Abstract
Sorting nexin 5 (SNX5) belongs to the SNX family, which is composed of a diverse group of proteins that mediate trafficking of plasma membrane proteins, receptors, and transporters. SNX5 is important in the resensitization of the dopamine D1-like receptor (D1R). D1R is uncoupled from its effector proteins in hypertension and diabetes, and treatment of diabetes restores D1R function and insulin receptor (IR) expression. We tested the hypothesis that the D1R and SNX5 regulate IR by studying the expression, distribution, dynamics, and functional consequences of their interaction in human renal proximal tubule cells (hRPTCs). D1R, SNX5, and IR were expressed and colocalized in the brush border of RPTs. Insulin promoted the colocalization of SNX5 and IR at the perinuclear area of hRPTCs. Unlike SNX5, the D1R colocalized and coimmunoprecipitated with IR, and this interaction was enhanced by insulin. To evaluate the role of SNX5 and D1R on IR signaling, we silenced via RNA interference the endogenous expression of SNX5 or the D1R gene DRD1 in hRPTCs. We observed a decrease in IR expression and abundance of phosphorylated IR substrate and phosphorylated protein kinase B, which are crucial components of the IR signal transduction pathway. Our data indicate that SNX5 and D1R are necessary for normal IR expression and activity. It is conceivable that D1R and SNX5 may interact to increase the sensitivity to insulin via a positive regulation of IR and insulin signaling.
Essential hypertension affects about 30% of the United States population 18 years of age and older and is the most common risk factor for cardiovascular diseases (1, 2). The kidney, with input from several systems, including the endocrine, vascular, and nervous systems, plays an important role in the long-term control of blood pressure (BP), in part, through regulation of sodium balance and peripheral resistance (3, 4). Several natriuretic and antinatriuretic systems interact to maintain normal salt balance and BP (5, 6). One of the natriuretic systems is the renal dopaminergic system that decreases sodium transport in almost all the segments of the nephron (5–9).
Dopamine receptors belong to the rhodopsin-like family of G protein-coupled receptors, characterized by the presence of 7-transmembrane domains (5–9). Based on their differential effects on adenylyl cyclase (AC) activity, the 5 mammalian dopamine receptors are classified into the dopamine D1-like receptors (D1Rs) (D1R and D5R), which stimulate AC activity, and dopamine D2-like receptors (D2R, D3R, and D4R), which inhibit AC activity (5, 7, 9). All of the dopamine receptor subtypes are expressed in almost all segments of the nephron, including the renal proximal tubule (RPT) (9). Because the function of the D1R has been well studied in the RPT, we directed our studies in this nephron segment.
Upon agonist stimulation of the D1R receptor, the activated D1R oligomers dissociate into monomers, couple with Gsα to stimulate AC activity, and recruit G protein-coupled receptor kinase 4 to phosphorylate the third cytoplasmic loop and C-terminal tail of D1R. This may or may not be followed by the binding of adaptor proteins, such as the β-arrestins, leading to the internalization of the activated receptors through the invagination of the plasma membrane (9–11). Once internalized, the receptors, in vesicles termed “early endosomes,” are sorted by sorting nexins (SNXs) and follow divergent pathways; the receptors are either sorted into recycling endosomes for their return to the cell membrane (recycling and resensitization) or in late endosomes, which target the receptors to lysosomes or proteasomes for their subsequent degradation (12, 13).
The SNX family is involved in vesicular trafficking and recycling of G protein-coupled receptors (12, 14, 15). SNX1, SNX2, and SNX4 have been reported to regulate the cellular trafficking of receptors for epidermal growth factor, insulin, platelet-derived growth factor, and leptin (15). In addition, we found that the recycling of D5R and D1R is also regulated by SNX1 and SNX5, respectively (12, 16). In a separate study, we found that SNX5 also positively regulates the expression and activity of the insulin degrading enzyme; selective renal silencing of SNX5 in Wistar-Kyoto rats increases the circulating insulin levels (our unpublished data). Insulin increases sodium reabsorption in the RPT and has vasodilatory actions (17, 18). There are several levels at which insulin receptor (IR) action can be regulated, including the overall expression level. IR expression is down-regulated in the kidney and pressure-natriuresis and insulin-induced vasodilation are impaired in obese Zucker rats (18–20). Streptozotocin-induced hyperglycemia, because of hypoinsulinemia, reduces the expression of renal IR (20). However, although mice with targeted deletion of renal epithelial IR have increased BP, renal sodium excretion is also decreased (21). Thus, there seems to be a disconnect between the effect of insulin on renal sodium transport and vascular resistance. In diabetes, the stimulatory effect of insulin on sodium transport may persist while its ability to decrease vascular resistance is impaired (19, 22). Insulin treatment has been reported to decrease D1R expression but also to increase D5R expression in rat RPT cells (RPTCs) (23, 24). By contrast, fenoldopam, a D1R-like receptor agonist (both D1R and D5R), has been shown to decrease IR expression in vascular smooth muscle cells (VSMCs), but its effect in RPTCs is unknown (25). Therefore, we designed studies to test the hypothesis that SNX5 and D1R interact to regulate IR expression in human RPTCs (hRPTCs).
Materials and Methods
Cell lines
Immortalized hRPTCs (passage, <20) (26) were cultured at 37°C in 95% O2/5% CO2 in DMEM containing Nutrient Mixture F-12 (DMEM/F12) (Invitrogen, Life Technologies), supplemented with 10% fetal bovine serum (Sigma-Aldrich), 5% penicillin-streptomycin (Invitrogen, Life Technologies), and epidermal growth factor (10 ng/mL) (Sigma-Aldrich).
Western blotting
Total cell lysates were prepared using radioimmunoprecipitation assay lysis buffer with a cocktail of protease and phosphatase inhibitors (Thermo Fisher Scientific) on ice. Proteins in the cell lysates were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel, followed by western blotting (27). The dilutions of the antibodies were: SNX5 (1:500; Santa Cruz Biotechnology, Inc), IRβ (against the β-subunit of IR, 1:500; Santa Cruz Biotechnology, Inc), D1R (1:500, proprietary rabbit polyclonal), phospho-protein kinase B (p-PKB) (Pathscan Multiplex Western Cocktail I, 1:1000; Cell Signaling Technology), total PKB (t-PKB) (1:1000; Abcam), phosphorylated IR substrate 1 (p-IRS1) (1:500; Abcam), and total IRS1 (t-IRS1) (1:500; Abcam). The secondary antibodies were obtained from Santa Cruz Biotechnology, Inc or LI-COR (1:10 000). The bands were analyzed by either ImageJ or LI-COR.
Immunofluorescence confocal microscopy
The cells, grown on glass coverslips, were fixed for 20 minutes with 4% cold paraformaldehyde, permeabilized for 5 minutes with 0.05% Triton X-100, and immunostained with appropriately diluted antibodies against SNX5 (rabbit, 1:400; Santa Cruz Biotechnology, Inc), IRβ (mouse, 1:500; Santa Cruz Biotechnology, Inc), or D1R (goat, 1:200; Santa Cruz Biotechnology, Inc) for 1 hour at room temperature or overnight at 4°C. The secondary antibody (Alexa Fluor 488 and Alexa Fluor 568, 1:200; Invitrogen, Life Technologies) staining was performed at room temperature for 1 hour. Colocalization of SNX5 and IR, and D1R and IR was analyzed by MetaMorph image analysis software.
