Abstract
Although the existence of a massive projection from the caudal intralaminar nuclei of the thalamus (i.e., the centromedian and parafascicular nuclei, CM/PF) to the striatum is well documented, the effects of CM activation upon striatal cells remain poorly understood. Therefore, we studied the effects of electrical stimulation of CM on the electrophysiological activity of striatal neurons, and on striatal levels of gamma-aminobutyric acid (GABA) and acetylcholine in rhesus monkeys. Striatal cells did not respond to single pulse stimulation (bipolar biphasic stimulation, 175–500 µA), but the large majority of recorded neurons responded to burst stimulation (100 Hz, 1 sec, 150–175 µA) of CM, often with a delay of tens of milliseconds. Striatal phasically active neurons, which likely correspond to projection neurons, responded mainly with increases in firing (13/28 cells), while tonically active neurons (likely cholinergic interneurons), often showed combinations of increases and decreases in firing (24/46 cells). In microdialysis studies, CM stimulation lead to a reduction of striatal acetylcholine levels. This effect was prevented by addition of the GABA-A receptor antagonist gabazine to the microdialysis fluid. We conclude that CM stimulation frequently results in striatal response patterns with excitatory and inhibitory components. Under the conditions chosen here, the specific patterns of striatal responses to CM stimulation are likely the result of striatal processing of thalamic inputs. Through these indirect effects, local CM stimulation may engage large portions of the striatum. These effects may be relevant in the interpretation of the therapeutic effects of CM-stimulation for the treatment of neurologic disorders.
Keywords: phasically active neuron, tonically active neuron, extracellular recording, microdialysis, acetylcholine
INTRODUCTION
There is strong anatomical evidence that thalamic activity may act to regulate the neuronal activity in the basal ganglia in primates and rodents (Smith et al., 2004; Lacey et al., 2007). While projections to the basal ganglia arise from many different thalamic nuclei, the main source of thalamostriatal projections originates in the caudal intralaminar thalamic complex (Smith et al., 2004). In primates, the intralaminar complex comprises the centromedian and parafascicular nuclei (CM and PF, respectively, see, Vogt & Vogt, 1941; Powell & Cowan, 1956; Smith et al., 2004), which send a massive, topographically organized projection to the striatum, and less prominent projections to extrastriatal basal ganglia nuclei (Smith et al., 2004). Through these projections, CM and PF are part of segregated basal ganglia-thalamostriatal loops (Sidibe et al., 2002; Smith et al., 2004) that involve projections from the medial CM to the sensorimotor putamen (Sadikot et al., 1992b; Sidibe et al., 1997; Sidibe et al., 2002), and from the PF to the associative and limbic striata (Sadikot et al., 1992a; Parent & Parent, 2005). In this study, we assessed electrophysiologic features of the CM-putamenal projection system in monkeys.
The most prominent striatal targets of CM efferents are dendrites of medium-sized spiny projection neurons (MSNs, Sadikot et al., 1992b; Sidibe & Smith, 1996) and cholinergic striatal interneurons (Meredith & Wouterlood, 1990; Lapper & Bolam, 1992; Sidibe & Smith, 1999). In monkeys, parvalbumin- and somatostatin-containing GABAergic interneurons also receive significant inputs from CM (Sidibe & Smith, 1999).
The functional effects of these strong anatomical connections between CM and striatal neurons remain poorly characterized. It is known that chemical inactivation of CM in monkeys greatly reduces reward-related responses of tonically active neurons (TANs, Matsumoto et al., 2001), a group of striatal interneurons that is thought to correspond to cholinergic cells (Wilson et al., 1990; Aosaki et al., 1995; Kawaguchi et al., 1995). In rats, chemical disinhibition of PF reduces acetylcholine release in the striatum, likely via activation of striatal GABAergic mechanisms (Zackheim & Abercrombie, 2005).
Despite the paucity of information regarding the functional interactions between CM/PF and the striatum, these nuclei have been empirically identified as targets for deep brain stimulation therapies for disorders such as the Tourette syndrome or Parkinson’s disease (for a review see Wichmann & Delong, 2006; Kringelbach et al., 2007). In order to gain a better understanding of the role of the thalamostriatal system in the functional circuitry of the basal ganglia, we studied the impact of electrical stimulation of CM on the electrical activity of phasically active neurons (PANs, likely corresponding to MSNs) and TANs in the putamen of rhesus monkeys. We also studied the effects of CM stimulation on striatal acetylcholine and GABA release. Our findings demonstrate that electrical CM stimulation induces complex and widespread excitatory and inhibitory responses in striatal projection neurons and TANs in the monkey putamen.
