Graphical abstract
Abbreviations: α-LA, α-lactalbumin; BAMLET, bovine α-lactalbumin made lethal to tumor cells; DLS, dynamic light scattering; EPR, enhanced permeability and retention; FA, fatty acid; FoA, folic acid; HAMLET, human α-lactalbumin made lethal to tumor cells; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; NP, nanoparticles; OA, oleic acid; PMS, phenazine methosulfate; SEM, scanning electron microscopy
Keywords: BAMLET, Cancer therapy, Fatty acid, HAMLET, α-Lactalbumin, Oleic acid
Highlights
-
•
We synthesized three different BAMLET complexes consisting of oleic acid coupled to bovine α-lactalbumin.
-
•
Oleic acid micelles alone are tumoricidal at equimolar concentrations of oleic acid bound in the BAMLET complexes.
-
•
α-Lactalbumin is non-toxic to cells even when delivered to their cytoplasm.
-
•
Both, BAMLET and oleic acid micelles showed no selective cytotoxicity to cancer cells.
Abstract
Lipid–protein complexes comprised of oleic acid (OA) non-covalently coupled to human/bovine α-lactalbumin, named HAMLET/BAMLET, display cytotoxic properties against cancer cells. However, there is still a substantial debate about the role of the protein in these complexes. To shed light into this, we obtained three different BAMLET complexes using varying synthesis conditions. Our data suggest that to form active BAMLET particles, OA has to reach critical micelle concentration with an approximate diameter of 250 nm. Proteolysis experiments on BAMLET show that OA protects the protein and is probably located on the surface, consistent with a micelle-like structure. Native or unfolded α-lactalbumin without OA lacked any tumoricidal activity. In contrast, OA alone killed cancer cells with the same efficiency at equimolar concentrations as its formulation as BAMLET. Our data show unequivocally that the cytotoxicity of the BAMLET complex is exclusively due to OA and that OA alone, when formulated as a micelle, is as toxic as the BAMLET complex. The contradictory literature results on the cytotoxicity of BAMLET might be explained by our finding that it was imperative to sonicate the samples to obtain toxic OA.
1. Introduction
Discovering effective cancer treatments is a priority in medical research. Traditional treatment by chemotherapy often still utilizes combinations of very cytotoxic drugs that primarily kill all metabolically active cells [1]. Since some normal cells also divide rapidly, most treatments involving chemotherapy have a low pharmaceutical index and frequently produce severe side effects [1–3]. Taking this into consideration, there is a strong need to develop more selective cancer drugs. For example, nano-sized delivery systems are being developed to promote drug accumulation at the tumor site by the enhanced permeability and retention (EPR) effect [4,5].
Targeted delivery systems are also being developed that exploit other hallmarks of cancer. Such hallmarks include differences in the cell membrane composition including the fatty acid (FA) composition [6]. Some FA have key roles in regulating cell processes [7] and thus have potential in bio-therapeutic and pharmaceutical applications. Specifically, over the last three decades, different studies have characterized the pharmacological and biological [8,9] role of FA and their application in cancer treatments [10,11]. Unsaturated FA are internalized by receptor-mediated mechanisms or by diffusion across the cell membranes [12] and have been shown to influence inflammation, apoptosis, and growth inhibition processes [13–15]. The mechanism of FA that causes cell death involves apoptosis and necrosis [16]. Unsaturated FA also promote the modification of immune responses, changes in membrane composition, and cell fluidity [17,18]. The ω-3 and ω-6 FA have been the most studied antineoplastic agents [14,19] and only few studies also explored ω-9 monounsaturated FA. A few years ago, Martins de Lima and Curi-Boaventura demonstrated that ω-9 monounsaturated FA could bind to peroxisome-proliferator-activated receptors to produce lipid peroxidation and reactive oxygen species [20,21].
OA is a ω-9 monounsaturated FA that was discovered in the 1990ths by Svanborg and co-workers to be associated with human α-lactalbumin (LA) in breast milk to form a complex they called HAMLET (Human Alpha lactalbumin Made LEthal to Tumor cells) because it was characterized by potent cytotoxic activity [22]. Subsequent papers also reported that this complex was selectively cytotoxic to malignant cells but spared healthy ones [23–25]. Follow-up studies of HAMLET revealed that the OA moieties are bound to a denatured form of α-LA [26,27]. Svanborg and co-workers also conducted experiments to form HAMLET-like complexes using cis- and trans-mono- and polyunsaturated FA with the purpose of determining if other FA could produce similar cytotoxic complexes [28]. Their results showed that the two cis-monounsaturated FA, OA (C18:1:9 cis) and vaccenic acid (C18:1:11 cis), killed cancer cells when coupled to α-LA while trans- and polyunsaturated FA were unable to do so. The study also claimed that OA in the absence of α-LA is not tumoricidal. Highlighting the importance of the protein in the tumor toxicity, another study concluded that the denaturation of the protein is the key to the novel anti-tumoral effect of the HAMLET complex [29].
However, recently published papers report contradictory results on the toxicity of α-LA and OA in HAMLET-like complexes. In essence, several works interpret their data to show that none of the individual HAMLET components (α-LA and OA) alone were able to activate cell death [30]. In contrast, other articles pointed out that OA is the key for the potent cytotoxicity and cell internalization [31–34]. Other research groups still attribute the cytotoxicity to the partially unfolded structure of α-LA [35,36], yet other studies associate the tumoricidal action to the lipid factor but highlight that OA is not as toxic at the same concentration than OA in HAMLET-like complexes [33] or even claim that free OA is basically inactive at the concentration at which HAMLET causes cell death [36]. We can surmise that even today there is a vivid ongoing discussion as to the contributions of OA and α-LA to the cytotoxicity of HAMLET and HAMLET-like complexes.
Another point recently raised in the literature is with respect to the specificity of the HAMLET-like complexes to only target cancer cells. Some recent studies continue suggesting that HAMLET-like complexes are selectively cytotoxic by activating caspase-independent apoptosis, necrosis, and macroautophagy in cancer cells [25,27,36]. However, Brinkmann and co-workers showed that non-cancer derived primary cells were indiscriminately killed after exposure to BAMLET and that some normal cells could even be more sensitive to it than cancer cells [31].
