Abstract
Circadian disruption has become a significant factor contributing to the epidemics of obesity and insulin resistance. However, interventions to treat metabolic dysfunctions induced by circadian disruptions are limited. The ovarian hormone, estrogen, produces important antiobesity and antidiabetic effects in female animals and has profound effects on daily behavioral rhythms. Here, we show that in female mice depleted with endogenous estrogens, a jet-lag paradigm induced visceral fat accumulation and systemic insulin resistance, which were associated with altered expression of multiple circadian genes in the visceral fat depot. Interestingly, all these jet-lag-induced deficits were completely rescued in female mice supplemented with exogenous estrogens. We further examined 24-hour oscillations of circadian genes in adipose tissues in female mice with estrogen depletion or replacement and showed that expression levels of the circadian gene, period circadian protein homolog 2, oscillate in visceral adipose tissue in an estrogen-dependent manner. Together, our results indicate that estrogens interact with the intrinsic circadian clock in adipose tissue and prevent abnormal lipid accumulation caused by circadian disruptions.
Obesity is a serious global health problem due to its increasing prevalence and comorbidities. Circadian clocks align behavioral and biochemical processes with the day/night cycle and play important roles in the regulation of energy and glucose homeostasis (1). The circadian disruption caused by shift work, jet-lag (JL) or circadian activity disturbance, a common occurrence in the modern society, has become a significant factor contributing to the epidemics of obesity and insulin resistance (2–4). In rodents, misalignment of day/night cycles leads to increased body weight (5, 6), hyperglycemia (5), and hyperinsulinemia (6). However, the mechanisms for this strong connection are not fully understood and interventions to treat or prevent metabolic dysfunctions induced by circadian disruptions are limited.
The circadian clocks are operated by molecular feedback loops of circadian genes (7, 8). In particular, the core circadian genes, namely brain and muscle Arnt-like 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK), form heterodimer transcription factors to drive expression of period circadian protein homolog (PER)1–PER3 and cryptochrome (CRY)1–CRY2. PERs and CRYs then bind to the BMAL1/CLOCK complex and inhibit their transcription activity. The alternating activation and suppression of the BMAL1/CLOCK positive loop and the PER/CRY negative loop partly contribute to the circadian oscillation. Interestingly, mutations in circadian genes, including BMAL1, CLOCK, and CRY2, have been associated with obesity and metabolic syndrome in humans (9–11).
The ovarian hormone, estrogen, has been shown to suppress food intake and produce important antiobesity and antidiabetic effects in female animals. For example, ovariectomy (OVX) in female animals leads to hyperphagia, increased body weight and hyperadiposity (12–14), effects that are prevented by estrogen replacement (15). Further, mice with mutations in the gene encoding estrogen receptor (ER)α are obese (16). Importantly, estrogens also regulate fat distribution. Females distribute relatively more fat in sc depot, whereas males have more fat stored in visceral depot (17–19). Estrogens may account for this sexual dimorphism, because the differences in fat distribution between premenopausal women and age-matched men are abolished in postmenopausal females and age-matched males (20). Further, preferential increases in visceral fat distribution vs sc fat distribution have been observed in mice with ERα mutations (21).
Interestingly, accumulating evidence indicates that estrogens have profound effects on circadian rhythms in females. For example, female hamsters show phase advances of locomotor activity and higher total activity during stages of proestrus and estrus relative to diestrus, and estrogen treatment advances the daily onset of activity (22). Similar effects were also observed in rats (23, 24). Recent studies provided molecular evidence that estrogens regulate the oscillation of circadian genes in various tissues, including the brain, liver, kidney, uterus, and ovary (25–27). These findings led us to hypothesize that estrogens may interact with the intrinsic circadian pacemakers and prevent metabolic deficits evoked by circadian disruptions.
In the current study, we first examined the metabolic consequences in OVX female mice in responses to a chronic JL paradigm, in the presence or the absence of estrogen replacement. Because we observed that the combination of JL and estrogen depletion led primarily to increased adiposity in mice, we then systemically examined the effects of estrogens on 24-hour oscillations of circadian genes in the white adipose tissue (WAT).
Materials and Methods
Mice
Female C57Bl6 mice (∼8 wk of age) were obtained from Baylor College of Medicine. All mice were maintained under standard pathogen-free conditions of 2–5 per cage at 22°C–24°C with a humidity of 50%–70%, a 12-hour light, 12-hour dark cycle (light on at 6 am and off at 6 pm) if no specific light/dark conditions were described in the text. The mice were fed standard chow (2919; Harlan-Teklad). All mouse experiments were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
OVX and estrogen replacement
Whole-body fat mass and lean mass were measured using quantitative magnetic resonance (QMR) before OVX surgeries, and these data were used to ensure that mice subjected to different treatments and circadian conditions (in the JL study) had comparable baseline fat mass and lean mass (see figure 2 below). As we described before (28), mice were anesthetized with isoflurane and received bilateral OVX, followed by sc implantations of pellets containing 17β-estradiol (0.5 μg/d per mouse for 90 d, OVX+E) or containing vehicle (OVX+V). Note that the dose of 17β-estradiol was chosen based on our previous observations that this dose prevented OVX-induced body weight gain but did not produced any body weight loss compared with the baseline body weight (28). These mice were then were used for the JL study or used to detect the circadian gene oscillation in fat tissues, as outlined below. Terminal analyses revealed that OVX+V mice displayed expected uterine atrophy which confirmed successful 17β-estradiol depletion, whereas OVX+E mice showed significantly heavier uterus than OVX+V mice (OVX+E, 162.60 ± 25.46 mg vs OVX+V, 10.36 ± 1.00 mg; P < .0001), indicating sufficient 17β-estradiol supplement.
