fetal sex steroid and maternal gonadotropin milieu
Gestational progesterone, which has ovarian and placental origins, is essential for pregnancy maintenance and specifically for placental growth and function as well as development of the fetus (46). Progesterone also plays a role in regulating maternal secretion of nutrients, growth factors as well as immunosuppressive agents for successful embryo development (47). Reduction in maternal progesterone results in IUGR offspring as documented in many species (48–51). The finding that gestational T reduces progesterone (this study) and induces IUGR (22, 52) and LBW offspring (28) is consistent with this premise. Currently the mechanism by which reduced progesterone culminates in IUGR is unclear. Whether the impact of gestational T treatment in reducing progesterone is at the corpus luteum or placental level is unclear. Because gestational T treatment advances placental differentiation, whereas reducing maternal progesterone levels and cotreatment with androgen antagonist prevents both these compromises (reference 33 and this study), it is tempting to speculate that the reduction in progesterone may originate at the placental level. In pregnant PCOS women, who have elevated maternal androgen levels, a compromise in placental steroidogenesis is evident, although there was no statistically significant decrease in progesterone (10). Considering that sheep corpus luteum remains functional throughout the gestation (53) and luteectomy as late as day 70 of gestation reduces maternal progesterone levels (54), the possibility of progestogenic compromise originating at the level of the corpus luteum cannot be ruled out. Because placental progesterone is sufficient to maintain gestation from day 50 of gestation (55) and reduction in progesterone after luteectomy at day 70 of gestation did not result in IUGR, to what extent the reduction in progesterone observed in this study contributes to the IUGR evidenced at day 140 of gestation (22, 52) is uncertain. In the absence of interventions to restore progesterone, if the lower progesterone and IUGR (22, 52) evidenced in prenatal T-treated sheep is cause and effect or an association cannot be ascertained.
The finding that gestational T treatment did not increase estradiol concentrations in the maternal milieu is consistent with previous findings (22). Implication of paradoxical increase in estradiol levels in the maternal circulation of animals cotreated with T and androgen antagonist is unclear. The increase in fetal estradiol in gestational T-treated animals is also consistent with previous findings in female offspring of gestational T-treated sheep (22). The finding that cotreatment with flutamide or rosiglitazone did not reverse the increase in fetal estradiol indicates that any reversal in reprogrammed events in postnatal life in these intervention groups is not due to increased estrogen. Absence of increase in estrone in the maternal circulation of gestational T-treated sheep in the present study is discordant with our previous findings (22) and may relate to the high variability in responsiveness evidenced in the gestational T-treated group. The findings of elevated fetal estrogens in all these groups are indicative of increased aromatase activity. Earlier studies have found that substrate availability is the main regulator of aromatase activity in the ovine fetal compartment (56). Increased Cyp19, the encoding gene for aromatase, in fetal ovaries exposed to gestational T treatment supports this premise (57). The finding that gestational interventions (androgen antagonist or insulin sensitizer) did have an impact on postnatal reproductive function (V.P., A.V.-L., C. Herkimer, B.A.S., J. Moeller, E. Beckett, R. Sreedharan, unpublished data) without altering fetal increase in estradiol levels, supports modulation via androgenic or metabolic pathways or placental nutrient transfer. Indeed, gestational T-treatment induces maternal hyperinsulinemia (this study) and placental compromise (33).
The impact of T excess was limited to T and estrogen in the fetal circulation and did not include the examination of androgen and estrogen metabolites or conjugates reflecting inactive forms of these steroids destined for excretion. Considering that these measures were undertaken at a single time point, it is unclear whether other changes in fetal steroids will become evident later in gestation, when ovarian follicular differentiation is more complete. Some of the subtle changes described in the current study at the maternal level may be the culprit in the development of the PCOS-like phenotype in the prenatally T-treated females.
Maternal LH and FSH levels are generally low during pregnancy. Gestational T treatment had no impact on maternal LH and FSH levels. The observed LH and FSH values are similar to that reported previously (58). The low level of gonadotropins seen in control animals is likely the result of elevated progesterone that exerts negative feedback on the gonadotropins (58, 59). Lack of increase in the gestational T-treated group from gestational day 65, when a reduction in progesterone levels is evident indicates that the negative feedback threshold is not passed.