Immunohistochemistry of kidney samples was performed following standard procedures. The human kidney sections were obtained from a nonpathological section of a healthy male human kidney (Imgenex/Novus Biologicals, LLC). The rat kidneys were obtained from our rats bred at Georgetown University. The slides were deparaffinized in xylene in dry oven at 60°C for 1 hour and then washed twice for 3 minutes in 100%, 95%, and 75% ethanol, followed by a 5-minute wash in tap water for hydration. The slides were boiled in sodium citrate buffer (pH 6.0) for 3 minutes for antigen retrieval, cooled to room temperature and then blocked in 1% BSA blocking buffer for 30 minutes at room temperature. Double-staining (overnight at 4°C) with the next pairs of antibodies (1:50), SNX5 and IR and D1R and IR was performed, after which the secondary antibodies were added and incubated for 1 hour at room temperature. The slides were mounted on glass slides using ProLong Gold antifade reagent with 4',6-diamidino-2-phenylindole, and the tissues were examined using an LSM 510 laser scanning confocal microscope.
All animals used in this study were bred and maintained at the animal facilities at Georgetown University. The studies were conducted in accordance with National Institutes of Health guidelines for the ethical treatment and handling of animals in research, and approved by the Georgetown University Institutional Animal Care and Use Committee.
Coimmunoprecipitation
The cell lysates, in buffer 1, were precleared using 50 μL of protein A/G Plus-Agarose (Santa Cruz Biotechnology, Inc) for 30 minutes with rocking at 4°C. Uniform amounts (800 μg) of protein were immunoprecipitated (Immunoprecipitation kit; Roche Applied Science) with the primary antibody (2 μg) specific for the protein of interest for 2 hours. Then 50 μL of Protein G beads were added, and the mixture was incubated with rocking overnight at 4°C. Normal IgG was used as a negative control. Fifty microliters of 2× Laemmli sample buffer (Bio-Rad Laboratories, Inc) was added to the beads. Finally, the samples were boiled for 5 minutes and subjected to western blotting.
RNA preparation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using an RNeasy plus mini kit (QIAGEN Sciences). The samples were treated with deoxyribonuclease I to remove the contaminating DNA from the total RNA sample. Equal amounts of RNA (1 μg) were loaded for cDNA synthesis using Tetro cDNA Synthesis kit (Bioline USA, Inc). One microliter of cDNA was used as a template for the reaction, and the standard curve was obtained for each pair of primers. RT-quantitative polymerase chain reaction was then conducted using a 7900HT Fast Real-Time PCR machine and SYBR Green PCR Master Mix (Applied Biosystems, Life Technologies). Relative amounts of SNX5 and IR were normalized by β-actin. Thermal cycler settings were: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, 59°C for 1 minute, 95°C for 15 seconds, and 60°C for 15 seconds.
The gene-specific primer pairs were: SNX5 (PubMed accession number AF121855) forward (5′-ACGTTTCAGAGCCCAGAGTT-3′) and SNX5 reverse (5′-TCGAGGACCATCAAAGTCG-3′); IR (PubMed accession number BC117172) forward (5′-AGACCTTGGAAATTGGGAACT-3′) and IR reverse (5′-TCTGACAAGCAGAGTTTGGG-3′); and β-actin forward (5′-ACCTGTACGCCAACACAGTG-3′) and β-actin reverse (5′-ACACGGAGTACTTGCGCTCA-3′).
siRNA transfection and shRNA stable transfections
The hRPTCs were transfected with SNX5 siRNA using Lipofectamine RNAiMAX Transfection Reagent (Santa Cruz Biotechnology, Inc). Nontransfected (NT), transfection reagent only, and scrambled siRNA-transfected hRPTCs were used as negative controls. The cells were harvested after 48 hours for RNA extraction, which was quantified by RT-qPCR or after 72 hours to quantify the protein of interest by western blotting.
Alternatively, SNX5 shRNA or DRD1 shRNA was also transfected (FuGENE 6 transfection reagent; Promega) into hRPTCs. Clones were isolated by adding puromycin 1 day after transfection and incubating for 14 days at 37°C. We tested individually several shRNAs on their ability to silence the expression of SNX5 or DRD1. However, we only used the shRNA that gave the best down-regulation of the expression (SNX5 shRNA, 49.3 ± 0.1%; DRD1 shRNA, 73.5 ± 0.01%).
Förster resonance energy transfer (FRET) microscopy
A 1:10 dilution of labeled antibodies was used for FRET. The fluorophore pairs (Invitrogen, Life Technologies) used for FRET imaging were Alexa Fluor 555 (the acceptor dipole) conjugated with D1R antibody and pIR-Alexa Fluor 488 (the donor dipole). Seven images were acquired for each FRET analysis with an Olympus Fluoview FV300 laser scanning confocal microscope equipped with a ×60/1.4 NA objective, an Argon (488 nm) and HeNe (543 nm) laser, emission filters of 515/50 nm, and a 590-nm long pass filter (28).
Statistical analysis
Results are reported as mean ± SEM. Significant differences between 2 groups were determined by Student's t test. Significance among groups was determined by one-way factorial ANOVA followed by Student-Newman-Keuls post hoc test. P < .05 was considered statistically significant.
Results
D1R, SNX5, and IR colocalize in several nephron segments, including the RPT
The distribution of SNX5 and IR was studied in human and rat kidneys. Both SNX5 and IR colocalized in all the tubules of the human kidney. However, IR, but not SNX5, was expressed in glomeruli (Figure 1A). In the rat kidney, their colocalization was observed only in the brush border and apical membranes of RPTs (Figure 1B), which was unlike that observed in the human kidney where both SNX5 and IR were evenly distributed throughout the cell, including the brush border and apical membranes of RPTs.
Figure 1.