Taking into consideration the extensive level of controversial data and knowledge gap concerning the structure–function relationship of HAMLET/BAMLET, we optimized three different reaction conditions to construct various BAMLET complexes using bovine α-LA and OA (Fig. 1). The structure, size, morphology, and cytotoxicity of these BAMLET complexes were scrutinized and compared to activity profiles of preparations of the free OA.
Fig. 1.
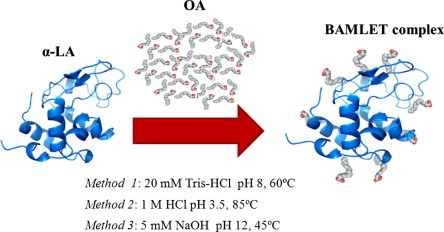
Schematic representation of the syntheses conditions for the coupling of OA moieties to bovine α-LA to obtain BAMLET used in this work. For details on the properties of the complexes prepared by the three methods please see Table 1.
2. Results and discussion
2.1. Synthesis and characterization of BAMLET complexes
In order to be able to compare various BAMLET complexes, we adapted several methods from the literature [37–39] and optimized them to improve the coupling reaction of OA to α-LA (Fig. 1). The main differences in the reaction conditions were the temperatures (45 °C, 60 °C, and 85 °C) and pH values (2, 8, and 12) employed. According to the literature increased temperature should lead to better loading with OA because more denatured α-LA molecules expose more of the hydrophobic core where most FA binding is anticipated [40]. Similarly, the pH should also influence the protein conformation, which is important since the coupling of OA to α-LA is largely based on the hydrophobic effect [41]. At the end of the syntheses, all the complexes were submitted to 1-step of ultra-sonication for 1 min at 400 W and then lyophilized. The three complexes were named α-LA-OA HCl 85, NaOH 45, and Tris–HCl 60 based on the synthesis conditions employed (Table 1).
Table 1.
Characterization of the synthesized BAMLET complexes.
| Sample | OA/α-LAa (mol/mol) | Diameter (nm)b | % Polydispersityc |
|---|---|---|---|
| α-LA-OA Tris–HCl 60 | 11 ± 3 | 227 ± 14 | 13.2 |
| α-LA-OA NaOH 45 | 9 ± 2 | 256 ± 17 | 33.9 |
| α-LA-OA HCl 85 | 4 ± 1 | 292 ± 11 | 28.7 |
| OAd | n/a | 198 ± 9 | 10.9 |
Number of OA molecules attached to each α-LA on average determined.
Obtained by dynamic light scattering (DLS). BAMLET complexes were suspended in filtered nanopure water.
Polydispersity is a measure of the homogeneity of the NP.
Free OA dispersed in filtered nanopure water by sonication. The values are the mean and the error values are the calculated S.D.
After performing the BAMLET syntheses, the coupling of OA to α-LA was confirmed by fluorescence emission spectroscopy (for details see Supplementary Data). We precisely determined the amount of OA bound to α-LA (mol/mol) in each of the synthesized BAMLET complexes by a colorimetric assay. The results confirmed the presence of OA in each BAMLET complex and also show that the amount of OA depended on the details of the coupling reaction (Table 1). In general, neutral and high pH values resulted in better loading of OA to α-LA.
The size of drug formulation is extremely important for delivery purposes to tumors because of the so-called EPR effect [42]. Particles with a diameter of about 50–500 nm accumulate in tumors because the blood vessels have relatively large gaps due to the leaky vasculature. Previous studies did not evaluate the size and shape of HAMLET or BAMLET complexes. We characterized both, the size and shape of the BAMLET complexes formed using the three different methods by dynamic light scattering (DLS) and scanning electron microscopy (SEM). All complexes, independent of the method used, produced spherical NP of around 250 nm in diameter (Table 1 and Fig. 2A). We assume that these NP resemble micelles, because lipids have the feasibility of incorporate hydrophobic or hydrophilic drugs when reaching the critical micellar concentration [43].
Fig. 2.
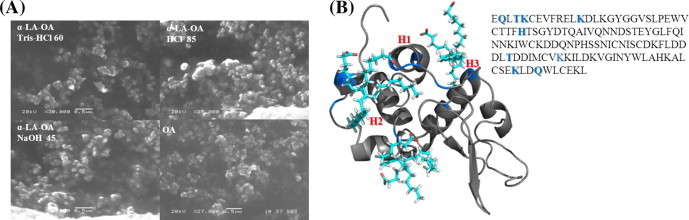
(A) SEM images of the three BAMLET complexes synthesized. In agreement with DLS measurements (Table 1) the images display spherical micellar NP. (B) Modeling of OA binding to α-LA in the BAMLET complex. The blue colored residues were found to be responsible for OA interactions with the α-LA surface.
To get an idea about the possible self-assembly of OA surrounding the protein, we performed computational modeling studies of the interaction of OA with α-LA. Using the YASARA molecular modeling suite, we docked 6 molecules of OA (4DQ3) to the apo bovine α-LA (1F6R). α-LA is a 14 kD (123 aa) protein stabilized by a Ca+2 binding loop at the interface between the α- and β-domains. The large α-domain is composed of three α-helices and the small β-domain of three antiparallel β-pleated sheets [28,44]. The results from the simulation demonstrate OA binding sites on the surface of α-LA. The OA molecules bound here by hydrophobic interactions point the polar head to the aqueous environment (Fig. 2B). In addition, we found that OA molecules are interacting mostly with the helical lobe and near to the Ca+2 binding site, at the interface of the two lobes, as proposed by others [45,46]. Furthermore, some solvent-exposed polar and charged residues on the α-LA surface (Lys 5, 13, 93, 114, and His 32) expectedly interact with the negatively charged COO− of OA. In contrast, somewhat counterintuitive, the polar residues Gln 2, 117 and Thr 4, 86 interact with the non-polar hydrocarbon chain of the OA. One has to keep in mind that additional OA binding could involve the hydrophobic core area of α-LA after partial denaturation.
