Abstract
Glucose is an essential nutrient that directly regulates the expression of numerous genes in liver and adipose tissue. The carbohydrate response element–binding protein (ChREBP) links glucose as a signaling molecule to multiple glucose-dependent transcriptional regulatory pathways, particularly genes involved in glycolytic and lipogenic processes. In this study, we used chromatin immunoprecipitation followed by next-generation sequencing to identify specific ChREBP binding targets in liver and white adipose tissue. We found a large number of ChREBP binding sites, which are attributable to 5825 genes in the liver, 2418 genes in white adipose tissue, and 5919 genes in both tissues. The majority of these target genes were involved in known metabolic processes. Pathways in insulin signaling, the adherens junction, and cancers were among the top 5 pathways in both tissues. Motif analysis revealed a consensus sequence CAYGYGnnnnnCRCRTG that was commonly shared by ChREBP binding sites. Putative ChREBP binding sequences were enriched on promoters of genes involved in insulin signaling pathway, insulin resistance, and tumorigenesis.
Glucose is perhaps a most important and, in some tissues, the preferred fuel for cellular metabolism. After a meal rich in carbohydrates, blood glucose increases sharply, which induces pancreatic β-cells to release insulin. The insulin stimulates glucose uptake in the peripheral tissues such as muscle, liver, and adipose tissues via insulin receptor-mediated signaling pathways while it represses hepatic glucose production. Insulin also up-regulates sterol regulatory element binding protein-1c (SREBP-1c), the major lipogenic transcription factor in the liver that orchestrates the conversion of glucose into triglyceride for storage and subsequent export (1). When the blood glucose level drops during fasting, glucagon released from the pancreatic β-cells stimulates the liver (and kidney to a lesser extent) to produce glucose especially for the brain, which prefers to use glucose for metabolism, maintaining whole-body glucose homeostasis.
In addition to its effects on hormones such as insulin and glucagon, glucose can also directly modulate the expression of key enzymes in glycolytic and lipogenic pathways independent of the insulin-SREBP-1c axis (2). Studies of these glucose-regulated genes identified a sequence motif called carbohydrate response element (ChoRE) that is present in the promoter of many glycolytic and lipogenic genes (3, 4). Using synthetic oligonucleotides containing ChoRE sequence, Yamashita et al (5) isolated a new transcription factor, the carbohydrate response element–binding protein (ChREBP) from rat liver extracts (5). ChREBP, a 98-kDa protein, is a basic helix-loop-helix/leucine zipper transcription factor that belongs to the Mondo family.
The activity of ChREBP is determined by its phosphorylation state and subcellular localization (6). In addition to phosphorylation/dephosphorylation regulation, our structure-function analysis of ChREBP protein revealed an intramolecular inhibition in low glucose conditions through a glucose-sensing module that renders ChREBP responsive to glucose (7–9). The glucose-sensing module is conserved across all Mondo family genes and encompasses 2 subdomains: a low-glucose inhibitory domain (LID) and a glucose-response activation conserved element. At low glucose concentrations, the LID inhibits the transactivation activity of glucose-response activation conserved element, whereas in the presence of abundant glucose, the inhibition of LID-mediated inhibition is released, probably through the glucose metabolite, glucose-6-phosphate, and ChREBP is activated (8).
Recently, it has been proposed that in pancreatic β-cells, sorcin, a penta-EF-hand protein, binds to ChREBP and sequesters it in the cytosol under low glucose concentrations, but when stimulated with glucose and accompanied by calcium ion influx in β-cells, the conformation of sorcin changes, releasing ChREBP to enter into the nucleus (10, 11). Thus, the activity of ChREBP appears to be regulated by multiple factors in different types of cells. ChREBP does not work alone; it regulates glucose-responsive genes in the form of a heterodimeric complex with a partner, Max-like protein X (Mlx) (3, 12, 13). For this reason, ChREBP is also known as Mlx-interacting protein-like (Mlxipl), and the transactivation and inhibitory activities of ChREBP appear to be associated with the heterodimer complex (12–15).
The functional importance of ChREBP was further highlighted by the phenotype of ChREBP-knockout mice (16–18). Although these mice survive without a functional ChREBP, they exhibit suppressed lipogenesis and glycolysis; they are intolerant to high sucrose and high fructose diets and have reduced epididymal and brown fat mass compared to their wild-type littermates (16, 18).
Because liver and adipose tissue are the 2 major lipogenic tissues in mice, we reasoned that ChREBP must play a major function in these 2 organs/tissues. In this study, we have identified the putative genes controlled by ChREBP in these 2 tissues by a genome-wide analysis using chromatin immunoprecipitation followed by next-generation sequencing (ChIP-seq) technology. We believe that use of this unbiased global approach will enable us to further define the general and distinctive roles of ChREBP in these 2 tissues.
Materials and Methods
Animals
C57BL/6 male mice were purchased from The Jackson Laboratory and were maintained in the accredited pathogen-free Baylor College of Medicine transgenic mouse facility. All experiments were performed after approval of the protocol by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
ChIP-seq
Three-month-old male C57BL/6J mice were fasted for 24 hours followed by feeding with a high-carbohydrate fat-free diet (catalog number 901683; MP Biomedicals) for 18 hours. Mice were killed, and liver and white adipose tissue (WAT) were removed and frozen immediately in liquid nitrogen. Frozen tissues were shipped to Genpathway, Inc for further processing and ChIP-seq analysis as outlined below.
ChIP
Frozen tissue samples were submersed in PBS with 1% formaldehyde, cut into small (∼1 mm3) pieces with a razor blade, and incubated at room temperature for 15 minutes. Fixation was stopped by the addition of 0.125 M glycine (final). The tissue pieces were then treated with a Tissue-Tearor and finally spun down and washed twice in PBS. Chromatin was isolated from the sample by addition of 5 to 10 mL of lysis buffer containing PIPES, Igepal, phenylmethylsulfonylfluoride, and protease inhibitor cocktail, followed by disruption with a Dounce homogenizer. Samples were pelleted by centrifugation and resuspended in a buffer containing sodium deoxycholate, SDS, and Triton X-100. Lysates were sonicated using a Misonix Sonicator 3000 equipped with a microtip to shear the DNA to an average length of 300 to 500 bp. Lysates were cleared by centrifugation, and the chromatin suspensions were transferred to new tubes and stored at −80°C. The preceding procedure was directly applied to liver tissues. The fat tissue was processed following a similar protocol, with additional modifications as described (19).
For preparation of input genomic DNA, 2 aliquots of 10 to 25 μL each (approximately of each chromatin preparation) were removed and treated with RNase for 1 to 2 hours at 37°C, proteinase K for 3 hours at 37°C, and 65°C heat for at least 6 hours to overnight for de-cross-linking. DNAs were purified by phenol-chloroform extraction and ethanol precipitated. Pellets were resuspended in TE (10mM Tris–1mM EDTA) buffer. The resulting DNAs were quantified on a Nanodrop spectrophotometer. Extrapolation to the original chromatin volume allowed determination of the yield for each chromatin preparation (as measured by the DNA content).
Before use in ChIP, protein A agarose beads (Invitrogen) were preblocked using blocking proteins and nucleic acids for 3 hours. For each ChIP reaction, an aliquot of chromatin (20–30 μg) was precleared with 30 μL of preblocked protein A agarose beads for 1 to 2 hours. ChIP reactions were set up using precleared chromatin and ChREBP antibody (NB400-135; Novus Biologicals) in a buffer containing sodium deoxycholate and were incubated overnight at 4°C. Preblocked protein A agarose beads were added, and incubation at 4°C was continued for another 3 hours. Agarose beads containing the immune complexes were washed 2 times each with a series of buffers consisting of the deoxycholate sonication buffer, high salt buffer, LiCl buffer, and TE buffer. An SDS-containing buffer was added to elute the immune complexes from the beads, and the eluates were subjected to RNase treatment at 37°C for 20 minutes and proteinase K treatment at 37°C for 3 hours. Cross-links were reversed by incubation overnight at 65°C, and ChIP DNAs were purified by phenol-chloroform extraction and ethanol precipitation. The quality of ChIP enrichment was assayed by quantitative PCR (qPCR) using primers against candidate binding sites (Supplemental Table 1). Input DNA was queried at the same sites in parallel. Statistical analysis was performed using t test and a significance level of .05.