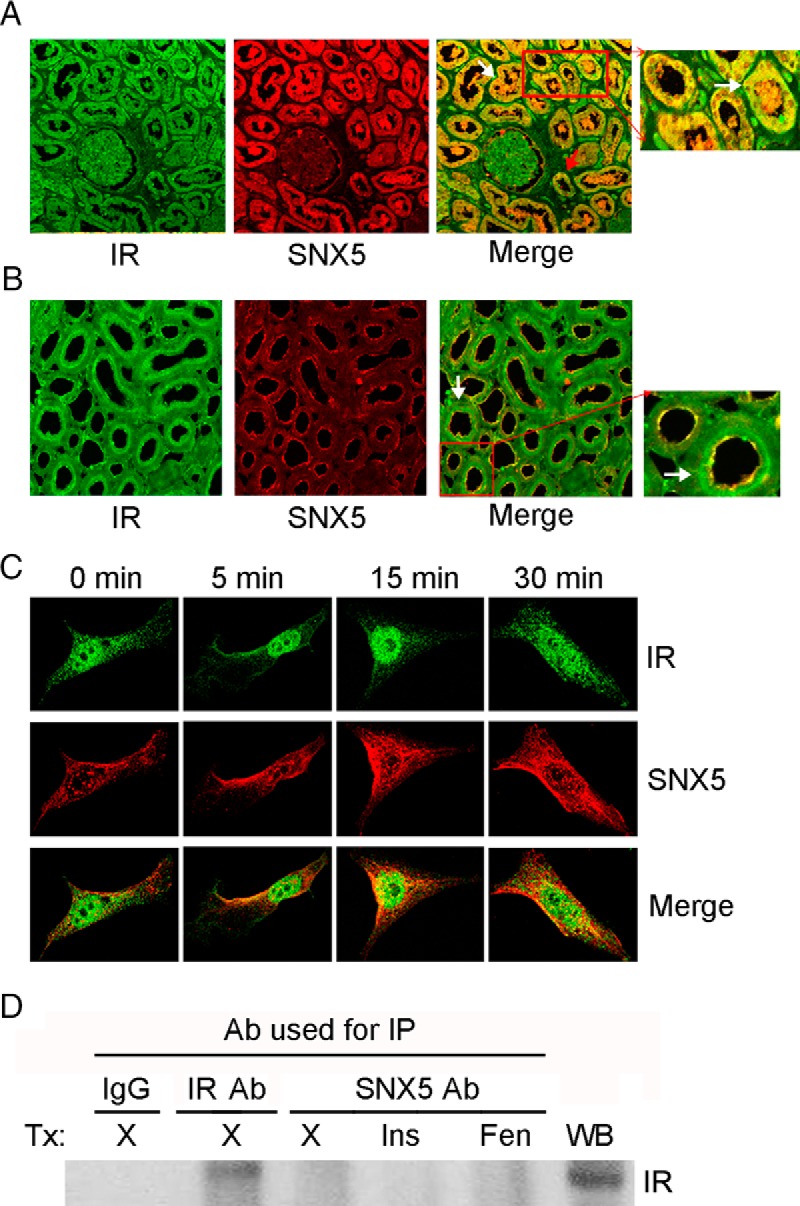
Colocalization of SNX5 and IR in hRPTCs and human and rat kidney sections and lack of coimmunoprecipitation of SNX5 and IR in hRPTCs. A, IR and SNX5 colocalized (yellow, merge images) in RPTs (white arrows) and collecting ducts (red arrow) of human kidney (×100). The inset shows a selected area under higher magnification. B, IR and SNX5 colocalized (yellow, merge images) at the brush border of RPTs (white arrows) in rat kidney (×200). IR was also expressed in subapical areas in rat kidney. The inset shows a selected area under higher magnification. C, In hRPTCs, IR colocalized (yellow, merge images) weakly with SNX5 in the basal state and increased in the perinuclear area after 15 and 30 minutes of insulin (100nM) treatment. D, SNX5 and IR did not coimmunoprecipitate in hRPTCs in the basal state or after insulin (Ins) (100nM/30 min) or D1-like receptor agonist, fenoldopam (Fen) (10−6M/30 min), treatment (Tx). WB, western blotting; X, vehicle; Ab, antibody. SNX5 Ab was used for immunoprecipitation (IP) and IR Ab was used for WB.
The cellular distribution of SNX5 and IR was also studied by laser scanning confocal microscopy in hRPTCs. There was minimal colocalization of SNX5 and IR in the basal state; colocalization of SNX5 and IR in the perinuclear area increased after 15 and 30 minutes of insulin (100nM) treatment (Figure 1C). We have previously reported that SNX5 and D1R colocalize and coimmunoprecipitate in hRPTCs (12). However, there was no coimmunoprecipitation between SNX5 and IR in the basal state or even after insulin (100nM/30 min) or fenoldopam (10−6M/30 min) treatment (Figure 1D).
IR expression in SNX5-depleted hRPTCs
Even though SNX5 and IR did not coimmunoprecipitate, we determined whether SNX5 can still regulate IR by studying hRPTCs in which endogenous SNX5 expression was decreased by transfection with SNX5 shRNA. First, the protein expression of SNX5 in hRPTCs was determined by immunoblotting using a SNX5 antibody, the specificity of which was confirmed by the absence of the expected SNX5 band (∼47–51 kDa) when the SNX5 antibody was preincubated with the immunizing peptide (Figure 2A). Next, immunoblotting showed that SNX5 protein expression was decreased by 49.3 ± 0.1% in SNX5 shRNA-transfected hRPTCs (Figure 2B) that was associated with a 70% decrease in IR protein expression (Figure 2C). The SNX5 shRNA-mediated decrease in IR protein expression could be related to a decrease in transcription because IR mRNA expression, quantified by qRT-PCR, was decreased by approximately 40% with approximately 80% decrease in SNX5 expression (Figure 2, D and E). Silencing either SNX5 or DRD1 did not result in any change in the expression of other closely associated proteins, such as SNX6 and D5R, respectively (Figure 2F), indicating the lack of off-target effects from the shRNA transfection.
Figure 2.
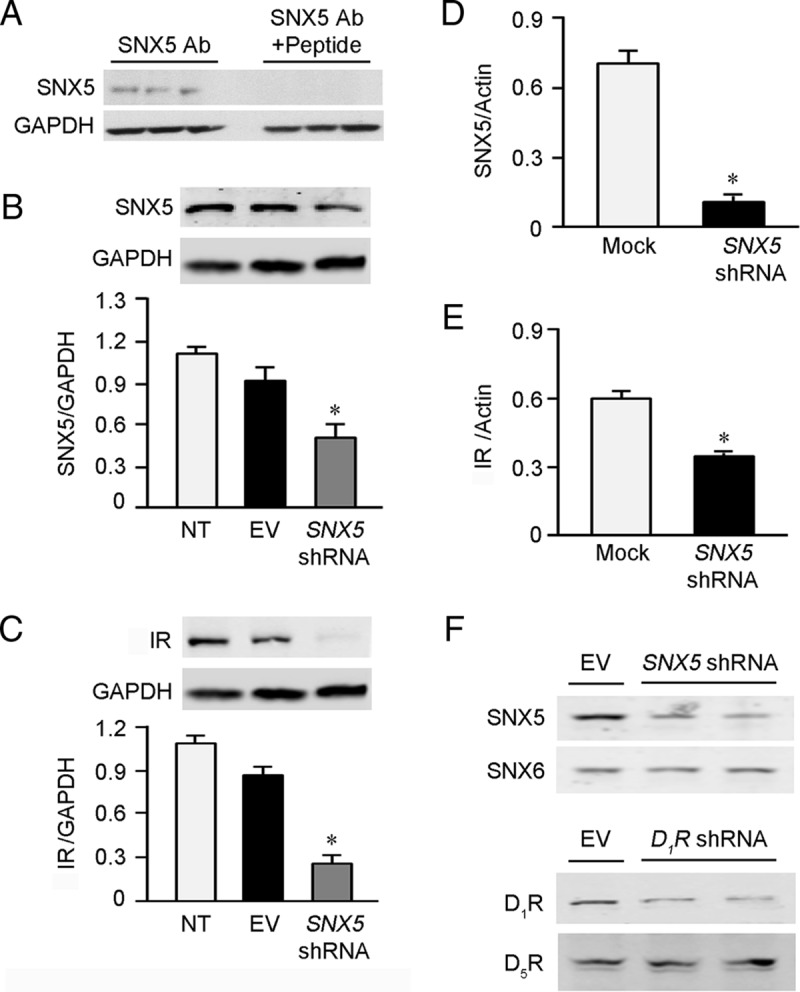
IR expression in SNX5-depleted hRPTCs. A, SNX5 (47–51 kDa) band could not be detected when the SNX5 antibody was preblocked by the immunizing peptide, indicating SNX5 antibody specificity to SNX5. B and C, The protein expressions of SNX5 (B) and IR (C) were decreased in SNX5-depleted hRPTCs compared with NT and EV-transfected hRPTCs (n = 3; *, P < .05 vs others, one-way ANOVA, Student-Newman-Keuls test). D and E, SNX5 (D) and IR (E) mRNA expressions, quantified by real-time PCR (qRT-PCR), were decreased in SNX5-depleted hRPTCs (n = 6; *, P < .05 vs mock, Student's t test). F, The protein expressions of SNX5 and SNX6 in SNX5 shRNA-transfected hRPTCs and the protein expressions of D1R and D5R in DRD1 shRNA-transfected hRPTCs compared with EV-transfected hRPTCs. Ab, antibody; EV, empty vector; NT, nontransfected.
