Abstract
The appropriate control of synthesis and secretion of the gonadotropin hormones LH and FSH by pituitary gonadotropes is essential for the regulation of reproduction. The hypothalamic neuropeptide GnRH is the central regulator of both processes, coordinating secretion with transcription and translation of the gonadotropin hormone subunit genes. The MAPK family of second messengers is strongly induced in gonadotropes upon GnRH stimulation, and multiple pathways activate these kinases. Intracellular reactive oxygen species participate in signaling cascades that target MAPKs, but also participate in signaling events indicative of cell stress. The NADPH oxidase (NOX)/dual oxidase (DUOX) family is a major enzymatic source of intracellular reactive oxygen, and we show that GnRH stimulation of mouse primary pituitary cells and the LβT2 gonadotrope cell line elevates intracellular reactive oxygen via NOX/DUOX activity. Mouse pituitary and LβT2 cells abundantly express NOX/DUOX and cofactor mRNAs. Pharmacological inhibition of NOX/DUOX activity diminishes GnRH-stimulated activation of MAPKs, immediate-early gene expression, and gonadotropin subunit gene expression. Inhibitor studies implicate the calcium-activated DUOX family as a major, but not exclusive, participant in GnRH signaling. Knockdown of DUOX2 in LβT2 cells reduces GnRH-induced Fshb, but not Lhb mRNA levels, suggesting differential sensitivity to DUOX activity. Finally, GnRH pulse-stimulated FSH and LH secretion are suppressed by inhibition of NOX/DUOX activity. These results indicate that reactive oxygen is a potent signaling intermediate produced in response to GnRH stimulation and further suggest that reactive oxygen derived from other sources may influence the gonadotrope response to GnRH stimulation.
Gonadotropin-releasing hormone I (GnRH) secreted from hormone-producing neurons of the hypothalamus binds to its cognate receptor on the gonadotropes of the anterior pituitary and activates signaling cascades that promote intracellular calcium mobilization and activation of protein kinase C (PKC) isoforms, both of which lead to increased expression and release of the gonadotropin hormones LH and FSH (1–3). These early events activate MAPK1/3 (also designated ERK2 and ERK1, respectively), p38 MAPK, and c-Jun N-terminal kinase (JNK) (4, 5). Activation of MAPK1/3 by GnRH depends on SRC and dynamin-dependent phosphorylation of RAF by PKC, which then signals via MAPK kinase (MEK) family members to robustly phosphorylate the target MAPKs (6). The activation of MAPK1/3 occurs in response to growth factor stimulation, mitogens, and environmental stresses such as osmotic stress, heat shock, or UV light (7, 8). Both JNK and p38 MAPKs are also activated by inflammatory cytokines and heat shock (9, 10). These triggers of MAPKs are associated with reactive oxygen species (ROS) generation (11). Although ROS can activate MAPK pathways in other systems, GnRH-induced MAPK activation via ROS signaling is not documented.
In gonadotropes, GnRH-activated MAPKs translocate to the nucleus to activate factors that increase Lhb and Fshb transcription, such as the immediate-early response genes c-Fos, c-Jun, Egr1, and Atf3 (1, 4, 12). c-FOS and c-JUN proteins heterodimerize to form the activator protein 1 (AP1) transcription factor, which induces the transcription of the Fshb gene (13). AP1 is a redox-sensitive transcription factor that is regulated by ROS-mediated MAPK activation (14). EGR1, which regulates Lhb and its own transcription, is directly induced by ROS via MAPK1/3 and JNK pathways (15, 16). ATF3 contributes to GnRH responsiveness of Cga transcription by forming a heterodimer with JUN (17). The Atf3 gene is induced by TNF-α, phorbol esters, and H2O2 (18). Thus, immediate-early response genes are modulated by ROS signaling either directly or via ROS-mediated MAPK activation (19). However, the signaling pathways of oxidative stress in the pituitary are poorly understood.
The superoxide radical, hydroxyl radical, and hydrogen peroxide, collectively known as ROS, are constantly produced by a number of cellular metabolic and regulatory events and have been identified as important contributors to many signaling pathways (20, 21). A major source of intracellular ROS is the NADPH oxidase and dual oxidase family of enzymes, which catalyze the reduction of O2 using cytosolic NADPH as an electron donor or directly reduce it using Ca2+, generating superoxide or peroxide (22). Seven members of the NADPH oxidase (NOX)/dual oxidase (DUOX) family have been identified: Nox1-5 and Duox1 and Duox2. These are differentially expressed in several tissues and induced by different stimuli (23). The activation of NOX enzymes requires coordinated assembly with the transmembrane protein p22phox and the recruitment of 3 cytosolic proteins, the small G protein RAC, p47phox, and p67phox. DUOX enzymes produce H2O2 in response to elevated intracellular Ca2+ without association with cytosolic factors. The NOX family is activated by phorbol 12-myristate 13-acetate (PMA) in cultured aortic endothelial cells, demonstrating their activation by PKC activity (24). NOX/DUOX-derived ROS also contribute to a wide range of pathological process such as hypertension, ischemia/reperfusion damage, and immunosuppression (22). Furthermore, NOX/DUOX-derived ROS are considered important components of thyroid hormone biosynthesis and reproduction (22, 25–28). The expression of NOX isoforms is reported in testis, ovary, prostate, and uterus (22). NOX-derived ROS may be important for spermatogenesis, fertilization, and regulation of luteal function (29–31), and their action suggests they may play a role in GnRH signaling.
Here we show expression of NOX isoforms and subunits in the pituitary and the LβT2 gonadotrope cell line. We demonstrate that ROS are generated in response to GnRH through NOX/DUOX activity. We also demonstrate that GnRH-induced MAPK1/3 and JNK activation, induction of Atf3, Egr1, c-Fos, and c-Jun mRNAs, and gonadotropin subunit Fshb and Lhb gene expression depends on NOX/DUOX activity. Finally, sustained pulsatile secretion of gonadotropins in response to GnRH stimulation is reduced by blockade of NOX/DUOX activity. We conclude that NOX/DUOX-derived ROS are essential for GnRH signaling in gonadotropes, linking PKC activation to downstream induction of MAPK signaling and gene expression.
Materials and Methods
Cell culture
The male mouse LβT2 gonadotrope cell line was maintained in high-glucose HEPES-buffered DMEM supplemented with penicillin/streptomycin and 10% fetal bovine serum. The cells were maintained at 37°C in a humidified atmosphere of 5% CO2. For PKC down-regulation by PMA (Calbiochem), LβT2 cells were pretreated with serum-free DMEM containing 1 μM PMA for 16 hours.
Primary mouse pituitary cell culture
Whole pituitaries were dissected from wild-type C57BL/6 male or female mice at 8 to 9 weeks of age. Females were cohoused, but estrous stage was not determined due to subsequent culture in the absence of exogenous steroids. Mice were killed using CO2 asphyxiation followed by cervical dislocation, in accordance with University of California, San Diego Institutional Animal Care and Use Committee regulations. Whole pituitaries were isolated into ice-cold PBS and then dispersed as described previously (32). After plating in 48-well Costar tissue culture plates (Corning Life Sciences) precoated with poly-d-lysine (Sigma-Aldrich) to aid adherence, the cells were allowed to recover for at least 48 hours at 37°C and 5% CO2 in culture medium before studies were conducted.
ROS measurement
To detect intracellular ROS accumulation in primary pituitary and LβT2 cells, cells grown on chamber slides coated with poly-d-lysine were treated with 10 nM GnRH for 30 minutes and then with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2-DCFDA) (Life Technologies) at 10 μM for 30 minutes. In NOX/DUOX inhibitor experiments, cells were pretreated with 5 μM diphenylene iodonium (DPI) for 30 minutes before GnRH addition. Wide-field fluorescence images were obtained using a Nikon TE2000-U microscope (Nikon America Inc) equipped with an X-Cite 120PC collimated light source (Lumen Dynamics Group Inc) and a GFP-3035B filter set (Semrock) using a CoolSNAP EZ monochrome camera (Photometrics Inc). Images were captured and analyzed using Nikon NIS-Elements BR software (Nikon America Inc). Fluorescence intensity measurements and object counting were obtained from 5 different fields of view through 3 experimental repeats using Nikon NIS-Elements BR software.
RT-PCR and quantitative real-time PCR
Expression of NOX isoforms and cofactors was detected by RT-PCR and quantitative PCR of total RNA isolated from pituitary tissue of C57BL/6 mice or LβT2 cells using the UltraClean Tissue & Cell RNA Isolation Kit (MoBio Laboratories, Inc), according to the manufacturer's instructions. cDNA was synthesized using qScript cDNA SuperMix per the manufacturer's instructions (Quanta Biosciences). Quantitative real-time PCR was performed with KAPA SYBR Green (KAPA Biosystems) and specific primer sequences (Supplemental Table 1) using the MyiQ Single-Color Real-Time Detection System (Bio-Rad Laboratories Inc). A melt curve was performed after each PCR run to ensure that a single product was amplified, and the sizes of the products were verified using agarose gel electrophoresis. Four independent determinations of mRNA content were made, and the relative transcript levels were determined using the 2−ΔΔCT method with GAPDH as an endogenous control (33).
