Abstract
Pathways that stimulate β-cell regeneration remain of great clinical interest, yet effective therapeutic avenues that promote survival or reconstitution of β-cell mass remain elusive. Using a mouse model with inducible β-cell apoptosis followed by adiponectin-mediated regeneration, we aimed to identify key molecules boosting β-cell viability. In the regenerating pancreatic islets, we examined changes within the transcriptome and observed an extensive up-regulation of genes encoding proteins involved in lipid transport and metabolism. The most prominent targets were further confirmed by quantitative PCR and immunofluorescence. Among the upstream regulators predicted by pathway analysis of the transcriptome, we detected enhanced levels of 2 key transcription factors, Hepatocyte Nuclear Factor 4α and Peroxisome Proliferator-Activated Receptorα. Our data suggest that improving pancreatic islet lipid metabolism as an important antilipotoxic phenomenon to boost β-cell regeneration. This is primarily mediated by the adipokine adiponectin that exerts its action on both the beta-cell directly as well as on the adipocyte. Adiponectin induces lipid metabolism gene expression in regenerating islets through Hepatocyte Nuclear Factor 4α and Peroxisome Proliferator-Activated Receptorα. Adiponectin also modulates leptin levels via preserving adipose tissue mass in the insulinopenic state.
The regeneration of insulin-producing β-cells remains as a promising clinical strategy for diabetes mellitus. However, after β-cell failure in type 1 and late-stage type 2 diabetes, the spontaneous recovery of β-cell mass is very limited. As a terminally differentiated cell type with a specific function in insulin production, the β-cell has a relatively low rate of proliferation and differentiation in adults (1). Moreover, β-cells are susceptible to cytotoxic environmental factors (2), such as hyperglycemia and hyperlipidemia, both resulting directly from insulin deficiency. Glucolipotoxicity may impair the function of β-cells and induce apoptosis, thereby hindering their survival and proliferation (3). The key signaling pathways that can effectively enhance β-cell regeneration remain to be identified.
As an abundant hormone secreted from adipocytes, adiponectin exerts pleiotropic beneficial effects on metabolism, such as insulin-sensitizing, antioxidative, antiinflammatory and, antilipotoxic properties (4). In the context of β-cells, adiponectin displays a potent prosurvival role in tissue culture studies (5, 6). Our laboratory reported that adiponectin reduces the lipotoxic ceramides via a ceramidase activity, mediated by adiponectin receptors, and protection of β-cells from apoptosis (7).
Recently, we demonstrated in vivo that under insulin deficiency, adiponectin is critical for minimal systemic lipid homeostasis, and increased circulating adiponectin is sufficient to mitigate lipotoxicity and promote β-cell proliferation (8). To investigate the molecular signaling that mediates β-cell regeneration in the presence of adiponectin overexpression, we analyzed the gene expression profile in the regenerating pancreatic islets by high-throughput RNA sequencing (RNA-seq) and confirmed the most regulated targets at the levels of cDNA and protein. The adiponectin-promoted β-cell regeneration associated with marked up-regulation of lipid transport and metabolism genes, which might be mediated by the extrinsic hormone leptin and the activation of transcription factors Hepatocyte Nuclear Factor 4 (HNF4) α and Peroxisome Proliferator-Activated Receptor (PPAR) α. Taken together, our results suggest that adiponectin promotes β-cell regeneration by improving islet lipid metabolism. We conclude that both systemic and local interventions can ameliorate lipotoxicity in β-cells and enhance their viability and recovery.
Materials and Methods
Mice
Breeding, maintenance, and B/B Homodimerizer (AP20187) treatment of mouse strains are as previously described (8). Only male mice were used in this study, as female mice show a sexually dimorphic response in sensitivity to dimerizer-induced β-cell ablation and in their potential for β-cell regeneration (data not shown). Body fat mass was measured with a Bruker Minispec mq10. All protocols for mouse use and euthanasia were reviewed and approved by the Institutional Animal Care and Use Committee of The University of Texas Southwestern (UTSW) Medical Center.
Arginine tolerance test
Mice were fasted for 4–6 hours and subjected to an ip injection of L-arginine (1 mg/g body weight). Tail blood was collected at 0, 5, 15, and 30 minutes and prepared for plasma. Insulin and C-peptide 2 were measured with ELISA kits (EZRMI-13K and EZRMCP2-21K; Millipore).
Immunohistochemistry
Tissues were freshly collected and processed for paraffin sections as previously described (8). Primary antibodies used included: insulin (1:500, A0564; Dako), phospho-Erk (1:500, 9101; Cell Signaling), phospho-Akt/protein kinase B (1:150, 4060; Cell Signaling), NK6 homeobox 1 (Nkx6.1) (1:40, sc-15027; Santa Cruz Biotechnology, Inc), musculoaponeurotic fibrosarcoma transcription factor A (MafA) (1:50, sc-66958; Santa Cruz Biotechnology, Inc), fatty acid binding protein 1 (Fabp1) (1:100, 5352; Cell Signaling), major urinary protein (Mup) (1:50, sc-66976; Santa Cruz Biotechnology, Inc), apolipoprotein (Apo)B (1:100, ab20737; Abcam), and ApoE (1:50, sc-6384; Santa Cruz Biotechnology, Inc). Terminal deoxynucleotidyl transferase dUTP nick end labeling assays were performed with the in situ cell death detection kit, tetramethylrhodamine red (12156792910; Roche). Quantitation of signal area and density was performed with ImageJ.