ChIP-seq
ChIP DNA was amplified by following the Illumina ChIP-seq DNA Sample Prep Kit protocol. In brief, DNA ends were polished and 5′ phosphorylated using T4 DNA polymerase, Klenow polymerase, and T4 polynucleotide kinase. After addition of 3′-A to the ends using the Klenow fragment (3′–5′ exonuclease minus), Illumina genomic adapters were ligated, and the sample was size fractionated (200–250 bp) on a 2% agarose gel. After a final PCR amplification step (18 cycles, Phusion polymerase), the resulting DNA libraries were quantified and tested by qPCR at the same specific genomic regions as those for the original ChIP DNA to assess the quality of the amplification reactions. DNA libraries were sequenced on a Genome Analyzer II. Sequences (36-nucleotide reads) were aligned to the mouse genome (National Center for Biotechnology Information [NCBI] Build 37.1/mm9) using ELAND (Illumina pipeline) software. The analysis only uses unique mapped reads with no more than 2 mismatches.
Analysis of ChIP-seq result
We used model-based analysis of ChIP-seq (MACS) to do the peak detection after the alignment. MACS produced the tag size shifting and used a dynamic Poisson distribution to simulate the local biases (20). MACS used the predicted fragment size to shift and extend the tags to generate wiggle format files, which stored the signal profiling information and were visualized by the Affymetrix Integrated Genome Browser (21) or UCSC Genome Browser (22). The maximal distance for protein-DNA interaction was set at 10 kb. Target genes were reported and annotated. In addition, the distance from the peak center to the transcription start site (TSS) and the score of the peak were also listed.
We conducted GO (Gene Ontology) slim classification of genes by the web-based GEne SeT AnaLysis Toolkit (WebGestalt) (23). Pathway analysis was performed using the National Institutes of Health DAVID (Database for Annotation, Visualization, and Integrated Discovery) (24). For motif finding, we used MEME Suite (25) with the default settings except that the expected motif site is any repetitions and the find uncentered regions option is selected. Logos of different motifs were generated from MEME-ChIP analysis. We used Regulatory Sequence Analysis Tools to generate the final consensus sequence logo and predict the ChoRE sequence in ChIP-seq intervals (26).
Results
Genome-wide analysis of ChREBP binding sites in liver and WATs
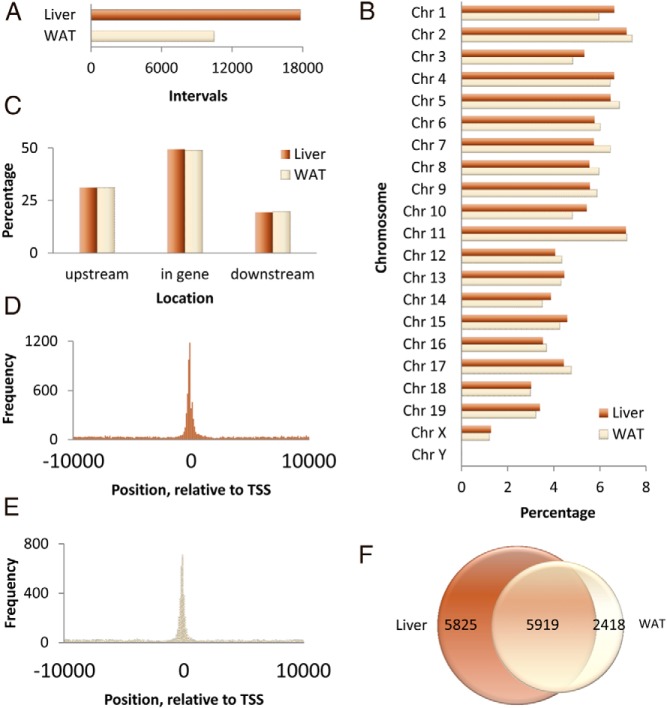
Male C57BL/6J mice were fasted for 24 hours and refed a high carbohydrate diet for 18 hours before they were killed. Liver and epididymal WAT were processed for ChIP-Seq using an antibody against ChREBP protein as described in Materials and Methods. This antibody recognizes both the α and β (27) isoforms of ChREBP. The total number of ChREBP DNA binding sites or ChIP interval was 17 802 in the liver and 10 477 in the WAT (Figure 1A). We found that the frequency of ChREBP binding sites is highest on chromosome 2 (7.39% of WAT ChIP intervals and 7.16% of liver ChIP intervals) and lowest on chromosome Y (0.01% of WAT ChIP intervals and 0.02% of liver ChIP intervals) (Figure 1B).
Figure 1.
Characteristics of anti-ChREBP ChIP-enriched DNA fragments in mouse liver and WAT after fasting followed by refeeding with a high-sucrose diet. A, Number of intervals presented in ChIP-seq of liver and WAT. B, Percentage of intervals located on each mouse chromosome in liver and WAT. C, Percentage of intervals located upstream of gene and downstream of gene in liver and WAT. D, Frequency of ChIP intervals in the liver specimen from the position −10 kb to 10 kb relative to the TSS of each gene. E, Frequency of ChIP intervals in the WAT specimen from the position −10 kb to 10 kb relative to the TSS of each gene. F, Number of genes related to intervals in liver and WAT.
Of these binding sites, about one third (31.11% in the liver and 31.21% in the WAT) are located upstream of the transcription initiation site (Figure 1C). About half (49.45% in the liver and 49.00% in the WAT) are found within the genes. The rest of the binding sites are located in the distal intergenic region (19.44% in the liver and 19.79% in the WAT). Binding sites are especially enriched in the close vicinity region of the TSSs in both liver (Figure 1D) and WAT (Figure 1E). Approximately one fifth (21.82% in the liver and 20.90% in the WAT) of them fall on the proximal promoter region roughly defined as 250 bp around the transcription initiation site.
These binding sites are attributable to 8337 and 11 744 genes in the WAT and liver, respectively, which include 5919 genes that are common between these 2 tissues (Figure 1F). Because there are multiple binding sites in a gene, the total number of binding sites is much larger than the total number of genes.
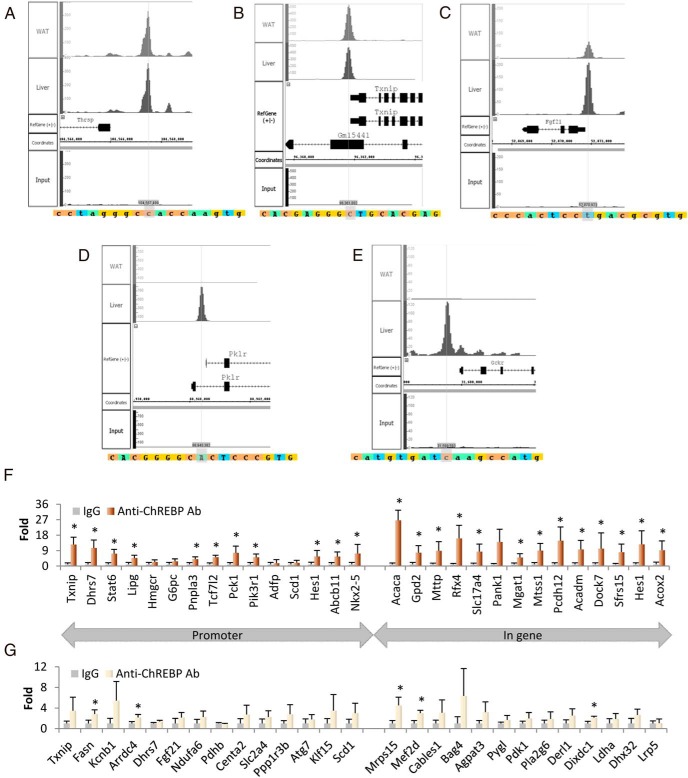
Validation of ChIP-seq data
We determined whether previously identified ChoREs of target genes are present in our ChIP interval sequences. The peaks of ChREBP binding in 5 representative genes are shown in Figure 2. ChREBP binds to the promoters of thyroid hormone–responsive (Thrsp; also known as S14) and thioredoxin-interacting protein (Txnip) genes in both the liver and WAT with similar peak distribution (Figure 2, A and B), whereas ChREBP preferentially binds to the promoters of fibroblast growth factor 21 (Fgf21) in both liver and WAT but with a higher preference for the liver (Figure 2C). Interestingly, the promoters of liver-type pyruvate kinase (Pklr) and glucokinase regulatory protein (Gckr) show binding by ChREBP exclusively in the liver and not at all in WAT (Figure 2, D and E). As expected, all of these ChoRE sequences reside in ChIP-seq peak areas.