JL study
JL and regular cycles (RCs)
After a 7-day recovery from the OVX+V/E surgeries, mice were divided to be subjected to either 9 repeats of the JL or RCs (n = 6–7 mice per drug treatment per circadian condition). As described before (29), 1 7-day JL cycle is comprised of the advanced phase (d 1–3, 10 pm/10 am light and 10 am/10 pm dark to induce 8 h advance in time) and the normal phase (d 4–7, 6 am/6 pm light and 6 pm/6 am dark). The transitions time was always at 9 am on day 1 (from the normal phase to the advanced phase) or day 4 (from the advanced phase to the normal phase). RCs are constantly at normal phase (6 am/6 pm light and 6 pm/6 am dark) (Figure 1A). Body weight and food intake were measured before the OVX surgeries and at the transition time points between the advanced phase and normal phase until the end of the study (9 wk after JL cycles). As described below, various measures were performed at different cycles but always on day 5. This ensured that at the time of these measures, JL mice had been in the normal phase for at least 24 hours.
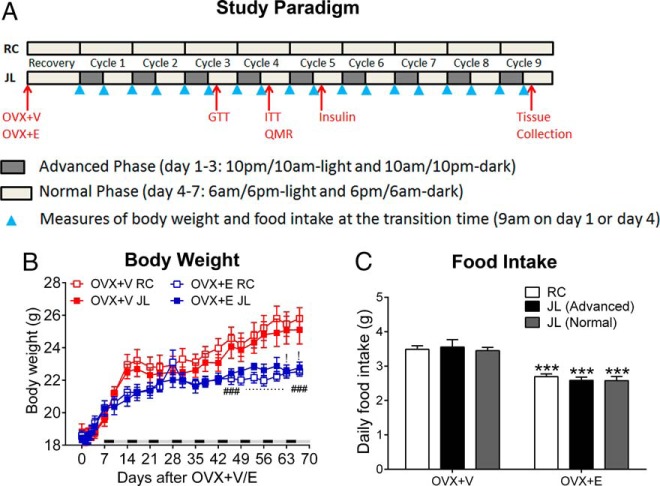
Figure 1.
A, Paradigm of the JL cycles and RCs. B, Body weight of female OVX+V or OXV+E mice subjected to JL or RC cycles; n = 6–7 per group. Data are presented as mean ± SEM. !, P < .05 between OVX+V JL and OVX+E JL; ###, P < .001 between OVX+V RC and OVX+E RC in two-way ANOVA analyses followed by post hoc Bonferroni tests. C, Averaged daily food intake of RC mice and JL mice (during advanced phase or normal phase); n = 6–7 per group. Data are presented as mean ± SEM. ***, P < .001 between OVX+V and OVX+E at the same circadian phase in two-way ANOVA analyses followed by post hoc Bonferroni tests.
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
The GTT and ITT were performed between 12 pm to 3 pm on day 5 of cycle 3 and cycle 4, respectively. GTT and ITT procedures have been described before (21, 30, 31). Briefly, for GTT, mice were fasted for overnight and then received an ip injection of glucose bolus (1 g/kg). Similarly, for ITT, mice were fasted for 2 hours (from 10 am to 12 pm to ensure empty stomachs) and then received an ip injection of insulin bolus (0.75 U/kg body weight). Blood glucose was measured from the tail blood using a glucometer at indicated time points in the figures.
Serum insulin
Tail blood was collected at 12 pm on day 5 in cycle 5, after a 2-hour fast to ensure empty stomachs. Blood samples were processed to obtain serum and insulin was measured using the ELISA kit (Crystal Chem, Inc), as we did before (30).
Terminal analyses
On day 5 in cycle 9 (from 1 to 4 pm, Zeitgeber time [ZT]13–ZT16 during the normal phase), these mice were deeply anesthetized and sacrificed. Gonadal WAT (gWAT) and inguinal WAT (iWAT) were isolated and weighed. One piece of these fat pads was fixed with 10% formalin overnight and then stored in 50% ethanol; 1 piece of these fat pads and the livers were quickly stored at −80°C and subjected to quantitative real-time PCR analyses as described below. The fixed fat pads were sent to Comparative Pathology Laboratory at Baylor College of Medicine, where the tissues were embedded with paraffin, sectioned and stained with hematoxylin and eosin (H&E). To determine adipocyte size in WAT tissues, pictures of the H&E staining were obtained using a Leica DM5500 compound epifluorescence microscope equipped with a CCD camera and analyzed using ImageJ software. A total of 800–1000 cells from each sample were included in the analysis.