Immunofluorescence
LβT2 cells were seeded on chamber slides coated with poly-d-lysine to aid adherence and fixation at a density of 50 000 cells/cm2 in culture medium. After serum deprivation for 16 hours, cells were pretreated with 10 μM DPI for 30 minutes and then with added 100 nM GnRH for 5 minutes. Cells were immediately fixed for 15 minutes in 4% paraformaldehyde in PBS, rehydrated with a brief wash of 20 nM Tris (pH 7.4) and 150 mM NaCl (Tris-buffered saline [TBS]), and then permeabilized with TBS supplemented with 0.25% Triton X-100 for 10 minutes. After changing into TBS-0.1% Triton X-100, cells were blocked with 1% BSA for 1 hour at room temperature. Phosphorylated MAPK1/3 was detected using mouse monoclonal antibody SC-7383 (Santa Cruz Biotechnologies). Secondary staining was accomplished using Alexa Fluor 488–conjugated goat anti-mouse IgG (Life Technologies) for 1 hour. Slides were washed 3 times in PBS and mounted with Vectashield (Vector Laboratories) mounting medium with 4′,6-diamidino-2-phenylindole.
Western blotting
Cell lysates or nuclear/cytosolic fractions prepared using the Nuclear/Cytosol Fraction Kit (Biovision) were separated by electrophoresis on 10% (MAPK1/3, stress-activated protein kinase [SAPK]/JNK, and NOX2) or 6% (DUOX1/2) SDS-PAGE gels (Mini-PROTEAN; Bio-Rad Laboratories) and transferred to nitrocellulose. Membranes were blocked with BSA or nonfat skim milk. Antibodies against phosphorylated MAPK1/3 (SC-7388; Santa Cruz Biotechnology, Inc), total MAPK1/3 (SC-94; Santa Cruz Biotechnology, Inc), phosphorylated SAPK/JNK (Thr183/Tyr185, catalog no. 9251; Cell Signaling Technology, Inc), total SAPK/JNK (catalog no. 9252; Cell Signaling Technology, Inc), NOX2 (SC-74514; Santa Cruz Biotechnology, Inc), DUOX1 (PA5-31374; Thermo Fisher Scientific Inc), DUOX2 (PA1-46354; Thermo Fisher Scientific Inc), and β-actin (A5441; Sigma-Aldrich) were incubated for 16 hours. Blots were developed using a 1:2000 dilution of biotinylated secondary antibody (Santa Cruz Biotechnology, Inc) and ChemiGlow chemiluminescence (Alpha Innotech) and visualized using the FluorChem Q Imaging System (Cell Biosciences). Quantitative densitometry was performed by using AlphaView Q software (Cell Biosciences). Data from at least 3 independent experiments were normalized to total MAPK1/3 or total SAPK/JNK and reported as fold change relative to the control.
Transfection and luciferase assay
Serum-starved LβT2 cells were transfected with a pGL3-basic luciferase reporter gene containing either the −398-bp murine Fshb or the −1.8-kbp rat Lhb promoters. All transfections were performed with FuGENE 6 (Promega) according to the manufacturer's instructions. The cells were plated at a density of 2.75 × 105 cells/cm2 and incubated for 24 hours before transfection. Reporter genes were cotransfected with a pGL3-basic reporter plasmid containing the herpesvirus thymidine kinase promoter and a substitution of the luciferase coding sequence with β-galactosidase, pGL3-TK-Gal, to serve as an internal control (34, 35). Transfected cells were preincubated with 5 μM DPI or 10 mM N-acetyl cysteine (NAC) for 30 minutes and then incubated with 10 nM GnRH (Sigma-Aldrich) for 5 hours. The cells were harvested with Reporter Lysis Buffer (Promega), and lysates were assayed using the Luciferase Assay system (Promega) and Galacto-Light Plus system (Applied Biosystems), respectively. Luminescence was measured in a Veritas microplate luminometer (Turner BioSystems).
Suppression by lentiviral short hairpin (sh) RNA)
Knockdowns were performed using MISSION Lentiviral shRNA knockdown (Sigma-Aldrich). The shRNA-encoding plasmids used were TRCN0000252145, TRCN0000252146, and TRCN0000252147 for mouse Duox1 mRNA and TRCN0000076653, TRCN0000076654, and TRCN000007665 for mouse Duox2 mRNA, respectively, as well as control lentiviral particles bearing a nontargeting shRNA (SHC001V). LβT2 cells were cultivated in 24-well plates and transduced with multiplicity of infection of 3 transducing units per cell for 2 days. Afterward, LβT2 cells were serum starved overnight and then incubated with 10 nM GnRH for 5 hours. Fshb and Lhb mRNA were analyzed by quantitative real-time PCR, and the reduction of DUOX1 and DUOX2 proteins was examined by Western blotting.
GnRH pulse stimulation and secretion analysis
Primary mouse pituitary cells isolated from 9-week-old cohoused females not assessed for estrous stage were cultured on Cytodex 3 microcarrier beads (GE Healthcare). After 2 days, cell suspensions were changed to serum-free DMEM with antibiotics for 16 hours. Cells were then loaded into perifusion columns and equilibrated for 40 minutes in serum-free DMEM supplemented alone or with 5 μM DPI at a flow rate of 200 μl/min. Subsequently, cells were pulsed for 2 minutes at a 10 nM GnRH peak pulse concentration with a 58-minute interpulse interval for 4 hours. Perifusion culture and pulse methodology were described previously (36). Five-minute fractions of approximately 1 ml were collected. Both FSH and LH were measured by a MILLIPLEX MAP Kit (Mouse Pituitary Magnetic Bead Panel, catalog no. MPTMAG-49K; EMD Millipore Co) following the manufacturer's instruction.
Statistical analysis
All experiments were repeated at least 3 times independently, and reported values are presented as the means ± SEM. Statistical analysis was conducted using JMP software (SAS Institute) on raw values or values optimally Box-Cox transformed to correct for heteroscedasticity. Data were evaluated by multifactor ANOVA and testing with the indicated post hoc comparison test.
Results
GnRH stimulation elevates intracellular ROS via NOX/DUOX activity
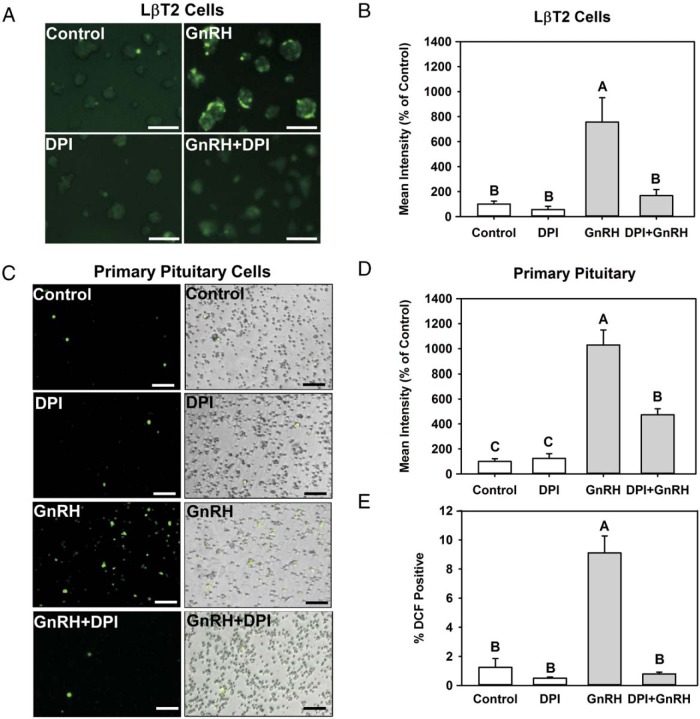
We evaluated the production of GnRH-induced intracellular ROS in both the LβT2 gonadotrope cell line and mouse primary pituitary cells. Intracellular ROS production was measured by the conversion of CM-H2-DCFDA to the fluorescence product dichlorofluorescein. LβT2 cells and female mouse primary pituitary cells were incubated in the presence of vehicle (control) or 10 nM GnRH for 30 minutes and then treated with 10 μM CM-H2-DCFDA for 30 minutes. Cells were analyzed by fluorescent microscopy (Figure 1, A and C), and digital images were quantified for intensity of fluorescence (Figure 1, B and D). GnRH treatment induces dichlorofluorescein fluorescence in LβT2 cells (Figure 1A) and mouse primary pituitary cells (Figure 1C). Approximately 10% of total cells in primary cultures responded to GnRH, corresponding to the approximate population of gonadotropes (Figure 1E). The intensity of fluorescence was significantly increased by exposure to GnRH treatment in LβT2 cells, as well as mouse primary pituitary cells (Figure 1, B and D). Based on these observations, we conclude that GnRH treatment induces intracellular ROS in both LβT2 cells and mouse primary pituitary cells.
Figure 1.
GnRH stimulation induces intracellular ROS production via NOX/DUOX activity. A–D, Serum-starved LβT2 cells (A) and female mouse primary pituitary cells (C) were preincubated with vehicle (dimethylsulfoxide) or 5 μM DPI for 30 minutes. Afterwards, cells were exposed to either vehicle (control) or 10 nM GnRH for 30 minutes and then treated with 10 μM CM-H2-DCFDA for 30 minutes. Intracellular ROS generation was visualized by fluorescence microscopy (A and C) and quantified by image analysis (B and D). E, Percentages of cells responsive to GnRH stimulation were quantified and reported relative to total cell numbers. In LβT2 cells, GnRH alone stimulated levels 756% over control, whereas DPI-treated cells responded only 168%. DPI alone fluoresced 84% of control, but this was not significantly different. In primary pituitary cells, DPI significantly inhibited GnRH stimulation 1024% over control with GnRH vs 474% over control in DPI+GnRH-treated cells. Results represent means ± SEM from 3 independent experiments normalized with the control. Scale bars correspond to 150 μm. Letters represent significant differences (P < .05) by ANOVA followed by the Tukey multiple comparison test. Groups with different letters are significantly different from each other.