In vivo studies of nano-sized micellar formulations have demonstrated protection of the drug against proteolytic degradation [47]. If our idea on a micelle-like arrangement of the lipids on the protein surface were to be correct, there should be protection of α-LA against proteolysis afforded by the lipid. We employed the protease trypsin in this assay because it cuts the peptide backbone next to Lys residues, which are involved in OA binding according to our molecular modeling results (Fig. 2). The BAMLET complexes and α-LA as control were incubated with trypsin for up to 72 h and the fluorescence emission intensity at 305–440 nm monitored to assess the protein conformation during the digest (Fig. 3) [48]. The fluorescence intensity should decrease because Trp residues become more solvent exposed thus enabling internal conversion pathways to relax from the excited state [49]. As expected, α-LA lost fluorescence emission intensity upon exposure to the proteolytic digest (Fig. 3). In contrast, OA improved the stability of α-LA toward proteolytic degradation in the BAMLET complex as predicted by our model. The decrease in the fluorescence was the least pronounced in α-LA-OA Tris–HCl 60 which had the most OA molecules bound (Table 1). The slightest protection was afforded by α-LA-OA HCl 85 with the least bound molecules of OA (Table 1).
Fig. 3.
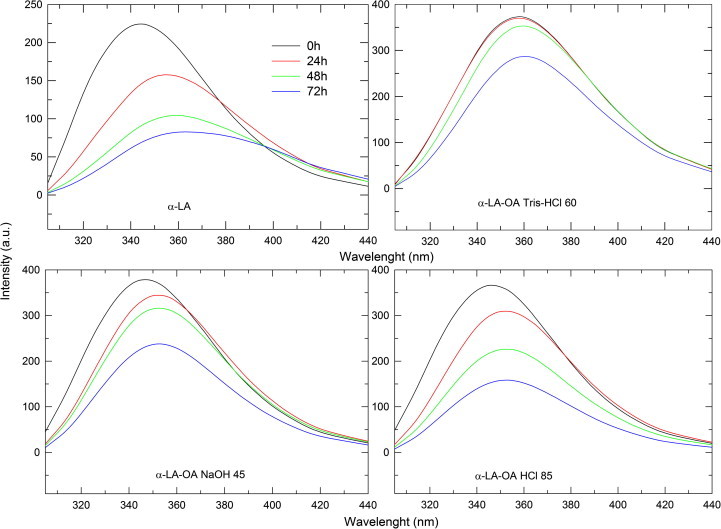
Fluorescence emission spectra (λexc = 295 nm) of the BAMLET complexes [0.5 mg/ml] after incubation with 3 mg of trypsin for 72 h.
In summary, the experiment confirms the results obtained from the theoretical simulation of the BAMLET complex in which OA affords proteolytic protection by shielding the protein surface.
2.2. Identification of the cytotoxic component in the BAMLET complexes
After the characterization of the synthesized complexes, we performed MTS viability assays of HeLa cells after treatment with BAMLET complexes. We found that all three synthesized complexes showed similar toxicities (see Supplementary Data). In the following, we aimed at identifying the cytotoxic component in the BAMLET complexes by using the individual components, namely the free OA and α-LA alone. We speculated that there must discrepancies in how previous studies employed OA because in many instances the isolated FA was reported not to induce cell death [22–29]. A recent study elucidated the importance of some of the experimental conditions when working with isolated FA [31]. For example, it was found that the use of glass vials instead of plastic vials is imperative for FA or FA-protein complex preparation to avoid adhesion problems. We suspected the dispersion method to be of major importance when working with FA and we tested the importance of good dispersion of the FA in cytotoxicity assays. We used OA without intense dispersion and after intense dispersion using sonication for two minutes at 30 °C (Branson 3510R-MT, 42 kHz, 130 W). We selected a standard MTS-based cell viability assay after incubating them with these preparations of OA (Fig. 4A). Our results confirm the importance of the dispersion method. Cell viability was still ca. 50% for the sample used without sonication while it dropped to only few percent when sonication was used to disperse the FA. The use of ethanol to improve OA solubility in the medium did not cause an increase in cell death in comparison with the samples without the ethanol. This observation indicates that likely micelles formed by OA are the cytotoxic compound, not individual OA molecules.
Fig. 4.
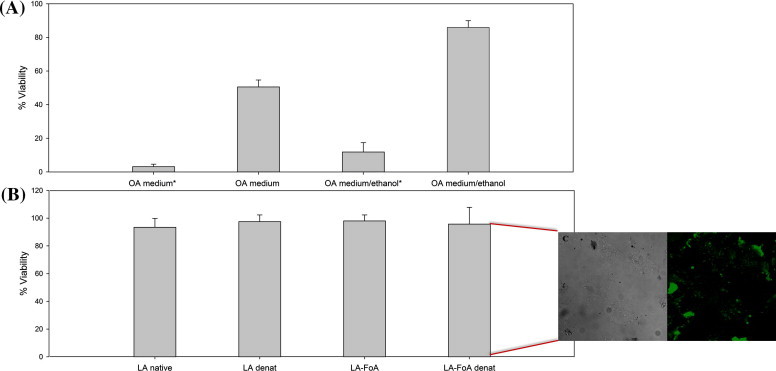
HeLa cell viability after 6 h of incubation with free OA and α-LA. (A) MTS of differently prepared OA samples. OA labeled with the * were prepared using sonication prior to cell incubation. (B) MTS of differently prepared α-LA samples (C). Cellular uptake of α-LA-FoA. OA (120 μM) and α-LA (117 mM) were adjusted to the same concentration of the synthetized BAMLET. The values are the mean and the error values are the calculated S.D.
Next, we verified whether α-LA or its denatured states had any active role in the cytotoxicity. Native α-LA was employed as control and compared to its heat-treated form obtained at 60 °C. Both formulations did not show any cytotoxicity (Fig. 4B). One could argue that this was due to the fact that α-LA is a membrane impermeable protein. Binding of OA, in theory, could cause the protein to be transported into the cell and then subsequently cause cytotoxicity. We therefore tested if the internalization of α-LA into the cell per se could cause cell death.