IRS1 and PKB phosphorylation in SNX5-depleted hRPTCs
IRS1 is an adaptor protein, the phosphorylation of which initiates downstream insulin signaling pathways (29, 30). The amount of p-IRS1 was decreased in SNX5 shRNA-transfected hRPTCs that is in contrast to the increased expression of t-IRS1 in SNX5 shRNA-transfected hRPTCs, relative to NT and empty vector (EV)-transfected hRPTCs (Figure 3A). In the basal state, the ratio of p-PKB to t-PKB was also decreased in SNX5 siRNA-treated hRPTCs (Figure 3, B and C). Insulin (100nM/30 min), as expected, increased the ratio of p-PKB to t-PKB expression that was decreased by silencing SNX5 with SNX5 siRNA (Figure 3D).
Figure 3.
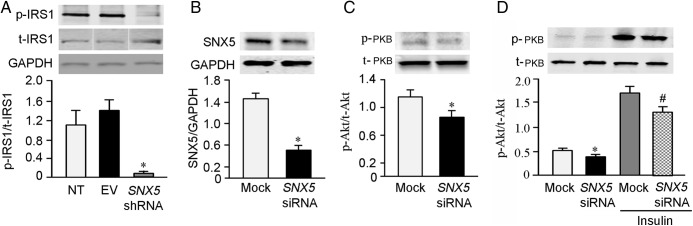
IRS1 and PKB phosphorylation in SNX5-depleted hRPTCs. A, The expression of t-IRS1was increased but p-IRSI was decreased in SNX5 shRNA-depleted hRPTCs, resulting in a decrease in the ratio of p-IRSI to t-IRSI (n = 3; *, P < .05 vs others, one-way ANOVA, Student-Newman-Keuls test). B and C, The protein expressions of SNX5 (B) and the ratio of p-PKB to t-PKB (C) were decreased in SNX5 siRNA-depleted hRPTCs (n = 3; *, P < .05 vs mock, Student's t test). D, Basal p-PKB expression was decreased in SNX5 siRNA-depleted hRPTCs, whereas p-PKB expression was markedly increased by insulin (100nM/30 min), which was also decreased in SNX5 siRNA-depleted hRPTCs (n = 4; *, P < .05 vs mock, Student's t test; #, P < .05, vs mock+insulin, Student's t test). EV, empty vector; NT, nontransfected.
Colocalization and coimmunoprecipitation of D1R and IR in hRPTCs
To determine the possibility of D1R, SNX5, and IR interaction, the expression of D1R and IR in human and rat kidneys was also examined. Both D1R and IR were observed at the brush border of RPTs where colocalization was also observed (Figure 4, A and B), similar to the colocalization between SNX5 and IR in the rat kidney but more restricted than that found in the human kidney (Figure 1, A and B). We next studied the interaction between D1R and IR by determining their cellular distribution in hRPTCs by laser scanning confocal microscope. There was some colocalization between D1R and IR at the perinuclear area in the basal state that was increased with insulin (100nM) treatment, reaching the maximum at 30 minutes (Figure 4C). To increase the resolution of the interaction between D1R and IR, FRET microscopy was employed (28, 31). There was FRET between IR and D1R after incubation with insulin (100nM/30 min), which coincided with the time of maximum colocalization observed with confocal microscopy; the efficiency of energy transfer between D1R and IR was around 30% (Figure 4D). Unlike the absence of a physical interaction between SNX5 and IR (Figure 1D), there was physical interaction between D1R and IR in hRPTCs. Coimmunoprecipitation of D1R and IR, observed in the basal state, was increased with insulin (100nM/30 min) treatment (Figure 4E).
Figure 4.
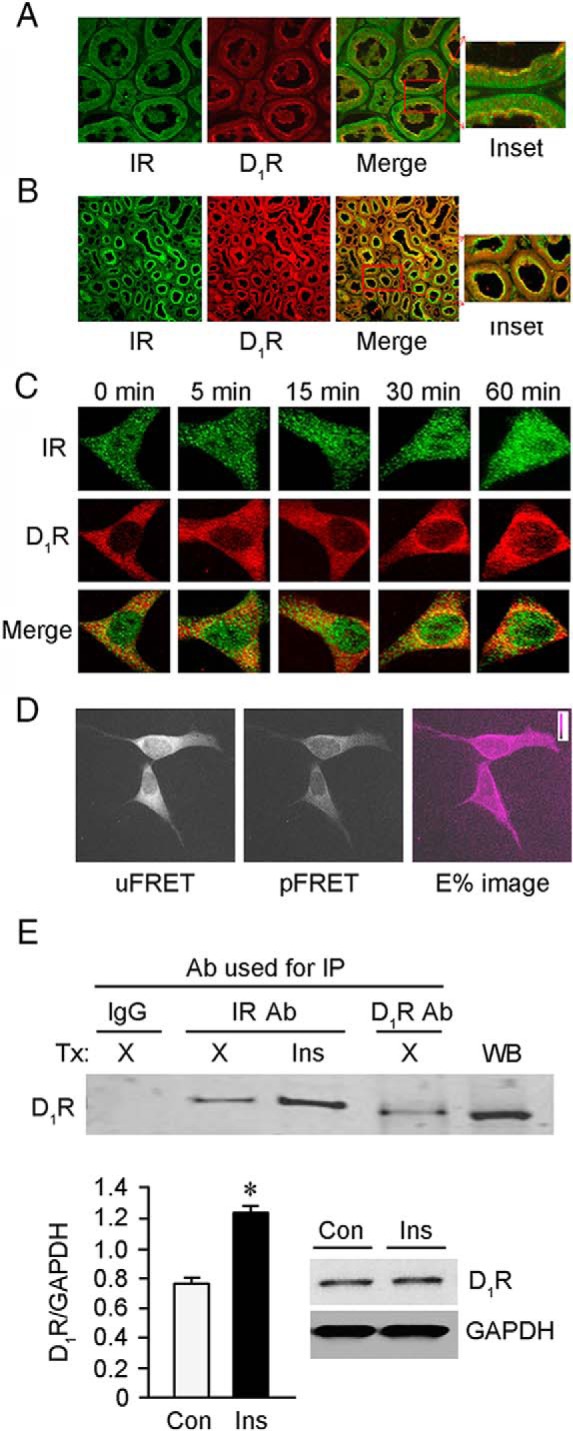
Colocalization and coimmunoprecipitation of D1R and IR in hRPTCs and in kidney sections. A and B, D1R and IR colocalized (yellow, merge images) at the brush borders of RPTs in human (A) and rat (B) kidneys. C, D1R colocalized (yellow, merge images) with IR at the perinuclear area in the basal state. Their colocalization increased with insulin treatment, reaching a maximum at 30 minutes. D, Alexa Fluor 488-labeled IR was used as the donor, whereas Alexa Fluor 555-labeled D1R was used as the acceptor in the FRET dipole. The images (from left to right) are uFRET or uncorrected FRET, pFRET or processed or pure FRET, and E% or efficiency of energy transfer is around 30%. E, D1R and IR coimmunoprecipitated and the amount of which was increased by insulin (Ins, 100nM/30 min) treatment (Tx). X, vehicle; Ab, antibody; IP, immunoprecipitation; Con, control. D1R Ab was used for WB. The immunoblots to the right of the bar graphs indicate uniform expression of D1R in the cells, with or without insulin treatment.