NADPH oxidases are a major source of intracellular ROS produced in response to extracellular stimuli (23). The NADPH oxidases are activated by PKC through phosphorylation of signaling complex subunits p47phox and RAC (22, 23). Because PKC isoforms are activated by the GnRH receptor (37, 38), we determined whether GnRH-induced ROS production in LβT2 cells and mouse primary pituitary cells was dependent on NOX/DUOX activity. Cells were preincubated with the NADPH oxidase inhibitor DPI at 5 μM for 30 minutes. The cells were subsequently treated with vehicle or 10 nM GnRH for 30 minutes and loaded with CM-H2-DCFDA for 30 minutes. Increased fluorescence induced by GnRH treatment was significantly diminished by DPI treatment in both LβT2 cells and mouse primary pituitary cells (Figure 1, A and C). Analysis of fluorescence intensity showed that DPI inhibition was significant (Figure 1, B and D). These results show that GnRH stimulation induces intracellular ROS via NOX/DUOX activity in both LβT2 and mouse primary pituitary cells.
NOX/DUOX and regulatory subunits are regulated by GnRH
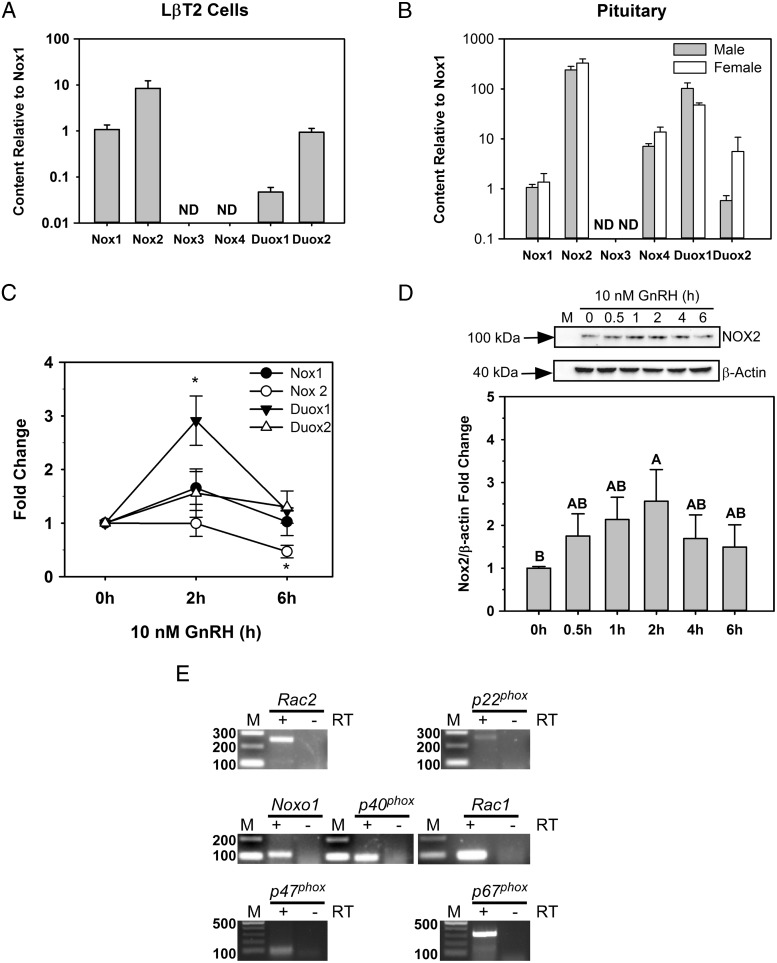
Six mouse homologs for NOX/DUOX, Nox1-4, Duox1, and Duox2, have been identified in a variety of nonphagocytic cells (23). We found that LβT2 cells, derived from male mice, express Nox1 and Nox2, as well as the calcium-regulated Duox1 and Duox2 (Figure 2A). The relative transcript level of Nox2 mRNA was approximately 8-fold greater than those of Nox1, Duox1, and Duox2 (Figure 2A). A similar pattern was found in whole pituitaries of both male and female mice. Expression of Nox2 mRNA was substantially higher than that of the other isoforms, and Duox1 was robustly expressed over Duox2 (Figure 2B). Nox3 and Nox4 mRNAs were undetectable in LβT2 cells or in whole pituitaries (Figure 2, A and B).
Figure 2.
Nox/Duox isoforms are expressed in LβT2 cells and mouse pituitary, and some are regulated by GnRH. A and B, Total RNA was isolated from LβT2 cells or whole pituitaries of female or male mice and reversed-transcribed into cDNA. Nox isoforms were identified by quantitative real-time PCR. Each Nox isoform mRNA is expressed normalized to the ratio of Nox 1 mRNA to Gapdh mRNA. C, Time course of the GnRH response. Serum-starved LβT2 cells were treated with 10 nM GnRH for the indicated times. Nox/Duox mRNA levels were analyzed by quantitative real-time PCR. Gapdh mRNA levels were used as internal controls for normalization across samples. The data are means ± SEM from 4 to 5 individual experiments with replicates. *, P < .05, significant difference from time 0 by the Dunnett test. D, Cells were treated with 10 nM GnRH for the indicated times. Clarified cell lysates were separated by SDS-PAGE and immunoblotted with an antibody for NOX2. The blots were then stripped and reprobed with an antibody to β-actin. Quantitative chemiluminescence was used to compare the levels of NOX2 relative to those of β-actin, and ratios were normalized to vehicle treatment (bottom panel). The histogram represents mean values ± SEM normalized to the vehicle control for 4 independent determinations. Letters indicate P < .05 by ANOVA followed by the Tukey multiple comparison test. Groups with different letters are significantly different from each other. E, Nox subunits and regulatory genes were assayed by RT-PCR using specific primers. −, no RT reaction; +, RT reaction; M, 100-bp DNA ladder.
Two of the NOX/DUOX family members are regulated at the mRNA level by GnRH in LβT2 cells. Nox2 mRNA was significantly reduced after 6 hours of GnRH stimulation and Duox1 mRNA was transiently increased after 2 hours of GnRH stimulation, returning to baseline by 6 hours (Figure 2C). In contrast to mRNA levels, only NOX2 protein was induced by GnRH treatment (Figure 2D). DUOX1 and DUOX2 proteins were also detected in LβT2 cells, but levels remained unchanged by GnRH treatment (Supplemental Figure 1).
Several regulatory subunits are required for activity of the NOX family (22). Activation of NOX1-3 requires the coordinated assembly of the transmembrane protein p22phox and the recruitment of cytosolic proteins RAC1/2 and the regulatory subunits p47phox, NOXO1, p40phox, and p67phox, all of which are detected in LβT2 cells (Figure 2E). Thus, LβT2 gonadotropes express the cofactors necessary for NOX2 activity.
GnRH-induced MAPK1/3 and JNK activation is attenuated by NOX/DUOX inhibition
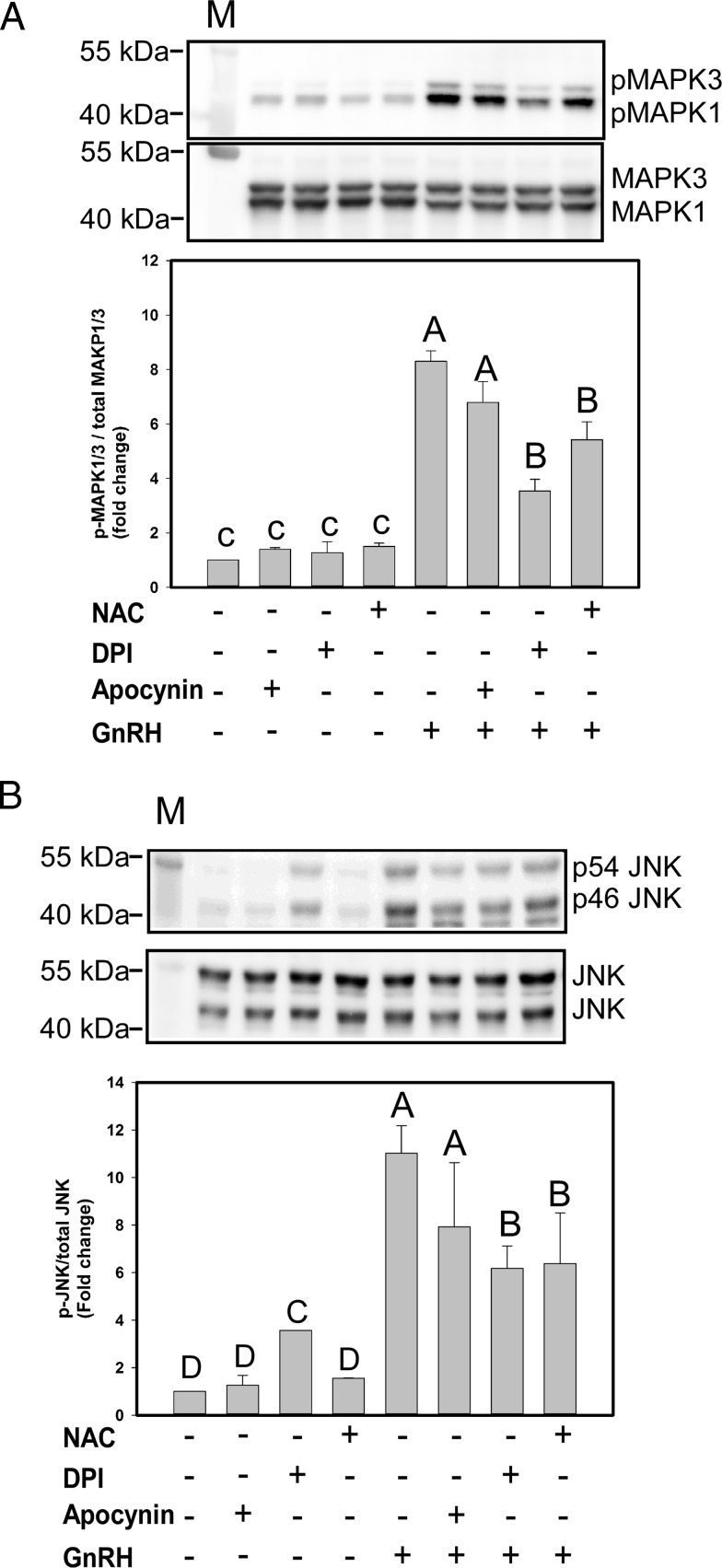
GnRH signaling via its receptor leads to activation of MAPK1/3, JNK, and p38 MAPK in gonadotropes (2). Activation of MAPK signaling cascades is essential for targeted activation of Lhb and Fshb gene expression. We tested whether NOX/DUOX-derived ROS induced by GnRH is necessary for MAPK activation in LβT2 cells. We used the NOX-specific inhibitor apocynin, the broad NOX/DUOX inhibitor DPI, and the general ROS scavenger NAC to distinguish the source of ROS. LβT2 cells were preincubated for 30 minutes with one of the compounds or vehicle and then treated with 10 nM GnRH for 5 minutes (for MAPK1/3) or 30 minutes (for JNK) based on the time to maximum activation by GnRH. Activation of both MAPK1/3 (Figure 3A) and JNK (Figure 3B) by GnRH treatment was significantly diminished by DPI and NAC treatment, but not by apocynin, suggesting a role for DUOX enzymes. None of the compounds inhibited GnRH-mediated p38 MAPK activation (data not shown). These results indicate that activation of MAPK1/3 and JNK by GnRH is at least partly dependent on NOX/DUOX-mediated ROS production.