Expression of various genes was measured in the frozen fat and liver tissues using the quantitative real-time PCR, as we described before (21). Briefly, tissues were homogenized with Qiazol Lysis reagent (QIAGEN). Total mRNAs were extracted using the RNeasy Lipid Tissue Mini kit (74804; QIAGEN) according to the instructions provided by the manufacturer. The total mRNAs were reverse transcribed to cDNAs using High Capacity cDNA Reverse Transcription kit (4368813; Applied Biosystems) according to the manufacturer's instructions. SYBR Green Real-Time PCR assays were performed according to published protocols (32). Normalized mRNA levels were expressed in arbitrary units obtained by dividing the averaged, efficiency-corrected values for sample mRNA expression by that for cyclophilin mRNA expression for each sample. The resulting values were expressed as fold change above average control levels. The primer sequences are as follow. Acetyl-coenzyme A (CoA) carboxylase α, F-ggcagctctggaggtgtatg and R-tccttaagctggcggtgtt; adipocyte protein 2 (AP2), F-gccaagcccaacatgatca and R-ttccacgcccagtttgaag; BMAL1, F-ccaagaaagtatggacacagacaaa and R-gcattcttgatccttccttggt; cluster of differentiation 36 (CD36), F-ggaactgtgggctcattgc and R-catgagaatgcctccaaacac; CLOCK, F-gaagaaacttttacaggcgttgttg and R- acgcaaggccgtcttctg; cyclophilin, F-tggagagcaccaagacagaca and R-tgccggagtcgacaatgat; CRY1, F-ctggcgtggaagtcatcgt and R-ctgtccgccattgagttctatg; CRY2, F-tgtcccttcctgtgtggaaga and R-gctccagcttggcttga; fatty acid synthase, F-gctgcggaaacttcaggaaat and R- agagacgtgtcactcctggactt; glucose-6-phosphatase (G6PC), F-gtggcagtggtcggagact and R-acgggcgttgtccaaac; phosphoenolpyruvate carboxykinase, F-cgcaagctgaagaaatatgacaa and R-tcgatcctggccacatctc; PER2, F-ggcattacctccgagtatatcgt and R-ggcgtccttcttacagtgaaag; PER3, F-ccttgctgtctaacactggttga and R-aaaattcttttatctactgggattcga; perilipin, F-tgctggatggagacctc and R-accggctccatgctcca; and stearoyl-CoA desaturase-1, F-ccggagaccccttagatcga and R-tagcctgtaaaagatttctgcaaacc.
Circadian gene expression in fat tissues
An independent cohort of female C57Bl6 mice received OVX+V or OVX+E surgeries at 8 weeks of age, as described above. One month after OVX+V or OVX+E surgeries, mice were deeply anesthetized and sacrificed at ZT1, ZT7, ZT13, and ZT19 (n = 4–5 per treat per time point). gWAT and iWAT were isolated and quickly stored at −80°C. Expression of circadian genes was measured using the quantitative real-time PCR, as described above.
Statistics
The data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism. Data were compared by one- or two-way ANOVA, followed by post hoc Bonferroni tests. P < .05 was considered to be statistically significant.
Results
Estrogen replacement prevents JL-induced metabolic deficits in OVX mice
We first assessed the role of estrogens on metabolic consequences induced by circadian disruptions in female mice. To this end, female C57Bl6 mice received bilateral OVX plus sc placebo pellets (OVX+V), or bilateral OVX plus sc 17β-estradiol pellets (0.5 μg/d lasting for 90 d, OVX+E). After recovery (1 wk), these mice were subjected to either RC or JL cycles, as described in Materials and Methods (Figure 1A).
We examined the role of 17β-estradiol and JL on metabolic parameters and their interactions using two-way ANOVA analyses. We first analyzed effects of 17β-estradiol (OVX+E vs OVX+V) in either RC or JL conditions. In both these circadian conditions, OVX+E mice showed significantly decreased body weight (Figure 1B), food intake (Figure 1C) and whole-body fat mass (Figure 2, A and B) compared with OVX+V mice, whereas the whole-body lean mass was comparable between OVX+E and OVX+V mice (Figure 2, C and D). Compared with OVX+V mice, both gWAT and iWAT in OVX+E mice showed significantly decreased weight and cell size (Figure 2, E–J). These findings are consistent with published reports (33, 34) and therefore validated our OVX+V and OVX+E models.
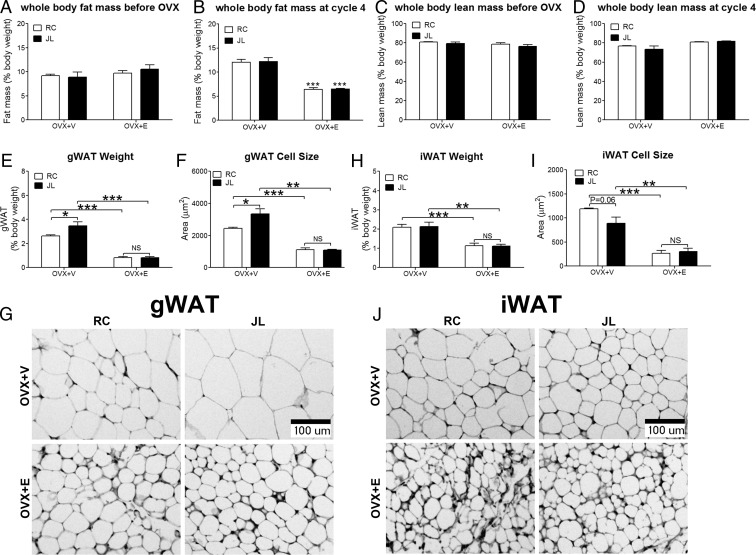
Figure 2.