RNA-seq and pathway analysis
Mouse islets were isolated as previously described (8). Total RNA was purified from isolated pancreatic islets with RNeasy Mini kit (74106; QIAGEN) plus on-column deoxyribonuclease digestion. The strand-specific whole transcriptome RNA-seq was perform on an Illumina HiSeq 2000 sequencer at UTSW McDermott Center Next Generation Sequencing Core. The reads were paired-end, 40 million in number, and 100 bp in length. The raw data were processed and subjected to differential expression analysis with cufflinks (University of Maryland Center for Bioinformatics and Computational Biology) by the UTSW McDermott Center Computational Biology/Bioinformatic Core. For fold change calculation, the 0 values in the cuffdiff-normalized fragments per kilobase of exon per million fragments mapped (FPKM) were set to 0.001, an arbitrary number close to and lower than the smallest positive values (0.006) in the data set. Genes with the cuffdiff-normalized FPKM value less than 2 in both genotypes, and those with a fold change between −1.5 and 1.5, were excluded for the next pathway analysis. The fold change data were subject to the core analysis of the ingenuity pathway analysis (IPA) program. The ingenuity knowledge base (genes only) was used as the reference set, with both direct and indirect relationships considered.
RT quantitative real-time PCR (qPCR)
The SuperScript II Reverse Transcriptase (18064-014; Invitrogen) and the RNaseOUT Recombinant Ribonuclease Inhibitor (10777-019; Invitrogen) were used for cDNA synthesis. qPCR was performed on a 7900HT Fast Real-Time PCR System (4329001; Applied Biosystems), with the Power SYBR Green PCR Master Mix (4368708; Applied Biosystems), and at least triplicate. See Supplemental Table 1 for primer sequences.
Leptin assay
Mouse tail blood samples were collected and prepared for serum. Leptin levels were assayed with an ELISA kit (EZML-82K; Millipore).
Statistical analysis
Two-tailed Student's t test was applied for all pairwise comparisons. Pearson product-moment correlation coefficients were calculated for correlation analysis. Statistical significance was accepted at P < .05.
Results
Adiponectin is essential for β-cell function in pancreatic islet beta-cell apoptosis through targeted activation of caspase 8 (PANIC-ATTAC) mice
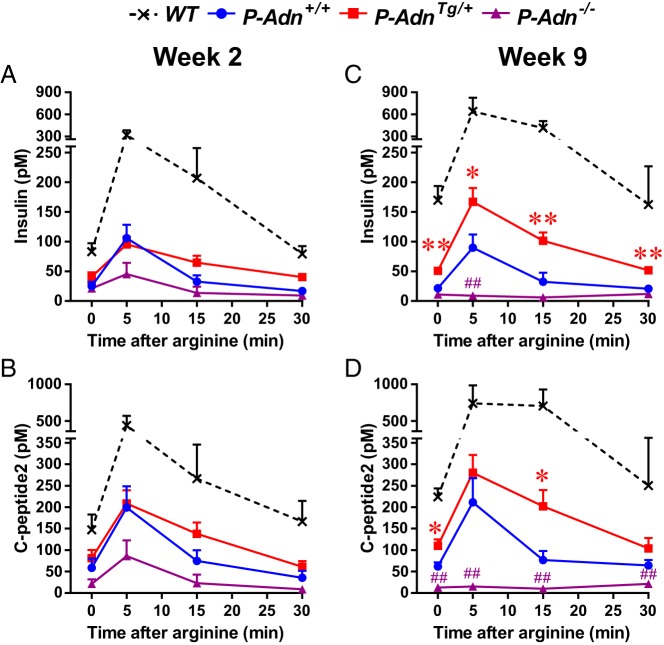
To further investigate the role of adiponectin in pancreatic β-cell function after ablation and regeneration, we used the homozygous PANIC-ATTAC transgenic mouse model (9) in combination with the adiponectin overexpressing transgene (10) or the targeted knockout of endogenous adiponectin gene (11). The 4 experimental genotypes, wild-type (WT), PANIC-ATTAC plus adiponectin WT (P-Adn+/+), PANIC-ATTAC plus adiponectin overexpression (P-AdnTg/+), or PANIC-ATTAC in the background of an adiponectin knockout (P-Adn−/−) were induced for severe β-cell apoptosis by activating the transgenic caspase 8 fusion protein with the chemical dimerizer AP20187 as described in our recent report (8). To assess the β-cell function, we performed arginine-stimulated insulin secretion experiments, a suitable test for first phase insulin secretion that has been used clinically as well (12). Within 2 weeks after the initial dimerizer administration, when β-cell mass was reduced to less than 15% of the euglycemic WT controls (8), all PANIC-ATTAC mice displayed a dramatic reduction in plasma insulin (Figure 1A) and C-peptide levels (Figure 1B) before and after arginine administration. There were no significant differences among the different adiponectin genotypes, ie, P-Adn+/+, P-AdnTg/+, and P-Adn−/− were all impaired, with a trend to a further deterioration in the adiponectin null background. Around 9 weeks after ablation, when partial β-cell mass recovery (to 29% of the WT controls) was observed in P-AdnTg/+ but not in P-Adn+/+ or P-Adn−/− mice (8), arginine-stimulated insulin secretion was significantly enhanced in adiponectin overexpressers but remained completely abolished in adiponectin knockouts (Figure 1, C and D). These data are consistent with our previous characterization of β-cell mass and glucose metabolism in the same mouse models (8), and further support that adiponectin is necessary for the maintenance, as well as sufficient for the improvement, of β-cell function in the PANIC-ATTAC mouse model.
Figure 1.