MATERIALS AND METHODS
Animals
Two rhesus monkeys (Macaca mulatta, 4.5 −5.5 kg) were used for these studies. The animals were housed under conditions of protected contact housing and maintained on a 12 h light/12 h dark cycle, with ad libitum access to food and water. All experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (Anonymous, 1996) and the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals (amended 2002). The studies were approved by the Institutional Animal Care and Use Committee of Emory University.
Surgical procedures and initial electrophysiological mapping
The animals were first trained to sit in a primate chair, adapt to the laboratory and permit handling by the experimenter. Under aseptic conditions and isoflurane gas anesthesia (1.5–3%), two stainless steel recording chambers (Crist Instruments, Hagerstown, MD) were then affixed on the same side of the animal’s skull with dental acrylic and bone screws. One chamber was aimed vertically at CM, while the other was directed at the putamen, angled 35° or 45° anteriorly from the vertical in the sagittal plane. Along with the recording chambers, a metal head holding bolt (Crist Instruments) was embedded into the acrylic cap to permit head stabilization during the subsequent recording and microdialysis procedures. After surgery, the animals were allowed to recover for one week before the start of recording and microdialysis sessions. During all experimental sessions, the monkeys remained awake, and were seated in a primate chair with their heads restrained. They were free to move their body and limbs.
In preparation for the stimulation and microdialysis sessions, we first generated electrophysiologic maps of the putamen and CM. A 20-ga stainless steel guide tube, mounted on a microdrive (MO95-S, Narishige, Tokyo, Japan) was lowered into the brain, piercing the dura mater. Tungsten electrodes (FHC, Bowdoinham, ME; Z = 0.5–1 MΩ@ 1 kHz) were then passed into the subcortical targets through the guide tube. Neuronal activity in the thalamus and putamen was recorded with standard extracellular electrophysiologic recording methods. The potentials were amplified (DAM-80, WPI, Sarasota, FL; MDA-2, BAK Electronics, Mount Airy, MD), filtered (400–6000 Hz, model 3700, Krohn-Hite, Brockton, MA), displayed on a digital oscilloscope (DL 1640; Yokogawa, Tokyo, Japan), and audio-amplified.
The striatum was identified by its location and the characteristic co-existence of PANs and TANs. To guide the identification of the CM nucleus, we relied on previously established criteria (Matsumoto et al., 2001; Minamimoto & Kimura, 2002; Minamimoto et al., 2005) These include the relation of CM to other structures (including easily identifiable basal ganglia structures, such as the globus pallidus, and the fact that the CM nucleus is located 3–6 mm below the thalamic surface, and is found at least 2 mm lateral to the midline). In addition, electrophysiological features of CM neurons were also used, including the finding of a lower neuronal firing rate than in the overlying mediodorsal nucleus of the thalamus (MD), a repetitive, burst-like, discharge pattern, and prominent responses to alerting stimuli (see, e.g., Matsumoto et al., 2001). We did not analyze the firing pattern of CM neurons in detail.
CM stimulation and striatal recordings
At a location chosen based on the results of the electrophysiological mapping, a concentric bipolar stimulation electrode (modified SNEX-100, RMI, Tujunga, CA; inter-contact distance 1 mm; outer diameter, 250 µm, Z = 25–35 kΩ@ 1 kHz) was inserted through the vertical chamber into the CM nucleus. In each animal, we used the same CM location for all stimulation experiments.
A recording electrode was then lowered into the striatum through the other recording chamber. Once a striatal neuron was isolated with sufficient quality, its baseline activity was recorded. Recordings at baseline and during CM stimulation (see below) were limited to periods when the monkey was sitting still. The minimal length of the baseline recordings was 30 s, but many cells were, in fact, recorded for longer periods (up to 5 minutes). After these baseline periods, we recorded the same neurons during CM stimulation with trains of biphasic square wave pulses (10 or 100 Hz; trains of 1, 10 or 100 pulses, 150–500 µA, 100µs/phase, randomized inter-train intervals of 2–3 seconds, bipolar stimulation). The trains of stimuli were delivered via a constant-current stimulus isolation device (BSI-2, BAK Electronics), and timed using a digital D/A computer interface (Power 1401 with Spike 2 interface, CED, Cambridge, UK). The A/D portion of the same interface was used to record the neuronal activity to computer disk (sampling rate, 25 kHz), along with digital time stamps of the stimulation, and voltage signals linearly related to the stimulation currents.