A, Baseline whole-body fat mass measured by QMR before OVX surgeries; n = 6–7 per group. Data are presented as mean ± SEM. B, Whole-body fat mass measured by QMR at cycle 4 (5 wk after OVX+V or OVX+E); n = 6–7 per group. Data are presented as mean ± SEM. ***, P < .001 between OVX+V and OVX+E at the same circadian phase in two-way ANOVA analyses followed by post hoc Bonferroni tests. C, Baseline whole-body lean mass measured by QMR before OVX surgeries; n = 6–7 per group. Data are presented as mean ± SEM. D, Whole-body lean mass measured by QMR at cycle 4 (5 wk after OVX+V or OVX+E); n = 6–7 per group. Data are presented as mean ± SEM. E, Weight of gWAT fat pads (normalized by total body weight) measured at cycle 9; n = 6–7 per group. Data are presented as mean ± SEM. F, Averaged adipocyte cell size in gWAT; n = 3 per group. Data are presented as mean ± SEM. G, Representative H&E histology of adipocytes in gWAT. H, Weight of iWAT fat pads (normalized by total body weight) measured at cycle 9; n = 6–7 per group. Data are presented as mean ± SEM. I, Averaged adipocyte cell size in iWAT; n = 3 per group. Data are presented as mean ± SEM. J, Representative H&E histology of adipocytes in iWAT. In E, F, H, and I: *, P < .05; **, P < .01; ***, P < .001; NS, no significance in two-way ANOVA analyses followed by post hoc Bonferroni tests.
We further analyzed effects of JL (JL vs RC) in mice treated with OVX+E or OVX+V. We found that JL had no effect on body weight, as JL and RC mice showed similar body weight (Figure 1B). Similarly, JL had no effect on food intake in mice treated with OVX+V or OVX+E (Figure 1C). JL also had no effect on whole-body fat mass and lean mass (Figure 2, A–D). Interestingly, JL significantly increased the weight and cell size of gWAT in OVX+V mice, whereas these effects were abolished in OVX+E mice (Figure 2, E–G). On the other hand, JL did not affect the weight or cell size of iWAT (Figure 2, H–J). Collectively, these results show that JL in OVX+V female mice led to increased visceral fat accumulation independent of food intake, which was rescued in OVX+E mice.
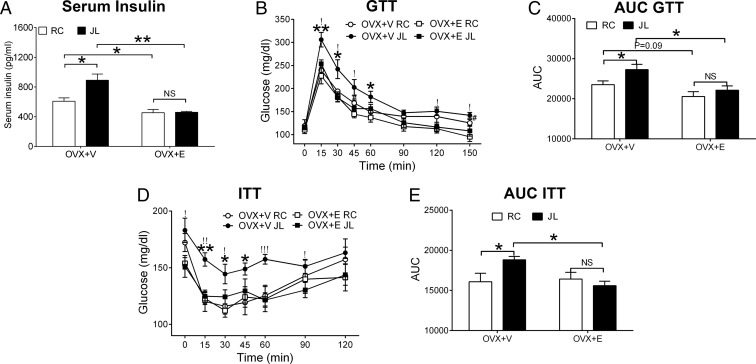
We found that JL induced hyperinsulinemia at fed condition in OVX+V mice, whereas this effect was abolished in OVX+E mice (Figure 3A). In the GTT and ITT, we found that JL induced glucose intolerance (Figure 3, B and C) and insulin resistance in OVX+V mice (Figure 3, D and E), the effects that were abolished in OVX+E mice. Thus, our data indicated that circadian disruptions in OVX mice led to insulin resistance and glucose intolerance; these JL-induced metabolic deficits were rescued in OVX+E mice.
Figure 3.
A, Serum insulin measured at cycle 5 from female OVX+V or OXV+E mice subjected to JL or RC; n = 6–7 per group. Data are presented as mean ± SEM. *, P < .05; **, P < .01; NS, no significance in two-way ANOVA analyses followed by post hoc Bonferroni tests. B, GTT performed at cycle 3; n = 6–7 per group. Data are presented as mean ± SEM. *, P < .05 or **, P < .01 between OVX+V JL and OVX+V RC; !, P < .05 between OVX+V JL and OVX+E JL; #, P < .05 between OVX+V RC and OVX+E RC in two-way ANOVA analyses followed by post hoc Bonferroni tests. C, Area under the curve (AUC) for GTT data in B; n = 6–7 per group. Data are presented as mean ± SEM. *, P < .05; NS, no significance in two-way ANOVA analyses followed by post hoc Bonferroni tests. D, ITT performed at cycle 4; n = 6–7 per group. Data are presented as mean ± SEM. *, P < .05 or **, P < .01 between OVX+V JL and OVX+V RC; !, P < .05; !!, P < .01; or !!!, P < .001 between OVX+V JL and OVX+E JL in two-way ANOVA analyses followed by post hoc Bonferroni tests. E, AUC for ITT data in D; n = 6–7 per group. Data are presented as mean ± SEM. *, P < .05; NS, no significance in two-way ANOVA analyses followed by post hoc Bonferroni tests.