Impact of prenatal T and interventions on maternal metabolic measures and fetal growth
Insulin is vital for fetal growth. Gestational exposure to excess T appears to be accompanied by increased maternal insulin IUGR (23, 26) and can co-occur with small-for-gestational-age babies in humans (12, 60). Our studies with sheep also found that gestational exposure to excess T increases insulin levels (this study) and leads to IUGR (22, 52) and LBW offspring (28), risk factors for adult-onset of disease. In mouse models, increases in maternal insulin results in higher fetal weight (61). In humans, the impact of maternal increases in insulin during gestational diabetes is evident as a U-shaped birth weight outcome (20). Recent clinical research found no association between high maternal insulin levels and birth size (62). Findings from our study are in concordance with studies in women with PCOS, in which euglycemia is maintained in maternal milieu conceivably by increased insulin production to overcome insulin resistance in the face of high plasma T during gestation (26). PCOS women, however, usually have an increased risk of gestational diabetes with its attendant hyperglycemia and hyperinsulinemia (63–65). Gestational T-treated monkey dams also exhibit transient gestational hyperglycemia (23). The fact that cotreatment with androgen antagonist or insulin sensitizer prevented the increase in maternal insulin secretion in our sheep model is suggestive of involvement of both androgenic and insulin signaling pathways and a common mediary in modulating maternal insulin secretion.
The finding that fetal weight at day 90 was not different between the four groups is consistent with our earlier reports (33). In contrast, IUGR is evident on gestational day 140 (22, 52). Previous studies have documented that growth exacerbation occurs during the last trimester of gestation in sheep when growth rate increases exponentially (46, 66).
Impact of prenatal T and interventions on maternal and fetal amino acids, acylcarnitines, and FFAs
Nutrient transport across the placental barrier plays a major role in fetal development and growth. Deficient nutrient availability to the fetus can be the origin of IUGR pregnancies (67), such as seen after prenatal exposure to excess T (22, 52). The deficient nutrient supply to the fetus may be reflective of defect(s) in placental transport or lack of maternal nutrients available. Gestational T treatment was found to reduce maternal medium-/long-chain acylcarnitines (C12 and C14), transition substrates of mitochondrial fatty acid oxidation (68). Most disease states associated with defects in β-oxidation are accompanied by increased acylcarnitine species (69); specifically, plasma long-chain acylcarnitine levels were found to be higher in obese and insulin-resistant humans relative to lean controls (70). Our findings of reduction in medium-chain acylcarnitines and accompanying hyperinsulinemia parallel what is reported in gestational diabetic pregnancies; lower total circulating acylcarnitines are found in the third trimester of gestational diabetic pregnancies (45). The significance of these findings is unclear. Acylcarnitine accumulation likely reflects an imbalance in delivery of fatty acids into the mitochondria and the efficiency in oxidation. It has been suggested that acylcarnitine species accumulation could be mediating increases in insulin resistance (71), although this remains controversial. Gestational T treatment of female monkeys has been found to diminish fetal but not maternal FFA levels in late gestation (23).
The reversal in acylcarnitines by cotreatment with the insulin sensitizer may be a function of reversal in hyperinsulinemia evident in this group. These findings are consistent with the concept that the insulin resistant-state during gestation plays a role in maternal metabolic adaptations (72). Alternatively, this reversal could be a direct effect of the insulin sensitizer on lipid metabolism via the peroxisome proliferator-activated receptor-γ (73, 74). The fact that reduction in maternal medium chain acylcarnitines did not influence the fetal environment suggests that there are redundancies in the maternal/placental transport system that protects the fetal compromise or, alternatively, that the placental exchange has the plasticity to be modified to compensate for the maternal deficit (67). Interestingly, low-birth-weight pregnancies are associated with reduced circulating acylcarnitines in cord blood at the time of delivery (75–77). Therefore, it is possible that the maternal changes we observed in acylcarnitines during midgestation could lead to alterations in the fetal environment closer to term. Similarly, the lack of changes in amino acids or FFAs in the fetal environment at day 90 of gestation (end of second third of gestation), although reflective of lack of changes in maternal environment at this time point, does not necessarily predict the array of metabolic alterations that may take place later during gestation leading up to the IUGR phenotype evident at day 140 of gestation (term ∼147 days). The fact that placental differentiation is already impaired at day 90 of gestation in T pregnancies, with IUGR not being evident until very close to term (33), indicates the remarkable capacity that the placenta has to buffer external insults.