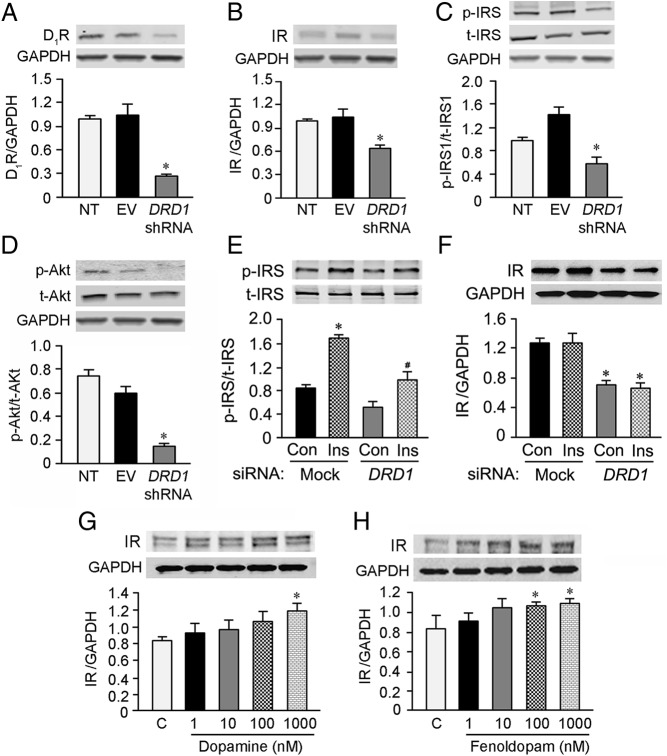
IR expression in the D1R gene DRD1 shRNA-transfected hRPTCs
To determine whether D1R can regulate IR, DRD1 shRNA was transfected into the hRPTCs to silence the expression of endogenous D1R. D1R expression was decreased by 73.5 ± 0.01% in DRD1 shRNA-transfected hRPTCs (Figure 5A). This was associated with a 40% decrease in IR and the ratio of p-IRS1 to t-IRS1 protein expression (Figure 5, B and C) and a 77% decrease in the ratio of p-PKB to t-PKB expression (Figure 5D). It should be noted that SNX5 depletion also decreased basal IR and the ratio of p-IRSI to t-IRSI (Figures 2C and 3A), and decreased the ratio of p-PKB to t-PKB in basal state and minimized the increase in p-PKB to t-PKB caused by insulin treatment (Figure 3, C and D). The results also showed that the ratio of p-IRS1 to t-IRS1 expression was increased after insulin treatment, the increase of which was diminished in DRD1 siRNA-treated cells (Figure 5E). However, there was no difference in IR expression after acute (30 min) insulin stimulation between mock siRNA- and DRD1 siRNA-treated cells (Figure 5F), even though IR expression was decreased by DRD1 siRNA, in agreement with the data shown in Figure 5B. Furthermore, we found that the expression of IR was increased after 24 hours of treatment with dopamine or fenoldopam, the D1-like receptor agonist (Figure 5, G and H), which also confirmed the positive role of D1R in the regulation of IR.
Figure 5.
IR expression in DRD1 shRNA-transfected hRPTCs. A and B, The expressions of D1R (A) and IR (B) were decreased when D1R was silenced in DRD1 shRNA-transfected hRPTCs (n = 3; *, P < .05 vs others, one-way ANOVA, Student-Newman-Keuls test). C, The ratio of p-IRS1 to t-IRS1 was decreased in DRD1 shRNA-transfected hRPTCs compared with NT and EV-transfected hRPTCs. By contrast, t-IRS expression was not affected (n = 3; *, P < .05 vs others, one-way ANOVA, Student-Newman-Keuls test). D, p-PKB expression was decreased, whereas t-PKB expression was not significantly affected in DRD1 shRNA-transfected hRPTCs compared with NT and EV-transfected hRPTCs (n = 3; *, P < .05 vs others, one-way ANOVA, Student-Newman-Keuls test). E, The ratio of p-IRS1 to t-IRS1 was increased in mock siRNA-transfected hRPTCs treated with insulin (100nM/30 min), which was also increased, but to a lesser extent, in DRD1-depleted hRPTCs treated with insulin (100nM/30 min) (n = 3; *, P < .05 vs others, one-way ANOVA, Student-Newman-Keuls test; #, P < .05, vs control treated with DRD1 siRNA, Student's t test). F, The expression of IR was decreased in DRD1 siRNA-transfected hRPTCs, as previously shown; however, insulin (100nM/30 min) had no effect on IR expression in mock or DRD1 siRNA-transfected hRPTCs (n = 3; *, P < .05 vs others, one-way ANOVA, Student-Newman-Keuls test), indicating that short-term insulin treatment does not affect IR expression. G and H, IR expression was increased by dopamine (G) or fenoldopam (H) treatment with the indicated concentrations in hRPTCs (n = 3–4; *, P < .05, vs control, one-way ANOVA, Student-Newman-Keuls test). EV, empty vector; NT, nontransfected.
We have reported that SNX5 physically interacts with D1R and SNX5 is involved in D1R trafficking (12, 16). Because the D1R physically interacts with both SNX5 and IR but SNX5 does not directly interact with IR, it is possible that SNX5 regulates IR through its interaction with and regulation of D1R. Therefore, we studied the colocalization of D1R, SNX5, and IR. There was a slight colocalization of these 3 proteins at the perinuclear area in the basal state that increased with insulin (100nM) treatment, peaking at 30 minutes and returning to baseline at 60 minutes of insulin treatment (Figure 6, A–C).
Figure 6.