Adiponectin is critical for functional β-cell reserve in PANIC-ATTAC mice. Mice with 4 different genotypes: WT, homozygous P-Adn+/+, adiponectin heterozygous transgenic (P-AdnTg/+), or adiponectin knockout transgenic (P-Adn−/−) mice were exposed to dimerizer, and subjected to arginine tolerance test 2 (A and B) or 9 (C and D) weeks later. Plasma insulin (A and C) and C-peptide 2 (B and D) are presented as the mean ± SEM; n = 4–11 mice per condition. *, P < .05; **, P < .01 for P-AdnTg/+ vs P-Adn+/+; ##, P < .01 for P-Adn+/+ vs P-Adn−/−.
Adiponectin increases β-cell proliferation in PANIC-ATTAC mice
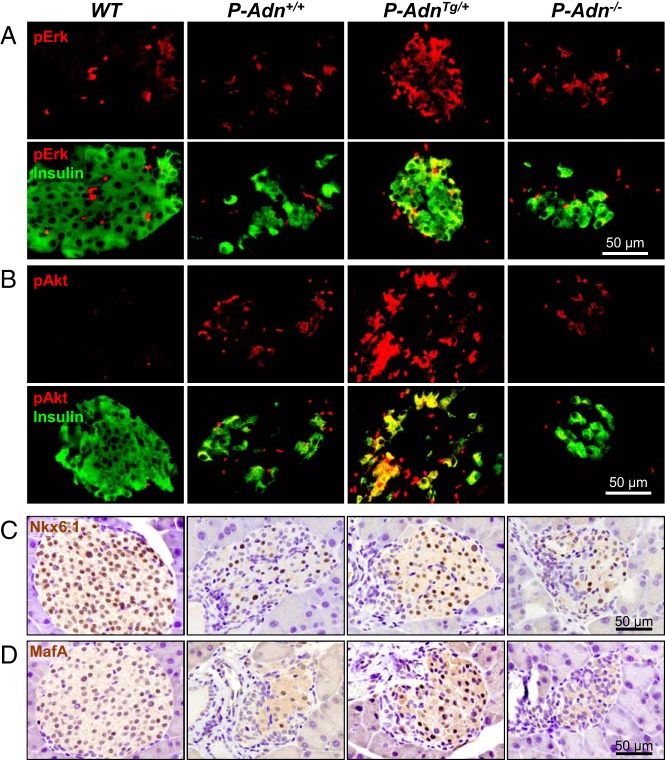
Adiponectin overexpressing mice showed enhanced β-cell proliferation at 5 weeks after dimerizer, as revealed by antigen identified by monoclonal antibody Ki-67 (Ki-67) and bromodeoxyuridine labeling (8). As early as 2 weeks after dimerizer, we detected increased phospho-Erk (Figure 2A and Supplemental Figure 1A) and phospho-Akt (Figure 2B and Supplemental Figure 1B) signals in insulin-positive cells by immunofluorescence. The activated Erk, and particularly phospho-Akt are consistent with proliferative activity in β-cells (6), and may support the mitogenesis in the PANIC-ATTAC model as observed 3 weeks later (8). Terminal deoxynucleotidyl transferase dUTP nick end labeling assay showed no difference in β-cell apoptosis among the P-Adn+/+, P-AdnTg/+, and P-Adn−/− mice (Supplemental Figure 1C).
Figure 2.
Adiponectin stimulates β-cell proliferation in PANIC-ATTAC mice. A and B, Representative immunofluorescence of phosphorylated Erk (pErk) (A) and Akt (pAkt) (B) in pancreatic islets of mice 2 weeks after initial dimerizer treatment. Upper panels, pErk or pAkt signal (red) only. Lower panels, Merged with insulin (green). C and D, Representative immunostaining (brown) of Nkx6.1 (C) and MafA (D) in pancreatic islets of mice 5 weeks after initial dimerizer treatment, counterstained with hematoxylin (blue).
To examine whether adiponectin can also drive β-cell differentiation in regenerating islets, we performed immunostaining with antibodies to Nkx6.1 (Figure 2C) and MafA (Figure 2D), 2 of the key transcription factors expressed in mature β-cells and their precursors (13). Compared with the euglycemic WT controls, P-Adn+/+, and P-Adn−/− mice displayed more than an 85% reduction in Nkx6.1-positive or MafA-positive cells in islets, whereas P-AdnTg/+ mice showed approximately a 2-fold recovery relative to the P-Adn+/+ islets (Supplemental Figure 1, D and E). We wanted to test whether we find any insulin-negative cells with Nkx6.1 or MafA expression but were unable to detect any (Supplemental Figure 1F and data not shown). This is not overly surprising, because the insulin genes are direct transcriptional targets for both of these 2 transcription factors.
