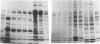Abstract
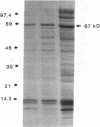
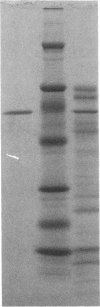
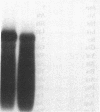
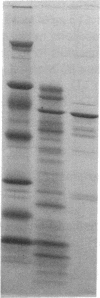
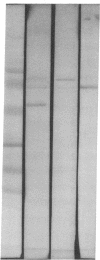
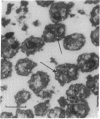
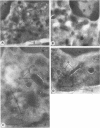
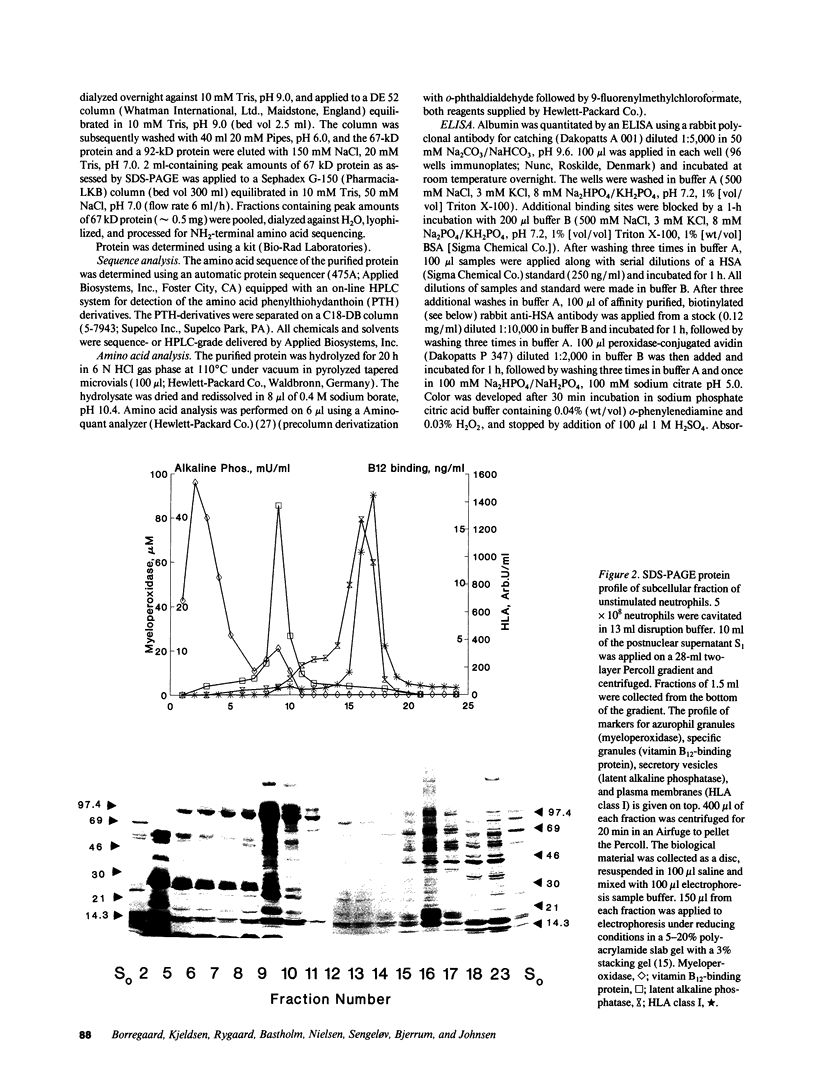
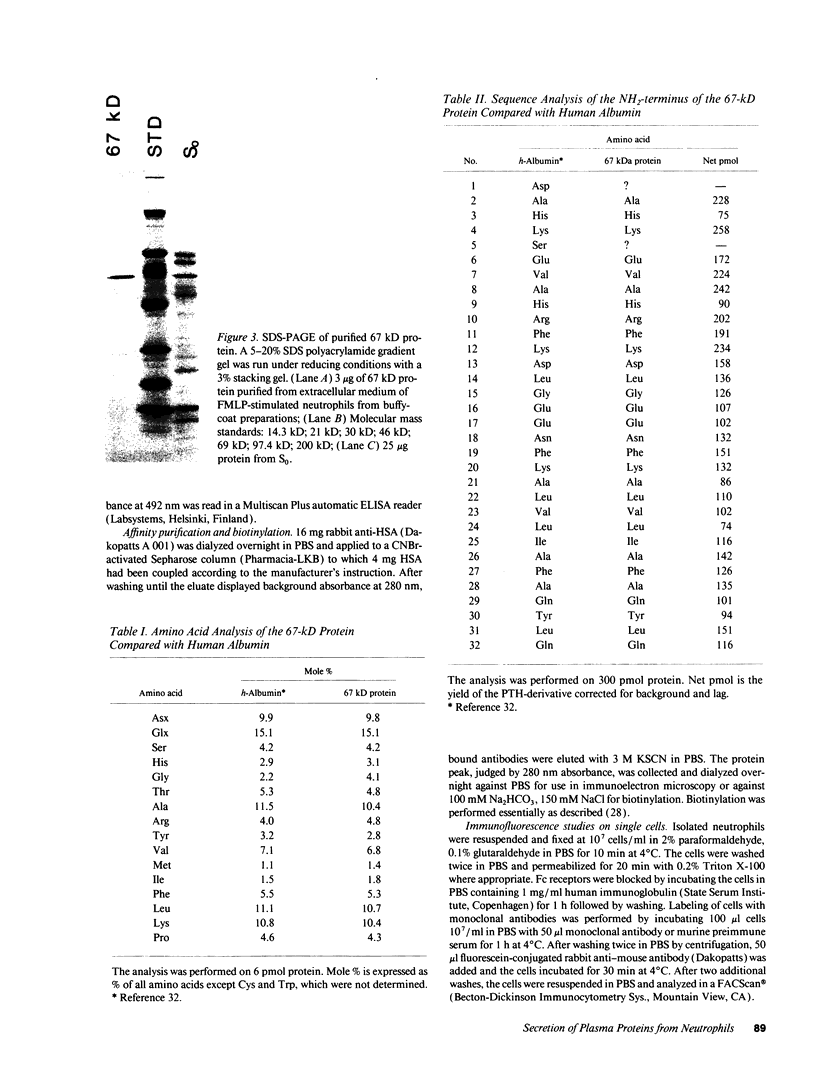
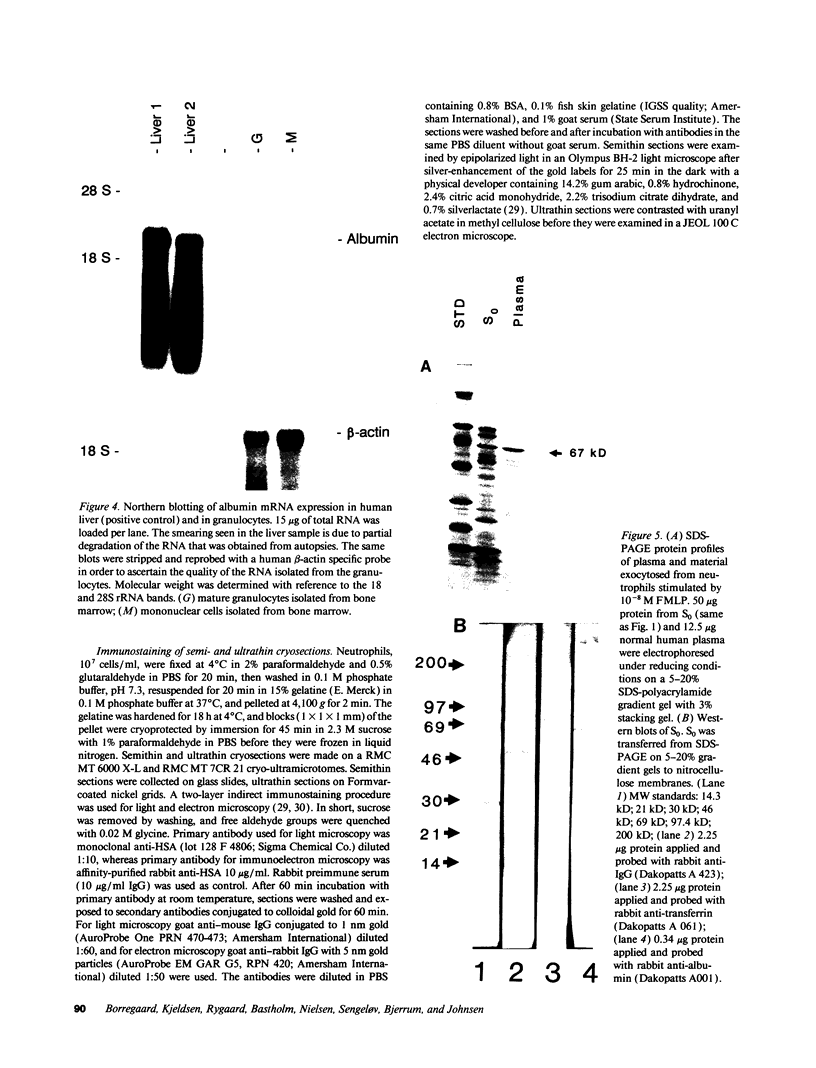
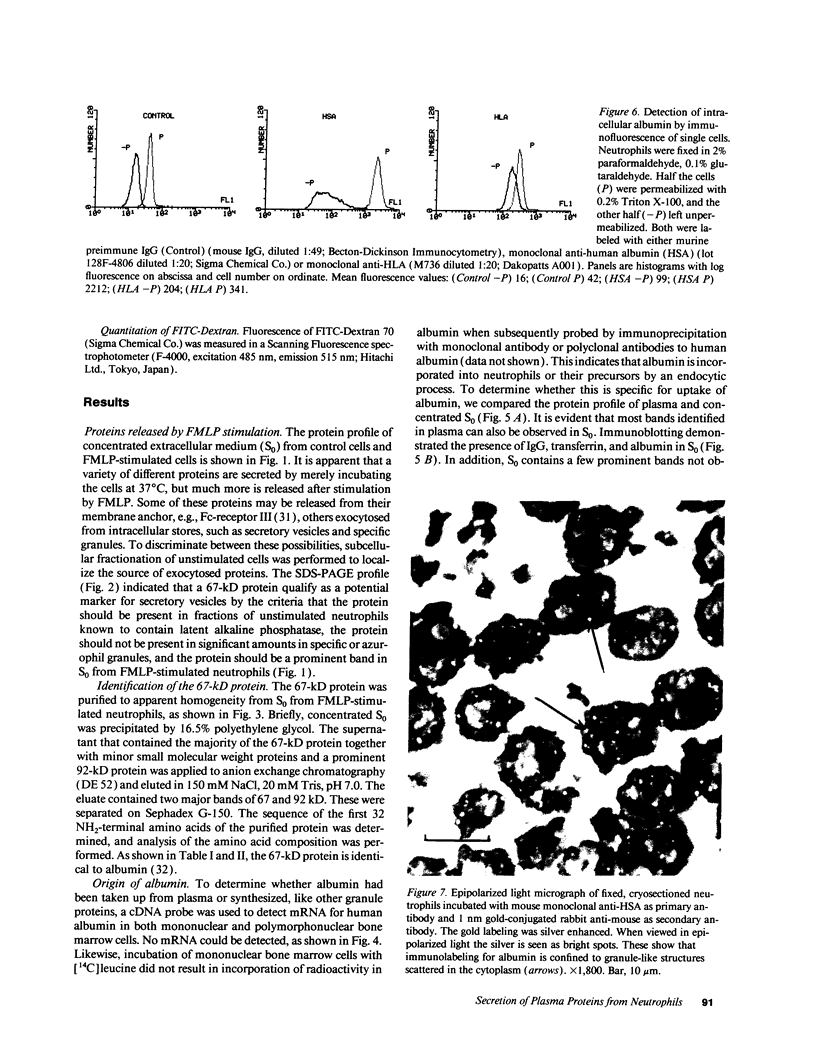
In search for matrix proteins released from secretory vesicles of human neutrophils, a prominent 67-kD protein was identified in the extracellular medium of neutrophils stimulated by the chemotactic peptide, FMLP. The protein was purified to apparent homogeneity and partially sequenced. The sequence of the first 32 NH2-terminal amino acids was identical to the sequence of albumin. mRNA for human albumin could not be detected in bone marrow cells, nor could biosynthetic labeling of albumin be demonstrated in bone marrow cells during