Analysis of electrophysiological data
Only neurons that were identified by physiological criteria as belonging to the striatum, and were isolated with sufficient quality were considered for further off-line analysis.
To remove stimulation artifacts from the neuronal recordings, we used a modified version of a previously described digital averaging method (Wichmann, 2000), implemented in Matlab (Mathworks Inc., Natick, MA). For each cell, a family of artifact templates was generated by averaging the waveforms of all available artifacts, and clustering them with a k-means algorithm. The artifacts were then subtracted from the record at the appropriate stimulation times. Artifact components that remained in the record even after the artifact removal procedure were replaced by brief zero-value segments. In such cases, similar brief zero-value segments were inserted in the non-stimulated portions of the records, so that the likelihood of detecting spikes was similarly diminished during stimulated and non-stimulated portions of the record. The zero-segment length never exceeded 1.5 ms.
After artifact removal, the data was imported back into the Spike2 environment for spike detection and sorting, accomplished through a waveform matching algorithm, combined with principal component analysis. Inter-spike interval (ISI) distribution histograms were constructed to analyze the recording quality for each spike train objectively.
For each experiment, we constructed peri-train histograms (50 ms bins), aligned to the start of the stimulation segment. The one-second epoch of data preceding the stimulation trains was chosen as the pre-stimulation epoch. The effects of CM stimulation on striatal cell firing were analyzed along different time scales. Thus, we compared the entire epoch of the stimulation train against the pre-stimulation epoch, using trial-by-trial, paired comparisons (Wilcoxon signed-rank test, p < 0.05). Cells were classified as showing significant increases, decreases or no changes in their firing rate.
To analyze stimulation effects of shorter duration, the peri-train histograms were analyzed with 50-ms bin resolution. Responses were considered significant (p < 0.05) if two consecutive 50 ms bins during the stimulation were either both above the 78th percentile, or both below the 22nd percentile of the pre-stimulation baseline activity. Responses were classified into increases or decreases of firing, or combinations of both.
Classification of striatal cells into PANs or TANs
A definitive classification of the recorded striatal cells into distinct groups that correlate with the histologically identifiable cell types (DiFiglia et al., 1976; Bishop et al., 1982; Tepper & Bolam, 2004) is not possible from in vivo extracellular recording studies.
However, based on their basal firing rates and response patterns, cells can be divided into two main electrophysiologic classes, PANs and TANs, likely corresponding to MSNs and cholinergic interneurons, respectively (see, e.g., Kimura, 1986; Hikosaka et al., 1989; Aosaki et al., 1994; Apicella et al., 1997; Blazquez et al., 2002). Using the published criteria, we classified cells with average basal firing rates below 1.5 spikes/s as PANs, and cells with average basal firing rates of 2–10 spikes/s as TANs.
CM stimulation and striatal microdialysis
In a separate series of experiments, the levels of GABA and acetylcholine were measured in the striatum before, during and after CM stimulation. Bipolar stimulation electrodes were placed into CM, as described above. Microdialysis probes (modified CMA-11, CMA, North Chelmsford, MA, 3 mm cuprophane membrane, molecular weight cut-off, 6 kD) were inserted into the striatum through a 22-gauge guide cannula. To avoid extensive tissue damage from repeated probe insertions, the total number of microdialysis penetrations was kept to a minimum and tracts were separated by at least 1 mm. The microdialysis experiments were temporally separated by at least 24 hours.
At the beginning of the experiment, the guide cannula with a fitting stylet was lowered into the brain with the microdrive, so that the tip of the cannula was located 1 mm above the striatal target. The stylet was then removed and the microdialysis probe inserted. The probes were perfused with artificial cerebrospinal fluid (aCSF) at 2 µl/min. The aCSF [comprised of (in mM) 143 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 1 Na2HPO4] was pH-adjusted to 7.2–7.4 and filtered with a 0.2 µm pore size nylon membrane (Fisher Scientific, Hampton, NH). After a 2–3 hour stabilization period, samples were collected every 10 minutes. Formic acid (Sigma-Aldrich, St. Louis, MO; 0.2 M, 10 µl) was added to each sample as a preservative. The first three samples were used as baseline. Then, CM stimulation was applied, using those parameters that were effective in the electrophysiological experiments (100 Hz, 100 pulses, 150 µA, 100 µs/phase, with 2–3 seconds random inter-train intervals) was given during sample #4, followed by collection of three additional samples. To allow for more reliable acetylcholine measurements, in some experiment the level of acetylcholine was elevated by the addition of neostigmine (100 nM, Sigma, Damsma et al., 1988; Damsma & Flentge, 1988; Moor et al., 1998). In a separate series of experiments, the GABA-A receptor antagonist gabazine (SR-95531 hydrobromide, 10 µM, Tocris Cookson, Ellisville, MO) was added to the perfusate, 10 minutes prior to the beginning of sample collection until the end of the experiment. All drugs were dissolved in aCSF. At the end of collection, dialysates were immediately frozen, stored at −80°C and later assayed.