We modified α-LA with folic acid (FoA) since HeLa cells overexpress the FoA receptor [50] using EDC/NHS crosslinking chemistry [51]. The internalization of α-LA-FoA was confirmed by confocal microscopy using FITC to label α-LA (Fig. 4C) (for more details, see Supplementary Data). Again, no cytotoxicity was observed (Fig. 4B). In conclusion, none of our data support the notion that the structure of α-LA is particularly important for the cytotoxicity. Regardless of its structure per se it does not have any intrinsic cytotoxic activity.
Having established that OA alone displays cytotoxicity when treated by sonication, next we compared the cytotoxicity of OA versus OA in BAMLET complex after sonication (Fig. 5). We selected the 120 μM BAMLET (α-LA-OA Tris–HCl 60) complex because it has the most OA bound of the complexes prepared (Table 1), and in addition was the most stable complex in the trypsin environment (Fig. 3). Our results show similar lethal concentrations (LC) upon HeLa cell incubation with OA alone at comparable concentrations to OA bound in the BAMLET complex (Fig. 5A). Thus, OA cytotoxicity is not modified or enhanced by binding to the protein – if anything the OA micelles are slightly more cytotoxic when presented to the cells as demonstrated by DAPI & PI confocal analysis (Fig. 5B). OA micelles-treated cells showed a PI signal increment in the cells indicative of late apoptosis compared to BAMLET-treated cells showing early apoptosis (Fig. 5B).
Fig. 5.
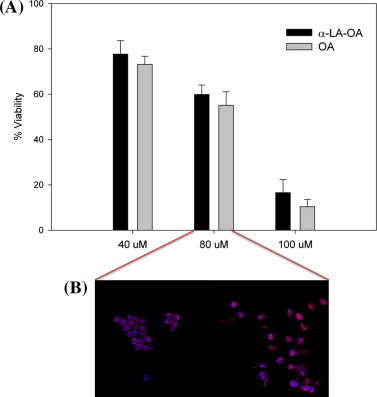
LC50 determination of BAMLET (α-LA-OA Tris–HCl 60) and OA after 6 h of incubation. (A) HeLa cell viability assay at different concentrations (40, 80, 100 μM) of α-LA-OA Tris–HCl 60 and OA. The values are the mean and the error values are the calculated S.D. (B) Confocal microscopy of HeLa cells treated with 80 μM of α-LA-OA Tris–HCl 60 and with 80 μM of OA alone. All the samples were prepared by sonication in MEM for 2 min at an energy setting of 130 W.
2.3. BAMLET cytotoxicity in cancer and normal cell lines
It has been claimed in numerous studies that HAMLET/BAMLET complexes specifically only kill cancer cells while leaving regular cells unharmed [22–29]. However, this notion has been challenged in recent reports in which it is stated that HAMLET/BAMLET complexes are not selectively cytotoxic to cancer cells [31,52]. Looking into this controversy, we decided to test the cytotoxicity of the BAMLET (α-LA-OA Tris–HCl 60) complex on normal (Cho-K1 and NIH/3T3) and cancer (HeLa and A-549) cell lines (Fig. 6). The results demonstrate that there was no selectivity afforded by associating OA with the protein towards cancer cells. The cytotoxicity of 120 μM of OA alone or in the BAMLET complex was quite similar in the different cell lines even though the cell lines showed some differences in sensitivity towards OA and BAMLET. It is interesting, however, that normal cells seem less sensitive towards OA and BAMLET than cancer cells. This indicates that it perhaps is possible to use OA and BAMLET as medication in cancer applications after optimizing the conditions further.
Fig. 6.
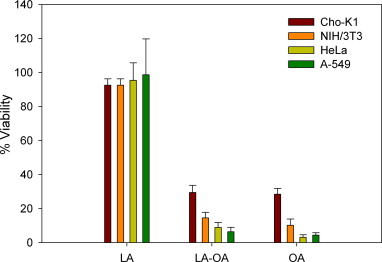
Non-selective cytotoxicity of BAMLET (α-LA-OA Tris–HCl 60) and OA towards normal cells (Cho-K1 and NIH/3T3) and cancer cells (HeLa and A-549) after 6 h of incubation. The values are the mean of quadruplicate measurements and the error values are the calculated S.D.
In summary, through this study, we found that OA is the cytotoxic component of the BAMLET/HAMLET complexes. Native or unfolded α-LA lacked any tumoricidal activity, even when delivered to the cytoplasm of the HeLa cancer cells. The data highlight that α-LA just serves as the carrier of OA molecules and no synergistic activity of FA and α-LA was detected because the free FA alone was as effective as the FA bound to BAMLET. Furthermore, BAMLET and OA micelles both also killed normal cells, incompatible with the notion of BAMLET acting solely on cancer cells. However, the cancer cell lines employed herein were more sensitive towards the drugs than the normal cell lines. While this might be a hint for a potential therapeutic window in the use of BAMLET or OA micelles in cancer treatment, many more studies are needed to explore possibilities to improve the potential drugs by attaching targeting ligands or by using other proteins as carriers. For instance, there are studies ongoing in our laboratory investigating the coupling of OA to therapeutically-active proteins to increase the efficacy of these complexes by exploiting synergies.
3. Experimental procedures
3.1. Materials
Calcium depleted Type III α-lactalbumin from bovine milk (α-LA), a fatty acid quantitation kit (MAK044), trypsin from porcine pancreas, oleic acid (OA, >99% purity), were purchased from Sigma Aldrich (St. Louis, MO). The centrifugal filtration system (Millipore Amicon cut off 3–5 kD,) was purchased from Thermo Fisher Scientific (Hudson, New Hampshire). HeLa and NIH/3T3 cells, serum, and culture media were purchased from the American Type Culture Collection (Manassas, VA). A-549 and Cho-K1 cells were cordially donated from Dr. Gabriel Barletta’s lab at University of Puerto Rico-Humacao. All other chemicals were of analytical grade and from various commercial suppliers and used without further purification. All samples were analyzed in quadruplicate.