Figure 2.
Validation of anti-ChREBP ChIP-seq results in mouse liver and WAT. A–E, The presence of known ChoRE sequences on the ChIP-seq peaks. We explored the ChIP-seq data using the Integrated Genome Browser and demonstrated the presence of known ChoRE sequences at the summit of ChIP-seq peaks of Thrsp (A), Txnip (B), Fgf21 (C), Pklr (D), and Gckr (E). F and G, Fold enrichment of 29 ChIP DNA fragments in liver (F) and 27 fragments in WAT (G). We performed anti-ChREBP ChIP in a separate group of mice and randomly evaluated ChIP-enriched DNA fragments located within the promoter (15 genes in liver and 14 genes in WAT) or in the gene (14 genes in liver and 13 genes in WAT) using qPCR. The error bars are SD. *, P < .05. Ab, antibody.
To more thoroughly validate the ChIP-seq results, we fasted and refed another group of C57BL/6J mice (n = 4) and performed ChIP with the same ChREBP antibody in the isolated liver and WAT samples, using preimmune rabbit IgG as an additional control. We randomly selected 13 to 15 ChREBP binding regions each from the liver and adipose tissues, a total of 56 genomic regions, to validate the ChIP-seq results using qPCR (Figure 2, F and G). In the promoter regions of the hepatic genes chosen, 11 of 15 ChREBP binding sites are enriched in the DNA samples immunoprecipitated with ChREBP antibody (Figure 2F) compared with that with control rabbit IgG. In the 14 intronic or intergenic regions of the liver genes that we tested, all but 1 of the ChREBP-binding sites were enriched in the DNA samples immunoprecipitated with ChREBP antibody compared with that with control rabbit IgG (Figure 2F). Although most of the 27 regions of the adipose genes were more than 2-fold enriched, only 5 regions reached statistical significance because of substantial variability (Figure 2G).
Pathway analysis
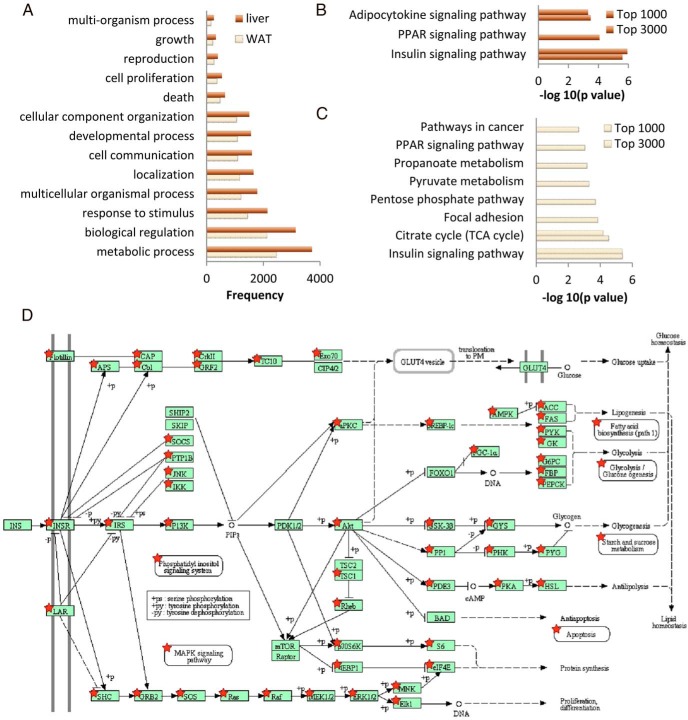
Gene set analysis revealed that most ChIP-based ChREBP target genes are involved in the metabolic process (72.02% and 70.66% of classifiable genes in the liver and WAT, respectively) and biological regulation (60.84% and 61.24% of classifiable genes in the liver and WAT, respectively) (Figure 3A and Supplemental Figure 1, A and B).
Figure 3.
Classification and pathway analysis of genes related to ChREBP binding. A, Frequency of genes identified by anti-ChREBP ChIP-seq that are involved in biological processes. We generated the list of biological processes from WebGestalt using GO slim classification. B and C, Enriched pathways identified by DAVID for the top 1000 and top 3000 genes with high peak intensity in liver and WAT, respectively. D, Genes in the KEGG (Kyoto Encyclopedia of Genes and Genomes) insulin signaling pathway that are related to ChREBP binding (★).
We next performed pathway analysis of the genes that exhibited ChREBP binding to obtain a global picture of biological pathways that may be regulated by ChREBP. Table 1 shows biological pathways relevant to ChIP-seq–based ChREBP target genes in liver and WAT. Three pathways with significant numbers of genes exhibiting ChREBP binding that are among the top 5 pathways in both adipose and liver tissues are the insulin signaling pathway, the adherens junction, and pathways in cancers. Pathways involved in metabolism (citrate cycle, propanoate, and purine metabolism) are among the ChREBP-regulated pathways in both liver and WAT. Various signaling pathways, including MAPK, ErbB, Wnt, neurotrophin, and adipocytokine signaling, were also among the list in both tissues. Genes involved in RNA and protein degradation were also represented, which suggests possible regulation by ChREBP.
Table 1.
Pathway Analysis of Gene Lists by DAVID
| Tissue | Term | Count | P Value | Benjamini |
|---|---|---|---|---|
| Liver | mmu04520: Adherens junction | 54 | 3.0E–09 | 5.8E–07 |
| Liver | mmu04910: Insulin signaling pathway | 77 | 4.8E–06 | 4.7E–04 |
| Liver | mmu05200: Pathways in cancer | 156 | 7.8E–06 | 5.0E–04 |
| Liver | mmu04120: Ubiquitin mediated proteolysis | 75 | 1.2E–05 | 5.6E–04 |
| Liver | mmu00380: Tryptophan metabolism | 29 | 1.5E–05 | 6.0E–04 |
| Liver | mmu00250: Alanine, aspartate, and glutamate metabolism | 23 | 4.1E–05 | 1.3E–03 |
| Liver | mmu04010: MAPK signaling pathway | 128 | 5.7E–05 | 1.6E–03 |
| Liver | mmu04920: Adipocytokine signaling pathway | 41 | 8.2E–05 | 2.0E–03 |
| Liver | mmu00410: β-Alanine metabolism | 18 | 1.1E–04 | 2.1E–03 |
| Liver | mmu05220: Chronic myeloid leukemia | 45 | 1.0E–04 | 2.2E–03 |
| Liver | mmu04144: Endocytosis | 100 | 1.3E–04 | 2.3E–03 |
| Liver | mmu04142: Lysosome | 64 | 1.5E–04 | 2.4E–03 |
| Liver | mmu00280: Valine, leucine, and isoleucine degradation | 30 | 2.1E–04 | 3.1E–03 |
| Liver | mmu00310: Lysine degradation | 27 | 3.9E–04 | 5.3E–03 |
| Liver | mmu04722: Neurotrophin signaling pathway | 67 | 5.0E–04 | 6.5E–03 |
| Liver | mmu04012: ErbB signaling pathway | 48 | 5.6E–04 | 6.7E–03 |
| Liver | mmu00640: Propanoate metabolism | 21 | 7.2E–04 | 8.2E–03 |
| Liver | mmu04912: GnRH signaling pathway | 52 | 7.7E–04 | 8.2E–03 |