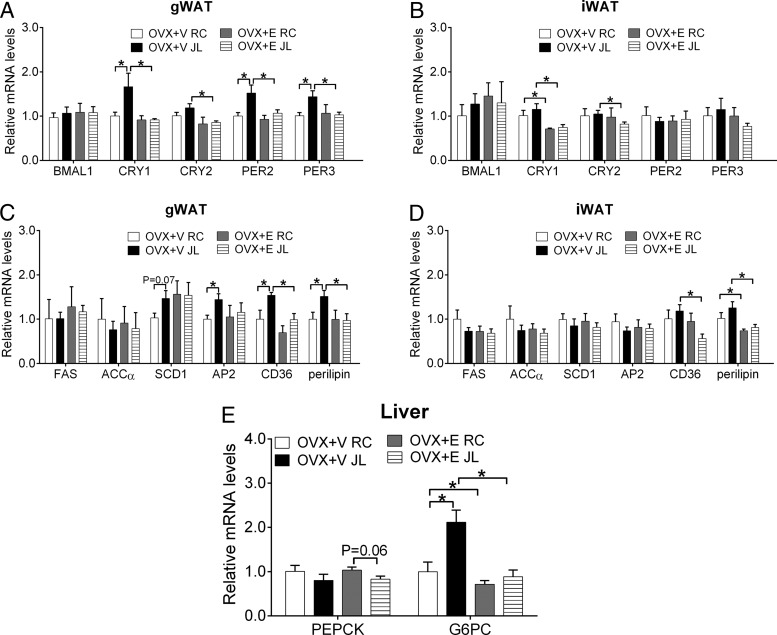
We analyzed expression levels of circadian genes in gWAT and iWAT at ZT13–ZT16. Expression BMAL1 in gWAT was comparable in all 4 groups of mice (Figure 4A). Interestingly, in OVX+V mice, JL significantly elevated gWAT levels of CRY1, PER2, and PER3 compared with RC; in OVX+E mice; however, levels of these genes were comparable between RC and JL conditions (Figure 4A). In addition, we observed that gWAT CRY2 in OVX+E JL mice were significantly lower than in OVX+V JL mice (Figure 4A). On the other hand, JL did not significantly alter these circadian genes in iWAT, although we observed that OVX+E mice showed significantly suppressed CRY1 and CRY2 expression under RC and/or JL conditions compared with OVX+V mice (Figure 4B).
Figure 4.
Relative mRNAs of indicated genes in gWAT (A and C), iWAT (B and D) and liver (E) from female OVX+V or OXV+E mice subjected to JL or RC. Note that tissues were isolated at ZT13–ZT16 during the normal phase of the 9th cycle; n = 6–7 per group. Data are presented as mean ± SEM. *, P < .05 in two-way ANOVA analyses followed by post hoc Bonferroni tests.
We further analyzed expression levels of genes related to lipid metabolism in gWAT and iWAT. Several different patterns were observed in gWAT genes. Thus, fatty acid synthase and acetyl-CoA carboxylase α were comparable among the 4 treatment groups (Figure 4C). Expression of stearoyl-CoA desaturase-1 trended to increase in JL OVX+V mice compared with RC OVX+V mice (Figure 4C). Interestingly, JL significantly increased expression of AP2, CD36, and perilipin in OVX+V mice, whereas these effects were abolished in OVX+E mice (Figure 4C). On the other hand, JL failed to significantly alter these genes in iWAT (Figure 4D). Notably, we found that CD36 and perilipin levels in iWAT were significantly reduced in OVX+E mice compared with OVX+V mice under the same circadian condition (Figure 4D).
We also analyzed expression levels of genes related to glucose metabolism in liver among the 4 groups. We found that JL trended to decrease phosphoenolpyruvate carboxykinase in OVX+E mice without reaching statistical significance (Figure 4E). Interestingly, expression of G6PC was significantly elevated by JL in OVX+V mice, whereas these effects were abolished in OVX+E mice (Figure 4E). Notably, G6PC levels were significantly reduced in OVX+E mice compared with OVX+V under the same circadian condition (Figure 4E).