3.2. BAMLET complexes synthesis
The three different procedures were adapted from the literature and used to synthetize the BAMLET complex:
Method 1 (Tris–HCl 60) [37,53]. Briefly, α-LA was dissolved in 20 mM Tris–HCl at pH 8 to a final concentration of 600 μM and the solution heated to 60 °C. Then, 40 mol of OA was added to the solution followed by 2 min of sonication at 30 °C (Branson 3510R-MT, 42 kHz, 130 W). The reaction mixture was stirred at 60 °C for 30 min, cooled under tap water, acidified to pH 3.5, and stirred for 24 h at 4 °C. The complex is referred to as α-LA-OA Tris–HCl 60.
Method 2 (HCl 85) [39,54]. Briefly, α-LA was dissolved in 1 M HCl and by stirring for 24 h at room temperature to a final concentration of 600 μM. Next, the solution was heated to 85° and then 40 mol of OA previously dissolved in 1 ml of ethanol was added to the solution followed by 2 min of sonication at 30 °C (Branson 3510R-MT, 42 kHz, 130 W). The reaction was completed by constant stirring for 30 min at 85 °C and cooling under tap water and stirred for 24 h at 4 °C. The complex is referred to as α-LA-OA HCl 85.
Method 3 (NaOH 45) [38]. α-LA was dissolved in 5 mM NaOH and 1 mM EDTA at pH 12 to a final concentration of 600 μM. The solution was heated to 45 °C and then 40 mol of OA dissolved in 1 ml of ethanol was added followed by sonication for 2 min at 30 °C (Branson 3510R-MT, 42 kHz, 130 W). After stirring for 30 min at 45 °C the reaction was cooled under tap water to room temperature, acidified with HCl to pH 3.5, and stirred for 24 h at 4 °C. The complex is referred to as α-LA-OA NaOH 45.
All the reactions following Methods 1–3 were neutralized to pH 7 and finally ultra-sonicated (Branson Ultrasonics 450) for 1 min at 400 W. Unbound OA was removed by a centrifugal purification system against 50% ethanol/50% nanopure water (HERMLET Labnet Z 323 k, 8000 rpm), and against nanopure water, and then lyophilized (LABCONCO FREEZONE 6 freeze dry system) for 48 h. Finally, all the synthetized complexes were stored at −20 °C in glass-amber vials.
3.3. FA quantification
We employed a commercially available kit from Sigma Aldrich according to the manufacturer instructions and as previously described [31]. The samples were diluted in 50 μL of assay buffer and analyzed in a 96-well plate. Palmitic acid was used as standard. 2 μL Acyl-CoA synthetase was added to each well to convert FAs to their CoA derivatives. The CoA-FA were subsequently oxidized with the concomitant generation of color. The reaction outcome was quantified by measuring the absorbance at 570 nm using a microplate reader (Thermo Scientific Multiskan FC).
3.4. Dynamic light scattering (DLS)
Particle sizes of the BAMLET complexes were determined by DLS using a DynaPro Titan by dispersing 0.5 mg in 2 ml of water and subject this suspension to ultra-sonication (Branson Ultrasonics 450) for 3 min at 400 W. The instrument was calibrated using 46 ± 2 nm (Cat. No. 3300A) and 300 ± 6 nm (Cat. No 3050A) nanospheres™ size standards (Thermo Fisher Scientific; Hudson, New Hampshire).
3.5. Scanning electron microscopy (SEM)
SEM of BAMLET complexes and FAs micelles was performed using a JEOL 5800LV scanning electron microscope at 20 kV by dispersing 0.5 mg of the complexes in 500 μL of water followed by ultra-sonication at 400 W for 3 min. Then, some small drops of each suspension were put on a carbon tape and air-dried. After 24 h the samples were coated with gold for 10 s using a Denton Vacuum DV-502A.
3.6. Proteolytic degradation assay
Proteolytic degradation was performed as described by us [51,55]. In this case intrinsic fluorescence was used to measure structural changes as stated by others [28,48]. In brief, for tryptic degradation, 0.5 mg/ml of α-LA and BAMLET complexes were prepared in 20 mM Tris–HCl at pH 8 with 2% (v/v) of ethanol and incubated for 20 min at 20 °C. Then, 4 mg of trypsin was added to 1 ml of each solution and incubated at 37 °C. The degradation of α-LA was determined by fluorescence measurements after 0 h, 24 h, 48 h, and 72 h of incubation. Fluorescence emission spectra (λem = 305–440 nm) were obtained using λexc = 295 nm excitation with a fluorimeter (Varian CARY Eclipse fluorescence spectrophotometer) using a quartz cuvette with 1-cm path length.
3.7. Theoretical molecular docking analysis
YASARA software was used to perform all the calculations. The BAMLET complex model was created using the apo bovine α-LA (1F6R) and 6 molecules of OA (4DQ3) from RCSB Protein Data Bank. The system was pre-equilibrated by the steepest descent minimization using AMBER03 force field at 25 °C with a cutoff of 7.86431 Å. The BAMLET model was solvated in a cubic box with the size of 20 Å and filled with explicit water molecules (0.997 g/ml of density and 1.4 Å of radius). Periodic boundary conditions were applied in all directions and the system was neutralized by adding counter ions replacing the water molecules. The energy-minimized system was subjected to 50 ps of equilibration. The docking calculations were carried out using AutoDock 4.2 to compute the free energy of binding (Docking runs = 50; Cluster RMSD = 5.00 Å) [56,57]. Five different docking jobs were run using initial positions, orientation and torsions of the ligand moieties randomly set. The final structure with all the ligand moieties bound to the potential binding sites was further refined by simulated annealing minimization using the following conditions: AMBER03, 60 °C, cutoff of 7.86431 Å, same solvated cubic box dimension and conditions.