| Liver | mmu04610: Complement and coagulation cascades | 42 | 9.0E–04 | 9.2E–03 |
| Liver | mmu00071: Fatty acid metabolism | 28 | 1.1E–03 | 9.2E–03 |
| Liver | mmu04310: Wnt signaling pathway | 74 | 9.8E–04 | 9.5E–03 |
| Liver | mmu03018: RNA degradation | 35 | 1.0E–03 | 9.6E–03 |
| Liver | mmu04330: Notch signaling pathway | 30 | 1.5E–03 | 1.2E–02 |
| Liver | mmu05215: Prostate cancer | 48 | 1.5E–03 | 1.2E–02 |
| Liver | mmu00970: Aminoacyl-tRNA biosynthesis | 26 | 1.9E–03 | 1.4E–02 |
| Liver | mmu05214: Glioma | 36 | 2.1E–03 | 1.5E–02 |
| Liver | mmu00140: Steroid hormone biosynthesis | 27 | 2.8E–03 | 2.0E–02 |
| Liver | mmu04510: Focal adhesion | 92 | 3.2E–03 | 2.2E–02 |
| Liver | mmu00230: Purine metabolism | 75 | 3.4E–03 | 2.2E–02 |
| Liver | mmu00020: Citrate cycle (TCA cycle) | 20 | 4.1E–03 | 2.5E–02 |
| Liver | mmu00982: Drug metabolism | 40 | 4.0E–03 | 2.5E–02 |
| Liver | mmu00980: Metabolism of xenobiotics by cytochrome P450 | 36 | 4.1E–03 | 2.5E–02 |
| Liver | mmu05016: Huntington disease | 85 | 4.7E–03 | 2.7E–02 |
| Liver | mmu00330: Arginine and proline metabolism | 30 | 4.8E–03 | 2.7E–02 |
| Liver | mmu04530: Tight junction | 65 | 5.3E–03 | 2.9E–02 |
| Liver | mmu04110: Cell cycle | 62 | 5.5E–03 | 2.9E–02 |
| Liver | mmu00561: Glycerolipid metabolism | 27 | 6.2E–03 | 3.2E–02 |
| Liver | mmu05212: Pancreatic cancer | 38 | 6.4E–03 | 3.2E–02 |
| Liver | mmu04720: Long-term potentiation | 37 | 7.0E–03 | 3.3E–02 |
| Liver | mmu05223: Non-small cell lung cancer | 30 | 6.9E–03 | 3.4E–02 |
| Liver | mmu05412: Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 39 | 7.7E–03 | 3.6E–02 |
| Liver | mmu04320: Dorso-ventral axis formation | 15 | 8.5E–03 | 3.8E–02 |
| Liver | mmu04350: TGF-β signaling pathway | 44 | 8.4E–03 | 3.8E–02 |
| Liver | mmu00650: Butanoate metabolism | 22 | 9.1E–03 | 3.9E–02 |
| Liver | mmu00510: N-Glycan biosynthesis | 26 | 9.6E–03 | 4.1E–02 |
| Liver | mmu04916: Melanogenesis | 49 | 1.1E–02 | 4.5E–02 |
| WAT | mmu04910: Insulin signaling pathway | 64 | 2.4E–08 | 4.5E–06 |
| WAT | mmu04510: Focal adhesion | 81 | 2.4E–07 | 2.3E–05 |
| WAT | mmu05200: Pathways in cancer | 111 | 3.0E–05 | 1.1E–03 |
| WAT | mmu00230: Purine metabolism | 62 | 2.8E–05 | 1.3E–03 |
| WAT | mmu04520: Adherens junction | 36 | 2.5E–05 | 1.6E–03 |
| WAT | mmu05222: Small cell lung cancer | 38 | 6.8E–05 | 1.9E–03 |
| WAT | mmu04142: Lysosome | 49 | 6.6E–05 | 2.1E–03 |
| WAT | mmu00280: Valine, leucine, and isoleucine degradation | 24 | 1.3E–04 | 3.2E–03 |
| WAT | mmu04010: MAPK signaling pathway | 90 | 3.0E–04 | 6.3E–03 |
| WAT | mmu04120: Ubiquitin-mediated proteolysis | 52 | 3.5E–04 | 6.7E–03 |
| WAT | mmu04722: Neurotrophin signaling pathway | 50 | 3.9E–04 | 6.8E–03 |
| WAT | mmu04920: Adipocytokine signaling pathway | 30 | 4.6E–04 | 7.3E–03 |
| WAT | mmu04110: Cell cycle | 49 | 5.1E–04 | 7.6E–03 |
| WAT | mmu00640: Propanoate metabolism | 17 | 5.6E–04 | 7.6E–03 |
| WAT | mmu04310: Wnt signaling pathway | 55 | 6.3E–04 | 8.0E–03 |
| WAT | mmu03320: PPAR signaling pathway | 33 | 9.7E–04 | 1.2E–02 |
| WAT | mmu04530: Tight junction | 50 | 1.1E–03 | 1.2E–02 |
| WAT | mmu04012: ErbB signaling pathway | 35 | 1.4E–03 | 1.5E–02 |
| WAT | mmu04912: GnRH signaling pathway | 38 | 1.5E–03 | 1.5E–02 |
| WAT | mmu00020: Citrate cycle (TCA cycle) | 16 | 3.0E–03 | 2.8E–02 |
Abbreviation: TCA, tricarboxylic acid.
To further focus on genes with higher peak values at the binding sites, we selected subsets of genes for pathway analysis. In the liver, when we sorted the intervals by their peak values and selected the top 3000 intervals for pathway analysis, we identified 3 biological pathways with significant number of genes represented (Figure 3B): the signaling pathways insulin signaling, peroxisome proliferator–activated receptor (PPAR) signaling, and adipocytokine signaling. When we picked the top 1000 instead of 3000 intervals and repeated the analysis, we narrowed the choices to the insulin and adipocytokine pathways.
In the WAT, the insulin signaling pathway and citrate cycle are enriched in the top 3000 or 1000 intervals with the highest peak values (Figure 3C). Additional pathways involved in focal adhesion, pentose phosphate metabolism, pyruvate metabolism, propanoate metabolism, PPAR signaling, and cancer are enriched in the cutoff of 3000 but not 1000.
We demonstrated close relationships between potential ChREBP target genes and insulin signaling in Figure 3D. For genes enriched in the top 1000 intervals, ChREBP may regulate Prkag2, Acaca, Acacb, G6pc, Ppp1r3c, Ppp1r3b, Sorbs1, Pygl, Fasn, Pik3r1, and Shc4 genes in both tissues (Table 2). Irs2 and Gys1 were presented in the top 1000 gene list in liver and top 3000 in WAT, whereas Pck1 was in the group of the top 1000 in WAT and the top 3000 list in liver. Socs3, Cblb, Sos1, Pklr, Gys2, and Prkaa2 were not presented in the top 3000 WAT ChIP-seq result. Mknk2, Prkar2a, Eif4e, Prkaa1, Sh2b2, and Lipe were genes related to the insulin signaling pathway that were not enriched in the top 3000 liver ChIP-seq result.
Table 2.
