Abstract
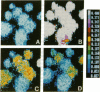
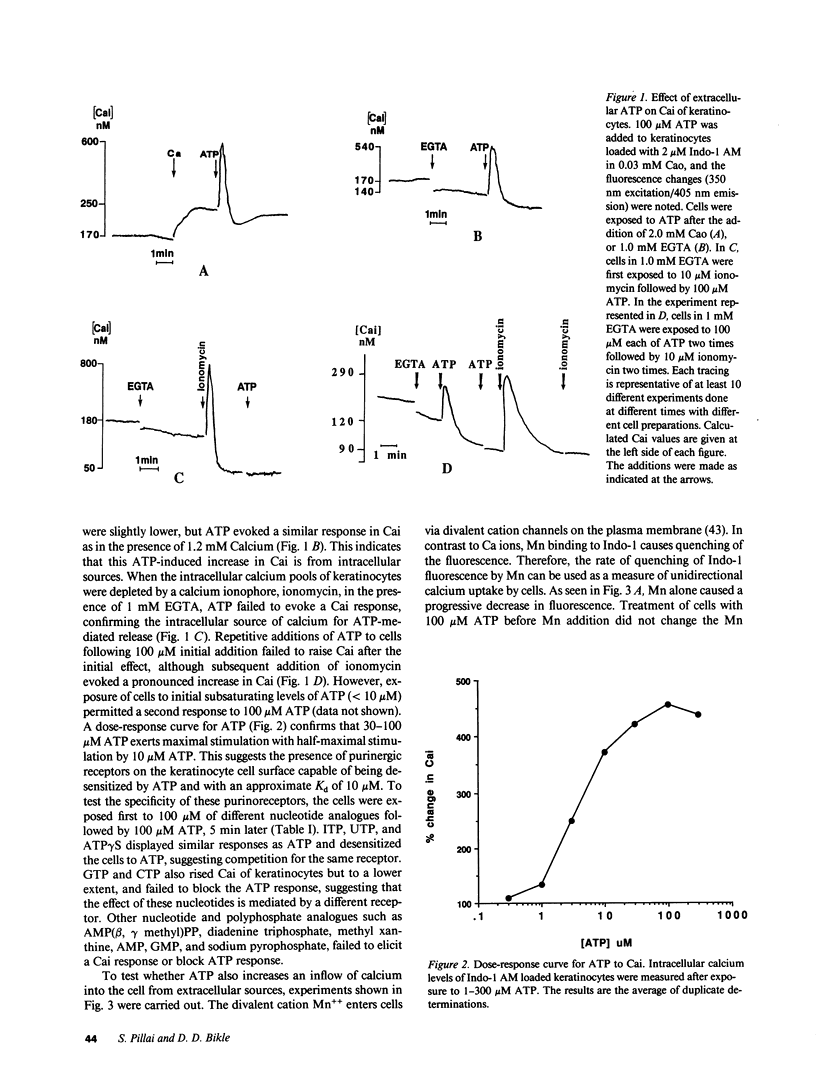
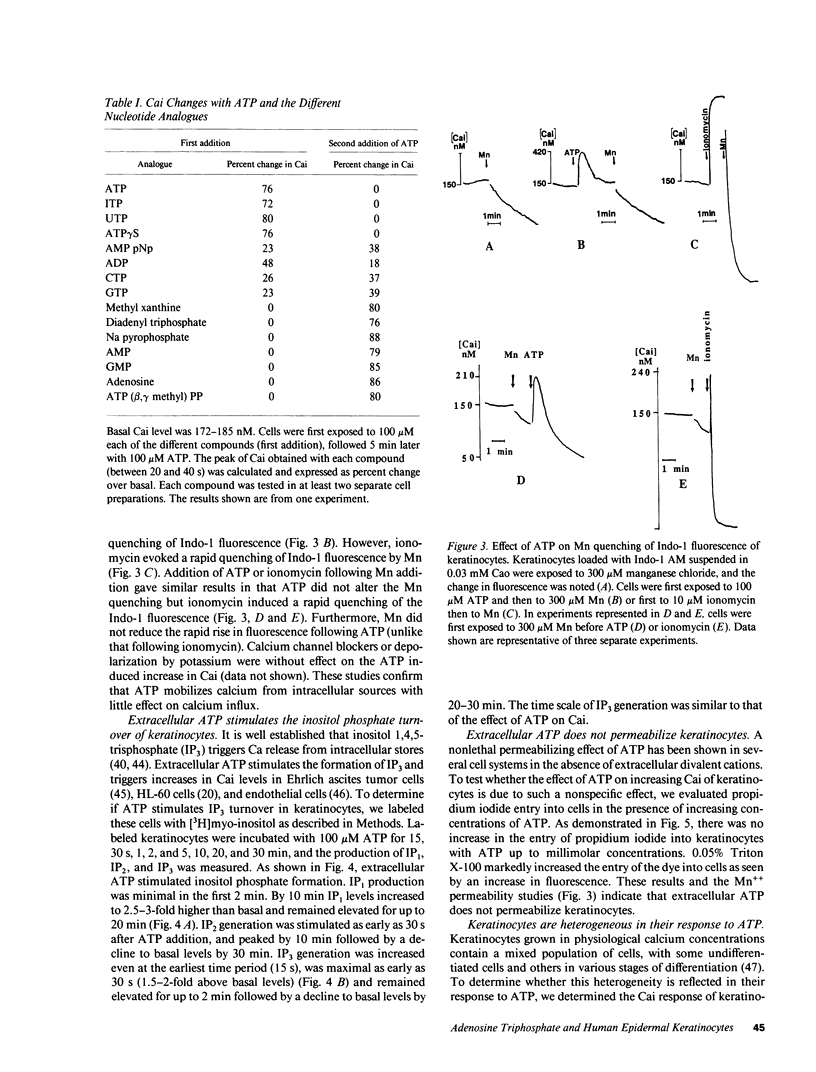
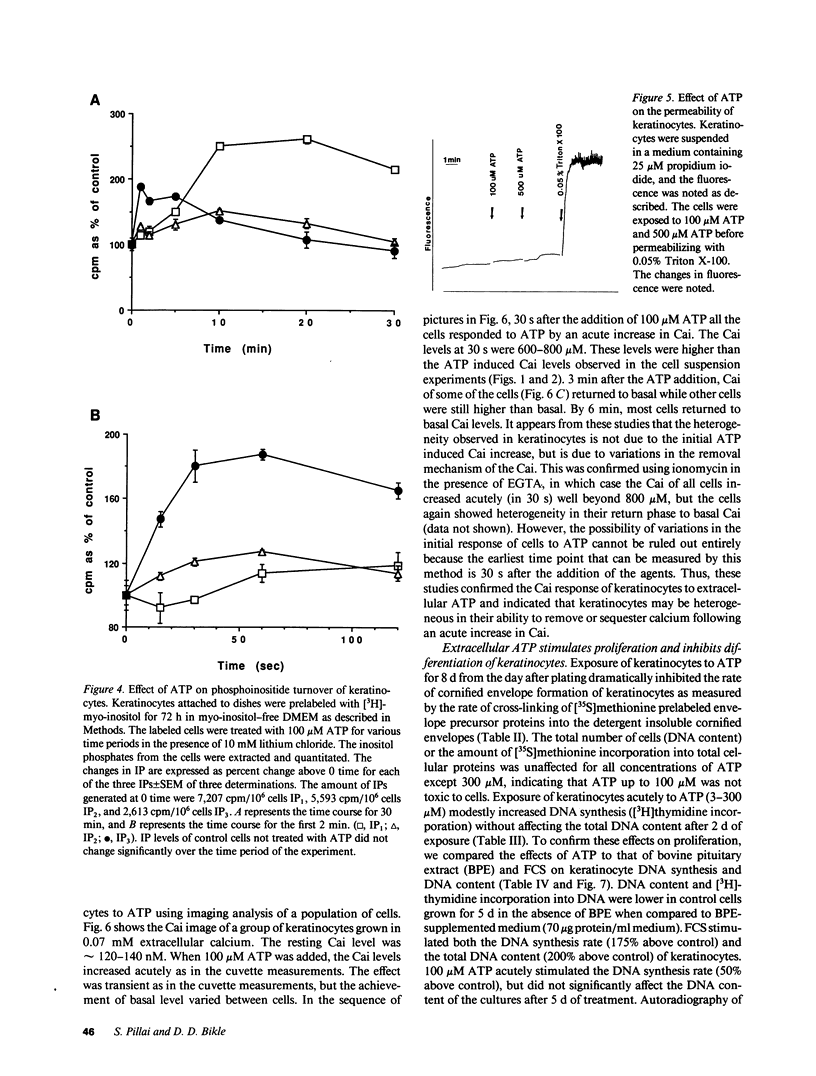
During wound healing, release of ATP from platelets potentially exposes the epidermis to concentrations of ATP known to alter cellular functions mediated via changes in inositol trisphosphate (IP3) and intracellular calcium (Cai) levels. Therefore, we determined whether keratinocytes respond to ATP with a rise in Cai and IP3 and whether such increases are accompanied by a change in their proliferation and differentiation. Changes in Cai were measured in Indo-1-loaded neonatal human foreskin keratinocytes after stimulation with extracellular ATP. Extracellular ATP evoked a transient and acute increase in Cai of keratinocytes both in the presence and in the absence of extracellular calcium. ATP also induced the phosphoinositide turnover of keratinocytes, consistent with its effect in releasing calcium from intracellular sources. ATP did not permeabilize keratinocytes, nor did it promote Ca influx into the cells. The half-maximal effect of ATP was at 10 microM, and saturation was observed at 30-100 microM. UTP, ITP, and ATP gamma S were as effective as ATP in releasing Cai from intracellular stores and competed with ATP for their response, whereas AMP and adenosine were ineffective, suggesting the specificity of P2 purinergic receptors in mediating the ATP response in keratinocytes. Single cell measurements revealed heterogeneity in the calcium response to ATP. This heterogeneity did not appear to be due to differences in the initial Cai response but to subsequent removal of increased Cai by these cells. ATP inhibited terminal differentiation of keratinocytes as measured by [35S]methionine incorporation into cornified envelopes and modestly stimulated incorporation of [3H]thymidine into DNA. Chelation of Cai by bis-(o-aminophenoxy)-N,N,N',N'-tetraacetic acid reduced basal Cai, blocked the Cai response to ATP, inhibited the basal rate of DNA synthesis, and blocked the ATP-induced increase in DNA synthesis. We conclude that extracellular ATP may be an important physiological regulator of epidermal growth and differentiation acting via IP3 and Cai.