Figure 3.
DPI and NAC inhibit GnRH-induced MAPK1/3 and JNK activation. Serum-starved LβT2 cells were preincubated with the NOX-specific inhibitor apocynin at 500 μM, the general NOX/DUOX inhibitor DPI at 5 μM, or the nonspecific ROS scavenger NAC at 10 mM for 30 minutes. Cells were then treated with 10 nM GnRH for 5 minutes (MAPK1/3) and 30 minutes (JNK). Both phosphophorylated (p) MAPK1/3 and phosphophorylated JNK were measured by Western blotting and quantitative chemiluminescence. A and B, top, Representative Western blot images using an antibody to phosphorylated MAPK1/3 or JNK and then stripped and reblotted with an antibody to total MAPK1/3 or total JNK. Reported values are determined by the combined ratio of phosphorylated MAPK1/3 to total MAPK1/3. Data show mean values ± SEM normalized to the vehicle control from 4 independent determinations. Letters represent significant differences (P < .05) by ANOVA followed by the Tukey multiple comparison test. Groups with different letters are significantly different from each other. M, Protein size marker.
GnRH-mediated activation of MAPK1/3 depends on both PKC- and calcium-mediated NOX/DUOX pathways
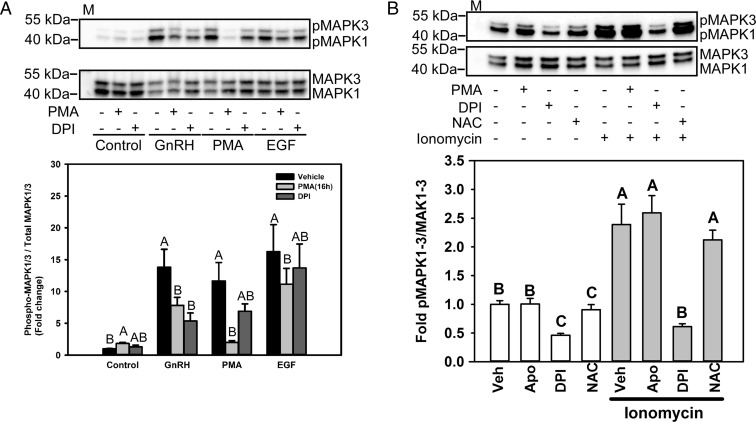
The GnRH and epidermal growth factor (EGF) receptor signaling cascades utilize different pathways to active MAPK1/3 in gonadotropes (39, 40). GnRH-mediated activation of MAPK1/3 depends on a complex PKC to MEK pathway that involves multiple intermediates including c-SRC and RAS (37, 41). Further, PKC activation of NOX activity may occur through direct activation of p47phox or through the intermediate action of c-SRC. In contrast, activation of MAPK1/3 by the EGF receptor pathway in immature αT3-1 gonadotrope cells utilizes the GRB-SOS-RAS-RAF-MEK signaling pathway but may also involve c-SRC (39). To determine the specificity of MAPK1/3 activation by GnRH through PKC-dependent NOX activation, rather than alternative pathways such as EGF receptor activation, we compared the sensitivity of GnRH- and EGF-mediated ERK activation to down-regulation of PKC activity. Prolonged PMA treatment for 16 hours causes down-regulation of conventional and novel but not atypical PKC isoforms in LβT2 cells and inhibits GnRH signaling (42, 43). Therefore, cells were pretreated with either vehicle (control), 1 μM PMA for 16 hours, or 5 μM DPI for 30 minutes and then were treated with either 10 nM GnRH, 100 nM PMA, or 100 nM EGF. Phosphorylated MAPK1/3 formed in response to treatment was analyzed by Western blotting (Figure 4A, top) and quantitative chemiluminescent analysis (Figure 4A, bottom). Individually, GnRH, PMA, and EGF treatments strongly stimulated the phosphorylation of MAPK1/3 (Figure 4A). Both GnRH- and PMA-induced MAPK1/3 activation was reduced by prolonged PMA treatment or treatment with the NOX/DUOX inhibitor DPI. Although EGF-stimulated MAPK1/3 activation was also inhibited by prolonged PMA, the NOX/DUOX inhibitor DPI had no significant effect on EGF-mediated MAPK1/3 activation, demonstrating ROS independence of EGF activation. The ability of EGF to stimulate MAPK1/3 in the presence of DPI also suggests that lack of stimulation by GnRH is not due to cytotoxic effects of the inhibitor.
Figure 4.
GnRH-mediated MAPK1/3 activation is regulated by the PKC/NOX pathway. A, Serum-starved LβT2 cells were preincubated with 1 μM PMA for 16 hours or with 5 μM DPI for 30 minutes. After treatment, the cells were incubated with 10 nM GnRH, 100 nM PMA, or 50 ng/mL EGF for 5 minutes. The cells were lysed and analyzed by Western blotting for phosphorylated (p) MAPK1/3. The blots were stripped and reblotted for total MAPK1/3. The graph shows the quantitative chemiluminescence results from 4 independent experiments. The data represent mean values ± SEM normalized to the vehicle control of 4 independent determinations. Data from vehicle or 5-minute GnRH, PMA, or EGF treatment groups were analyzed by ANOVA and post hoc testing with the Tukey multiple comparison test. Groups with different letters are significantly different from each other (P < .05) within treatment groups. B, Serum-starved LβT2 cells were preincubated with 500 μM apocynin, 5 μM DPI, or 10 mM NAC for 30 minutes. Cells were then treated with 1 μM ionomycin for 5 minutes. The cells were lysed and analyzed by Western blotting for phosphorylated MAPK1/3. The blots were stripped and reblotted for total MAPK1/3. The graph shows quantitative chemiluminescence results from 3 independent experiments. The data represent mean values ± SEM normalized to the vehicle control from 4 independent determinations. Groups with different letters are significantly different from each other (P < .05) by ANOVA followed by the Tukey multiple comparison test. M, Protein size marker.
GnRH-induced activation of MAPK1/3 is correlated with calcium flux in LβT2 cells. Intracellular calcium participates directly in ROS production by DUOX1 and DUOX2. To assess whether calcium flux alone can induce ROS production and MAPK1/3 activation, cells were treated with the calcium ionophore ionomycin and MAPK1/3 phosphorylation was determined by Western blotting. Ionomycin alone was capable of inducing MAPK1/3 phosphorylation (Figure 4B). The broad NOX/DUOX inhibitor DPI suppressed MAPK1/3 phosphorylation by ionomycin, but the NOX2-specific inhibitor apocynin and the ROS scavenger NAC had no effect, suggesting further that DUOX family members participate in MAPK1/3 activation via ROS production.