Adiponectin improves lipid transport and metabolism in regenerating PANIC-ATTAC islets
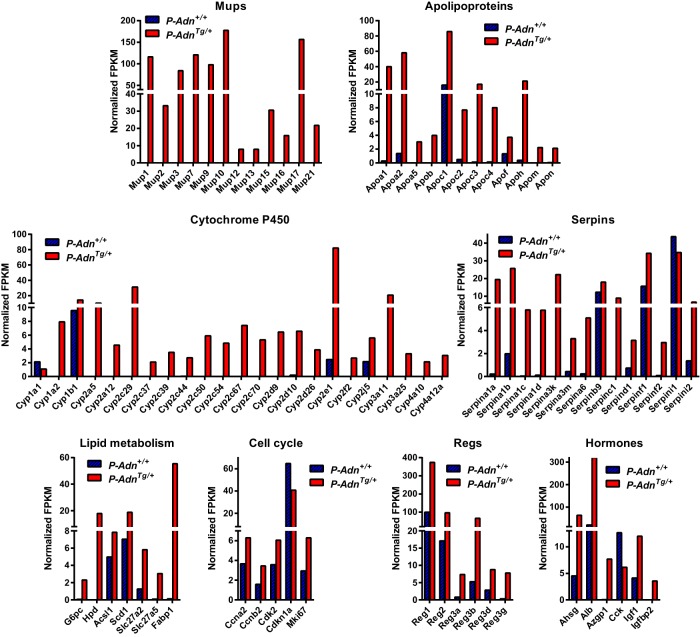
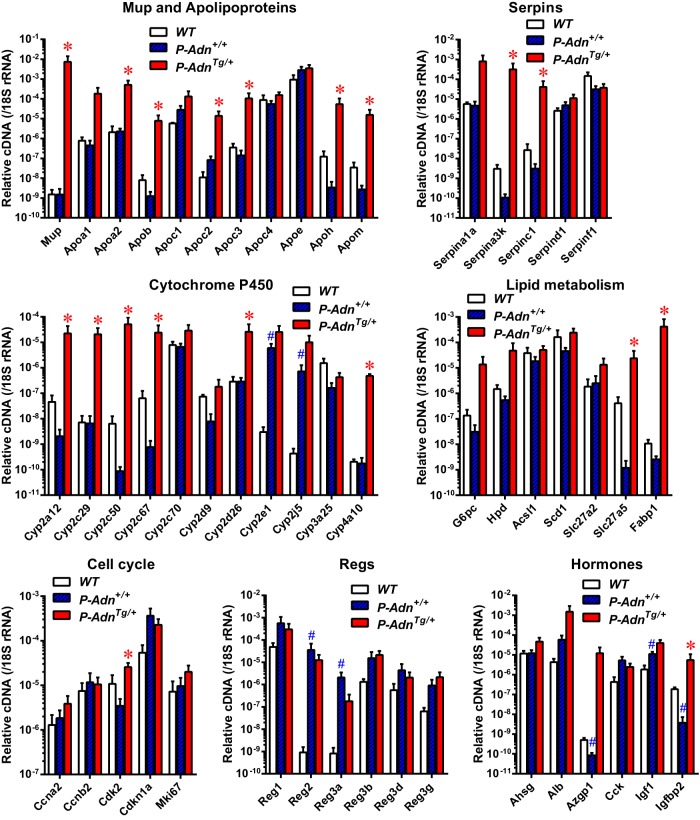
We have previously observed a general reduction of lipotoxic sphingolipids in the regenerating islets from P-AdnTg/+ mice (8). To gain a better understanding how adiponectin activates proliferation and diminishes lipotoxicity, we employed RNA-seq technology to survey the transcriptomes of islets isolated from P-Adn+/+ and P-AdnTg/+ mice. Interestingly, most of the gene expression differences between the 2 genotypes were seen at the level of a transcriptional up-regulation in P-AdnTg/+ islets (Supplemental Figure 2). This was not a generalized transcriptional enhancement, rather we observed a prominent induction in 4 gene families (Figure 3): Mups that serve as lipid transporters (14, 15); Apos, which are a well-established protein family of lipid transporters (16); cytochromes pigment 450 (P450); which are monooxygenases that support detoxification and lipid metabolism (17); and serpins, which are serine/cysteine peptidase inhibitors that regulate coagulation and inflammatory responses and tissue remodeling (18). Expression of the fatty acid transporter Fabp1 was induced to a high level in P-AdnTg/+ islets. Adiponectin also drove expression of key enzymes in nutrient metabolism, such as glucose-6-phosphatase, 4-hydroxyphenylpyruvate dioxygenase, and bile acyl-coenzyme A (CoA) synthetase (solute carrier family 27, member 5). Up-regulation of the cell cycle regulators cyclin A2, cyclin B2, and cyclin-dependent kinase 2, as well as inhibition of cyclin-dependent kinase inhibitor 1, supports enhanced proliferation in the adiponectin transgenic mice. Consistently, transcripts of Ki-67 and the regenerating islet-derived proteins were more abundant in the P-AdnTg/+ islets than in the P-Adn+/+ islets. Other markedly regulated genes included circulating proteins α-2-Heremans-Schmid-glycoprotein, albumin, zinc α-2-glycoprotein 1, and insulin-like growth factor binding protein 2. These findings are consistent with the RT-qPCR analysis of transcripts in the isolated islets (Figure 4). The induction of a partial hepatic gene expression profile in the β-cell that includes albumin and glucose phosphatase has previously been reported (19), although the functional relevance of these findings for the β-cell is unclear. Collectively, adiponectin promotes gene expression for lipid transport, metabolism, tissue remodeling, and cell proliferation in regenerating PANIC-ATTAC islets.
Figure 3.
Transcriptome survey of regenerating PANIC-ATTAC islets by RNA-seq. Transcript abundance as assessed by RNA-seq on total RNA of islets pooled from 8 P-Adn+/+ and 4 P-AdnTg/+ mice 5 weeks after initial dimerizer treatment, presented as FPKM normalized by cuffdiff across the samples.
Figure 4.
RT-qPCR analysis of gene expression for lipid transport and metabolism in regenerating PANIC-ATTAC islets. Pancreatic islets were isolated from WT, P-Adn+/+, and P-AdnTg/+ mice 5 weeks after initial dimerizer treatment, prepared for cDNA, and subjected to qPCR for the indicated genes. cDNA abundances were normalized against 18S rRNA. Data are presented as the mean ± SEM. n = 3–4 samples per genotype.