Genes in Enriched Pathways Identified by DAVID for Top 1000 and Top 3000 Genes in Liver and WAT
| Tissue | Category | Term | Genes | Count | P Value | Benjamini |
|---|---|---|---|---|---|---|
| Liver | Top 3000 | mmu04910: Insulin signaling pathway | PRKAG2, PPP1R3C, PPP1R3B, SORBS1, SOS1, FASN, GYS1, GYS2, PRKACA, PRKAA2, INSR, RAPGEF1, PIK3R1, SHC4, SREBF1, IRS2, IRS3, SOCS2, SOCS3, ACACA, FBP1, RAF1, PDE3A, ACACB, PCK1, CBLB, G6PC, CRKL, PYGL, GSK3B, PKLR, PTPN1 | 32 | 2.5E–06 | 4.6E–04 |
| Liver | Top 3000 | mmu03320: PPAR signaling pathway | SCD1, ACOX2, PPARA, SLC27A1, CPT1B, PPARD, ACADM, EHHADH, DBI, CPT1A, PCK1, ACSL1, APOA1, SORBS1, CYP7A1, APOA5, UBC, ACAA1A, SCP2, ACSL5 | 20 | 8.4E–05 | 7.8E–03 |
| Liver | Top 3000 | mmu04920: Adipocytokine signaling pathway | PPARA, CPT1B, IRS2, IRS3, SOCS3, LEPR, PRKAG2, ADIPOR2, ACACB, CPT1A, TRADD, PCK1, TNFRSF1B, G6PC, ACSL1, PRKAA2, ACSL5 | 17 | 3.3E–04 | 2.0E–02 |
| Liver | Top 1000 | mmu04910: Insulin signaling pathway | IRS2, SOCS3, PRKAG2, ACACA, ACACB, CBLB, G6PC, PPP1R3C, PPP1R3B, SORBS1, PYGL, SOS1, PKLR, FASN, GYS1, GYS2, PRKAA2, PIK3R1, SHC4 | 19 | 1.2E–06 | 1.8E–04 |
| Liver | Top 1000 | mmu04920: Adipocytokine signaling pathway | PPARA, G6PC, IRS2, ACSL1, SOCS3, PRKAG2, ADIPOR2, PRKAA2, ACACB, TRADD | 10 | 4.9E–04 | 3.6E–02 |
| WAT | Top 3000 | mmu04910: Insulin signaling pathway | PRKAG3, PRKAG2, HK2, MKNK2, PRKAR2B, PPP1R3C, PRKAR2A, EIF4EBP1, PPP1R3B, SORBS1, SOS2, FASN, GYS1, PRKAA1, SH2B2, INSR, RAPGEF1, PIK3R1, SHC4, IRS2, ACACA, RAF1, ACACB, PCK2, PCK1, PPP1CA, G6PC, EIF4E, PYGL, LIPE | 30 | 4.2E–06 | 7.5E–04 |
| WAT | Top 3000 | mmu00020: Citrate cycle (TCA cycle) | SDHB, PCX, ACO1, SUCLG2, IDH1, ACLY, PDHA1, DLAT, SUCLA2, PCK2, IDH3A, PCK1 | 12 | 3.0E–05 | 2.7E–03 |
| WAT | Top 3000 | mmu04510: Focal adhesion | BCAR1, COL3A1, PIP5K1C, ITGB5, PTEN, PXN, LAMB3, ITGB8, SOS2, COL6A1, RAPGEF1, PIK3R1, SHC4, EGFR, COL4A2, ACTN4, RAF1, CAPN2, VAV2, COL5A3, VWF, PPP1CA, LAMA4, LAMA3, ITGA6, LAMC3, CCND2, LAMB1–1, VEGFA, GRLF1, LAMC2, MYLK, PARVB, PARVA | 34 | 1.4E–04 | 8.5E–03 |
| WAT | Top 3000 | mmu00030: Pentose phosphate pathway | PGM2, TALDO1, PFKL, H6PD, PGD, PFKP, DERA, TKT, RPIA, GPI1 | 10 | 2.0E–04 | 8.9E–03 |
| WAT | Top 3000 | mmu00620: Pyruvate metabolism | PCX, AKR1B3, PKM2, ACYP2, ALDH2, ACACA, PDHA1, ACACB, GRHPR, DLAT, PCK2, PCK1 | 12 | 5.0E–04 | 1.8E–02 |
| WAT | Top 3000 | mmu00640: Propanoate metabolism | ACADM, SUCLG2, ALDH2, ACACA, ECHS1, ACACB, HIBCH, SUCLA2, PCCB, PCCA | 10 | 6.7E–04 | 2.0E–02 |
| WAT | Top 3000 | mmu03320: PPAR signaling pathway | SCD1, ACOX1, SCD2, ACADM, RXRA, PPARG, PCK2, DBI, ADIPOQ, CPT1A, PCK1, ACSL1, SORBS1, UBC, FABP4, UBB, PLTP, ANGPTL4 | 17 | 9.3E–04 | 2.1E–02 |
| WAT | Top 3000 | mmu05200: Pathways in cancer | TRAF1, WNT5B, APC2, PPARG, ZBTB16, KIT, GLI2, PTEN, TCF7L2, ACVR1C, MAX, LAMB3, SOS2, RALB, RUNX1, AXIN2, TRAF5, PIK3R1, TRP53, EGFR, TCF7, COL4A2, EPAS1, RXRA, TGFBR2, CYCS, RUNX1T1, RAF1, SMAD3, FGF22, SMAD2, FGF21, RB1, STAT1, DAPK1, JUP, LAMA4, LAMA3, ITGA6, PIAS4, LAMC3, VEGFA, LAMB1–1, LAMC2 | 44 | 2.2E–03 | 4.3E–02 |
| WAT | Top 1000 | mmu04910: Insulin signaling pathway | PRKAG2, ACACA, MKNK2, ACACB, PCK1, PRKAR2A, G6PC, PPP1R3C, EIF4E, PPP1R3B, SORBS1, PYGL, FASN, PRKAA1, SH2B2, LIPE, PIK3R1, SHC4 | 18 | 4.3E–06 | 6.3E–04 |
| WAT | Top 1000 | mmu00020: Citrate cycle (TCA cycle) | SDHB, ACO1, SUCLG2, IDH1, PDHA1, DLAT, IDH3A, PCK1 | 8 | 6.8E–05 | 5.0E–03 |
Abbreviation: TCA, tricarboxylic acid.
Motif analysis
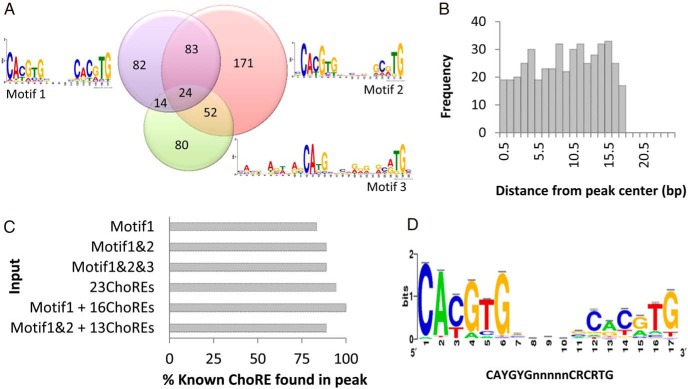
We observed that most of the ChoREs in the promoter of well-established ChREBP target genes are located at the summit (tip) of the ChIP-seq peaks (Figure 2, A–E); we therefore extracted 50-bp sequence at the tips of the top 1000 peaks from liver ChIP-seq data and top 1000 peaks of WAT ChIP-seq data for motif discovery. Three motifs containing 17-bp ChoRE or a ChoRE-like sequence were found in a quarter of these peak sequences (506 sequences) (Figure 4A): (1) 2 CAYGYG separated by 5 nucleotides (203 sequences), (2) CAYGYK at one end and (5′-)CAYGY at the other end (330 sequences), and (3) (5′-)CABG at one end and (5′-)CAY E-box half-site at the other end (170 sequences). These ChoRE or ChoRE-like motifs are distributed throughout the 50-bp peak region (Figure 4B).
Figure 4.
Enriched ChREBP binding motifs in ChIP-seq results. A, ChREBP binding motifs recognized by analysis of the top 1000 peak sequences and their frequencies. We used MEME-CHIP to analyze the 50-bp sequences of the top 1000 peak sequences from ChIP-seq in liver and WAT and identified 3 ChoRE-like motifs. B, Frequency of distance between the discovered ChoRE-like sequence and ChIP-seq peak center. C, Evaluation of ChoRE prediction models. We used 6 different set of sequences to validate ChoRE prediction by ensuring the presence of 18 mouse ChoRE sequences on Pklr, Gckr, Pnpla3, G6pc, G0s2, Thrsp, Fasn, Acaca, Txnip, Slc2a4, Fgf21, Klf10, Pc, Hif1a, Bhlhe40, Mlxipl, and Mid1ip1 (2 sequences) genes. D, Consensus ChREBP binding sequence. We used Regulatory Sequence Analysis Tools to generate the ChoRE sequence logo with 219 sequences from the best prediction model (Motif1 + 16 known ChoREs).
We next searched for ChoREs in the rest of ChIP-seq data using enriched sequences from those top 1000 peaks. We validated our ChoRE prediction by ensuring the presence of 18 mouse ChoRE sequences, retrieved from known ChREBP target genes or conserved sequence prediction, which displayed binding peaks in our study in liver (Pklr, Gckr, and Pnpla3), WAT (G6pc and G0s2), or both tissues (Thrsp, Fasn, Acaca, Txnip, Slc2a4, Fgf21, Klf10, Pc, Hif1a, Bhlhe40, Mlxipl, and Mid1ip1 [2 sequences]) (27–38).