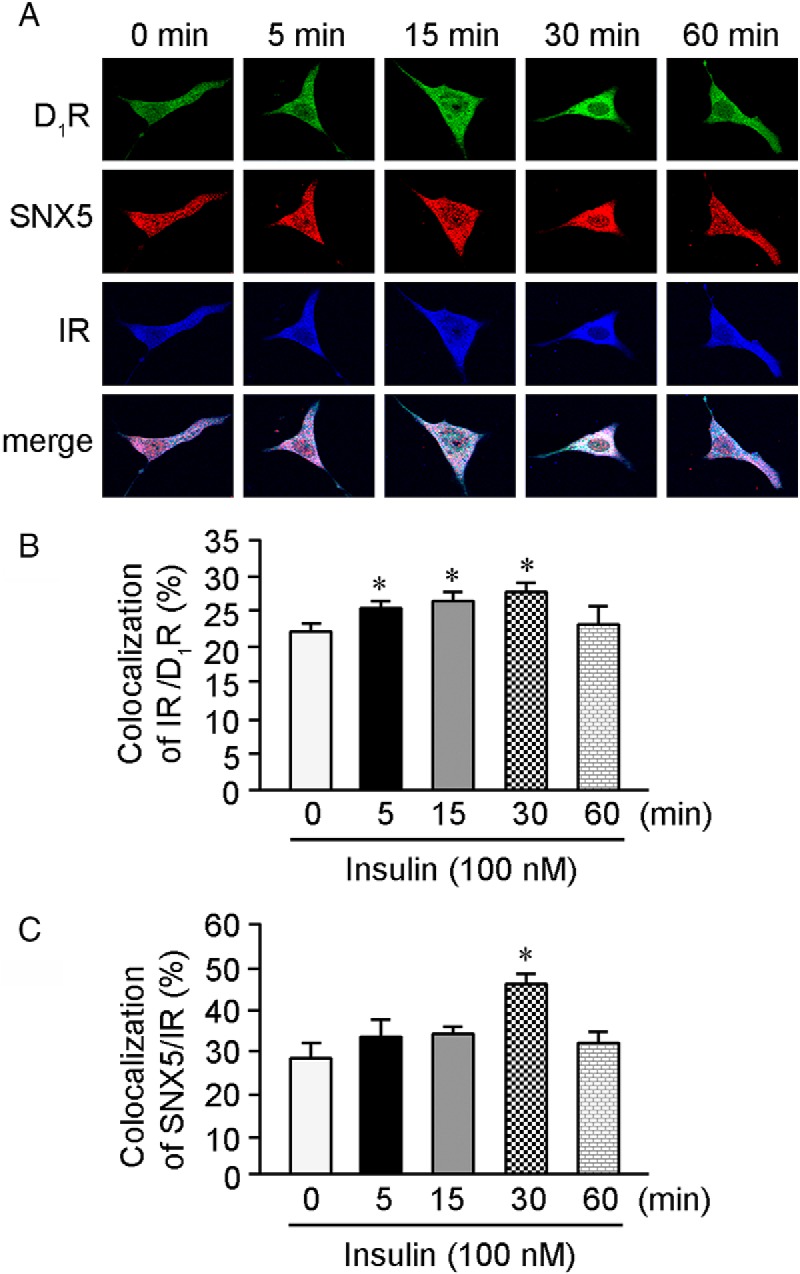
Colocalization and quantification of colocalization among D1R, SNX5, and IR in hRPTCs. A, D1R (green), SNX5 (red), and IR (blue) colocalized (white, merge images) in hRPTCs. Insulin treatment gradually increased their colocalization at the perinuclear area, reached a maximum at 30 minutes and decreased at 60 minutes. B, The bar graph shows that the colocalization of D1R and IR gradually increased from 5 to 30 minutes and decreased at 60 minutes with insulin treatment (n = 4; *, P < .05 vs control, one-way ANOVA, Student-Newman-Keuls test). C, The bar graph shows that the increase in colocalization of SNX5 and IR reached significance at 30 minutes and decreased at 60 minutes with insulin treatment (n = 3; *, P < .05 vs control, one-way ANOVA, Student-Newman-Keuls test).
Discussion
Considerable progress has been made towards the elucidation of the cellular mechanisms of membrane receptor trafficking and signaling by SNXs. SNX1, SNX2, SNX4, SNX5, and SNX6 have been reported to bind to epidermal growth factor receptor, platelet-derived growth factor receptor, leptin receptor, and IR (15, 32, 33). We have also reported that the D5R signaling involves SNX1, whereas D1R signaling involves SNX5 (12, 16). In a separate study, we found that SNX5 positively regulates the expression of the insulin degrading enzyme in hRPTCs and silencing of the SNX5 results in an increase in circulating insulin levels (our unpublished data). Whether or not SNX5 regulates other components of the insulin signaling pathway was not determined.
The current studies showed that SNX5 and IR colocalize in RPTs and RPTCs, but there is no coimmunoprecipitation between SNX5 and IRβ. However, SNX5 and IR have functional interactions. We found that SNX5 protein expression is decreased in the kidney of obese, insulin-resistant Zucker rats (our unpublished data) and renal IR expression is also decreased in these rats (20). Therefore, it is possible that SNX5 is involved in the regulation of renal IR expression. Our studies showed that SNX5 regulates the protein expression of IR, in part via gene transcription, in hRPTCs. We did not study the mechanism of SNX5-dependent transcription although it has been reported that SNX6, which is structurally and functionally close to SNX5, interacts with breast cancer metastasis suppressor 1 both in the cytoplasm and the nucleus and enhances breast cancer metastasis suppressor 1-dependent transcriptional repression (34, 35). Whether or not a similar mechanism is involved in the SNX5 regulation of IR transcription remains to be determined.
The mechanism by which SNX5 regulates IR expression probably does not involve direct physical interaction between the 2 proteins. However, it is possible that another structural protein may be involved in the interaction between SNX5 and IR, possibly a scaffolding, anchoring, or docking protein. The D1R has been identified as a novel partner of SNX5 (12) and may play the role of interacting protein in the SNX5-mediated regulation of IR.
Our data in proximal tubule of kidneys and hRPTCs showed the colocalization of D1R and IR. Moreover, FRET microscopy was performed to narrow down the resolution (200nM) of the confocal laser scanning microscopy because the distance over which FRET can occur is limited to 1–10 nm. The pFRET signal between D1R and IR provides further evidence that D1R and IR are in close proximity. Coimmunoprecipitation between D1R and IR, observed in the basal state, is increased with insulin treatment. Therefore, unlike the absence of a physical interaction between SNX5 and IR, there is a physical interaction between D1R and IR. These observations suggest that D1R interacts with IR and raises the possibility that the D1R may serve to tether SNX5 and IR, because the D1R physically interacts with both SNX5 and IR. Additionally, SNX5, D1R, and IR colocalize in hRPTCs, and their colocalization is also increased with insulin treatment, providing the evidence that SNX5 regulates IR through the D1R because the D1R physically interacts with both SNX5 and IR.
Numerous studies have described alterations in renal D1R function in hypertension and diabetes. The D1R-like receptor agonist fenoldopam improves the decreased insulin sensitivity and impaired natriuresis in streptozotocin-induced type 2 diabetes in rats (36, 37). By contrast, high insulin levels decrease D1R protein expression in the plasma membrane of RPTCs (38). Furthermore, insulin increases D1R serine phosphorylation, which contributes to receptor desensitization, mediated in a PI3K-dependent manner, which increases protein kinase C activity and G protein-coupled receptor kinase 2 membranous translocation (38). Moreover, fenoldopam decreases IR protein expression in VSMCs (25), which is opposite to our findings that D1R actually increases IR protein expression in hRPTCs, suggesting that the regulation may be tissue specific. PD128907, a D3R agonist, decreases IR protein expression in VSMCs, which is tissue specific, because D3R stimulation does not affect IR protein expression in RPTCs (39). These observations indicate a close link between dopamine and IR. Our data may give an explanation, although only partially, why hypertension is frequently present in humans with diabetes (40).