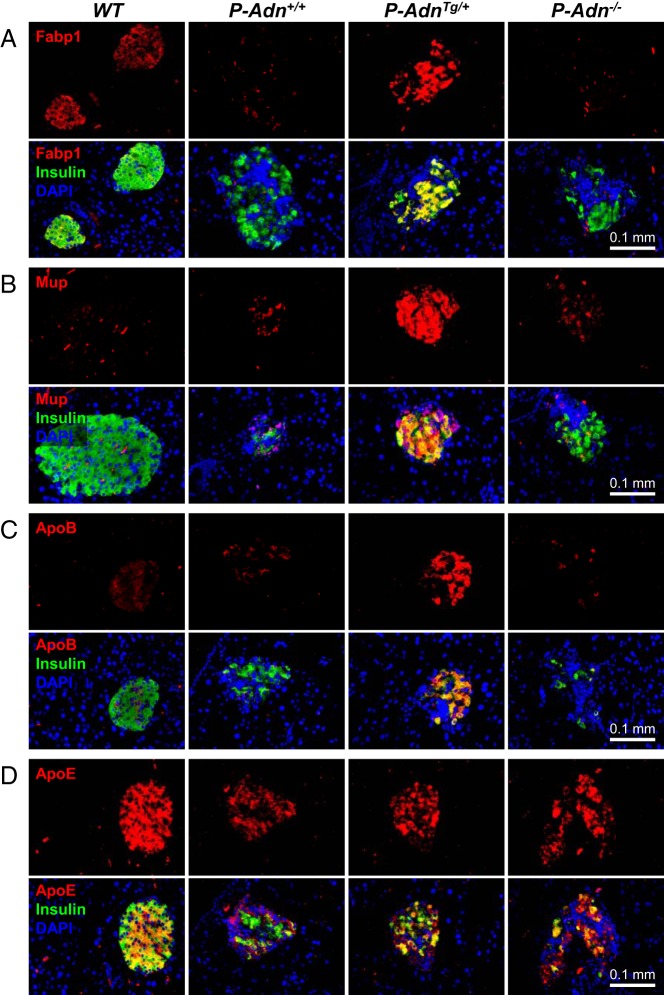
To further confirm the up-regulation of lipid transporters, we examined the islet protein levels of Fabp1 (Figure 5A and Supplemental Figure 3A), Mup (Figure 5B and Supplemental Figure 3B), and ApoB (Figure 5C and Supplemental Figure 3C) by immunofluorescence. During regeneration, P-AdnTg/+ islets displayed stronger signals of these 3 proteins than the WT, P-Adn+/+, and P-Adn−/− islets. Consistent with the unchanged transcript abundance (Figure 4), ApoE immunofluorescence signals were independent of adiponectin genetic manipulations (Figure 5D and Supplemental Figure 3D). These data suggest that the adiponectin-mediated transcriptional regulations were translated to protein levels and functionally improve local lipid metabolism in islets.
Figure 5.
Adiponectin induces lipid transport proteins in regenerating PANIC-ATTAC islets. Representative immunofluorescence of Fabp1 (A), Mup (B), ApoB (C), and ApoE (D) in pancreatic islets of mice 5 weeks after initial dimerizer treatment. Upper panels, Fabp1 (A), Mup (B), ApoB (C), or ApoE (D) signal (red) only. Lower panels, Merged with insulin (green) and DAPI (blue).
Dichotomous antilipotoxic effects of adiponectin in regenerating PANIC-ATTAC islets
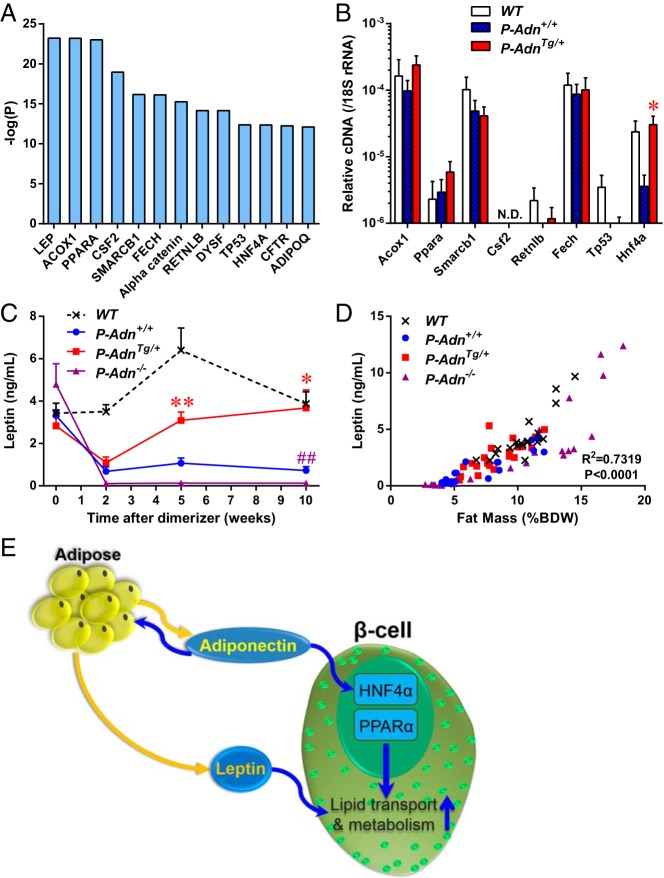
To investigate the signaling pathways responsible for the transcriptional changes in the P-AdnTg/+ islets, we subjected the RNA-seq results to IPA. Among the predicted upstream regulators (Figure 6A), we examined the most prominently affected intracellular molecules by RT-qPCR (Figure 6B). Compared with P-Adn+/+, the adiponectin overexpressing P-AdnTg/+ islets demonstrated increases in transcription factors HNF4α, PPARα and the downstream enzyme Acox1. These factors may contribute to the regulation of Mups, Apos, and cytochrome P450 family members, as well as Fabp1 and metabolic enzymes. Noticeably, the mRNA level of HNF4α decreased by 85% in the lipotoxic, not regenerating P-Adn+/+ islets compared with WT controls, and exhibited a significant increase (8.5-fold) in the regenerating P-AdnTg/+ islets. The adipokine leptin is the predicted upstream regulator with the lowest overlap P value (5.96 × 10−24) (Figure 6A), and this may be responsible for the expression of cytochrome P450 family members, some Apos, and others. We measured the circulating leptin levels at different stages of regeneration (Figure 6C). In all 3 PANIC-ATTAC groups, the leptin levels dropped rapidly after β-cell ablation (as of wk 2). From that point onward, the adiponectin transgenic mice showed a 2.8-fold increase in leptin levels at the regeneration stage (wk 5), and they reached a level comparable with the euglycemic WT mice after recovery stage (wk 10). In contrast, no improvement in circulating leptin levels was observed in P-Adn+/+ and P-Adn−/− mice. Notably, at the end of the observation period, P-Adn−/− leptin levels were significantly lower than the P-Adn+/+ (0.12 ± 0.04 vs 0.73 ± 0.18 ng/mL). As a hormone mainly produced by adipose tissue, the leptin levels in blood were positively correlated with adipose tissue mass among all 4 experimental genotypes and through all 4 stages (Figure 6D and Supplemental Figure 4), suggesting altered leptin levels as secondary outcomes of fat mass changes. Consistent with our previous findings (8), adiponectin plays an essential role in adipocyte lipid uptake and fat mass maintenance. Leptin is also tightly modulated by adiponectin under insulinopenic conditions. The recovery of circulating leptin levels preceded the β-cell mass recovery in adiponectin transgenic mice, and might be one of the major driving forces to keep the lipotoxic environment at bay within the β-cells.
Figure 6.