We compared detection of these sequences using six different models (Figure 4C): (1) motif 1 alone (203 sequences), (2) motifs 1 and 2 (426 sequences), (3) combined all 3 ChoRE motifs (506 sequences), (4) 23 experimentally verified ChoRE sequences present in mouse, rat, or human, (5) motif 1 plus 16 experimentally verified ChoREs (219 sequences; 7 of 23 known ChoREs were already present in motif 1), and (6) motif 1 and 2 plus 13 experimentally verified ChoREs (439 sequences; 10 of 23 known ChoREs were already present in motifs 1 and 2). Hif1a and Pnpla3 ChoREs are absent when we use input ChoRE sequences from motif 1 alone or motifs 1 and 2 but present in other types of analysis. Thrsp ChoRE cannot be detected by motif 1 alone. Analysis using these 23 known ChoREs failed to detect the mouse Fgf21 ChoRE sequence. The combination of sequences from motif 1 and 16 experimentally verified ChoRE sequences was able to detect all of the 18 mouse ChoRE sequences presented in our peak sequences. Addition of the sequences of motif 2 in analysis worsened the sensitivity of the ChoRE detection. We therefore use sequences from motif 1 and 16 known ChoREs to generate a CAYGYGnnnnnCRCRTG consensus sequence for ChREBP binding (Figure 4D). The selected putative ChREBP target genes and their ChoREs are listed in Table 3.
Table 3.
List of Selected Genes and Predicted ChoRE Sequences on Their Promoters
| Gene | Chromosome | Location |
Position From TSS | Sequence | |
|---|---|---|---|---|---|
| TSS | Predicted ChoRE | ||||
| Pklr | 3 | 88940544 | 88940374 | −170 | CACGGGGCACTCCCGTG |
| Thrsp | 7 | 104566019 | 104567432 | −1413 | CACTTGGTGGCCCTAGG |
| Fasn | 11 | 120685860 | 120692357 | −6497 | CATGCGCTGCAGGCATG |
| Fasn | 11 | 120685860 | 120685682 | 179 | CGCGTGGCCCGCGCGAG |
| Acaca | 11 | 84008939 | 83986684 | −22 255 | CATGTGAAAACACTGTG |
| Txnip | 3 | 96361879 | 96361794 | −85 | CACGAGGGCTGCACGAG |
| G6pc | 11 | 101229043 | 101226090 | −2953 | CATATACCGAGCACATG |
| Slc2a4 | 11 | 69761691 | 69762434 | −743 | CACGCGGCCAGCACATG |
| G0S2 | 1 | 195099381 | 195099524 | −143 | GGCGTGCACTACTCGTG |
| Gckr | 5 | 31599953 | 31599585 | −368 | CATGTGATCAAGCCATG |
| Klf10 | 15 | 38230461 | 38230605 | −144 | CAGCCGCTGATCACGCG |
| Pnpla3 | 15 | 83998245 | 83998006 | −239 | AGAGTGCCACCGGCGTG |
| Pnpla3 | 15 | 83998245 | 83998103 | −142 | CACGCGGGAGGGACATG |
| Pc | 19 | 4510471 | 4510107 | −364 | CACGTGCTACCCGAGTG |
| Hif1aa | 12 | 75008853 | 75008741 | −112 | CAGCTGGGGCTCAGCTG |
| Fgf21 | 7 | 52870859 | 52870931 | −72 | CACGCGTCAGGAGTGGG |
| Mid1ip1 | X | 10294490 | 10294124 | −366 | CACGTGCATCCGGCATG |
| Mid1ip1 | X | 10294490 | 10294113 | −377 | CACCCCATGTCCACGTG |
| Bhlhe40 | 6 | 108610622 | 108610365 | −257 | CACGTGAGGCTCATGTG |
| Bhlhe40 | 6 | 108610622 | 108609766 | −856 | CAGGTGCGCGCGGCGTG |
| Bhlhe40 | 6 | 108610622 | 108609425 | −1197 | CGCGTCCGGGCCACGTG |
| Gapdh | 6 | 125115600 | 125117145 | −1545 | CACGGGCCACACAGCTG |
| Gapdh | 6 | 125115600 | 125115846 | −246 | CACACGCTTGGTGCGTG |
| Gpd1 | 15 | 99548023 | 99546539 | −1484 | CAGGAGGTCTGCACATG |
| Mlxipl | 5 | 135565759 | 135565916 | +158 | CGCGTGGAGCTCAGGTG |
| Mlxipl | 5 | 135582760 | 135577105 | −5655 | CACGAGGTGGGAGGCTG |
| Acacb | 5 | 114615526 | 114611660 | −3866 | CATGAGGTTTGCACGTG |
| Ppp1r3b | 8 | 36438794 | 36438672 | −142 | CACGTGGTAGAGTGCTG |
| Ppp1r3c | 19 | 36811093 | 36811282 | −189 | CACGTTCCAGGGGCAGG |
| Pygl | 12 | 71328669 | 71334944 | −6275 | CACATGCTTGGCACATG |
| Gys2 | 6 | 142421628 | 142421467 | +162 | CACGCTTCATCCAGCTG |
| Fbp1 | 13 | 62989595 | 62995044 | −5449 | GACAGGCTAAGCACGTG |
| Fbp1 | 13 | 62989595 | 62995033 | −5438 | CACGTGCACTCAAGTTG |
| Irs2 | 8 | 11008429 | 11011390 | −2961 | CGTGAGCACAGCACGTG |
| Irs3 | 5 | 138086941 | 138094849 | −7908 | CTCATGCAGTTCACGTG |
| Srebf1 | 11 | 60034105 | 60036424 | −2319 | CGGGCGCTGGGCACGTG |
| Mtor | 4 | 147822690 | 147822408 | −282 | CACACGGTCACAGCGTG |
| Raf1 | 6 | 115626652 | 115626695 | −43 | CACATTCCAGGCTCGAG |
| Adipor2 | 6 | 119367500 | 119368809 | −1309 | CATCGGGCTGTCACGTG |
| Adipor2 | 6 | 119367500 | 119368798 | −1298 | CACGTGTCCTGCCAGTG |
| Hnf1a | 5 | 115421070 | 115421169 | −99 | CATGAGGCCTGCACTTG |
| Nfe2l2 | 2 | 75542697 | 75543365 | −668 | CACGCTGCAAACTCGTG |
| Klf15 | 6 | 90412619 | 90413530 | 912 | CATTCGCCACCCCCGTG |
| Sult1e1a | 5 | 88020635 | 88030255 | −9620 | CATGTCAGATCCACAAC |
| Rtp4a | 16 | 23610004 | 23606874 | −3130 | CACGTGAAGGAGATGGG |
| Slc34a2a | 5 | 53440591 | 53439394 | −1197 | TCCGTGGTGGTCACGTG |
| Lgals3bpa | 11 | 118263244 | 118263395 | −151 | CACAGGGCGCTTGGGTG |
| Ehhadha | 16 | 21787906 | 21788287 | −381 | CAGCTGGAAGGACCGTG |
| Cav1a | 6 | 17257639 | 17257641 | +3 | CAGGCGCTCTCTGCCTG |
| Cxcl1a | 5 | 91320270 | 91319967 | −303 | CACGTGCACATAACGCG |
| Junba | 8 | 87502646 | 87510120 | −7474 | CGCGTGCGTCGTTCGTG |
Glucose-repressed genes identified by Ma et al (29) in rat primary hepatocytes.
To explore ChREBP binding motifs on the glucose-repressed genes, we adopted the published list of 38 rat glucose-repressed genes in primary hepatocytes for additional analysis (29). Fifteen genes are present in our mouse liver ChIP-seq data. There are 11 genes with peak sequences in the promoter region, 9 having putative conventional ChoREs located at the summit of ChIP-seq peak. Information on the predicted ChoRE sequences of these 9 genes (Sult1e1, Rtp4, Slc34a2, Lgals3bp, Ehhadh, Cav1, Cxcl1, Junb, and Hif1a) is presented in Table 3.