Nuclear MAPK1/3 and immediate-early gene activation are dependent on NOX/DUOX activity
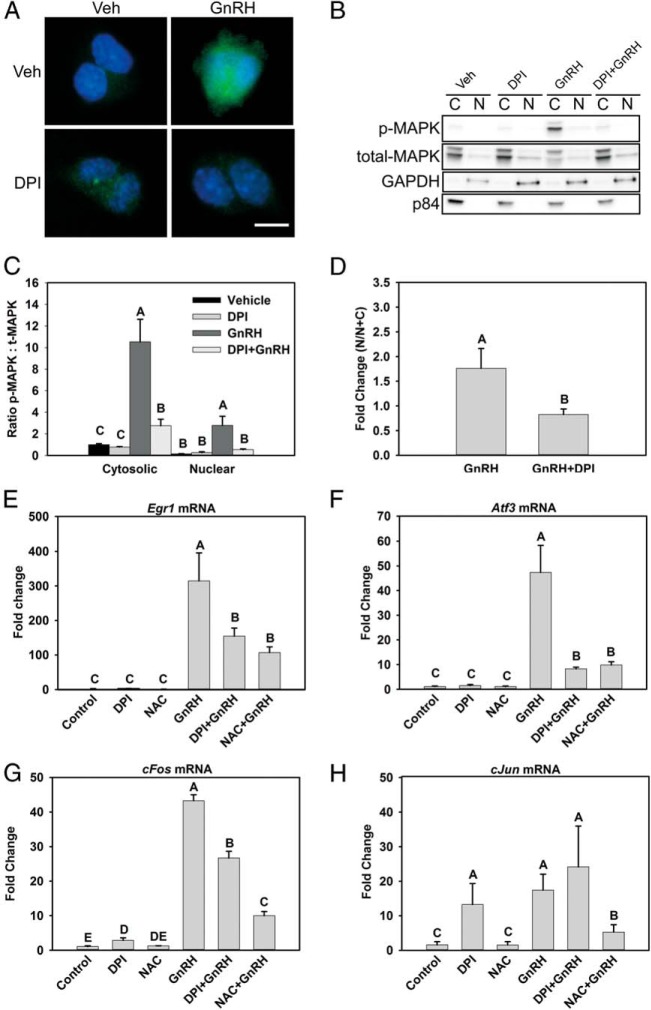
Although most activated MAPK1/3 is cytosolic in LβT2 cells, acute GnRH treatment induces transient activation and translocation of phosphorylated MAPK1/3 into the nucleus (35), and this is a major signaling component mediating the regulation of transcription in response to GnRH. GnRH activates both cytosolic translation factors (32, 35, 41, 44–46) and nuclear transcription factors (47) via MAPK1/3 activation. We examined the impact of NOX/DUOX inhibition on both the cytosolic and nuclear MAPK1/3 contents. Blockade of ROS production reduced both pools dramatically (Figure 5, A and B). However, quantification of phosphorylated MAPK1/3 in nuclear and cytosolic subcellular fractions (Figure 5C) suggested that, in addition to overall suppression, nuclear content was disproportionately suppressed. Examination of the proportion of nuclear MAPK1/3 to the total detected (Figure 5D) revealed that nuclear localization was proportionately lower in DPI-treated cells, indicating a greater impact on nuclear targets of MAPK1/3 action. GnRH-induced MAPK signaling activates expression of the immediate-early genes (IEGs) c-Jun, c-Fos, Atf3, and Egr1. The GnRH-induced increases in Egr1, Atf3, and c-Fos mRNA levels were significantly reduced in DPI- and NAC-treated LβT2 cells stimulated with GnRH (Figure 5, D–F). Notably, GnRH-induced c-Jun levels were attenuated in the presence of the ROS scavenger NAC but not with DPI treatment (Figure 5G). DPI treatment alone was found to induce the expression of c-Jun mRNA, but it was not further induced by GnRH. Treatment with NAC abolished the increase in c-Jun mRNA in response to GnRH, as with the other IEGs examined. These results demonstrate that GnRH-induced increases in the IEG mRNA level are regulated via NOX/DUOX-mediated ROS signaling, but that different mechanisms may control c-Jun expression.
Figure 5.
NOX/DUOX inhibition suppresses nuclear MAPK1/3 and IEG induction by GnRH. A, MAPK1/3 activation in both nuclear and cytosolic compartments was examined by immunocytochemical analysis of LβT2 cells treated with 10 nM GnRH for 30 minutes with or without preincubation with the NOX/DUOX inhibitor DPI. Cells were fixed and stained with an antibody to phosphorylated MAPK1/3 and Alexa Fluor 488–conjugated secondary antibody (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole-containing mounting medium before visualization (blue) B, LβT2 cells treated similarly were harvested and fractionated into nuclear (N) and cytosolic (C) fractions and evaluated by Western blotting for abundance of phosphorylated (p) MAPK1/3. C, Blots were stripped and reblotted for total MAPK1/3 and quantified by chemiluminescence. Blots of subcellular fractions were also probed with antibodies for GAPDH and p84 to distinguish separation of cytosolic and nuclear fractions, respectively. D, Nuclear localization of phosphorylated MAPK1/3 appeared disproportionately reduced by DPI, so the fold increase in nuclear phospho-MAPK1/3 was specifically evaluated in vehicle and DPI-treated samples. To analyze IEG mRNA responses, serum-starved LβT2 cells were preincubated with 10 mM NAC or 5 μM DPI for 30 minutes. After treatment, the cells were incubated with 10 nM GnRH for 1 hour. Real-time PCR was performed using specific primers for Egr1 (E), Atf3 (F), c-Fos (G), and c-Jun (H). GAPDH mRNA was used to normalize samples, and each gene is shown relative to the vehicle control. Data are reported as means ± SEM from 3 independent experiments. Letters represent significant differences (P < .05) by ANOVA followed by the Tukey multiple comparison test. Groups with different letters are significantly different from each other.
Gonadotropin subunit gene promoter activity and mRNA levels are regulated by NOX/DUOX activity
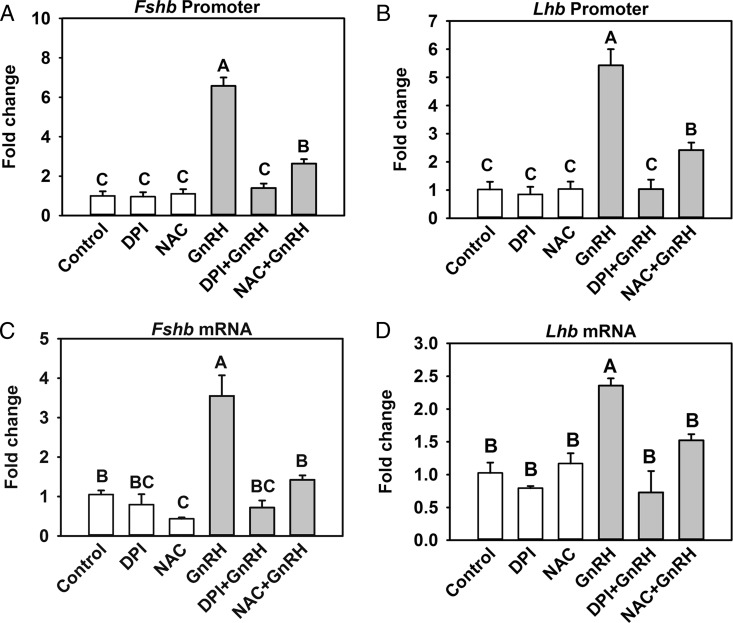
Activation of IEGs by MAPK1/3 and JNK plays a major role in regulating Fshb and Lhb transcription. We examined whether NOX/DUOX activity, as determined by DPI inhibition, or the increase in ROS, as determined by NAC quenching, influences Fshb or Lhb promoter activity and mRNA levels in LβT2 cells. The activity of the Fshb and Lhb promoters was significantly increased by GnRH treatment compared with that in vehicle-treated cells but was attenuated by both DPI and NAC (Figure 6, A and B). We also tested whether ROS affected the changes in endogenous Fshb and Lhb mRNA levels that occur in response to GnRH treatment. Both Lhb and Fshb mRNA were significantly increased by GnRH treatment over that in untreated controls, and both responses were attenuated by DPI and NAC (Figure 6, C and D). Thus, our results strongly suggest that GnRH induction of Fshb and Lhb promoter and mRNA occur via NOX/DUOX-mediated ROS signaling.
Figure 6.
GnRH-induced activation of Fshb and Lhb promoter activity and mRNA is ROS dependent. A and B, LβT2 cells transiently transfected with a −398-bp murine Fshb promoter (A) or a −1.8-kb rat Lhb (B) luciferase reporter plasmid were serum starved for 16 hours. Transfected cells were pretreated with 10 mM NAC and 5 μM DPI for 30 minutes and then treated with 10 nM GnRH for 5 hours. Luciferase values were internally normalized using a cotransfected β-galactosidase reporter to control for transfection efficiency and expression. Data are presented as means ± SEM from 5 independent experiments. Letters represent significant differences (P < .05) by ANOVA followed by the Tukey multiple comparison test. Groups with different letters are significantly different from each other. C and D, For analysis of Fshb and Lhb mRNA expression, serum-starved LβT2 cells were preincubated with 10 mM NAC and 5 μM DPI for 30 minutes and then treated with 10 nM GnRH for 5 hours in accordance with the maximal response to GnRH stimulation. Real-time PCR was performed using specific primers for Fshb (C) and Lhb (D). Gapdh mRNA was used as an internal control for normalization across samples. Data shown are the means ± SEM from 3 independent experiments. Letters represent significant differences (P < .05) by ANOVA followed by the Tukey multiple comparison test. Groups with different letters are significantly different from each other.