Relating the steroid and metabolic milieus, the main association of steroids with metabolites was found between maternal progesterone and short-chain acylcarnitines. Very little is known about the steroid regulation of fatty acid β-oxidation; thus, the physiological explanation for this particular finding is unclear, but the loss of this correlation in the gestational T-treated group could be driven by the reduction in maternal circulating progesterone. This is supported by the fact that this correlation was reversed in the T-Flut (progesterone levels similar to the C group) but not the T-Rosi group (progesterone levels similar to the T group). Alternatively, the loss of correlation between maternal progesterone and short-chain acylcarnitines could be a direct effect of the gestational T treatment on short-chain acylcarnitines via insulin. Pregnancy-driven changes in the acylcarnitines profile have been described in women (78) and are related to changes in maintenance of insulin and glucose homeostasis and fatty acid β-oxidation requirements throughout pregnancy in humans (79), although the specific mechanism remains to be elucidated. Similarly, the negative correlation found between fetal estradiol and acylcarnitines in the control group and the loss in the gestational T-treated group could be due to either the increased estradiol in female fetuses or a direct effect of gestational T treatment on acylcarnitines. Further studies are required to elucidate the potential role of steroids on metabolic pathways controlling mitochondrial energy pathways during pregnancy.
As evident from the above discussion, potential mechanisms by which T influences metabolites and subsequent fetal growth restriction are complex. Because androgen receptors are expressed in several metabolic tissues, T has the potential to act at any of these sites to alter biosynthesis, uptake, transport or degradation of metabolites, and influence the maternal metabolic homeostasis. Evidence supports T administration diminishes lipolysis (80), stimulates protein synthesis while decreasing protein degradation, and improves the reutilization of amino acids in the muscle, promotes the commitment of pluripotent stem cells into the myogenic lineage, and inhibits their differentiation into the adipogenic lineage (81) and inhibits adipogenesis (82). Effects can be mediated at the level of placental nutrient supply, which in turn is affected by state of placental differentiation, blood supply, and transporter abundance as well as the synthesis and metabolism of nutrients/hormones by the uteroplacental tissues (83). Our studies show placental development is compromised (33) and a previous study in rodents found prenatal T to affect placental transport (84). The hormonal and metabolic factors (amino acids, FFAs, and acylcarnitines) considered in this study are all implicated in the control of fetal growth. For instance, progesterone regulates the expression of several growth factors that are involved in fetal development at the level of the uterus and placenta (47). Fatty acid metabolism is also a critical player relative to placental function and fetal development. Because compromise in enzymes involved in fatty acid oxidation leads to fetal growth restriction (85), observed changes in maternal acylcarnitines may be indicative of an imbalance in fatty acid oxidation, thus contributing to the fetal growth restriction.
The correlation between the maternal and fetal metabolites carried out in this study was performed as a potential way to identify a maternal biomarker that could serve as a surrogate of fetal compromise. However, gestational T treatment did not lead to corresponding alterations in fetal metabolites as was seen in the maternal milieu. These may arise later in gestation because IUGR is not evident in this animal model until day 140 of gestation (52). Future studies are warranted to address the metabolic developmental changes that occur throughout gestation and at which time points the fetal milieu may be reflective of the maternal milieu.
Acknowledgments
We thank Douglas Doop for his expert animal care, facility management, and help with the generation of the experimental animals and Carol Herkimer, Evan M. Beckett, Jacob Moeller, and Stephanie Stout for assistance with the prenatal treatments. We are grateful to Chunhai Ruan for help with the amino acids and acylcarnitines analysis and Arun Das for help with the free fatty acid measures. We are thankful to Tanu Soni for her help in the statistical analysis of the metabolomics.