incubation with [14C]leucine. Immunofluorescence studies on single cells demonstrated the presence of intracellular albumin in fixed permeabilized neutrophils. Light microscopy of immunogold-silver-stained cryosections visualized albumin in cytoplasmic "granules." The morphology of these was determined by immunoelectron microscopy as vesicles of varying form and size. Subcellular fractionation studies on unstimulated neutrophils demonstrated the presence of albumin in the low density pre-gamma and gamma-regions that contain secretory vesicles, but are devoid of specific granules and azurophil granules. Albumin was readily released from these structures during activation of neutrophils with inflammatory mediators. Immunoblotting demonstrated the presence of immunoglobulin and transferrin along with albumin in exocytosed material from stimulated neutrophils. This indicates that secretory vesicles are unique endocytic vesicles that can be triggered to exocytose by inflammatory stimuli.
Full text
PDF

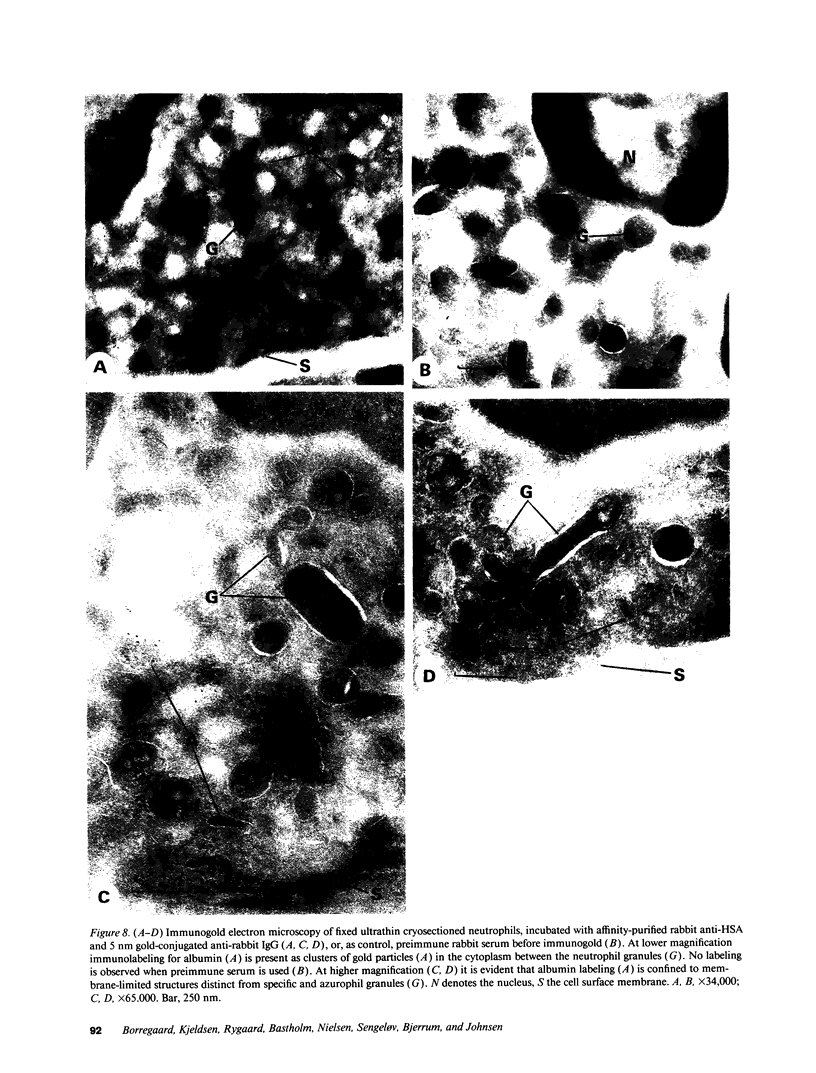