Discussion
Liver and adipose tissues are 2 important lipid-handling tissues in mammals. Although liver is the major organ for fatty acid and triglyceride synthesis, adipose tissues are the main organs for lipid storage. It is, therefore, not surprising that of all the genes (14 162 genes) that exhibit ChREBP binding sequences by our analysis, a large majority (5919 genes) were present in both liver and WATs (Figure 1F). In comparison with a recent study in HepG2 human hepatocellular carcinoma cell model (39), our ChIP-seq experiment using mouse liver has identified a 15 times larger number of genes. This discrepancy may possibly be explained by differences in species and properties of cancer vs noncancer cells.
Gene set analysis of our data revealed that ChIP-based ChREBP target genes are involved in different metabolic processes and biological regulation (Figure 3A). These putative ChREBP target genes encompass diverse biochemical pathways. Only a handful of these pathways have been documented in the past to be regulated by ChREBP, for example, fatty acid metabolism and the citrate cycle (Table 1). Cancer-related pathways, including those involved in cell cycle and notch signaling, were significantly enriched in our study. Genes involved in the adherens junction were also enriched. Dysregulation of the cell-cell adherens junction is closely related to invasion and metastasis of cancer (40). Our findings underline the potential importance of ChREBP as a player in tumorigenesis and cancer progression (41–43).
The insulin signaling pathway is among the top 3 pathways in both liver and WAT. In addition, this is the only pathway identified in the top 1000 intervals with the highest peak values in both liver and WAT. A few of their members have been described as ChREBP target genes involved in glucose metabolism and lipogenesis, ie, Acaca, G6pc, Fasn, and Pklr. Here we showed that ChREBP binds to a number of additional genes within this pathway (Table 2), including Prkag2, Acacb, Ppp1r3c, Ppp1r3b, Sorbs1, Pygl, Pik3r1, Shc4, Irs2, Socs3, Cblb, Sos1, Gys1, Gys2, Prkaa2, Mknk2, Pck1, Prkar2a, Eif4e, Prkaa1, Sh2b2, and Lipe. Srebf1, which encodes the lipogenic transcription factor SREBP-1c, is another key gene in this pathway that is among those in the top 3000 intervals in the liver (Table 2). This observation is largely consistent with a recent study using a HepG2 human hepatocellular carcinoma cell model to analyze the ChREBP targets (39), which demonstrated that glucose up-regulates SREBP-1c expression and binding of ChREBP to the Srebf1 gene may mediate high glucose-induced expression of SREBP-1c. Human and mouse Srebf1 promoters in this region are conserved, and the sequence of the validated human Srebf1 promoter fragment falls within the mouse Srebf1 promoter sequence resulting from our ChIP-seq in the liver. We predicted putative ChoRE (Table 3) on the mouse Srebf1 promoter and promoters of genes in the insulin signaling pathway (Mtor, Raf1, Acacb, Ppp1r3b, Ppp1r3c, Pygl, Gys2, Fbp1, Irs2, and Irs3), as well as promoters of important genes related to insulin resistance and diabetes (Adipor2, Hnf1a, Nfe2l2, and Klf15) (44–47). The functional activity of these predicted ChoREs should be further validated because binding may not necessarily affect transcription activity. For example, ChREBP binding to Gpd1 ChoRE was previously demonstrated and also supported by our data; however, neither the natural Gpd1 promoter with this ChoRE sequence nor the artificial promoter containing 2 copies of Gpd1 ChoRE responded to the presence of high glucose (29).
The consensus sequences CAYGnGnnnnnCnCRTG and CABGTGnnCnGnGnSTG were previously proposed in rat liver and the human HepG2 cell line, respectively (29, 39). Our motif analysis of ChIP-seq data reveals similar ChoRE sequences consisting of 2 E-boxes with a sequence signature of (5′-)CAYGYG that are separated by 5 nucleotides. It has been shown that promoters containing 2 perfect CACGTG motifs separated by 5 nucleotides conferred a very strong response to high glucose in primary rat hepatocytes. Spacing of 4 nucleotides between these CACGTG motifs completely abolished induction of promoter activity by high glucose, whereas spacing by 6, 7, or 15 nucleotides resulted in a weak glucose response (4). Our recent study in the 832/13 rat insulinoma cell line further provided corroborative evidence that ChREBP does not activate promoters containing 2 E-boxes with a 4-bp spacer (32). It is not known whether spacing lengths other than 5 nucleotides could enable a response to ChREBP.
Our analysis successfully detected all previously verified or predicted ChoRE sequences (Table 3) except the Gcgr promoter (48), which did not display a binding peak in our ChIP-seq result. We also found additional ChREBP binding sequences on Fasn, Pnpla3, Bhlhe40, Gapdh, and Mlxipl. The ChREBP binding to ChoRE located on the distal promoter of rat Fasn was well characterized. It also has been shown by ChIP that ChREBP binds to the mouse proximal Fasn promoter (49). Our analysis reveals the putative ChoRE sequence located in that binding region as shown in Table 3. A functional ChoRE sequence on the mouse Pnpla3 promoter was previously reported (30). The mutated ChoRE-containing Pnpla3 promoter did not respond to ChREBP/Mlx at all. For that reason, another ChoRE-like sequence that we detected between that ChoRE and the TSS (Table 3) is probably inactive. Serial deletion experiments revealed the functional ChoRE of the mouse Bhlhe40 promoter (37). The 5′ deletion of the promoter fragment, which contains one of our putative ChoREs, diminished the responsiveness of mouse Bhlhe40 promoter activity to a dominant active form of ChREBP. Thus, this sequence may function as a ChoRE. Another ChoRE-like sequence that we predicted was not included in that study. The mouse Gapdh promoter also contained a ChoRE sequence (50). Activity of the Gapdh promoter in response to glucose was not completely abolished when that sequence was manipulated. Another Gapdh ChoRE identified in our study might be responsible for the rest of the glucose response. The β-isoform of Mlxipl or ChREBP in liver and adipose tissues as well as ChoRE on its promoter was recently described (27). The α isoform of Mlxipl may also have a ChoRE on the promoter because its expression was influenced by fasting and refeeding in mice. It is possible that our predicted ChoRE on the distal promoter of the α isoform of Mlxipl may serve this function.
The most commonly reported action of ChREBP is up-regulation of target genes; however, as noted in Table 3, in a minority of cases, the transcription factor may repress specific target genes. Close scrutiny of the negatively and positively regulated target genes has failed to reveal a consistent difference between them, suggesting that a ChoRE marks a gene target but does not necessarily determine whether the gene is subject to up- or down-regulation. It is plausible that other cis- or trans-acting factors contribute to the direction of ChREBP-mediated gene regulation, which is a subject for future investigation.
In summary, genome-wide analysis of ChREBP binding sites in the liver and WAT reveals the optimal consensus ChREBP binding sequence CAYGYGnnnnnCRCRTG and demonstrates that ChREBP is a major transcription factor that binds to large numbers of putative hepatic and/or adipose target genes involved in several cellular processes, including several key metabolic pathways, the adherens junction, the insulin signaling pathway, and tumorigenesis.
Acknowledgments
This work was supported by the National Institutes of Health (Grants R01-HL051586/DK105527 to L.C., R01-DK84495 to B.C., and R01HG007538 and CPRIT RP110471 to W.L.), the Diabetes Research Center (Grant P30 DK079638 to L.C.), the Betty Rutherford Chair in Diabetes Research at Baylor St. Luke's Medical Center in Houston, Texas, the Frank and Cindy Liu Family Foundation, the Cunningham Family Charitable Fund, and the T.T. & W.F. Chao Global Foundation. NP was supported in part by a Discovery-Based Development Grant from the National Science and Technology Development Agency, Thailand, and the Siriraj Research Fund (R015433020) from the Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- chromatin immunoprecipitation
- ChIP-Seq
- chromatin immunoprecipitation followed by next-generation sequencing
- ChoRE
- carbohydrate response element
- ChREBP
- carbohydrate response element–binding protein
- LID
- low-glucose inhibitory domain
- MACS
- model-based analysis of ChIP-Seq
- Mlx
- Max-like protein X
- PPAR
- peroxisome proliferator–activated receptor
- qPCR
- quantitative PCR
- SREBP-1c
- sterol regulatory element binding protein-1c
- TSS
- transcription start site
- WAT
- white adipose tissue.