DUOX2 is necessary for GnRH-mediated Fshb mRNA induction
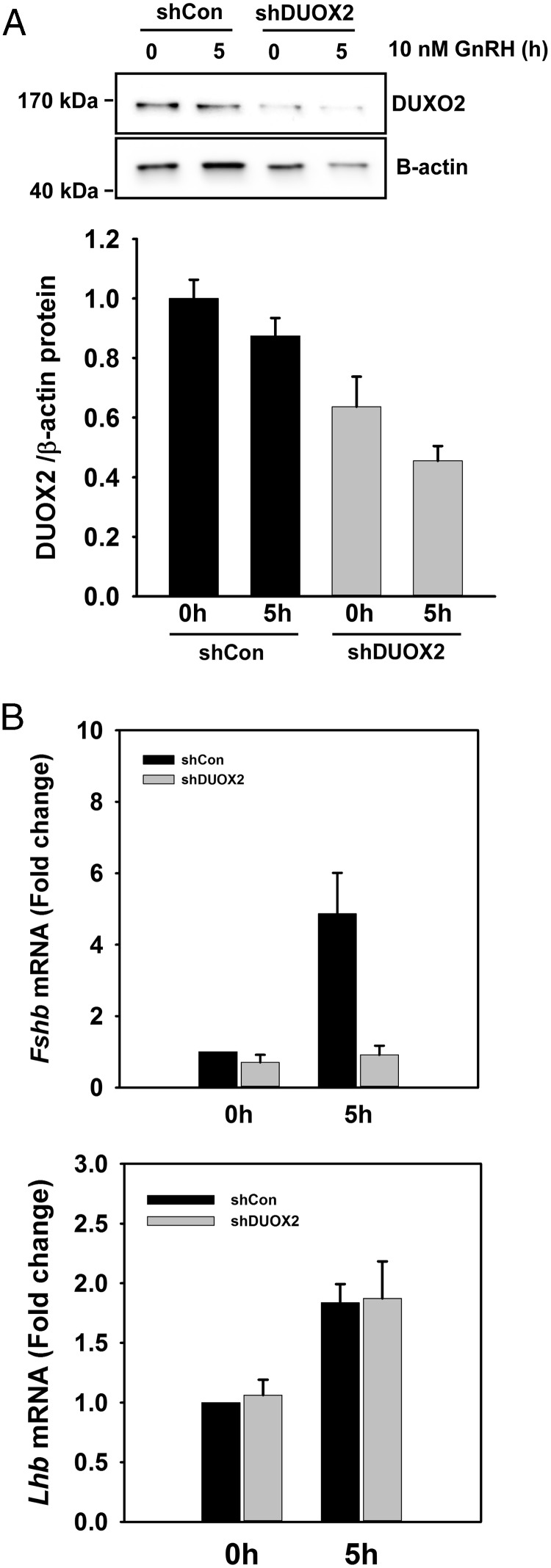
In the results above, the NOX2-selective inhibitor apocynin slightly reduced MAPK1/3 and JNK activation in response to GnRH. However, the general NOX/DUOX inhibitor DPI dramatically reduced MAPK activation, Fshb and Lhb mRNA, and Fshb and Lhb promoter activity in response to GnRH. The blockade of ionomycin-induced MAPK activation with DPI supports the role of DUOX-dependent ROS generation in gonadotropes. Although Duox1 mRNA was significantly increased by GnRH, its protein level remained unchanged (Supplemental Figure 1). Both mRNA and protein levels of DUOX2 remained unchanged by GnRH treatment. We examined the contribution of each DUOX isoform in regulating gonadotropin gene expression by examining the effect of shRNA knockdown on GnRH-stimulated increases in Lhb or Fshb mRNA. DUOX1-targeted shRNAs had no effect on the DUOX1 protein levels (data not shown). However, shRNA targeting of DUOX2 significantly reduced the protein level (Figure 7A). Suppression of DUOX2 protein also significantly reduced the Fshb mRNA response to GnRH stimulation, but Lhb mRNA was not significantly affected (Figure 7B). These results indicate that DUOX2 mediates GnRH-induced Fshb gene expression. Although these results suggest that DUOX species are largely responsible for ROS action in response to GnRH, a contribution from other NOX/DUOX species cannot be ruled out.
Figure 7.
Suppression of DUOX2 reduces GnRH-induced Fshb mRNA. LβT2 cells were transduced with lentiviral shRNA targeting mouse Duox2 for 2 days. Transduced cells were starved for 16 hours and then stimulated with 10 nM GnRH for 5 hours. A, DUOX2 protein was measured by Western blotting. Both unstimulated 0-hour and 5-hour GnRH-stimulated DUOX2 levels were significantly reduced in comparison to those of control vector-transduced cells. B, Fshb and Lhb mRNA was analyzed by quantitative real-time PCR. Gapdh mRNA was used as an internal control for normalization across samples. Relative transcript levels were determined using the ΔΔCT method. Fshb mRNA was not significantly induced by GnRH in shDUOX2-transduced cells, but Lhb mRNA was unaffected.
GnRH pulse-induced FSH and LH secretion require NOX/DUOX activity
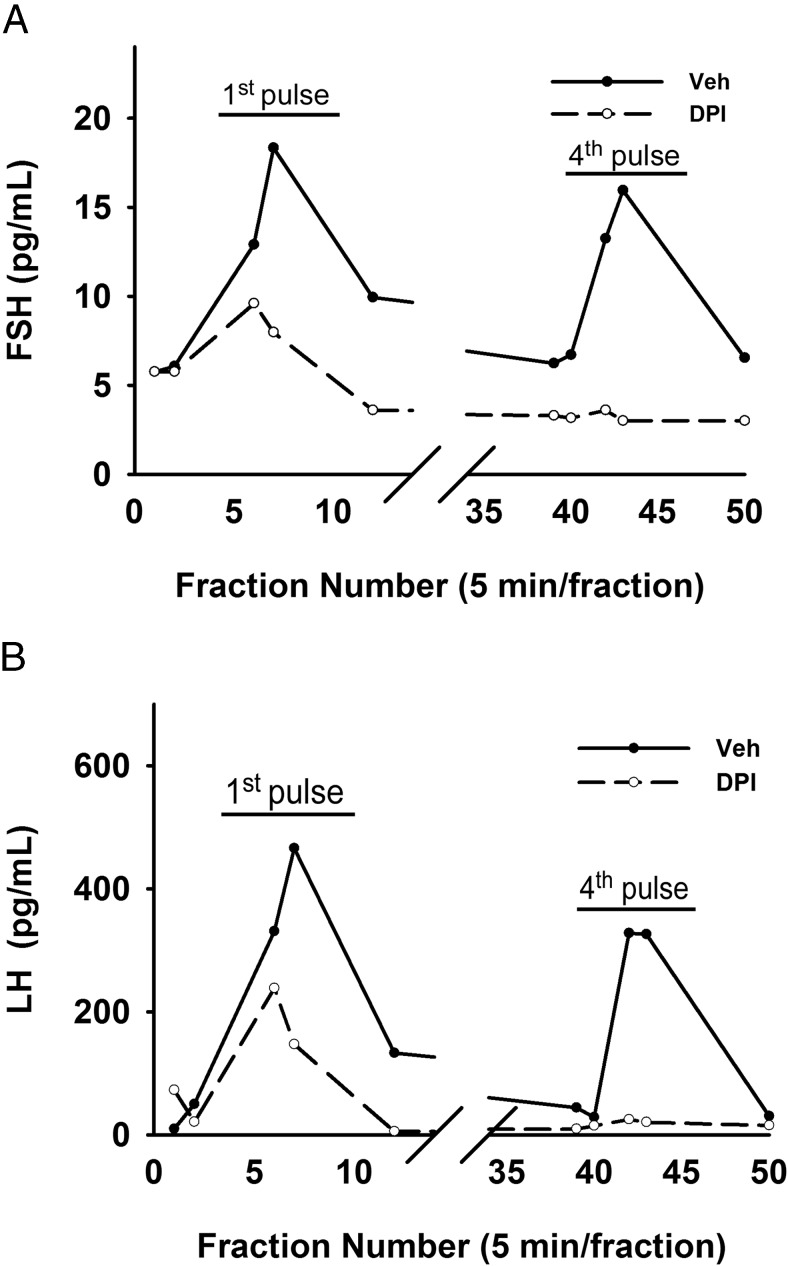
The above results demonstrate that NOX/DUOX activity is necessary for GnRH-induced expression of gonadotropin genes and may be necessary for GnRH-induced changes in gonadotropin synthesis or secretion. Under pulsatile stimulation, repeated demands for gonadotropin secretion will require an appropriate sustained synthetic response. Previous work has established that both gene expression and protein synthesis are regulated by GnRH in gonadotropes via MAPK1/3 (4) and that MAPK1/3 is necessary for female fertility (48). Loss of ROS may then lead to decreased gonadotropin production and the GnRH-induced secretory response. To test this possibility, we examined the consequence of reduced NOX/DUOX activity on the gonadotropin secretory response. Female mouse primary pituitary cells in perifusion culture were pulsed hourly with 10 nM GnRH for 4 hours in the presence of vehicle or the NOX/DUOX inhibitor DPI. The LH and FSH secretory response was measured from 5-minute fractions of the perifusate. The first GnRH pulse strongly induced FSH and LH secretion in untreated cells, but these levels were reduced by approximately 50% in the DPI-treated cells (Figure 8). Within 4 hours, DPI-treated cells were unable to mount a secretory response to GnRH stimulation, supporting a central role of NOX/DUOX activity in maintenance of GnRH sensitivity.
Figure 8.
FSH and LH secretion is suppressed by the NOX/DUOX inhibitor DPI. Primary pituitary cells isolated from 9- to 10-week-old randomly cycling female mice were cultured on Cytodex 3 microcarrier beads for 2 days. After serum starving for 16 hours, cells were loaded into perifusion columns and pretreated with vehicle or 5 μM DPI for 40 minutes and then pulsed for 2 minutes at 58-minute intervals at 10 nM peak GnRH for 4 hours. Fractions of 5-minute length were collected, and FSH (A) and LH (B) concentrations were measured. Each representative chart shows the FSH and LH levels at the first and fourth GnRH pulse.