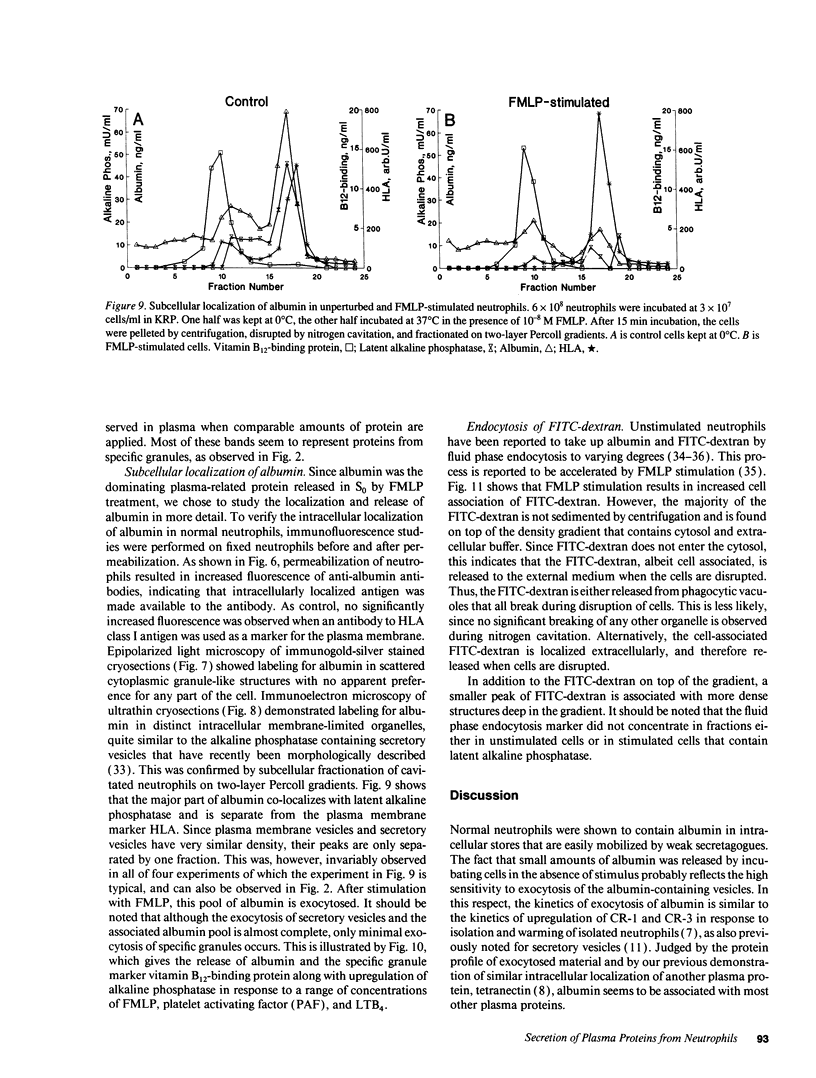
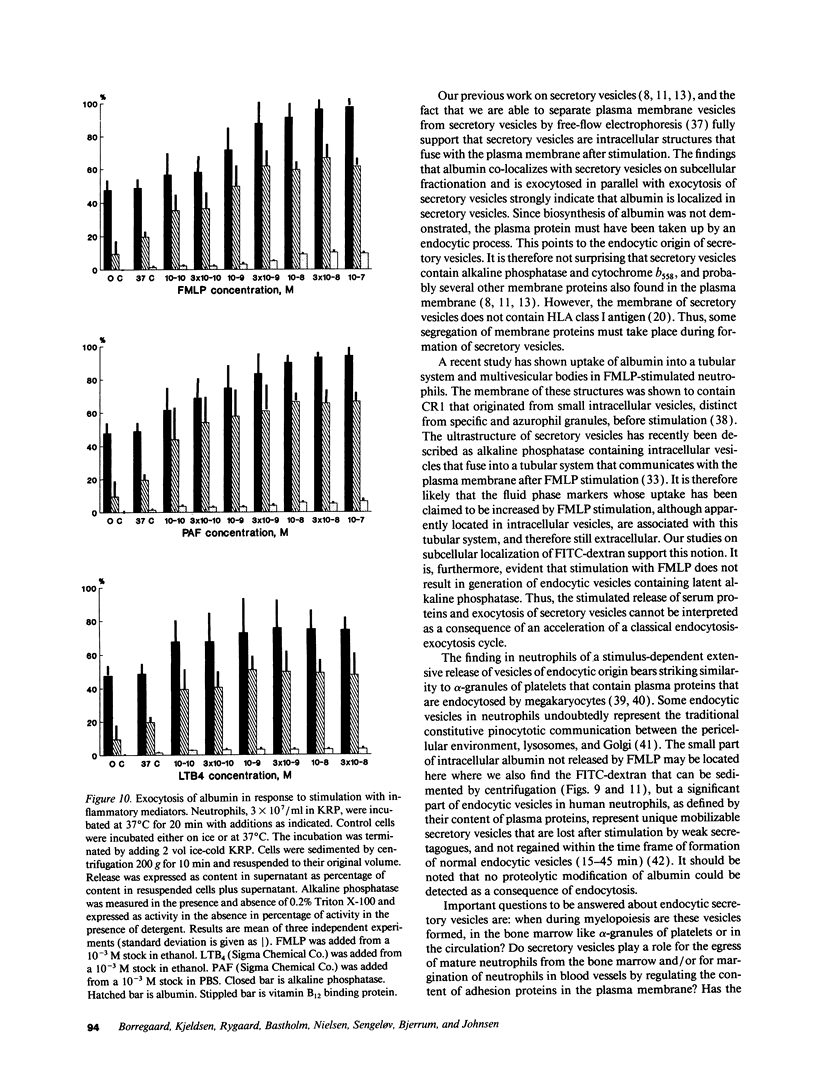
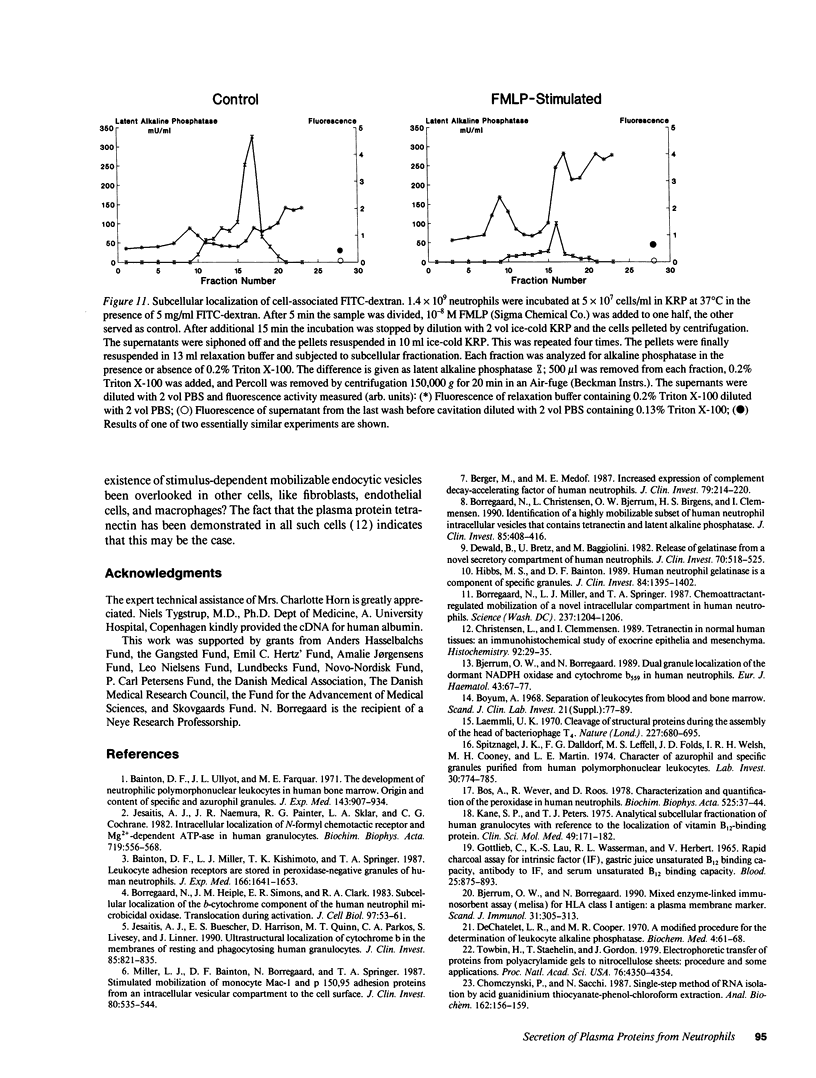

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastholm L., Nielsen M. H., Larsson L. I. Simultaneous demonstration of two antigens in ultrathin cryosections by a novel application of an immunogold staining method using primary antibodies from the same species. Histochemistry. 1987;87(3):229–231. doi: 10.1007/BF00492414. [DOI] [PubMed] [Google Scholar]
- Berger M., Medof M. E. Increased expression of complement decay-accelerating factor during activation of human neutrophils. J Clin Invest. 1987 Jan;79(1):214–220. doi: 10.1172/JCI112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Wetzler E. M., Welter E., Turner J. R., Tartakoff A. M. Intracellular sites for storage and recycling of C3b receptors in human neutrophils. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3019–3023. doi: 10.1073/pnas.88.8.