This work was supported by National Institutes of Health Grants P01 HD44232, DK089503, and U24 DK097153. Trainee support for B.A.S. was provided through National Institutes of Health Grant T32 DK071212-08.
Current Address: for A.V.-L.: Department of Animal Sciences, Michigan State University, East Lansing, MI 48824.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CV
- coefficient of variation
- DHEA
- dehydroepiandrosterone
- DOC
- deoxycorticosterone
- FFA
- free fatty acid
- GC
- gas chromatography
- IUGR
- intrauterine growth restriction
- LBW
- low birth weight
- PCOS
- polycystic ovary syndrome
- QC
- quality control.
References
- 1. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;(suppl 6):588S–595S. [DOI] [PubMed] [Google Scholar]
- 2. Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda). 2006;21:29–37. [DOI] [PubMed] [Google Scholar]
- 3. Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;11:3114–3127. [DOI] [PubMed] [Google Scholar]
- 4. Poston L. Developmental programming and diabetes—the human experience and insight from animal models. Best Pract Res Clin Endocrinol Metab. 2010;4:541–552. [DOI] [PubMed] [Google Scholar]
- 5. Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72:257–265. [DOI] [PubMed] [Google Scholar]
- 6. Bourguignon JP, Parent AS. Early homeostatic disturbances of human growth and maturation by endocrine disrupters. Curr Opin Pediatr. 2010;4:470–477. [DOI] [PubMed] [Google Scholar]
- 7. Witchel SF, Recabarren SE, González F, et al. Emerging concepts about prenatal genesis, aberrant metabolism and treatment paradigms in polycystic ovary syndrome. Endocrine. 2012;3:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vejrazkova D, Vcelak J, Vankova M, et al. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122–129. [DOI] [PubMed] [Google Scholar]
- 9. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;3:245–291. [DOI] [PubMed] [Google Scholar]
- 10. Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166:151–155. [DOI] [PubMed] [Google Scholar]
- 11. Allen DB, Hoffman GL, Fitzpatrick P, Laessig R, Maby S, Slyper A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J Pediatr. 1997;130:128–133. [DOI] [PubMed] [Google Scholar]
- 12. Sir-Petermann T, Hitchsfeld C, Maliqueo M, et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. [DOI] [PubMed] [Google Scholar]
- 13. Laitinen J, Taponen S, Martikainen H, et al. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 2003;27:710–715. [DOI] [PubMed] [Google Scholar]
- 14. Sadrzadeh S, Klip WAJ, Broekmans FJM, et al. Birth weight and age at menarche in patients with polycystic ovary syndrome or diminished ovarian reserve, in a retrospective cohort. Hum Reprod. 2003;18:2225–2230. [DOI] [PubMed] [Google Scholar]
- 15. Legro RS, Roller RL, Dodson WC, Stetter CM, Kunselman AR, Dunaif A. Associations of birthweight and gestational age with reproductive and metabolic phenotypes in women with polycystic ovarian syndrome and their first-degree relatives. J Clin Endocrinol Metab. 2010;95:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cresswell JL, Barker DJ, Osmond C, Egger P, Phillips DI, Fraser RB. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet. 1997;350:1131–1135. [DOI] [PubMed] [Google Scholar]
- 17. Davies MJ, March WA, Willson KJ, Giles LC, Moore VM. Birthweight and thinness at birth independently predict symptoms of polycystic ovary syndrome in adulthood. Hum Reprod. 2012;27:1475–1480. [DOI] [PubMed] [Google Scholar]
- 18. Mumm H, Kamper-Jørgensen M, Nybo Andersen AM, Glintborg D, Andersen M. Birth weight and polycystic ovary syndrome in adult life: a register-based study on 523,757 Danish women born 1973–1991. Fertil Steril. 2013;99:777–782. [DOI] [PubMed] [Google Scholar]
- 19. Uzelac PS, Li X, Lin J, et al. Dysregulation of leptin and testosterone production and their receptor expression in the human placenta with gestational diabetes mellitus. Placenta. 2010;3:581–588. [DOI] [PubMed] [Google Scholar]