Potential mediators of the adiponectin-modulated gene expression in regenerating PANIC-ATTAC islets. A, RNA-seq data from P-Adn+/+ and P-AdnTg/+ islets were processed with IPA to predict upstream regulators. P values of overlapping indicate the significance of the prediction. B, RT-qPCR analysis of indicated potential upstream regulators in islets isolated at the regeneration stage. cDNA abundance was normalized by 18S rRNA. N.D., not detected. n = 3–4 samples per genotype. C, Blood letpin in mice under fed status. n = 3–15 mice per condition. For B and C, data are presented as the mean ± SEM. *, P < .05; **, P < .01 for P-AdnTg/+ vs P-Adn+/+; ##, P < .01 for P-Adn−/− vs P-Adn+/+. D, Correlation of fed blood leptin levels and fat mass in mice through all stages of islet ablation and regeneration. n = 15–23 mice per genotype. E, Adiponectin improves lipid metabolism in the β-cell via both systemic and local pathways. As an adipocyte-derived hormone, adiponectin can improve lipid uptake and storage in adipose tissues, thereby increasing the production of leptin. Leptin exerts antilipotoxic effects on the β-cell. On the other hand, adiponectin may directly activate the transcription factors HNF4α and PPARα, which in turn induce the expression of genes encoding lipid transport and metabolism proteins.
Discussion
Recovery of functional β-cell mass can save diabetes patients from insulin dependence. As a first step towards understanding the signaling pathways controlling β-cell regeneration, we took advantage of one of our previously characterized mouse models, in which β-cell mass can be effectively ablated, followed by a partial recovery in the presence of transgenic adiponectin overexpression (8). Adiponectin stimulates early activation of signaling molecules Erk and Akt in β-cells, which may potentiate their proliferative activity as demonstrated by bromodeoxyuridine incorporation, Ki-67 expression (8), and modulation of cell cycle regulators. At the regeneration stage, transcriptome analysis of isolated islets reveals a prominent induction of gene families including Mups, Apos, cytochrome P450, serpins, and regenerating islet-derived proteins, as well as key enzymes for nutrient metabolism. Specific targets are further confirmed by RT-qPCR and immunofluorescence. The adipokine leptin, as well as transcriptional regulators HNF4α and PPARα, are predicted by pathway analyses as upstream regulators of the antilipotoxic programs observed, and their induction in the adiponectin overexpressing mice is confirmed. These data reveal the genetic modulations in β-cells that are key for their regeneration, and suggest that improvement in pancreatic islet lipid transport and metabolism can be a major driving force. Using agonists for adiponectin receptors or PPARα to ameliorate islet lipotoxicity may offer a therapeutic strategy for diabetic patients.
Our transcriptome-wide pathway analysis implies leptin as one of the signaling molecules responsible for the improved lipid metabolism in the islets of adiponectin transgenic mice. Previous studies suggested an antilipotoxic function of leptin (20–22). Adenoviral overexpression of leptin rescued the lipotoxic pathology in cardiomyocytes of the acyl-CoA synthase transgenic mouse model (23). Hyperleptinemia also prevents the gradual failure of islet transplants with lipotoxicity (24). Our in vivo studies suggest the adipose tissue might remotely regulate lipid metabolism in other tissues including pancreatic islets (8), and leptin could be one of the messengers. On the other hand, leptin has been reported to regulate β-cell mass, insulin production and secretion, although results from in vitro and in vivo experiments remain largely controversial (25, 26). Our data suggest that in the context of insulinopenia, the reinstated leptin levels do not inhibit β-cell proliferation or function. As mechanistic links in our mouse models, leptin levels are tightly correlated with adipose tissue mass, which is in turn regulated by adiponectin via its lipid uptake capability (8). We previously reported that β-cell ablation in the PANIC-ATTAC mouse model led to decreased fat mass and increased serum ketone bodies (8). Adiponectin overexpression restored both fat mass and leptin levels before the final recovery of β-cell mass, suggesting the improvements in adipocyte fat storage and systemic lipid homeostasis as contributing factors to β-cell regeneration. In contrast, the difference in ketone body levels between P-Adn+/+ and P-AdnTg/+ mice remained insignificant until β-cell mass recovery was observed (8), implicating the phenomenon as a secondary effect of the amelioration in insulin production and lipid metabolism.