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Cooney R. V., Hill T. D., Nilsson T., Arkhammar P., Berggren P. O. Extracellular ATP mobilizes intracellular Ca2+ in T51B rat liver epithelial cells: a study involving single cell measurements. Exp Cell Res. 1989 Mar;181(1):245–255. doi: 10.1016/0014-4827(89)90198-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Comparative studies of purinergic nerves. J Exp Zool. 1975 Oct;194(1):103–133. doi: 10.1002/jez.1401940108. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Neural nomenclature. Nature. 1971 Jan 22;229(5282):282–283. doi: 10.1038/229282d0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979 Nov;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Heppel L. A., Rozengurt E. Control of membrane permeability by external and internal ATP in 3T6 cells grown in serum-free medium. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2103–2107. doi: 10.1073/pnas.77.4.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., Cowen D. S., Meuller L. M. Activation of inositol phospholipid breakdown in HL60 cells by P2-purinergic receptors for extracellular ATP. Evidence for mediation by both pertussis toxin-sensitive and pertussis toxin-insensitive mechanisms. J Biol Chem. 1988 Dec 5;263(34):18108–18117. [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in calcium-mobilizing agonist responses. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:211–262. [PubMed] [Google Scholar]
- Gonzalez F. A., Gross D. J., Heppel L. A., Webb W. W. Studies on the increase in cytosolic free calcium induced by epidermal growth factor, serum, and nucleotides in individual A431 cells. J Cell Physiol. 1988 May;135(2):269–276. doi: 10.1002/jcp.1041350214. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Hennings H., Kruszewski F. H., Yuspa S. H., Tucker R. W. Intracellular calcium alterations in response to increased external calcium in normal and neoplastic keratinocytes. Carcinogenesis. 1989 Apr;10(4):777–780. doi: 10.1093/carcin/10.4.777. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hepler P. K. Calcium transients during mitosis: observations in flux. J Cell Biol. 1989 Dec;109(6 Pt 1):2567–2573. doi: 10.1083/jcb.109.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N. N., Wang D. J., Gonzalez F., Heppel L. A. Multiple signal transduction pathways lead to extracellular ATP-stimulated mitogenesis in mammalian cells: II. A pathway involving arachidonic acid release, prostaglandin synthesis, and cyclic AMP accumulation. J Cell Physiol. 1991 Mar;146(3):483–494. doi: 10.1002/jcp.1041460320. [DOI] [PubMed] [Google Scholar]