Estrogens regulate oscillations of circadian genes in gWAT
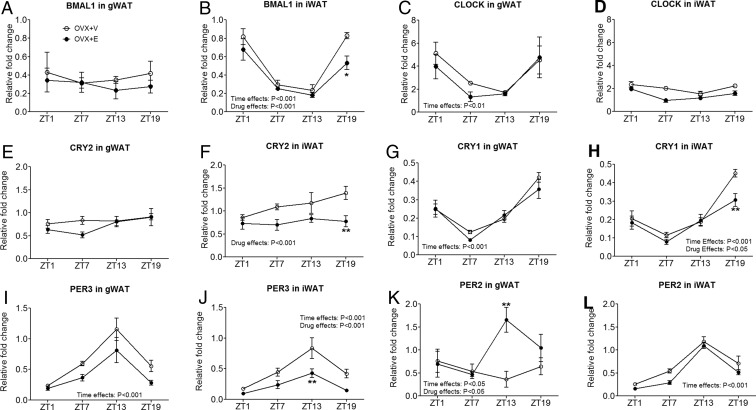
Estrogens have been shown to regulate the oscillation of circadian genes in reproductive and a number of nonreproductive tissues (25–27). Our observations from OVX females subjected to JL paradigm suggest a potential interplay between estrogenic actions and circadian clock in WAT. To address this, we examined 24-hour oscillations of the core circadian pacemakers (BMAL1 and CLOCK) in WAT from female C57Bl6 mice that were deprived with endogenous estrogens (OVX+V) or supplemented with 17β-estradiol (OVX+E). In gWAT, BMAL1 demonstrated a modest oscillation (Figure 5A), whereas a more robust oscillation was observed in CLOCK expression during 24-hour period (Figure 5C). Interestingly, the opposite pattern was observed in iWAT with a more robust oscillation in BMAL1 expression but a modest oscillation in CLOCK (Figure 5B). Importantly, these BMAL1/CLOCK expression patterns were largely comparable between OVX+V and OVX+E mice (Figure 5, A, C, and D), except that BMAL1 in iWAT was significantly decreased at ZT19 in OVX+E mice compared with OVX+V mice (Figure 5B).
Figure 5.
Levels of mRNAs of indicated genes at ZT1, ZT7, ZT13, and ZT19, in gWAT or iWAT of OVX+V or OVX+E female mice; n = 4–5 per group. Data are presented as mean ± SEM. Overall effects of drug (OVX+V vs OVX+E) or those of time (ZT1, ZT7, ZT13, and ZT19) in two-way ANOVA analyses are indicated as text. *, P < .05; **, P < .01 in two-way ANOVA analyses followed by post hoc Bonferroni tests.
We then examined expression pattern of CRY1, CRY2, PER2, and PER3, the direct transcriptional targets of the BMAL1/CLOCK1 complex. Several different patterns were observed. CRY2 expression in iWAT but not in gWAT oscillated in OVX+V mice (Figure 5, E and F); OVX+E mice showed significantly decreased CRY2 in iWAT at ZT19 compared with OVX+V mice (Figure 5F). CRY1 and PER3 oscillated in both gWAT and iWAT (Figure 5, G–J). OVX+E had no overall effects on the 24-hour oscillating patterns of these 2 genes, although CRY1 at ZT19 and PER3 at ZT13 were significantly decreased in OVX+E mice compared with OVX+V mice (Figure 5, H and J). Interestingly, PER2 expression showed different patterns in gWAT vs iWAT. In gWAT, PER2 was found to oscillate only in OVX+E mice, but this circadian pattern was significantly blunted in OVX+V mice (Figure 5K). On the other hand, PER2 in iWAT oscillated in both OVX+V and OVX+E mice (Figure 5L). These suggest that estrogen signals are required to maintain the normal oscillation of PER2 in gWAT but not in iWAT.
Discussion
The interactions of the circadian clocks and estrogens have been implicated in earlier studies showing different 24-hour locomotor rhythms at different estrus stages (22–24). However, questions remain regarding how the circadian clocks and estrogenic signals interact. On one hand, it has been reported that shift work can disrupt menstrual cycles in women (35), suggesting an impact of the circadian rhythm on the physiological functions of female sex hormones (including estrogens). On the other hand, estrogenic signals may also reciprocally influence the circadian clocks. Here, we focused on this latter aspect by systemically comparing the metabolic consequences induced by a chronic circadian-disrupting paradigm (JL) in female mice treated with OVX+V or OVX+E. The key finding is that circadian disruption in OVX+V mice led to increased fat accumulation preferentially in the visceral fat depot, which was associated with hyperinsulinemia, insulin insensitivity and glucose intolerance. Remarkably, none of these metabolic parameters was altered by circadian disruptions in OVX+E mice. Given that increased visceral fat is commonly associated with insulin resistance and glucose intolerance (36), one possibility is that insulin/glucose phenotypes observed in JL OVX+V mice may be attributed to the increased visceral fat accumulation. Consistent with a potential role of estrogens in adipose tissue in the regulation of insulin sensitivity, it has been recently reported that selective deletion of ERα from adipocytes in mice leads to insulin resistance associated with hyperadiposity (37).