In analogy, adiponectin might directly initiate signal transduction in β-cells, which awaits further investigation (6, 26). Interestingly, the adiponectin-mediated gene expression changes that we observed in pancreatic islets shows a lot of overlap with those reported in the liver (27). Adiponectin induces the expression of genes encoding lipid transport and metabolism components both in β-cells and in liver, including Apos, cytochrome P450 family members, acyl-CoA synthetase long-chain family member 1, stearoyl-CoA desaturase-1, and Fabp1. They also share some potential upstream regulators, such as HNF4α and PPARα. As a human maturity-onset diabetes of the young gene (28), HNF4α is required for β-cell expansion during pregnancy in mouse, as well as Erk activation (29). In a maturity-onset diabetes of the young-like diabetes mouse model with a triple knockout of transcription factors forkhead box O (FoxO) 1, FoxO3a, and FoxO4 in β-cells, HNF4α was predicted as the top transcriptional regulator in pancreatic islets (30). HNF4α is also a transcriptional activator of the PPARα gene (31). PPARα governs lipid metabolism by activating genes of lipid uptake, transport, and fatty acid oxidation (32). In the P-AdnTg/+ islets, the function of PPARα was demonstrated by activation of its downstream targets, including members of the cytochrome P450 family of proteins, Mups, Fabp1, ApoM, fatty acid transport protein 2 (solute carrier family 27, member 2), and 3-hydroxy-3-methylglutaryl-CoA synthase 2. Statistical analysis based on the gene regulation patterns suggest PPARα as the most possible transcriptional regulator for these changes. Interestingly, PPARα signaling pathways are augmented by adiponectin receptor 2 (33), and increase expression of adiponectin receptors 1 and 2 (34). These findings support a dichotomous role of adiponectin in lipid metabolism with effects in adipocytes as well as effects on the β-cell, with common signaling pathways in multiple tissues (Figure 6E).
These findings have implications for both type 1 and type 2 diabetes. It suggests that under conditions of a reduced systemic supply of insulin, maintain elevated adiponectin levels is particularly important. Thiazolidinediones effectively elevate adiponectin levels in circulation, and that elevation of adiponectin levels seems to be important for its antidiabetic actions (11). Thiazolidinediones also have a potent effect on the regeneration of β-cell mass in the model used here, the PANIC-ATTAC mouse (9). Another potent stimulator of adiponectin, fibroblast growth factor 21, has been shown to improve β-cell function and survival by activation of Erk1/2 and Akt signaling pathways (35), further highlighting the hierarchical relationship between activation of PPARγ through thiazolidinediones, induction of fibroblast growth factor 21, induction of adiponectin, targeting the β-cell in part through direct action, in part through indirect action via the adipocyte. Altogether, there is therefore significant potential in both the type 1 and type 2 diabetes arena for direct activation of the adiponectin receptors via small molecule agonists, such as the recently reported adiponectin receptor agonist (36). Future work will rely on a further optimization with respect to specificity, longevity and affinity for such low molecular weight agonists for an effective improvement of the regenerative potential of β-cell mass.
Acknowledgments
We thank The University of Texas Southwestern McDermott Center Next Generation Sequencing Core for RNA-seq service and the Computational Biology/Bioinformatic Core for data processing; The University of Texas Southwestern Molecular Pathology Core for tissue embedding and processing; Dr Roger H. Unger for helpful discussions; Dr Yingfeng Deng for introduction to and discussions on the IPA program; and Steven Connell for administrative and technical support.
This work was supported by National Institutes of Health Grants R01-DK55758, R01-DK099110, and P01-DK088761 (to P.E.S.); Juvenile Diabetes Research Foundation Grant JDRF 17-2012-36 (to P.E.S.); American Heart Association Postdoctoral Fellowship 11POST7240021 and a Naomi Berrie Research Fellowship from the Naomi Berrie Diabetes Center, Columbia University Medical Center (R.Y.); and an American Diabetes Association Postdoctoral Fellowship 7-11-MN-47 (to Q.A.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Akt
- protein kinase B
- Apo
- apolipoprotein
- CoA
- coenzyme A
- Fabp1
- fatty acid binding protein 1
- FoxO
- forkhead box O
- FPKM
- fragments per kilobase of exon per million fragments mapped
- HNF4
- Hepatocyte Nuclear Factor 4
- IPA
- ingenuity pathway analysis
- Ki-67
- antigen identified by monoclonal antibody Ki-67
- MafA
- musculoaponeurotic fibrosarcoma transcription factor A
- Mup
- major urinary protein
- Nkx6.1
- NK6 homeobox 1
- P450
- pigment 450nm
- PANIC-ATTAC
- pancreatic islet beta-cell apoptosis through targeted activation of caspase 8
- P-Adn+/+
- PANIC-ATTAC plus adiponectin WT
- P-Adn−/−
- PANIC-ATTAC in the background of an adiponectin knockout
- P-AdnTg/+
- PANIC-ATTAC plus adiponectin overexpression
- PPAR
- Peroxisome Proliferator-Activated Receptor
- qPCR
- quantitative real-time PCR
- RNA-seq
- RNA sequencing
- UTSW
- The University of Texas Southwestern
- WT
- wild type.