Because a decrease in SNX5 expression results in decreased phosphorylation of IRS1 and PKB in response to insulin treatment in SNX5-depleted hRPTCs, the decrease in IR expression upon SNX5 depletion in hRPTCs may be physiologically important. On the one hand, phosphorylated IRS acts as a second messenger within the cell to stimulate the transcription of insulin-regulated genes (41). Insulin-resistant, obese, and diabetic animals and humans have decreased IRS protein expression (42). On the other hand, PKB is a serine/threonine kinase that belongs to the protein kinase B family and is downstream of insulin and other signaling molecules such as epidermal growth factor, platelet-derived growth factor, fibroblast growth factor, and integrins (43). The optimal downstream responses to PKB occur at submaximal levels of PKB phosphorylation (44), indicating that a modest decrease in maximum PKB phosphorylation is unlikely to have an effect on downstream biological responses.
The mechanism through which SNXs regulate the insulin signaling pathway is not known. However, the impaired IRS-PI3K-3-phosphoinositide-dependent protein kinase insulin signaling with SNX5 depletion may contribute to the development of some diseases, such as hypertension and the metabolic syndrome, because the IRS-PI3K-3-phosphoinositide- dependent protein kinase pathway is involved in the stimulation of glucose metabolism and glycogen/lipid/protein synthesis, the so-called “metabolic effects” (45). Our study suggests a potential role of SNX5 in insulin signaling and may provide a promising target for the treatment of type 2 diabetes or insulin resistance.
There are limitations of the current study. First, most of the experiments were performed in vitro using immortalized hRPTCs. Whether or not the regulation of IR by SNX5 and D1R that we have described in cells apply to renal mechanisms in vivo has yet to be addressed. It will be important to extend this work to kidney-specific Snx5 knockout and Drd1 knockout mouse models. Second, dietary sodium intake may be a modulator of receptor expression. Studies have shown that depending on the animal model and tissue studied, the dietary sodium intake can regulate the expression of some receptors, including IR and D1R (46–50). For example, high-salt diet decreases the mRNA expression of IR in skeletal muscle and kidney of Sprague-Dawley rats but not fructose-fed rats (46). High-salt diet also decreases renal IR density and mRNA levels in all regions of the kidney in Wistar-Kyoto rats but not in spontaneously hypertensive rats (47). However, IR density in adipocytes is increased by high-salt diet in male Wistar rats (48). Sprague-Dawley rats fed a low-NaCl diet have decreased renal cortical and medullary D1R and D5R binding, whereas rats fed a high-NaCl diet show only a decrease in cortical D1R binding, without affecting D5R binding (49). If and how dietary salt affects SNX5 expression and distribution remain to be determined.
A simplified model of the interaction among SNX5, D1R, and IR is proposed and depicted in Figure 7. We suggest that both SNX5 and D1R regulate the expression of IR, and SNX5 regulates the protein expression of IR, in part by regulating its transcription. The mechanism is not due to direct physical interaction. However, D1R, which can directly interact with IR and regulate its protein expression, may act as the conduit to enable SNX5 to regulate IR. Indeed, SNX5 has been shown to directly interact with D1R (12). The coimmunoprecipitation of D1R and the colocalization of SNX5 and IR and SNX5 and D1R, as well as SNX5, IR and D1R are increased by insulin. Therefore, an interaction between SNX5 and D1R regulates the expression of IR in the absence or presence of insulin. This regulation of IR by SNX5 and D1R is important in the insulin signaling pathway.
Figure 7.
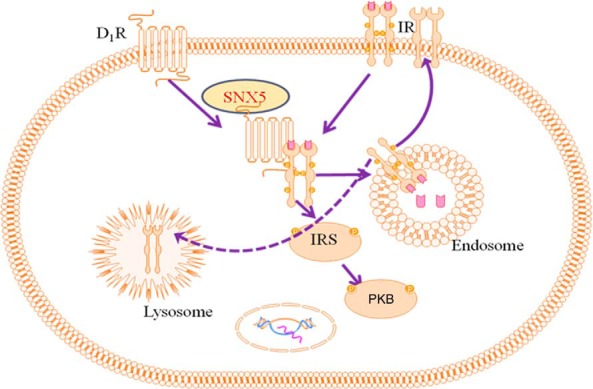
Proposed model for the role of SNX5 and D1R in the regulation of IR in hRPTCs. Both D1R and SNX5 regulate the expression of IR. SNX5 regulates the protein expression of IR, in part by regulating its transcription. D1R, which can directly interact with IR and regulate its protein expression, may act as the conduit to enable SNX5 to regulate IR.
Acknowledgments
These studies were supported in part by United States National Institutes of Health Grants R01HL092196, R37HL023081, and R01DK090918 and National Natural Science Foundation of China Grants 81100500 and 30925018.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- adenylyl cyclase
- BP
- blood pressure
- D1R
- dopamine D1-like receptor
- EV
- empty vector
- FRET
- Förster resonance energy transfer
- hRPTC
- human RPTC
- IR
- insulin receptor
- NT
- nontransfected
- p-PKB
- phospho-PKB
- p-IRS1
- phosphorylated IR substrate 1
- PI3K
- phosphatidylinositol-4,5-bisphosphate 3-kinase
- qRT-PCR
- quantitative real-time PCR
- RPT
- renal proximal tubule
- RPTC
- RPT cell
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- SNX
- sorting nexin
- t-PKB
- total PKB
- t-IRS1
- total IRS1
- VSMC
- vascular smooth muscle cell.