Alternatively, the insulin/glucose phenotypes may also partly result from dysfunctions of other metabolic tissues, eg, the liver. For example, it has been shown that estrogen replacement delays the phase and increases expression of PER1 mRNA in rat liver (26). In addition, genetic deletion of ERα in the liver blocks estrogenic actions to improve insulin sensitivity and to suppress hepatic glucose production in mice (38). Further, we found that expression of G6PC in the liver, a key enzyme for hepatic gluconeogenesis, were significantly elevated in JL OVX+V mice, but these alterations were not observed in JL OVX+E mice. Thus, our data also suggest a possibility that the insulin resistance observed in JL OVX+V mice may be attributed partly to impaired estrogen-circadian interactions in the liver.
It is surprising to us that the circadian disruption induced by JL cycles did not increase body weight and food intake even in OVX+V mice. It appears to be inconsistent with earlier reports that other circadian misalignments (eg, light during the night, or 20-h daily cycles) lead to rapid body weight gain and hyperphagia in male mice (5, 6). Of course, this discrepancy could be attributed, at least partly, to different paradigms used to disrupt the normal circadian cycles in these studies and/or to the sex difference. Another possibility is that the remarkable body weight gain and hyperphagia induced by estrogen depletion per se may have masked effects of circadian disruption on these metabolic parameters. In addition, it is important to point out that OVX+V and OVX+E female mice have either no estrogens or constant high estrogens. Although it is a powerful model to study functions of estrogens, potential effects of circadian disruption on estrogen synthesis and/or secretion may have been confounded in this model, which could also explain the lack of body weight and food intake effects.
Nevertheless, despite that no difference was observed in body weight and whole-body fat mass, significant increases in the weight and cell size of visceral fat depot (gWAT) were detected in OVX+V mice subjected to JL cycles vs OVX+V mice subjected to regular cycles. Importantly, these differences were completely rescued in OVX+E mice. Consistent with these changes in gWAT weight and cell size, we found that gWAT genes that are implicated in lipid trafficking (AP2) (39), transport (CD36) (40), and storage (perilipin) (41) were significantly elevated in JL OVX+V mice, but these alterations were normalized in JL OVX+E mice. Interestingly, these changes in gWAT, including weight, cell size and expression of lipid-related genes, were associated with alterations in expression of multiple circadian genes in gWAT. In particular, levels of CRY1, PER2, and PER3 in gWAT were elevated by the JL in OVX+V mice, but these elevations were normalized in OVX+E mice. Thus, our data indicate that these circadian genes may serve as molecular nodes to integrate the circadian cues and estrogenic signals in adipocytes. Notably, studies using genetic knockout mouse models have started to reveal important roles of the circadian genes in energy homeostasis and lipid metabolism. For example, it was recently reported that mice lacking CRY1 are more resistant to diet-induced obesity (42). Similarly, mice lacking PER2 are leaner compared with wild-type mice (43). Collectively, these observations suggest a possibility that multiple circadian genes in adipocyte may, at least partly, mediate the protective effects of estrogens on lipid metabolism during circadian disruptions.
Notably, although the weight, cell size and multiple gene expression in gWAT were synergistically affected by the combination of JL and estrogen depletion, these phenotypes were not observed in sc fat depot (iWAT). A similar fat depot specificity was also observed when we examined the effects of estrogens on circadian gene expression in gWAT and iWAT. Thus, we found that one of the circadian clock genes, PER2, oscillates in gWAT in an estrogen-dependent manner. Importantly, the 24-hour oscillations in PER2 expression in iWAT are comparable between OVX+V and OVX+E mice. Although it is compelling that the estrogen-circadian interactions play a more dominant role in the visceral fat depot than in the sc fat depot, the molecular basis accounting for this depot-specific action is not clear. One possibility is that estrogenic actions are dominant in visceral fat depot than in sc fat depot, and therefore, when estrogens are depleted, the visceral fat depot becomes more susceptible to metabolic challenges, eg, circadian disruptions. Supporting this, mice lacking ERα showed more robust increases in visceral fat than in sc fat (21). Notably, we observed a more robust BMAL1 oscillation but a modest CLOCK oscillation in gWAT. On the other hand, in iWAT, CLOCK oscillation appears to be more robust than that of BMAL1. Thus, these different oscillating patterns of core circadian genes could also partly contribute to the different estrogen-circadian interactions in these 2 fat depots.
The potent antiobesity and antidiabetic effects of estrogens have been well documented in animal models (15, 21, 33, 44, 45). Mutations in the ERα gene are also strongly associated with obesity and diabetes in humans (46, 47). In addition, the decline in circulating estrogens in postmenopausal women has been associated with development of obesity, type 2 diabetes and the metabolic syndrome (48). Interestingly, a large epidemiological study showed that when compared with age-matched women day workers, female shift workers have significantly higher prevalence for glucose intolerance only in older group (>60 y of age) but not in younger groups (49). These are consistent with our observations that circadian disruptions lead to metabolic deficits only in OVX+V mice, but not in OVX+E mice. One implication of these results is that the metabolic consequences of menopause in women could be manifested by concurrent circadian disruptions (eg, shift work or frequent international travel). Therefore, future studies are needed to further test the possibility that the estrogen replacement therapy could be used to prevent the metabolic dysfunctions, at least in postmenopausal women with frequent circadian disruptions.