- Huang N., Wang D. J., Heppel L. A. Extracellular ATP is a mitogen for 3T3, 3T6, and A431 cells and acts synergistically with other growth factors. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7904–7908. doi: 10.1073/pnas.86.20.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman C. M., Smith J. B., Silver M. J. Direct measurement of platelet secretion in whole blood. Thromb Res. 1979;16(3-4):335–344. doi: 10.1016/0049-3848(79)90081-1. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Deeney J. T., Corkey B. E. Cytosolic free calcium in adipocytes. Distinct mechanisms of regulation and effects on insulin action. J Biol Chem. 1989 Aug 5;264(22):12754–12757. [PubMed] [Google Scholar]
- Kennedy C., Burnstock G. ATP produces vasodilation via P1 purinoceptors and vasoconstriction via P2 purinoceptors in the isolated rabbit central ear artery. Blood Vessels. 1985;22(3):145–155. doi: 10.1159/000158592. [DOI] [PubMed] [Google Scholar]
- King I., Mella S. L., Sartorelli A. C. A sensitive method to quantify the terminal differentiation of cultured epidermal cells. Exp Cell Res. 1986 Nov;167(1):252–256. doi: 10.1016/0014-4827(86)90221-1. [DOI] [PubMed] [Google Scholar]
- Koshiyama H., Tashjian A. H., Jr Control of intracellular calcium redistribution by guanine nucleotides and inositol 1,4,5-trisphosphate in permeabilized GH4C1 cells. Endocrinology. 1991 Jun;128(6):2715–2722. doi: 10.1210/endo-128-6-2715. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lew D. P. Receptor signalling and intracellular calcium in neutrophil activation. Eur J Clin Invest. 1989 Aug;19(4):338–346. doi: 10.1111/j.1365-2362.1989.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C. The effects of ATP on platelets: evidence against the central role of released ADP in primary aggregation. Blood. 1975 Sep;46(3):309–320. [PubMed] [Google Scholar]
- Martin W., Cusack N. J., Carleton J. S., Gordon J. L. Specificity of P2-purinoceptor that mediates endothelium-dependent relaxation of the pig aorta. Eur J Pharmacol. 1985 Feb 5;108(3):295–299. doi: 10.1016/0014-2999(85)90452-2. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Defize L. H., de Laat S. W. Calcium in the action of growth factors. Ciba Found Symp. 1986;122:212–231. doi: 10.1002/9780470513347.ch13. [DOI] [PubMed] [Google Scholar]