References
- 1. Centers for Disease Control and Prevention (CDC). Vital signs: awareness and treatment of uncontrolled hypertension among adults–United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2012;61:703–709. [PubMed] [Google Scholar]
- 2. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 3. Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. [DOI] [PubMed] [Google Scholar]
- 4. Herrera M, Coffman TM. The kidney and hypertension: novel insights from transgenic models. Curr Opin Nephrol Hypertens. 2012;21:171–178. [DOI] [PubMed] [Google Scholar]
- 5. Chugh G, Pokkunuri I, Asghar M. Renal dopamine and angiotensin II receptor signaling in age-related hypertension. Am J Physiol Renal Physiol. 2013;304:F1–F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aperia A. 2011 Homer Smith Award: to serve and protect: classic and novel roles for Na+, K+ -adenosine triphosphatase. J Am Soc Nephrol. 2012;23:1283–1290. [DOI] [PubMed] [Google Scholar]
- 7. Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinto V, Pinho MJ, Soares-da-Silva P. Renal amino acid transport systems and essential hypertension. FASEB J. 2013;27:2927–2938. [DOI] [PubMed] [Google Scholar]
- 9. Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol. 2011;1:1075–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardner B, Liu ZF, Jiang D, Sibley DR. The role of phosphorylation/dephosphorylation in agonist-induced desensitization of D1 dopamine receptor function: evidence for a novel pathway for receptor dephosphorylation. Mol Pharmacol. 2001;59:310–321. [DOI] [PubMed] [Google Scholar]
- 11. Rankin ML, Marinec PS, Cabrera DM, Wang Z, Jose PA, Sibley DR. The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol. 2006;69:759–769. [DOI] [PubMed] [Google Scholar]
- 12. Villar VA, Armando I, Sanada H, et al. Novel role of sorting nexin 5 in renal D(1) dopamine receptor trafficking and function: implications for hypertension. FASEB J. 2013;27:1808–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarker S, Xiao K, Shenoy SK. A tale of two sites: how ubiquitination of a G protein-coupled receptor is coupled to its lysosomal trafficking from distinct receptor domains. Commun Integr Biol. 2011;4:528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng B, Ma YC, Ostrom RS, et al. RGS-PX1, a GAP for GαS and sorting nexin in vesicular trafficking. Science. 2001;294:1939–1942. [DOI] [PubMed] [Google Scholar]
- 15. Haft CR, de la Luz Sierra M, Barr VA, Haft DH, Taylor SI. Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol Cell Biol. 1998;18:7278–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Villar VA, Jones JE, Armando I, et al. Sorting nexin 1 loss results in D5 dopamine receptor dysfunction in human renal proximal tubule cells and hypertension in mice. J Biol Chem. 2013;288:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987;79:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658–H1666. [DOI] [PubMed] [Google Scholar]
- 19. Khan O, Riazi S, Hu X, Song J, Wade JB, Ecelbarger CA. Regulation of the renal thiazide-sensitive Na-Cl cotransporter, blood pressure, and natriuresis in obese Zucker rats treated with rosiglitazone. Am J Physiol Renal Physiol. 2005;289:F442–F450. [DOI] [PubMed] [Google Scholar]
- 20. Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrol. 2007;18:2661–2671. [DOI] [PubMed] [Google Scholar]
- 21. Tiwari S, Sharma N, Gill PS, et al. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci USA. 2008;105:6469–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manrique C, Lastra G, Sowers JR. New insights into insulin action and resistance in the vasculature. Ann NY Acad Sci. 2014;1311:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Umrani DN, Banday AA, Hussain T, Lokhandwala MF. Rosiglitazone treatment restores renal dopamine receptor function in obese Zucker rats. Hypertension. 2002;40:880–885. [DOI] [PubMed] [Google Scholar]
- 24. Yang J, Cui Z, He D, et al. Insulin increases D5 dopamine receptor expression and function in renal proximal tubule cells from Wistar-Kyoto rats. Am J Hypertens. 2009;22:770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng C, Han Y, Huang H, et al. D1-like receptors inhibit insulin-induced vascular smooth muscle cell proliferation via down-regulation of insulin receptor expression. J Hypertens. 2009;27:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felder RA, Sanada H, Xu J, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanada H, Jose PA, Hazen-Martin D, et al. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–1042. [DOI] [PubMed] [Google Scholar]
- 28. Li H, Armando I, Yu P, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whelan SA, Dias WB, Thiruneelakantapillai L, Lane MD, Hart GW. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked β-N-acetylglucosamine in 3T3-L1 adipocytes. J Biol Chem. 2010;285:5204–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li H, Li HF, Felder RA, Periasamy A, Jose PA. Rab4 and Rab11 coordinately regulate the recycling of angiotensin II type I receptor as demonstrated by fluorescence resonance energy transfer microscopy. J Biomed Opt. 2008;13:031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen LN, Holdren MS, Nguyen AP, et al. Sorting nexin 1 down-regulation promotes colon tumorigenesis. Clin Cancer Res. 2006;12:6952–6959. [DOI] [PubMed] [Google Scholar]
- 33. Liu H, Liu ZQ, Chen CX, Magill S, Jiang Y, Liu YJ. Inhibitory regulation of EGF receptor degradation by sorting nexin 5. Biochem Biophys Res Commun. 2006;342:537–546. [DOI] [PubMed] [Google Scholar]
- 34. Rivera J, Megías D, Bravo J. Sorting nexin 6 interacts with breast cancer metastasis suppressor-1 and promotes transcriptional repression. J Cell Biochem. 2010;111:1464–1472. [DOI] [PubMed] [Google Scholar]
- 35. Samant RS, Seraj MJ, Saunders MM, et al. Analysis of mechanisms underlying BRMS1 suppression of metastasis. Clin Exp Metastasis. 2000;18:683–693. [DOI] [PubMed] [Google Scholar]
- 36. Umrani DN, Goyal RK. Beneficial effects of fenoldopam treatment on renal function in streptozotocin-induced diabetic rats. Clin Exp Hypertens. 2002;24:207–219. [DOI] [PubMed] [Google Scholar]
- 37. Umrani DN, Goyal RK. Fenoldopam treatment improves peripheral insulin sensitivity and renal function in STZ-induced type 2 diabetic rats. Clin Exp Hypertens. 2003;25:221–233. [DOI] [PubMed] [Google Scholar]
- 38. Banday AA, Fazili FR, Lokhandwala MF. Insulin causes renal dopamine D1 receptor desensitization via GRK2-mediated receptor phosphorylation involving phosphatidylinositol 3-kinase and protein kinase C. Am J Physiol Renal Physiol. 2007;293:F877–F884. [DOI] [PubMed] [Google Scholar]
- 39. Huang H, Han Y, Wang X, et al. Inhibitory effect of the D(3) dopamine receptor on insulin receptor expression and function in vascular smooth muscle cells. Am J Hypertens. 2011;24:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Burgos-Lunar C, Jiménez-García R, Salinero-Fort MA, et al. Trends in hypertension prevalence, awareness, treatment and control in an adult type 2 diabetes Spanish population between 2003 and 2009. PLoS One. 2014;9:e86713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rose DW, Saltiel AR, Majumdar M, Decker SJ, Olefsky JM. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc Natl Acad Sci USA. 1994;91:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48:1359–1364. [DOI] [PubMed] [Google Scholar]
- 43. Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. [DOI] [PubMed] [Google Scholar]
- 44. Tan SX, Ng Y, Meoli CC, et al. Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J Biol Chem. 2012;287:6128–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeng XQ, Zhang CM, Tong ML, et al. Knockdown of NYGGF4 increases glucose transport in C2C12 mice skeletal myocytes by activation IRS-1/PI3K/AKT insulin pathway. J Bioenerg Biomembr. 2012;44:351–355. [DOI] [PubMed] [Google Scholar]
- 46. Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003;64:2163–2171. [DOI] [PubMed] [Google Scholar]
- 47. Sechi LA, Griffin CA, Giacchetti G, et al. Abnormalities of insulin receptors in spontaneously hypertensive rats. Hypertension. 1996;27:955–961. [DOI] [PubMed] [Google Scholar]
- 48. da Costa Lima NK, Lima FB, dos Santos EA, et al. Chronic salt overload increases blood pressure and improves glucose metabolism without changing insulin sensitivity. Am J Hypertens. 1997;10:720–727. [DOI] [PubMed] [Google Scholar]
- 49. Sharif NA, Nunes JL, Lake KD, et al. Chronic manipulation of dietary salt modulates renal physiology and kidney dopamine receptor subtypes: functional and autoradiographic studies. Gen Pharmacol. 1995;26:727–735. [DOI] [PubMed] [Google Scholar]
- 50. Ohbu K, Kaskel FJ, Kinoshita S, Felder RA. Dopamine-1 receptors in the proximal convoluted tubule of Dahl rats: defective coupling to adenylate cyclase. Am J Physiol. 1995;268:R231–R235. [DOI] [PubMed] [Google Scholar]