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerrum O. W., Borregaard N. Dual granule localization of the dormant NADPH oxidase and cytochrome b559 in human neutrophils. Eur J Haematol. 1989 Jul;43(1):67–77. doi: 10.1111/j.1600-0609.1989.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. W., Borregaard N. Mixed enzyme-linked immunosorbent assay (MELISA) for HLA class I antigen: a plasma membrane marker. Scand J Immunol. 1990 Mar;31(3):305–313. doi: 10.1111/j.1365-3083.1990.tb02773.x. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Christensen L., Bejerrum O. W., Birgens H. S., Clemmensen I. Identification of a highly mobilizable subset of human neutrophil intracellular vesicles that contains tetranectin and latent alkaline phosphatase. J Clin Invest. 1990 Feb;85(2):408–416. doi: 10.1172/JCI114453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Miller L. J., Springer T. A. Chemoattractant-regulated mobilization of a novel intracellular compartment in human neutrophils. Science. 1987 Sep 4;237(4819):1204–1206. doi: 10.1126/science.3629236. [DOI] [PubMed] [Google Scholar]
- Bos A., Wever R., Roos D. Characterization and quantification of the peroxidase in human monocytes. Biochim Biophys Acta. 1978 Jul 7;525(1):37–44. doi: 10.1016/0005-2744(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Cannarozzi N. A., Malawista S. E. Phagocytosis by human blood leukocytes measured by the uptake of 131I-labeled human serum albumin: inhibitory and stimulatory effects of cytochalasin B. Yale J Biol Med. 1973 Jun;46(3):177–189. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christensen L., Clemmensen I. Tetranectin immunoreactivity in normal human tissues. An immunohistochemical study of exocrine epithelia and mesenchyme. Histochemistry. 1989;92(1):29–35. doi: 10.1007/BF00495012. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Walter R. J., Pearson C. B., Becker E. L., Oliver J. M. Membrane activity and topography of F-Met-Leu-Phe-Treated polymorphonuclear leukocytes. Acute and sustained responses to chemotactic peptide. Am J Pathol. 