References
- 1. Bouwens L, Rooman I. Regulation of pancreatic β-cell mass. Physiol Rev. 2005;85(4):1255–1270. [DOI] [PubMed] [Google Scholar]
- 2. Bonora E. Protection of pancreatic β-cells: is it feasible? Nutr Metab Cardiovasc Dis. 2008;18(1):74–83. [DOI] [PubMed] [Google Scholar]
- 3. Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic β cell. Biochim Biophys Acta. 2010;1801(3):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rao JR, Keating DJ, Chen C, Parkington HC. Adiponectin increases insulin content and cell proliferation in MIN6 cells via PPARγ-dependent and PPARγ-independent mechanisms. Diabetes Obes Metab. 2012;14(11):983–989. [DOI] [PubMed] [Google Scholar]
- 6. Brown JE, Conner AC, Digby JE, et al. Regulation of β-cell viability and gene expression by distinct agonist fragments of adiponectin. Peptides. 2010;31(5):944–949. [DOI] [PubMed] [Google Scholar]
- 7. Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ye R, Holland WL, Gordillo R, et al. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife. 2014;3:e03851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang ZV, Mu J, Schraw TD, et al. PANIC-ATTAC: a mouse model for inducible and reversible β-cell ablation. Diabetes. 2008;57(8):2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Combs TP, Pajvani UB, Berg AH, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145(1):367–383. [DOI] [PubMed] [Google Scholar]
- 11. Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J Biol Chem. 2006;281(5):2654–2660. [DOI] [PubMed] [Google Scholar]
- 12. Sjostrand M, Carlson K, Arnqvist HJ, et al. Assessment of β-cell function in young patients with type 2 diabetes: arginine-stimulated insulin secretion may reflect β-cell reserve. J Intern Med. 2014;275(1):39–48. [DOI] [PubMed] [Google Scholar]
- 13. Gromada J, Franklin I, Wollheim CB. α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84–116. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Y, Jiang L, Rui L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J Biol Chem. 2009;284(17):11152–11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hui X, Zhu W, Wang Y, et al. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J Biol Chem. 2009;284(21):14050–14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet. 2009;10(2):109–121. [DOI] [PubMed] [Google Scholar]
- 17. Coon MJ. CYTOCHROME P450: nature's most versatile biological catalyst. Annu Rev Pharmacol Toxicol. 2005;45(1):1–25. [DOI] [PubMed] [Google Scholar]
- 18. Huntington JA. Serpin structure, function and dysfunction. J Thromb Haemost. 2011;9:26–34. [DOI] [PubMed] [Google Scholar]
- 19. Wu KJ. Activation of albumin and other liver-specific gene expression in fibroblast-pancreatic cell hybrids: different roles of transcription factors. Exp Cell Res. 1993;208(1):241–247. [DOI] [PubMed] [Google Scholar]
- 20. Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87(1):57–64. [DOI] [PubMed] [Google Scholar]
- 21. Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21(6):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Unger RH, Scherer PE, Holland WL. Dichotomous roles of leptin and adiponectin as enforcers against lipotoxicity during feast and famine. Mol Biol Cell. 2013;24(19):3011–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee Y, Naseem RH, Duplomb L, et al. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci USA. 2004;101(37):13624–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH. Metabolic mechanisms of failure of intraportally transplanted pancreatic β-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes. 2007;56(9):2295–2301. [DOI] [PubMed] [Google Scholar]
- 25. Marroquí L, Gonzalez A, Ñeco P, et al. Role of leptin in the pancreatic β-cell: effects and signaling pathways. J Mol Endocrinol. 2012;49(1):R9–R17. [DOI] [PubMed] [Google Scholar]
- 26. Dunmore SJ, Brown JE. The role of adipokines in β-cell failure of type 2 diabetes. J Endocrinol. 2013;216(1):T37–T45. [DOI] [PubMed] [Google Scholar]
- 27. Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci USA. 2012;109(36):14568–14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamagata K, Furuta H, Oda N, et al. Mutations in the hepatocyte nuclear factor-4[α] gene in maturity-onset diabetes of the young (MODY1). Nature. 1996;384(6608):458–460. [DOI] [PubMed] [Google Scholar]
- 29. Gupta RK, Gao N, Gorski RK, et al. Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev. 2007;21(7):756–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim-Muller JY, Zhao S, Srivastava S, et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab. 2014;20(4):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez-Jimenez CP, Kyrmizi I, Cardot P, Gonzalez FJ, Talianidis I. Hepatocyte nuclear factor 4α coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol Cell Biol. 2010;30(3):565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rakhshandehroo M, Knoch B, Müller M, Kersten S. Peroxisome proliferator-activated receptor α target genes. PPAR Res. 2010;2010:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–339. [DOI] [PubMed] [Google Scholar]
- 34. Tsuchida A, Yamauchi T, Takekawa S, et al. Peroxisome proliferator-activated receptor (PPAR)α activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARα, PPARγ, and their combination. Diabetes. 2005;54(12):3358–3370. [DOI] [PubMed] [Google Scholar]
- 35. Wente W, Efanov AM, Brenner M, et al. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55(9):2470–2478. [DOI] [PubMed] [Google Scholar]
- 36. Okada-Iwabu M, Yamauchi T, Iwabu M, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503(7477):493–499. [DOI] [PubMed] [Google Scholar]