References
- 1. Lee YS, Sohn DH, Han D, Lee HW, Seong RH, Kim JB. Chromatin remodeling complex interacts with ADD1/SREBP1c to mediate insulin-dependent regulation of gene expression. Mol Cell Biol. 2007;27:438–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meugnier E, Rome S, Vidal H. Regulation of gene expression by glucose. Curr Opin Clin Nutr Metab Care. 2007;10:518–522. [DOI] [PubMed] [Google Scholar]
- 3. Bergot MO, Diaz-Guerra MJ, Puzenat N, Raymondjean M, Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992;20:1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shih HM, Liu Z, Towle HC. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem. 1995;270:21991–21997. [DOI] [PubMed] [Google Scholar]
- 5. Yamashita H, Takenoshita M, Sakurai M, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA. 2001;98:9116–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci USA. 2001;98:13710–13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li MV, Chang B, Imamura M, Poungvarin N, Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes. 2006;55:1179–1189. [DOI] [PubMed] [Google Scholar]
- 8. Li MV, Chen W, Harmancey RN, et al. Glucose-6-phosphate mediates activation of the carbohydrate responsive binding protein (ChREBP). Biochem Biophys Res Commun. 2010;395:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li MV, Chen W, Poungvarin N, Imamura M, Chan L. Glucose-mediated transactivation of carbohydrate response element-binding protein requires cooperative actions from Mondo conserved regions and essential trans-acting factor 14-3-3. Mol Endocrinol. 2008;22:1658–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noordeen NA, Meur G, Rutter GA, Leclerc I. Glucose-induced nuclear shuttling of ChREBP is mediated by sorcin and Ca2+ ions in pancreatic β-cells. Diabetes. 2012;61:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leclerc I, Rutter GA, Meur G, Noordeen N. Roles of Ca2+ ions in the control of ChREBP nuclear translocation. J Endocrinol. 2012;213:115–122. [DOI] [PubMed] [Google Scholar]
- 12. Noordeen NA, Khera TK, Sun G, et al. Carbohydrate-responsive element-binding protein (ChREBP) is a negative regulator of ARNT/HIF-1β gene expression in pancreatic islet β-cells. Diabetes. 2010;59:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma L, Sham YY, Walters KJ, Towle HC. A critical role for the loop region of the basic helix-loop-helix/leucine zipper protein Mlx in DNA binding and glucose-regulated transcription. Nucleic Acids Res. 2007;35:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. da S X, Sun G, Qian Q, Rutter GA, Leclerc I. ChREBP regulates Pdx-1 and other glucose-sensitive genes in pancreatic β-cells. Biochem Biophys Res Commun. 2010;402:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120:4316–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burgess SC, Iizuka K, Jeoung NH, et al. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J Biol Chem. 2008;283:1670–1678. [DOI] [PubMed] [Google Scholar]
- 17. Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab. 2006;291:E358–E364. [DOI] [PubMed] [Google Scholar]
- 18. Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Musri MM, Gomis R, Parrizas M. Application of electrophoretic mobility shift assay and chromatin immunoprecipitation in the study of transcription in adipose cells. Methods Mol Biol. 2008;456:231–247. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Liu T, Meyer CA, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicol JW, Helt GA, Blanchard SG, Jr, Raja A, Loraine AE. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25:2730–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–W83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 25. Machanick P, Bailey TL. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas-Chollier M, Defrance M, Medina-Rivera A, et al. RSAT 2011: regulatory sequence analysis tools. Nucleic Acids Res 2011;39:W86–W91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herman MA, Peroni OD, Villoria J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Callaghan BL, Koo SH, Wu Y, Freake HC, Towle HC. Glucose regulation of the acetyl-CoA carboxylase promoter PI in rat hepatocytes. J Biol Chem. 2001;276:16033–16039. [DOI] [PubMed] [Google Scholar]
- 29. Ma L, Robinson LN, Towle HC. ChREBP · Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–28730. [DOI] [PubMed] [Google Scholar]
- 30. Dubuquoy C, Robichon C, Lasnier F, et al. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J Hepatol. 2011;55:145–153. [DOI] [PubMed] [Google Scholar]
- 31. Pedersen KB, Zhang P, Doumen C, et al. The promoter for the gene encoding the catalytic subunit of rat glucose-6-phosphatase contains two distinct glucose-responsive regions. Am J Physiol Endocrinol Metab. 2007;292:E788–E801. [DOI] [PubMed] [Google Scholar]
- 32. Poungvarin N, Lee JK, Yechoor VK, et al. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in β cell glucotoxicity. Diabetologia. 2012;55:1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009;583:2882–2886. [DOI] [PubMed] [Google Scholar]
- 34. Iizuka K, Takeda J, Horikawa Y. Krüppel-like factor-10 is directly regulated by carbohydrate response element-binding protein in rat primary hepatocytes. Biochem Biophys Res Commun. 2011;412:638–643. [DOI] [PubMed] [Google Scholar]
- 35. Pedersen KB, Buckley RS, Scioneaux R. Glucose induces expression of rat pyruvate carboxylase through a carbohydrate response element in the distal gene promoter. Biochem J. 2010;426:159–170. [DOI] [PubMed] [Google Scholar]
- 36. Isoe T, Makino Y, Mizumoto K, Sakagami H, et al. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int. 2010;78:48–59. [DOI] [PubMed] [Google Scholar]
- 37. Iizuka K, Horikawa Y. Regulation of lipogenesis via BHLHB2/DEC1 and ChREBP feedback looping. Biochem Biophys Res Commun. 2008;374:95–100. [DOI] [PubMed] [Google Scholar]
- 38. Tsatsos NG, Augustin LB, Anderson GW, Towle HC, Mariash CN. Hepatic expression of the SPOT 14 (S14) paralog S14-related (Mid1 interacting protein) is regulated by dietary carbohydrate. Endocrinology. 2008;149:5155–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeong YS, Kim D, Lee YS, et al. Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression. PLoS One. 2011;6:e22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mansouri M, Rose PP, Moses AV, Früh K. Remodeling of endothelial adherens junctions by Kaposi's sarcoma-associated herpesvirus. J Virol. 2008;82:9615–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tong X, Zhao F, Mancuso A, Gruber JJ, Thompson CB. The glucose-responsive transcription factor ChREBP contributes to glucose-dependent anabolic synthesis and cell proliferation. Proc Natl Acad Sci USA. 2009;106:21660–21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Airley RE, McHugh P, Evans AR, et al. Role of carbohydrate response element-binding protein (ChREBP) in generating an aerobic metabolic phenotype and in breast cancer progression. Br J Cancer. 2014;110:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calvisi DF, Wang C, Ho C, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bermúdez VJ, Rojas E, Toledo A, et al. Single-nucleotide polymorphisms in adiponectin, AdipoR1, and AdipoR2 genes: insulin resistance and type 2 diabetes mellitus candidate genes. Am J Ther. 2013;20:414–421. [DOI] [PubMed] [Google Scholar]
- 45. Pontoglio M. Hepatocyte nuclear factor 1, a transcription factor at the crossroads of glucose homeostasis. J Am Soc Nephrol. 2000;16(11 Suppl):S140–S143. [PubMed] [Google Scholar]
- 46. Seo HA, Lee IK. The role of Nrf2: adipocyte differentiation, obesity, and insulin resistance. Oxid Med Cell Longev. 2013;2013:184598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jung DY, Chalasani U, Pan N, et al. KLF15 is a molecular link between endoplasmic reticulum stress and insulin resistance. PLoS One. 2013;8:e77851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iizuka K, Tomita R, Takeda J, Horikawa Y. Rat glucagon receptor mRNA is directly regulated by glucose through transactivation of the carbohydrate response element binding protein. Biochem Biophys Res Commun. 2012;417:1107–1112. [DOI] [PubMed] [Google Scholar]
- 49. da Silva Xavier G, Rutter GA, Diraison F, Andreolas C, Leclerc I. ChREBP binding to fatty acid synthase and L-type pyruvate kinase genes is stimulated by glucose in pancreatic beta-cells. J Lipid Res. 2006;47:2482–2491. [DOI] [PubMed] [Google Scholar]
- 50. Hanke N, Scheibe RJ, Manukjan G, et al. Gene regulation mediating fiber-type transformation in skeletal muscle cells is partly glucose- and ChREBP-dependent. Biochim Biophys Acta. 2011;1813:377–389. [DOI] [PubMed] [Google Scholar]