- 20. Vambergue A, Fajardy I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J Diabetes. 2011;2:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taricco E, Radaelli T, Nobile de Santis MS, Cetin I. Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta. 2003;4:343–347. [DOI] [PubMed] [Google Scholar]
- 22. Veiga-Lopez A, Steckler TL, Abbott DH, et al. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abbott DH, Bruns CR, Barnett DK, et al. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299:E741–E751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210. [DOI] [PubMed] [Google Scholar]
- 25. Nicol LE, O'Brien TD, Dumesic DA, Grogan T, Tarantal AF, Abbott DH. Abnormal infant islet morphology precedes insulin resistance in PCOS-like monkeys. PLoS One. 2014;9:e106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. [DOI] [PubMed] [Google Scholar]
- 27. Mehrabian F, Kelishadi R. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J Res Med Sci. 2012;17:207–211. [PMC free article] [PubMed] [Google Scholar]
- 28. Manikkam M, Crespi EJ, Doop DD, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. [DOI] [PubMed] [Google Scholar]
- 29. Alexandre-Gouabau MC, Courant F, Moyon T, et al. Maternal and cord blood LC-HRMS metabolomics reveal alterations in energy and polyamine metabolism, and oxidative stress in very-low birth weight infants. J Proteome Res. 2013;12:2764–2778. [DOI] [PubMed] [Google Scholar]
- 30. Alvino G, Cozzi V, Radaelli T, Ortega H, Herrera E, Cetin I. Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatr Res. 2008;64:615–620. [DOI] [PubMed] [Google Scholar]
- 31. Lin G, Liu C, Feng C, et al. Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J Nutr. 2012;142:990–998. [DOI] [PubMed] [Google Scholar]
- 32. Favretto D, Cosmi E, Ragazzi E, et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem. 2012;402:1109–1121. [DOI] [PubMed] [Google Scholar]
- 33. Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A1, Padmanabhan V. Developmental programing: impact of testosterone on placental differentiation. Reproduction. 2014;148:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roberts CT, Kind KL, Earl RA, et al. Circulating insulin-like growth factor (IGF)-I and IGF binding proteins-1 and -3 and placental development in the guinea-pig. Placenta. 2002;23:763–770. [DOI] [PubMed] [Google Scholar]
- 35. Vonnahme KA, Hess BW, Hansen TR, et al. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod. 2003;69:133–140. [DOI] [PubMed] [Google Scholar]
- 36. Committee on the Nutrient Requirements of Small Ruminants NRC. Nutrient Requirements of Small Ruminants. Sheep, Goats, Cervids, and New World Camelids. Washington, DC: The National Academy Press; 2007. [Google Scholar]
- 37. Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ. Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology. 1995;62:248–258. [DOI] [PubMed] [Google Scholar]
- 38. Niswender GD, Reichert LE, Midgley AR, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. [DOI] [PubMed] [Google Scholar]
- 39. Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR., Jr Neuroendocrine control of follicle-stimulating hormone (FSH) secretion: I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology. 1997;138:424–432. [DOI] [PubMed] [Google Scholar]
- 40. Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2012;373:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tserng K-Y, Kliegman RM, Miettinen E-L, Kalhan SC. A rapid, simple, and sensitive procedure for the determination of free fatty acids in plasma using glass capillary column gas-liquid chromatography. J Lipid Res. 1981;22:852–858. [PubMed] [Google Scholar]
- 43. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. [DOI] [PubMed] [Google Scholar]