3.8. Cell culture
HeLa cells were maintained in accordance with the ATCC protocol. Briefly, HeLa, A-549, Chok-1, and NIH/3T3 cells were cultured in 75 cm2 flasks with minimum essential medium (MEM), Ham’s F-12 Nutrient Mixture, F-12K (Kaighn’s Modification of Ham’s F-12) and Dulbecco’s Modified Eagle Medium (DMEM), respectively and containing 1% l-glutamine, 10% fetal bovine serum (FBS), and 1% penicillin in a humidified incubator with 5% CO2 and 95% air at 37 °C. All experiments were conducted before cells reached 20 passages. In each passage, cells were washed twice with PBS, detached using trypsin, and suspended in supplemented medium.
3.9. Cell viability assay
Mitochondrial function was measured using the CellTiter 96 aqueous non-radioactive cell proliferation assay. All cell lines (5000 cells/well) were seeded in 96-well plates for 24 h. Cells were incubated with 100 μL of α-LA, OA, and BAMLET complexes for 6 h. The concentration of the BAMLET complexes added to each well was calculated based on the OA concentration determined in the complex. In other words, cell viability experiments were performed at the same OA concentration (120 μM) to allow for comparison with the free OA. Controls of the native and denatured α-LA alone were adjusted to the same concentration as the respective protein concentration (117 mM) in each complex.
All the samples were freshly prepared prior to the experiments. BAMLET complexes and OA were dispersed in the medium by 2 min of sonication (Branson 3510R-MT, 42 kHz, 130 W) prior to co-incubation with the cells except for the experiment with different preparations of OA. Next, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), and phenazine methosulfate (PMS) was added to each well (333 μg/ml MTS and 25 μM PMS). After 1 h, the absorbance at 492 nm was measured using the microplate reader. Cells treated with 2 μM staurosporine for 6 h were used as positive control and cells without treatment were used as negative control. The relative cell viability (%) was calculated by:
3.10. Apoptosis assay using confocal microscopy
HeLa cells (25,000 cells) were seeded and incubated in Lab-tek chambered coverglass (4-wells) as described above. The cells were incubated with 100 mM α-LA, and 80 μM of OA and BAMLET complexes at 37 °C for 6 h. For detection of apoptosis-dependent nuclear fragmentation, the cells were washed with PBS (1×) and incubated initially with DAPI (300 nM) and thereafter with PI (75 μM) for 5 min each. HeLa cells were then fixed using 3.7% formaldehyde. The coverslips were examined under a Zeiss laser-scanning microscope 510 using a 67× objective. Co-localization of DAPI and PI upon internalization into HeLa cells was determined, which is representative of highly condensed and fragmented chromatin in apoptotic cells. DAPI was excited at 405 nm and its emission detected at 420–480 nm. PI was excited at 488 nm and its emission detected above 505 nm.
Author contributions
Y.D. carried out all the experimental studies, contributed to the experimental strategy, analyzed data, and drafted the manuscript. M.M.C. and C.M.F. participated in the experimental design of in vitro studies and drafting the manuscript. J.H.R. and G.H. helped in the experimental part. K.G. conceived the study, participated in its design and coordination, and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Y.D., M.M.C. and C.M.F. are currently supported by fellowships from the PR NASA Space Grant Consortium (NNX10AM80H), NSF GK-12 Program (0841338) and NSF Institute for Functional Nanomaterials (EPS-01002410), respectively. Y.D., M.M.C., and C.M.F. were supported by fellowships from the NIH Research Initiative for Scientific Enhancement (RISE) Program (R25 GM061151), and the Bridge to the Doctorate Program (NSF AMP (HRD-0832961) at the University of Puerto Rico.
Appendix A. Supplementary data
This document file contains Supplementary material.
References
- 1.Eklund J.W., Trifilio S., Mulcahy M.F. Chemotherapy dosing in the setting of liver dysfunction. Oncology. 2005;19:1057–1063. [PubMed] [Google Scholar]
- 2.Naidu M.U., Ramana G.V., Rani P.U., Mohan I.K., Suman A., Roy P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis–complicating the treatment of cancer. Neoplasia. 2004;6:423–431. doi: 10.1593/neo.04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyama T., Nishikawa K., Takiguchi N., Tanabe K., Imano M., Fukushima R., Sakamoto J., Oba M.S., Morita S., Kono T., Tsuburaya A. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis. Cancer Chemo Pharmacol. 2014 doi: 10.1007/s00280-014-2440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control. Release. 2012;164:138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Madaan A., Singh P., Awasthi A., Verma R., Singh A.T., Jaggi M., Mishra S.K., Kulkarni S., Kulkarni H. Efficiency and mechanism of intracellular paclitaxel delivery by novel nanopolymer-based tumor-targeted delivery system, Nanoxel (TM) Clin. Transl. Oncol. 2013;15:26–32. doi: 10.1007/s12094-012-0883-2. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.A.C. Rustan, C.A. Drevon, Fatty Acids: Structures and Properties, in: eLS, John Wiley & Sons, Ltd.