Discussion
Understanding the targets and signaling pathways engaged by GnRH in gonadotropes is central to a full appreciation of the complexity of reproductive control. The gonadotropes of the anterior pituitary are of particular interest not only because of their role in interpreting and transmitting hypothalamic signals through decoding of GnRH pulses but also because of their exposure to the general circulation where they can be influenced by extrahypothalamic signals (36, 49, 50). Evidence for outside regulatory input is particularly strong in studies implicating the pituitary as the target of obesity-related suppression of gonadotropins (51, 52), of fatty acid signaling (53), and of insulin action (54, 55). Although it is evident that multiple factors can influence regulation of gonadotropin gene expression and secretion, the mechanistic details of how these factors alter gonadotropes or their response to GnRH is lacking.
The study of signaling cascades engaged by GnRH stimulation and activation of its cognate G protein–coupled receptor has continued to be of interest, and novel mechanisms of action continue to emerge, including signaling through prostaglandins, dynamin, and AMP kinase and through engagement of the unfolded protein response (32, 56–59). Our studies of the unfolded protein response in gonadotropes has led us to examine signaling components associated with high energy and protein synthesis demand. Among these components are ROS that act as both internal and autocrine/paracrine signal mediators associated with stress signaling in the endoplasmic reticulum and elevated mitochondrial oxidative activity. In this report, we show that ROS represent a key intermediate signaling molecule that is produced by both Ca2+- and PKC-dependent activation of NOX/DUOX family members. The activation of MAPK1/3, p38, and JNK by ROS is broadly described in other systems, and our results indicate that MAPK1/3 and JNK activation in gonadotropes involves ROS (60, 61).
ROS can be generated by incomplete mitochondrial oxidation of water and by enzymatic production from NADPH by the NOX proteins (60). The NOX/DUOX family members are the main source of rapid signal-mediated ROS production and play an important role in nonphagocytic cellular ROS signaling (19, 20). The expression of NOX/DUOX family members is cell and tissue specific, and they are associated with different subcellular compartments (22). We identified expression of Nox1, Nox2, Duox1, and Duox2 mRNA in mouse pituitary tissue and in the LβT2 gonadotrope cells, and similar patterns of expression were identified. These results show that gonadotrope cells are poised to use ROS signaling via both NOX and DUOX enzymes and can therefore generate ROS in response to a variety of stimuli. Generation of ROS can occur through kinase-mediated activation of the regulatory subunit p47phox, itself a target of c-SRC and PKC. DUOX1 and DUOX2 produce H2O2 directly via a 2-electron reduction of oxygen by Ca2+ and are thus regulated by the availability of intracellular Ca2+ (62). From a regulatory perspective, gonadotropes can generate ROS through the secretory pathway via increased intracellular calcium and activation of DUOX activity or by downstream signaling of the GnRH-receptor through activation of PKC and NOX activity. The ability of the general inhibitor DPI and to a lesser extent NAC to attenuate GnRH signaling, coupled with the minimal effectiveness of the NOX-selective inhibitor apocynin, suggests that the Ca2+-activated DUOX family members are probably major contributors to ROS production. Although the off-target effects of these inhibitors engenders caution in our interpretation (63, 64), the impact of DUOX2 siRNA knockdown on Fshb gene expression supports this general conclusion.
GnRH stimulates activation of MAPK1/3, JNK, and p38 MAPKs through the PKC pathway in gonadotropes (1). Activation of JNK and MAPK1/3 triggers activation of cap-dependent translation and increased expression of IEGs such as Egr1, Atf3, c-Jun, and c-Fos, which regulate both Lhb and Fshb promoters (12, 46). Our results in Figures 3 and 4 suggest that activation of MAPK 1/3, Egr1, and Atf3 is more sensitive to ROS inhibition than that of JNK, c-Fos, and c-Jun, but both Lhb and Fshb gene expression require ROS signaling. Central to the regulatory control of gonadotropin gene expression is promoter activation by IEGs whose expression and activity are regulated through MAPK activity. MAPK1/3 activation occurs through the canonical RAS-RAF-MEK cascade. The point of PKC contact with this cascade is not particularly clear. Earlier results indicated that GnRH-induced expression of the glycoprotein hormone α-subunit in the immature gonadotrope cell line αT3-1 is RAS dependent (41). As a target of GnRH receptor signaling, c-SRC is also a component of the upstream mediators of MAPK1/3 activation via RAS (57). However, RAS is described as either a target of or an upstream regulator of ROS (65, 66). Both GnRH and EGF are robust activators of MAPK1/3 in LβT2 cells (67, 68). GnRH action is sensitive to inhibition of NOX activity by DPI, but this has no effect on EGF action. Thus, ROS participates in the GnRH signaling cascade above the common point of RAS, or alternatively ROS acts through a cascade that is independent of the RAS-RAF-MEK pathway. The role of ROS in GnRH signaling may extend beyond direct MAPK signaling. The mechanisms of ROS action are not well defined, but the primary targets of H2O2 are protein tyrosine and dual-specificity phosphatases bearing active-site cysteine residues that are inactivated by oxidation (69). It is possible that ROS directly participate in regulation of activity through modulation of tyrosine and dual-specificity kinases, which control overall MAPK and tyrosine kinase activity and are known participants in MAPK regulation by GnRH (35, 70). The impact of ROS on expression of Atf3, c-Fos, Jun, and Egr1 mRNA and on promoter activation and the mRNA level of both Fshb and Lhb suggests that ROS participates in the major regulatory mechanisms engaged by GnRH in gonadotropes.
The participation of ROS in the GnRH signaling network is of general significance. Gonadotropes rely on interpretation of pulsatile GnRH that varies in frequency and amplitude throughout the ovulatory cycle for appropriate regulation of gene expression, and, consequently, fertility (2, 71–73). Blockade of the GnRH signaling response by NOX/DUOX inhibition does not block gonadotropin secretion to an initial pulse but prevents continued secretory responses to subsequent GnRH pulses (Figure 8). This finding suggests that ROS signaling is essential for maintenance of secretory capacity through increased gene expression and protein synthesis. Further, the paracrine action of ROS presents the possibility that exogenous ROS or ROS generated by other internal sources can influence signaling cascades and alter responses to GnRH (20). This implies that disorders of energy metabolism or activation of inflammatory signal pathways that also produce ROS can interfere with GnRH signal interpretation by gonadotropes. Alternative pathways of ROS generation may introduce noise into the GnRH signaling network. Chronic introduction of ROS by alternative sources may alter the appropriate induction and resolution of the periodic GnRH signals from the hypothalamus, thus causing misinterpretation of hypothalamic input that can then lead to inappropriate gonadotropin production or secretion. Further studies addressing the signaling cross talk between GnRH receptor and alternative sources of ROS will provide insight into the molecular and physiological impacts of oxidative stress on gonadotropin production.
Acknowledgments
We thank Ekaette Mbong for review and suggestions on this article.
This work was supported by the National Institutes of Health (Grants R01 HD 037568 and U54 HD012303 to M.A.L.). T. K. was supported by the National Institutes of Health (Grant T32 HD007203).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP1
- activator protein 1
- CM-H2-DCFDA
- 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- DPI
- diphenylene iodonium
- DUOX
- dual oxidase
- EGF
- epidermal growth factor
- IEG
- immediate-early gene
- JNK
- c-Jun N-terminal kinase
- MEK
- MAPK kinase
- NAC
- N-acetyl cysteine
- NOX
- NADPH oxidase
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- ROS
- reactive oxygen species
- SAPK
- stress-activated protein kinase
- sh
- short hairpin
- TBS
- Tris-buffered saline.