- 44. Kugler F, Graneis S, Schreiter PP, Stintzing FC, Carle R. Determination of free amino compounds in betalainic fruits and vegetables by gas chromatography with flame ionization and mass spectrometric detection. J Agric Food Chem. 2006;54:4311–4318. [DOI] [PubMed] [Google Scholar]
- 45. Pappa KI, Vlachos G, Theodora M, Roubelaki M, Angelidou K, Antsaklis A. Intermediate metabolism in association with the amino acid profile during the third trimester of normal pregnancy and diet-controlled gestational diabetes. Am J Obstet Gynecol. 2007;196:65.e1–e5. [DOI] [PubMed] [Google Scholar]
- 46. Bazer FW, Spencer TE, Thatcher WW. Growth and development of the ovine conceptus. J Anim Sci. 2012;90:159–170. [DOI] [PubMed] [Google Scholar]
- 47. Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–d1898. [DOI] [PubMed] [Google Scholar]
- 48. Parraguez VH, Urquieta B, De los Reyes M, González-Bulnes A, Astiz S, Muñoz A. Steroidogenesis in sheep pregnancy with intrauterine growth retardation by high-altitude hypoxia: effects of maternal altitudinal status and antioxidant treatment. Reprod Fertil Dev. 2013;25:639–645. [DOI] [PubMed] [Google Scholar]
- 49. Nisbet AD, Horne CH, Jandial V, Bremner RD, Cruickshank N, Sutcliffe RG. Measurement of plasma placental proteins and estriol in the detection of intrauterine growth retardation. Eur J Obstet Gynecol Reprod Biol. 1982;13:333–334. [DOI] [PubMed] [Google Scholar]
- 50. Laurin J, Persson PH, Fernlund P. The efficacy of biochemical assays in assessing an IUGR risk-group, preselected with ultrasound fetometry. Eur J Obstet Gynecol Reprod Biol. 1987;24:177–187. [DOI] [PubMed] [Google Scholar]
- 51. Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147:5568–5574. [DOI] [PubMed] [Google Scholar]
- 52. Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. [DOI] [PubMed] [Google Scholar]
- 53. Sawyer HR. Structural and functional properties of the corpus luteum of pregnancy. J Reprod Fertil. 1995;(suppl 49):97–110. [PubMed] [Google Scholar]
- 54. Al-Gubory KH, Solari A, Mirman B. Effects of luteectomy on the maintenance of pregnancy, circulating progesterone concentrations and lambing performance in sheep. Reprod Fertil Dev. 1999;11:317–322. [DOI] [PubMed] [Google Scholar]
- 55. Schombee CJA. Sensitivity of the corpus luteum towards prostaglandin F2α (PGF 2α) during the gestation period. Agricola. 1998–99;95–97. [Google Scholar]
- 56. France JT, Mason JI, Magness RR, Murry BA, Rosenfeld CR. Ovine placental aromatase: studies of activity levels, kinetic characteristics and effects of aromatase inhibitors. J Steroid Biochem. 1987;28:155–160. [DOI] [PubMed] [Google Scholar]
- 57. Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011;152:4974–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Al-Gubory KH, Blanc MR, Poirier JC, Solari A, Martinet J. Evidence that the corpus luteum of pregnancy contributes to the control of tonic secretion of LH in the ewe. J Reprod Fertil. 1989;85:125–131. [DOI] [PubMed] [Google Scholar]
- 59. Al-Gubory KH, Blanc MR, Martinet J. Role of the corpus luteum of pregnancy in controlling pituitary gonadotrophin secretion during the early postpartum period in the ewe. J Reprod Fertil. 1989;86:697–703. [DOI] [PubMed] [Google Scholar]
- 60. Ibáñez L, Potau N, Francois I, de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab. 1998;83:3558–3562. [DOI] [PubMed] [Google Scholar]
- 61. Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013;6:650–659. [PMC free article] [PubMed] [Google Scholar]
- 62. Kayemba-Kays S, Peters C, Geary MP, Hill NR, Mathews DR, Hindmarsh PC. Maternal hyperinsulinism and glycaemic status in the first trimester of pregnancy are associated with the development of pregnancy-induced hypertension and gestational diabetes. Eur J Endocrinol. 2013;168:413–418. [DOI] [PubMed] [Google Scholar]