Acknowledgments
This work was supported by National Institutes of Health Grants R01DK093587, R01DK101379, and P30 DK079638 (to Y.X.), R01DK092605 (to Q.T.), and R00DK099443 (to Z.S.); by an American Heart Association postdoctoral fellowship (P.X.); and by the National Natural Science Foundation of China Grant 81200623 (to L.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP2
- adipocyte protein 2
- BMAL1
- brain and muscle Arnt-like 1
- CD36
- cluster of differentiation 36
- CLOCK
- circadian locomotor output cycles kaput
- CoA
- coenzyme A
- CRY
- cryptochrome
- E
- 17β-estradiol
- ER
- estrogen receptor
- G6PC
- glucose-6-phosphatase
- GTT
- glucose tolerance test
- gWAT
- gonadal WAT
- H&E
- hematoxylin and eosin
- ITT
- insulin tolerance test
- iWAT
- inguinal WAT
- JL
- jet-lag
- OVX
- ovariectomy
- PER
- period circadian protein homolog
- QMR
- quantitative magnetic resonance
- RC
- regular cycle
- V
- vehicle
- WAT
- white adipose tissue
- ZT
- Zeitgeber time.
References
- 1. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. [DOI] [PubMed] [Google Scholar]
- 8. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. [DOI] [PubMed] [Google Scholar]
- 9. Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woon PY, Kaisaki PJ, Bragança J, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104:14412–14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond). 2008;32:658–662. [DOI] [PubMed] [Google Scholar]
- 12. Drewett RF. Sexual behaviour and sexual motivation in the female rat. Nature. 1973;242:476–477. [DOI] [PubMed] [Google Scholar]
- 13. Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. [DOI] [PubMed] [Google Scholar]
- 14. Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr. 2001;131:2351–2357. [DOI] [PubMed] [Google Scholar]
- 15. Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology. 2001;142:4751–4757. [DOI] [PubMed] [Google Scholar]
- 16. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bjorntorp P. Hormonal control of regional fat distribution. Hum Reprod. 1997;12(suppl 1):21–25. [DOI] [PubMed] [Google Scholar]
- 18. Björntorp P. Obesity. Lancet. 1997;350:423–426. [DOI] [PubMed] [Google Scholar]
- 19. Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795–803. [DOI] [PubMed] [Google Scholar]
- 20. Kotani K, Tokunaga K, Fujioka S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–202. [PubMed] [Google Scholar]
- 21. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. [DOI] [PubMed] [Google Scholar]
- 23. Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241:R62–66. [DOI] [PubMed] [Google Scholar]
- 24. Wollnik F, Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol Behav. 1988;43:389–396. [DOI] [PubMed] [Google Scholar]
- 25. Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S. The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci USA. 2006;103:5591–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura TJ, Moriya T, Inoue S, et al. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005;82:622–630. [DOI] [PubMed] [Google Scholar]
- 27. Nakamura TJ, Sellix MT, Kudo T, et al. Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels. Steroids. 2010;75:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu L, Yang Y, Xu P, et al. Steroid receptor coactivator-1 mediates estrogenic actions to prevent body weight gain in female mice. Endocrinology. 2013;154:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010;5:e10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu Y, Berglund ED, Sohn JW, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010;13:1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hill JW, Xu Y, Preitner F, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150:4874–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. [DOI] [PubMed] [Google Scholar]
- 34. Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Attarchi M, Darkhi H, Khodarahmian M, et al. Characteristics of menstrual cycle in shift workers. Glob J Health Sci. 2013;5:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008;34:317–327. [DOI] [PubMed] [Google Scholar]
- 37. Davis KE, D Neinast M, Sun K, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu L, Brown WC, Cai Q, et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maeda K, Cao H, Kono K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. [DOI] [PubMed] [Google Scholar]
- 40. Neculai D, Schwake M, Ravichandran M, et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature. 2013;504:172–176. [DOI] [PubMed] [Google Scholar]
- 41. Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem. 2009;326:15–21. [DOI] [PubMed] [Google Scholar]
- 42. Griebel G, Ravinet-Trillou C, Beeske S, Avenet P, Pichat P. Mice deficient in cryptochrome 1 (cry1 (−/−)) exhibit resistance to obesity induced by a high-fat diet. Front Endocrinol (Lausanne). 2014;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grimaldi B, Bellet MM, Katada S, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. [DOI] [PubMed] [Google Scholar]
- 45. Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gallagher CJ, Langefeld CD, Gordon CJ, et al. Association of the estrogen receptor-α gene with the metabolic syndrome and its component traits in African-American families: the Insulin Resistance Atherosclerosis Family Study. Diabetes. 2007;56:2135–2141. [DOI] [PubMed] [Google Scholar]
- 47. Sakka C, Efstathiadou ZA, Polyzos SA, Goutou M, Stakias N, Koukoulis GN. Associations of estrogen receptor α and β gene polymorphisms with sex steroid levels and body fat content in men. Exp Clin Endocrinol Diabetes. 2012;120:154–159. [DOI] [PubMed] [Google Scholar]
- 48. Allende-Vigo MZ. Women and the metabolic syndrome: an overview of its peculiarities. P R Health Sci J. 2008;27:190–195. [PubMed] [Google Scholar]
- 49. Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]