- 8.Hargrove J.L., Greenspan P., Hartle D.K. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp. Biol. Med. 2004;229:215–226. doi: 10.1177/153537020422900301. [DOI] [PubMed] [Google Scholar]
- 9.Costanzi S., Neumann S., Gershengorn M.C. Seven transmembrane-spanning receptors for free fatty acids as therapeutic targets for diabetes mellitus: pharmacological, phylogenetic, and drug discovery aspects. J. Biol. Chem. 2008;283:16269–16273. doi: 10.1074/jbc.R800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolakopoulou Z., Nteliopoulos G., Michael-Titus A.T., Parkinson E.K. Omega-3 polyunsaturated fatty acids selectively inhibit growth in neoplastic oral keratinocytes by differentially activating ERK1/2. Carcinogenesis. 2013;34:2716–2725. doi: 10.1093/carcin/bgt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolakopoulou Z., Shaikh M.H., Dehlawi H., Michael-Titus A.T., Parkinson E.K. The induction of apoptosis in pre-malignant keratinocytes by omega-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) is inhibited by albumin. Toxicol. Lett. 2013;218:150–158. doi: 10.1016/j.toxlet.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Hajri T., Abumrad N.A. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu. Rev. Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 13.Arita K., Kobuchi H., Utsumi T., Takehara Y., Akiyama J., Horton A.A., Utsumi K. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem. Pharmacol. 2001;62:821–828. doi: 10.1016/s0006-2952(01)00723-7. [DOI] [PubMed] [Google Scholar]
- 14.Calder P.C. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–374. doi: 10.3390/nu2030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Lin H., Gu Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. doi: 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cury-Boaventura M.F., Pompeia C., Curi R. Comparative toxicity of oleic acid and linoleic acid on Jurkat cells. Clin. Nutr. 2004;23:721–732. doi: 10.1016/j.clnu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Calder P.C., Yaqoob P., Harvey D.J., Watts A., Newsholme E.A. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem. J. 1994;300:509–518. doi: 10.1042/bj3000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheim D.E. Cytotoxicity of unsaturated fatty acids in fresh human tumor explants: concentration thresholds and implications for clinical efficacy. Lipids Health Dis. 2009;8:54. doi: 10.1186/1476-511X-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh S., Taketomi A., Harimoto N., Tsujita E., Rikimaru T., Shirabe K., Shimada M., Maehara Y. Antineoplastic effects of gamma linolenic Acid on hepatocellular carcinoma cell lines. J. Clin. Biochem. Nutr. 2010;47:81–90. doi: 10.3164/jcbn.10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins de Lima T., Cury-Boaventura M.F., Giannocco G., Nunes M.T., Curi R. Comparative toxicity of fatty acids on a macrophage cell line (J774) Clin. Sci. 2006;111:307–317. doi: 10.1042/CS20060064. [DOI] [PubMed] [Google Scholar]
- 21.Cury-Boaventura M.F., Gorjao R., de Lima T.M., Newsholme P., Curi R. Comparative toxicity of oleic and linoleic acid on human lymphocytes. Life Sci. 2006;78:1448–1456. doi: 10.1016/j.lfs.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Hakansson A., Zhivotovsky B., Orrenius S., Sabharwal H., Svanborg C. Apoptosis induced by a human milk protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8064–8068. doi: 10.1073/pnas.92.17.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallgren O., Gustafsson L., Irjala H., Selivanova G., Orrenius S., Svanborg C. HAMLET triggers apoptosis but tumor cell death is independent of caspases, Bcl-2 and p53. Apoptosis. 2006;11:221–233. doi: 10.1007/s10495-006-3607-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y.B., Wu W., Ding W. From HAMLET to XAMLET: the molecular complex selectively induces cancer cell death. Afr. J. Biotechnol. 2010;9:9270–9276. [Google Scholar]
- 25.Xiao Z., Mak A., Koch K., Moore R.B. A molecular complex of bovine milk protein and oleic acid selectively kills cancer cells in vitro and inhibits tumour growth in an orthotopic rat bladder tumour model. BJU Int. 2013;112:E201–210. doi: 10.1111/j.1464-410X.2012.11737.x. [DOI] [PubMed] [Google Scholar]
- 26.Liskova K., Kelly A.L., O’Brien N., Brodkorb A. Effect of denaturation of alpha-lactalbumin on the formation of BAMLET (bovine alpha-lactalbumin made lethal to tumor cells) J. Agric. Food Chem. 2010;58:4421–4427. doi: 10.1021/jf903901j. [DOI] [PubMed] [Google Scholar]
- 27.Mossberg A.K., Hun Mok K., Morozova-Roche L.A., Svanborg C. Structure and function of human alpha-lactalbumin made lethal to tumor cells (HAMLET)-type complexes. FEBS J. 2010;277:4614–4625. doi: 10.1111/j.1742-4658.2010.07890.x. [DOI] [PubMed] [Google Scholar]
- 28.Svensson M., Mossberg A.K., Pettersson J., Linse S., Svanborg C. Lipids as cofactors in protein folding: stereo-specific lipid–protein interactions are required to form HAMLET (human alpha-lactalbumin made lethal to tumor cells) Protein Sci. 2003;12:2805–2814. doi: 10.1110/ps.0231103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson-Kastberg J., Mossberg A.K., Trulsson M., Yong Y.J., Min S., Lim Y., O’Brien J.E., Svanborg C., Mok K.H. Alpha-Lactalbumin, engineered to be nonnative and inactive, kills tumor cells when in complex with oleic acid: a new biological function resulting from partial unfolding. J. Mol. Biol. 2009;394:994–1010. doi: 10.1016/j.jmb.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Spolaore B., Pinato O., Canton M., Zambonin M., Polverino de Laureto P., Fontana A. Alpha-Lactalbumin forms with oleic acid a high molecular weight complex displaying cytotoxic activity. Biochemistry. 2010;49:8658–8667. doi: 10.1021/bi1012832. [DOI] [PubMed] [Google Scholar]
- 31.Brinkmann C.R., Heegaard C.W., Petersen T.E., Jensenius J.C., Thiel S. The toxicity of bovine alpha-lactalbumin made lethal to tumor cells is highly dependent on oleic acid and induces killing in cancer cell lines and noncancer-derived primary cells. FEBS J. 2011;278:1955–1967. doi: 10.1111/j.1742-4658.2011.08112.x. [DOI] [PubMed] [Google Scholar]