- 63. de Wilde MA, Veltman-Verhulst SM, Goverde AJ, et al. Preconception predictors of gestational diabetes: a multicentre prospective cohort study on the predominant complication of pregnancy in polycystic ovary syndrome. Hum Reprod. 2014;29:1327–1336. [DOI] [PubMed] [Google Scholar]
- 64. Wang Y, Zhao X, Zhao H, et al. Risks for gestational diabetes mellitus and pregnancy-induced hypertension are increased in polycystic ovary syndrome. Biomed Res Int. 2013;2013:182582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Joham AE, Ranasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:E447–E452. [DOI] [PubMed] [Google Scholar]
- 66. Fowden AL, Moore T. Maternal-fetal resource allocation: co-operation and conflict. Placenta. 2012;33(suppl 2):e11–e15. [DOI] [PubMed] [Google Scholar]
- 67. Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregn. 2012;2012:179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oey NA, Ruiter JP, Attié-Bitach T, Ijlst L, Wanders RJ, Wijburg FA. Fatty acid oxidation in the human fetus: implications for fetal and adult disease. J Inherit Metab Dis. 2006;29:71–75. [DOI] [PubMed] [Google Scholar]
- 69. Rinaldo P, Cowan TM, Matern D. Acylcarnitine profile analysis. Genet Med. 2008;10:151–156. [DOI] [PubMed] [Google Scholar]
- 70. Mihalik SJ, Goodpaster BH, Kelley DE, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring). 2010;18:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Herrera E, Muñoz C, López-Luna P, Ramos P. Carbohydrate-lipid interactions during gestation and their control by insulin. Braz J Med Biol Res. 1994;27:2499–2519. [PubMed] [Google Scholar]
- 73. Xu Y, Wang Q, Cook TJ, Knipp GT. Effect of placental fatty acid metabolism and regulation by peroxisome proliferator activated receptor on pregnancy and fetal outcomes. J Pharm Sci. 2007;96:2582–2606. [DOI] [PubMed] [Google Scholar]
- 74. Smith SA. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem Soc Trans. 2002;30:1086–1090. [DOI] [PubMed] [Google Scholar]
- 75. Meyburg J, Schulze A, Kohlmueller D, Linderkamp O, Mayatepek E. Postnatal changes in neonatal acylcarnitine profile. Pediatr Res. 2001;49:125–129. [DOI] [PubMed] [Google Scholar]
- 76. Akisu M, Bekler C, Yalaz M, Hüseyinov A, Kültürsay N. Free carnitine concentrations in cord blood in preterm and full-term infants with intrauterine growth retardation. Pediatr Int. 2001;43:107–108. [DOI] [PubMed] [Google Scholar]
- 77. Horgan RP, Broadhurst DI, Walsh SK, et al. Metabolic profiling uncovers a phenotypic signature of small for gestational age in early pregnancy. J Proteome Res. 2011;10:3660–3673. [DOI] [PubMed] [Google Scholar]
- 78. Luan H, Meng N, Liu P, et al. Pregnancy-induced metabolic phenotype variations in maternal plasma. J Proteome Res. 2014;13:1527–1536. [DOI] [PubMed] [Google Scholar]
- 79. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(suppl 2):S112–S119. [DOI] [PubMed] [Google Scholar]
- 80. Zang H, Ryden M, Wahlen K, et al. 2007 Effects of testosterone and estrogen treatment on lipolysis signaling pathways in subcutaneous adipose tissue of postmenopausal women. Fertil Steril. 2007;88:100–106. [DOI] [PubMed] [Google Scholar]
- 81. Bhasin S, Taylor WE, Singh R, et al. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci. 2003;58:M1103–M1110. [DOI] [PubMed] [Google Scholar]
- 82. Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fowden AL, Ward JW, Wooding FPB, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572(Pt 1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mark PJ, Smith J, Waddell BJ. Placental and fetal growth retardation following partial progesterone withdrawal in rat pregnancy. Placenta. 2006;27:208–214. [DOI] [PubMed] [Google Scholar]
- 85. Shekhawat PS, Matern D, Strauss AW. Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: impact of expanded newborn screening on their diagnosis and management. Pediatr Res. 2005;57:78R–86R. [DOI] [PMC free article] [PubMed] [Google Scholar]