- Pillai S., Bikle D. D., Hincenbergs M., Elias P. M. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J Cell Physiol. 1988 Feb;134(2):229–237. doi: 10.1002/jcp.1041340208. [DOI] [PubMed] [Google Scholar]
- Pillai S., Bikle D. D., Mancianti M. L., Cline P., Hincenbergs M. Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol. 1990 May;143(2):294–302. doi: 10.1002/jcp.1041430213. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Potter D. D., Furshpan E. J., Landis S. C. Transmitter status in cultured rat sympathetic neurons: plasticity and multiple function. Fed Proc. 1983 Apr;42(6):1626–1632. [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Sharpe G. R., Gillespie J. I., Greenwell J. R. An increase in intracellular free calcium is an early event during differentiation of cultured human keratinocytes. FEBS Lett. 1989 Aug 28;254(1-2):25–28. doi: 10.1016/0014-5793(89)81002-6. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M. M., Crameri F. M., Slattery J. P., Millard P. J., Gonzalez F. A. Extracellular ATP and some of its analogs induce transient rises in cytosolic free calcium in individual canine keratinocytes. J Invest Dermatol. 1991 Aug;97(2):223–229. doi: 10.1111/1523-1747.ep12480162. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Borisy G. G. Intracellular free calcium and mitosis in mammalian cells: anaphase onset is calcium modulated, but is not triggered by a brief transient. J Cell Biol. 1989 Aug;109(2):627–636. doi: 10.1083/jcb.109.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. J., Huang N. N., Gonzalez F. A., Heppel L. A. Multiple signal transduction pathways lead to extracellular ATP-stimulated mitogenesis in mammalian cells: I. Involvement of protein kinase C-dependent and -independent pathways. J Cell Physiol. 1991 Mar;146(3):473–482. doi: 10.1002/jcp.1041460319. [DOI] [PubMed] [Google Scholar]
- Wang D. J., Huang N. N., Heppel L. A. Extracellular ATP shows synergistic enhancement of DNA synthesis when combined with agents that are active in wound healing or as neurotransmitters. Biochem Biophys Res Commun. 1990 Jan 15;166(1):251–258. doi: 10.1016/0006-291x(90)91938-o. [DOI] [PubMed] [Google Scholar]
- Watt F. M. Involucrin and other markers of keratinocyte terminal differentiation. J Invest Dermatol. 1983 Jul;81(1 Suppl):100s–103s. doi: 10.1111/1523-1747.ep12540786. [DOI] [PubMed] [Google Scholar]