- 32.Permyakov S.E., Knyazeva E.L., Khasanova L.M., Fadeev R.S., Zhadan A.P., Roche-Hakansson H., Hakansson A.P., Akatov V.S., Permyakov E.A. Oleic acid is a key cytotoxic component of HAMLET-like complexes. Biol. Chem. 2012;393:85–92. doi: 10.1515/BC-2011-230. [DOI] [PubMed] [Google Scholar]
- 33.Ho J.C., Storm P., Rydstrom A., Bowen B., Alsin F., Sullivan L., Ambite I., Mok K.H., Northen T., Svanborg C. Lipids as tumoricidal components of human alpha-lactalbumin made lethal to tumor cells (HAMLET): unique and shared effects on signaling and death. J. Biol. Chem. 2013;288:17460–17471. doi: 10.1074/jbc.M113.468405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoque M., Dave S., Gupta P., Saleemuddin M. Oleic acid may be the key contributor in the BAMLET-induced erythrocyte hemolysis and tumoricidal action. PLoS ONE. 2013;8:e68390. doi: 10.1371/journal.pone.0068390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu M., Sugiura Y., Nagaoka S., Kanamaru Y. IEC-6 intestinal cell death induced by bovine milk alpha-lactalbumin. Biosci. Biotechnol. Biochem. 2005;69:1082–1089. doi: 10.1271/bbb.69.1082. [DOI] [PubMed] [Google Scholar]
- 36.Ho C.S.J., Rydstrom A., Trulsson M., Balfors J., Storm P., Puthia M., Nadeem A., Svanborg C. HAMLET: functional properties and therapeutic potential. Future Oncol. 2012;8:1301–1313. doi: 10.2217/fon.12.122. [DOI] [PubMed] [Google Scholar]
- 37.Wen H., Rundgren I.M., Glomm W.R., Halskau Ø. Benchmarking different BAMLET-like preparations with respect to tryptophan exposure, interfacial activity, and effect on cell viability. J. Bioanal. Biomed. 2011;S5:003. [Google Scholar]
- 38.Permyakov S.E., Knyazeva E.L., Leonteva M.V., Fadeev R.S., Chekanov A.V., Zhadan A.P., Hakansson A.P., Akatov V.S., Permyakov E.A. A novel method for preparation of HAMLET-like protein complexes. Biochimie. 2011;93:1495–1501. doi: 10.1016/j.biochi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Li B., Du W., Jin J., Du Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated beta-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012;60:3477–3484. doi: 10.1021/jf300307t. [DOI] [PubMed] [Google Scholar]
- 40.Lazaridis T., Karplus M. Heat capacity and compactness of denatured proteins. Biophys. Chem. 1999;78:207–217. doi: 10.1016/s0301-4622(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 41.Law A.J., Leaver J. Effect of pH on the thermal denaturation of whey proteins in milk. J. Agric. Food Chem. 2000;48:672–679. doi: 10.1021/jf981302b. [DOI] [PubMed] [Google Scholar]
- 42.Maeda H., Nakamura H., Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Ogier J., Arnauld T., Carrot G., Lhumeau A., Delbos J.M., Boursier C., Loreau O., Lefoulon F., Doris E. Enhanced drug loading in polymerized micellar cargo. Org. Biomol. Chem. 2010;8:3902–3907. doi: 10.1039/c004134c. [DOI] [PubMed] [Google Scholar]
- 44.Permyakov E.A., Berliner L.J. Alpha-Lactalbumin: structure and function. FEBS Lett. 2000;473:269–274. doi: 10.1016/s0014-5793(00)01546-5. [DOI] [PubMed] [Google Scholar]
- 45.Chrysina E.D., Brew K., Acharya K.R. Crystal structures of apo- and holo-bovine alpha-lactalbumin at 2. 2-A resolution reveal an effect of calcium on inter-lobe interactions. J. Biol. Chem. 2000;275:37021–37029. doi: 10.1074/jbc.M004752200. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura T., Aizawa T., Kariya R., Okada S., Demura M., Kawano K., Makabe K., Kuwajima K. Molecular mechanisms of the cytotoxicity of human alpha-lactalbumin made lethal to tumor cells (HAMLET) and other protein–oleic acid complexes. J. Biol. Chem. 2013;288:14408–14416. doi: 10.1074/jbc.M112.437889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manjunath K., Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J. Control. Release. 2005;107:215–228. doi: 10.1016/j.jconrel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Themistou E., Singh I., Shang C., Balu-Iyer S.V., Alexandridis P., Neelamegham S. Application of fluorescence spectroscopy to quantify shear-induced protein conformation change. Biophys. J. 2009;97:2567–2576. doi: 10.1016/j.bpj.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakraborty S., Ittah V., Bai P., Luo L., Haas E., Peng Z. Structure and dynamics of the alpha-lactalbumin molten globule: fluorescence studies using proteins containing a single tryptophan residue. Biochemistry. 2001;40:7228–7238. doi: 10.1021/bi010004w. [DOI] [PubMed] [Google Scholar]
- 50.Holm J., Hansen S.I., Hoier-Madsen M., Korsbaek L., Beckmann H., Josefsen K. Ligand binding characteristics of a glycosylphosphatidylinositol membrane-anchored HeLa cell folate receptor epitope-related to human milk folate binding protein. Biosci. Rep. 2000;20:109–118. doi: 10.1023/a:1005567417123. [DOI] [PubMed] [Google Scholar]
- 51.Delgado Y., Morales-Cruz M., Hernández-Román J., Martínez Y., Griebenow K. Chemical glycosylation of cytochrome c improves physical and chemical protein stability. BMC Biochem. 2014;15:16. doi: 10.1186/1471-2091-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brinkmann C.R., Brodkorb A., Thiel S., Kehoe J.J. The cytotoxicity of fatty acid/α-lactalbumin complexes depends on the amount and type of fatty acid. Eur. J. Lipid Sci. Technol. 2013;115:591–600. [Google Scholar]
- 53.Ko S., Gunasekaran S. Preparation of sub-100-nm beta-lactoglobulin (BLG) nanoparticles. J. Microencapsul. 2006;23:887–898. doi: 10.1080/02652040601035143. [DOI] [PubMed] [Google Scholar]
- 54.Bengoechea C., Peinado Pardo I., McClements D. Formation of protein nanoparticles by controlled heat treatment of lactoferrin: factors affecting particle characteristics. Food Hydrocoll. 2011;25:1354–1360. [Google Scholar]
- 55.Méndez J., Morales Cruz M., Delgado Y., Figueroa C.M., Orellano E.A., Morales M., Monteagudo A., Griebenow K. Delivery of chemically glycosylated cytochrome c immobilized in mesoporous silica nanoparticles induces apoptosis in HeLa cancer cells. Mol. Pharm. 2014;11:102–111. doi: 10.1021/mp400400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar S. Comparative modeling and molecular docking of orphan human CYP4V2 protein with fatty acid substrates: insights into substrate specificity. Bioinformation. 2011;7:360–365. doi: 10.6026/97320630007360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document file contains Supplementary material.