References
- 1. Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11:177–199. [DOI] [PubMed] [Google Scholar]
- 2. Shacham S, Harris D, Ben-Shlomo H, et al. Mechanism of GnRH receptor signaling on gonadotropin release and gene expression in pituitary gonadotrophs. Vitam Horm. 2001;63:63–90. [DOI] [PubMed] [Google Scholar]
- 3. Naor Z, Harris D, Shacham S. Mechanism of GnRH receptor signaling: combinatorial cross-talk of Ca2+ and protein kinase C. Front Neuroendocrinol. 1998;19:1–19. [DOI] [PubMed] [Google Scholar]
- 4. Gur G, Bonfil D, Safarian H, Naor Z, Yaron Z. GnRH receptor signaling in tilapia pituitary cells: role of mitogen-activated protein kinase (MAPK). Comp Biochem Physiol B Biochem Mol Biol. 2001;129:517–524. [DOI] [PubMed] [Google Scholar]
- 5. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol. 2002;16:419–434. [DOI] [PubMed] [Google Scholar]
- 6. Shah BH, Farshori MP, Jambusaria A, Catt KJ. Roles of Src and epidermal growth factor receptor transactivation in transient and sustained ERK1/2 responses to gonadotropin-releasing hormone receptor activation. J Biol Chem. 2003;278:19118–19126. [DOI] [PubMed] [Google Scholar]
- 7. Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–174. [DOI] [PubMed] [Google Scholar]
- 8. Rubinfeld H, Seger R. The ERK cascade as a prototype of MAPK signaling pathways. Methods Mol Biol. 2004;250:1–28. [DOI] [PubMed] [Google Scholar]
- 9. Sarker KP, Biswas KK, Yamakuchi M, et al. ASK1–p38 MAPK/JNK signaling cascade mediates anandamide-induced PC12 cell death. J Neurochem. 2003;85:50–61. [DOI] [PubMed] [Google Scholar]
- 10. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 11. Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Binder AK, Grammer JC, Herndon MK, Stanton JD, Nilson JH. GnRH regulation of Jun and Atf3 requires calcium, calcineurin, and NFAT. Mol Endocrinol. 2012;26:873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding M, Li JJ, Leonard SS, et al. Vanadate-induced activation of activator protein-1: role of reactive oxygen species. Carcinogenesis. 1999;20:663–668. [DOI] [PubMed] [Google Scholar]
- 15. Aggeli IK, Beis I, Gaitanaki C. ERKs and JNKs mediate hydrogen peroxide-induced Egr-1 expression and nuclear accumulation in H9c2 cells. Physiol Res. 2010;59:443–454. [DOI] [PubMed] [Google Scholar]
- 16. Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol. 1999;19:2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol. 2005;19:2624–2638. [DOI] [PubMed] [Google Scholar]
- 18. Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2007;401:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. [DOI] [PubMed] [Google Scholar]
- 20. Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004;11:1163–1182. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333(Pt 2):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. [DOI] [PubMed] [Google Scholar]
- 23. Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. [DOI] [PubMed] [Google Scholar]
- 24. Kuroda J, Nakagawa K, Yamasaki T, et al. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. [DOI] [PubMed] [Google Scholar]
- 25. Massart C, Hoste C, Virion A, Ruf J, Dumont JE, Van Sande J. Cell biology of H2O2 generation in the thyroid: investigation of the control of dual oxidases (DUOX) activity in intact ex vivo thyroid tissue and cell lines. Mol Cell Endocrinol. 2011;343:32–44. [DOI] [PubMed] [Google Scholar]
- 26. Lacroix L, Nocera M, Mian C, et al. Expression of nicotinamide adenine dinucleotide phosphate oxidase flavoprotein DUOX genes and proteins in human papillary and follicular thyroid carcinomas. Thyroid. 2001;11:1017–1023. [DOI] [PubMed] [Google Scholar]
- 27. Milenkovic M, De Deken X, Jin L, et al. Duox expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J Endocrinol. 2007;192:615–626. [DOI] [PubMed] [Google Scholar]
- 28. Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2012;47:344–352. [DOI] [PubMed] [Google Scholar]
- 29. Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41:183–197. [DOI] [PubMed] [Google Scholar]
- 30. Baker MA, Aitken RJ. The importance of redox regulated pathways in sperm cell biology. Mol Cell Endocrinol. 2004;216:47–54. [DOI] [PubMed] [Google Scholar]
- 31. Kato H, Sugino N, Takiguchi S, Kashida S, Nakamura Y. Roles of reactive oxygen species in the regulation of luteal function. Rev Reprod. 1997;2:81–83. [DOI] [PubMed] [Google Scholar]
- 32. Do MH, Santos SJ, Lawson MA. GNRH induces the unfolded protein response in the LβT2 pituitary gonadotrope cell line. Mol Endocrinol. 2009;23:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 34. Breen KM, Thackray VG, Coss D, Mellon PL. Runt-related transcription factors impair activin induction of the follicle-stimulating hormone β-subunit gene. Endocrinology. 2010;151:2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen KA, Intriago RE, Upadhyay HC, Santos SJ, Webster NJ, Lawson MA. Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LβT2 gonadotropes. Endocrinology. 2010;151:4882–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone β promoter is determined by a negative feedback loop involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dobkin-Bekman M, Rahamin-Ben Navi L, Shterntal B, et al. Differential role of PKC isoforms in GnRH and phorbol 12-myristate 13-acetate activation of extracellular signal-regulated kinase and Jun N-terminal kinase. Endocrinology. 2010;151:4894–4907. [DOI] [PubMed] [Google Scholar]
- 38. Naor Z, Shacham S, Harris D, Seger R, Reiss N. Signal transduction of the gonadotropin releasing hormone (GnRH) receptor: cross-talk of calcium, protein kinase C (PKC), and arachidonic acid. Cell Mol Neurobiol. 1995;15:527–544. [DOI] [PubMed] [Google Scholar]
- 39. Shah BH, Soh JW, Catt KJ. Dependence of gonadotropin-releasing hormone-induced neuronal MAPK signaling on epidermal growth factor receptor transactivation. J Biol Chem. 2003;278:2866–2875. [DOI] [PubMed] [Google Scholar]
- 40. Reiss N, Llevi LN, Shacham S, Harris D, Seger R, Naor Z. Mechanism of mitogen-activated protein kinase activation by gonadotropin-releasing hormone in the pituitary of αT3-1 cell line: differential roles of calcium and protein kinase C. Endocrinology. 1997;138:1673–1682. [DOI] [PubMed] [Google Scholar]
- 41. Sosnowski R, Mellon PL, Lawson MA. Activation of translation in pituitary gonadotrope cells by gonadotropin-releasing hormone. Mol Endocrinol. 2000;14:1811–1819. [DOI] [PubMed] [Google Scholar]
- 42. Larivière S, Garrel G, Simon V, et al. Gonadotropin-releasing hormone couples to 3′,5′-cyclic adenosine-5′-monophosphate pathway through novel protein kinase Cδ and -ϵ in LβT2 gonadotrope cells. Endocrinology. 2007;148:1099–1107. [DOI] [PubMed] [Google Scholar]
- 43. Vasilyev VV, Lawson MA, Dipaolo D, Webster NJ, Mellon PL. Different signaling pathways control acute induction versus long-term repression of LHβ transcription by GnRH. Endocrinology. 2002;143:3414–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Do MH, Kim T, He F, et al. Polyribosome and ribonucleoprotein complex redistribution of mRNA induced by GnRH involves both EIF2AK3 and MAPK signaling. Mol Cell Endocrinol. 2014;382:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim T, Do MH, Lawson MA. Translational control of gene expression in the gonadotrope. Mol Cell Endocrinol. 2014;385:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen KA, Santos SJ, Kreidel MK, Diaz AL, Rey R, Lawson MA. Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LβT2. Mol Endocrinol. 2004;18:1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bliss SP, Miller A, Navratil AM, et al. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mistry DS, Tsutsumi R, Fernandez M, et al. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25:1387–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsaneva-Atanasova K, Mina P, Caunt CJ, Armstrong SP, McArdle CA. Decoding GnRH neurohormone pulse frequency by convergent signalling modules. J R Soc Interface. 2012;9:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pal L, Chu HP, Shu J, Topalli I, Santoro N, Karkanias G. In vitro evidence of glucose-induced toxicity in GnRH secreting neurons: high glucose concentrations influence GnRH secretion, impair cell viability, and induce apoptosis in the GT1–1 neuronal cell line. Fertil Steril. 2007;88:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ. Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab. 2008;93:2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sharma S, Morinaga H, Hwang V, et al. Free fatty acids induce Lhb mRNA but suppress Fshb mRNA in pituitary LβT2 gonadotropes and diet-induced obesity reduces FSH levels in male mice and disrupts the proestrous LH/FSH surge in female mice. Endocrinology. 2013;154:2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Navratil AM, Song H, Hernandez JB, et al. Insulin augments gonadotropin-releasing hormone induction of translation in LβT2 cells. Mol Cell Endocrinol. 2009;311:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhen S, Zakaria M, Wolfe A, Radovick S. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by insulin-like growth factor I in a cultured GnRH-expressing neuronal cell line. Mol Endocrinol. 1997;11:1145–1155. [DOI] [PubMed] [Google Scholar]
- 56. Bonfil D, Chuderland D, Kraus S, et al. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2004;145:2228–2244. [DOI] [PubMed] [Google Scholar]
- 57. Benard O, Naor Z, Seger R. Role of dynamin, Src, and Ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone. J Biol Chem. 2001;276:4554–4563. [DOI] [PubMed] [Google Scholar]
- 58. Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. 2012;55:783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Naor Z, Jabbour HN, Naidich M, et al. Reciprocal cross talk between gonadotropin-releasing hormone (GnRH) and prostaglandin receptors regulates GnRH receptor expression and differential gonadotropin secretion. Mol Endocrinol. 2007;21:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. [DOI] [PubMed] [Google Scholar]
- 61. McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. [DOI] [PubMed] [Google Scholar]
- 62. Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. [DOI] [PubMed] [Google Scholar]
- 63. Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tazzeo T, Worek F, Janssen L. The NADPH oxidase inhibitor diphenyleneiodonium is also a potent inhibitor of cholinesterases and the internal Ca2+ pump. Br J Pharmacol. 2009;158:790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tai P, Ascoli M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol Endocrinol. 2011;25:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hole PS, Pearn L, Tonks AJ, et al. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood. 2010;115:1238–1246. [DOI] [PubMed] [Google Scholar]
- 67. Armstrong J, Childs GV. Changes in expression of epidermal growth factor receptors by anterior pituitary cells during the estrous cycle: cyclic expression by gonadotropes. Endocrinology. 1997;138:1903–1908. [DOI] [PubMed] [Google Scholar]
- 68. Fowkes RC, Burch J, Burrin JM. Stimulation of extracellular signal-regulated kinase by pituitary adenylate cyclase-activating polypeptide in αT3-1 gonadotrophs. J Endocrinol. 2001;171:R5–R10. [DOI] [PubMed] [Google Scholar]
- 69. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. [DOI] [PubMed] [Google Scholar]
- 70. Armstrong SP, Caunt CJ, McArdle CA. Gonadotropin-releasing hormone and protein kinase C signaling to ERK: spatiotemporal regulation of ERK by docking domains and dual-specificity phosphatases. Mol Endocrinol. 2009;23:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Choi SG, Ruf-Zamojski F, Pincas H, Roysam B, Sealfon SC. Characterization of a MAPK scaffolding protein logic gate in gonadotropes. Mol Endocrinol. 2011;25:1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perrett RM, Voliotis M, Armstrong SP, et al. Pulsatile hormonal signaling to extracellular signal-regulated kinase: exploring system sensitivity to gonadotropin-releasing hormone pulse frequency and width. J Biol Chem. 2014;289:7873–7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ruf F, Hayot F, Park MJ, et al. Noise propagation and scaling in regulation of gonadotrope biosynthesis. Biophys J. 2007;93:4474–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]