1982 Aug;108(2):206–216. [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R. A modified procedure for the determination of leukocyte alkaline phosphatase. Biochem Med. 1970 Aug;4(1):61–68. doi: 10.1016/0006-2944(70)90103-1. [DOI] [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handagama P. J., Shuman M. A., Bainton D. F. Incorporation of intravenously injected albumin, immunoglobulin G, and fibrinogen in guinea pig megakaryocyte granules. J Clin Invest. 1989 Jul;84(1):73–82. doi: 10.1172/JCI114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs M. S., Bainton D. F. Human neutrophil gelatinase is a component of specific granules. J Clin Invest. 1989 Nov;84(5):1395–1402. doi: 10.1172/JCI114312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga T. W., de Haas M., Kleijer M., Nuijens J. H., Roos D., von dem Borne A. E. Soluble Fc gamma receptor III in human plasma originates from release by neutrophils. J Clin Invest. 1990 Aug;86(2):416–423. doi: 10.1172/JCI114727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Buescher E. S., Harrison D., Quinn M. T., Parkos C. A., Livesey S., Linner J. Ultrastructural localization of cytochrome b in the membranes of resting and phagocytosing human granulocytes. J Clin Invest. 1990 Mar;85(3):821–835. doi: 10.1172/JCI114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Naemura J. R., Painter R. G., Sklar L. A., Cochrane C. G. Intracellular localization of N-formyl chemotactic receptor and Mg2+ dependent ATPase in human granulocytes. Biochim Biophys Acta. 1982 Dec 17;719(3):556–568. doi: 10.1016/0304-4165(82)90246-x. [DOI] [PubMed] [Google Scholar]
- Kane S. P., Peters T. J. Analytical subcellular fractionation of human granulocytes with reference to the localization of vitamin B12-binding proteins. Clin Sci Mol Med. 1975 Aug;49(2):171–182. doi: 10.1042/cs0490171. [DOI] [PubMed] [Google Scholar]
- Keller H. U. Diacylglycerols and PMA are particularly effective stimulators of fluid pinocytosis in human neutrophils. J Cell Physiol. 1990 Dec;145(3):465–471. doi: 10.1002/jcp.1041450311. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Robinson J. M. A novel intracellular compartment with unusual secretory properties in human neutrophils. J Cell Biol. 1991 May;113(4):743–756. doi: 10.1083/jcb.113.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Adelman J., Bock S. C., Franke A. E., Houck C. M., Najarian R. C., Seeburg P. H., Wion K. L. The sequence of human serum albumin cDNA and its expression in E. coli. Nucleic Acids Res. 1981 Nov 25;9(22):6103–6114. doi: 10.1093/nar/9.22.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Schuster R. Determination of amino acids in biological, pharmaceutical, plant and food samples by automated precolumn derivatization and high-performance liquid chromatography. J Chromatogr. 1988 Oct 14;431(2):271–284. doi: 10.1016/s0378-4347(00)83096-0. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. Biotin-containing reagents. Methods Enzymol. 1990;184:123–138. doi: 10.1016/0076-6879(90)84267-k. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Petersen O. W., Olsnes S., Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]