Analysis of microdialysates
GABA concentrations in the dialysates were determined by pre-column derivatization and separation by reverse-phase high-performance liquid chromatography (HPLC, Rea et al., 2005). Ten µl of the dialysate samples were derivatized with o-phtaldialdehyde/mercaptoethanol reagent, mixed, and allowed to react for 2 minutes. Twenty µl of the mixture were then injected onto a reverse-phase 4.6 × 150 mm column (Hypersil, C18, 3 µm; Supelco, Bellefonte, PA). The mobile phase consisted of 30% methanol, 70 mM di-sodium hydrogen phosphate, 400 mM EDTA and 0.15% tetrahydrofluoran. Fluorescent detection was performed using a RF-10A XL detector (Shimadzu, Tokyo, Japan; excitation 350 nm, emission 450 nm). The lower limit of detection for GABA was 1 nM.
Acetylcholine concentrations were analyzed by HPLC separation with tandem mass spectrometry detection. Prior to analysis, acetylcholine samples were diluted 1:10 with a ringer-formic acid solution. Ten µl of an internal standard solution was added to 10 µl diluted dialysate sample. Fifteen µl of this mixture were injected onto the separation column (reverse-phase 150 × 2.00 mm, 4 µm, Synergi, Phenomenex, Torrance, CA). The mobile phase consisted of 5% acetonitrile, 0.3% trifluoroacetic acid and 20 mM ammoniumacetate in ultra purified water. Acetylcholine and the internal standard were detected using a mass spectrometer (API 4000, Applied Biosystems, Foster City, CA) equipped with a Turbo ion spray source (Applied Biosystems), and operated in positive mode. The lower limit of detection for acetylcholine was 0.1 nM.
Each drug or control experiment was done in quintuples. The GABA or acetylcholine measurements were normalized to the mean of the three baseline samples, and then averaged across the five experiments. The normalized baseline values in experiments with and without gabazine were compared using paired non-parametric statistics (Wilcoxon test). Differences at the p < 0.05 level were accepted as significant. The effects of CM stimulation were evaluated by comparing the average baseline value with the average of the (stimulated) sample #4 and the following sample (#5).
Histology
At the end of the experiments, the animals were killed with an overdose of pentobarbital (100 mg/kg), and perfused transcardially with cold oxygenated Ringer’s solution, followed by 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). The brains were removed from the skull and cut into 10 mm-thick blocks. Tissue sections (60 µm) were obtained with a vibratome and collected in cold phosphate-buffered saline (0.01M, pH 7.4). To verify the location of recording, stimulation and microdialysis probes, one out of each 6 serial sections was stained with cresyl violet, or with mouse antibodies against microtubule-associated protein 2 (MAP2, 1:1000, Chemicon-Millipore, Billerica, MA). To determine the lateral border of the CM/PF complex, one out of 6 thalamic serial sections was incubated with mouse antibodies against Calbindin-D-28K (1:5000, Sigma-Aldrich, Jones & Hendry, 1989). The labeling was visualized by the avidin-biotin-peroxidase method, using DAB as chromagen, according to a protocol described previously (Galvan et al., 2004).
RESULTS
Electrophysiologic studies
Patterns of striatal responses to CM stimulation
We examined the responses of neurons in the striatum to electrical stimulation of CM. Recordings were made throughout the post-commissural putamen. The electrical stimulation did not have overt behavioral effects.
In some experiments, we analyzed the electrophysiologic activity of striatal cells in response to single pulse stimulation of CM. Six cells (3 PANs and 2 TANs, 1 unclassified cell), were tested with a 175 µA, and 24 additional cells (12 PANs, 12 TANs) with 500 µA current strength. CM stimulation did not produce significant responses in these cases.
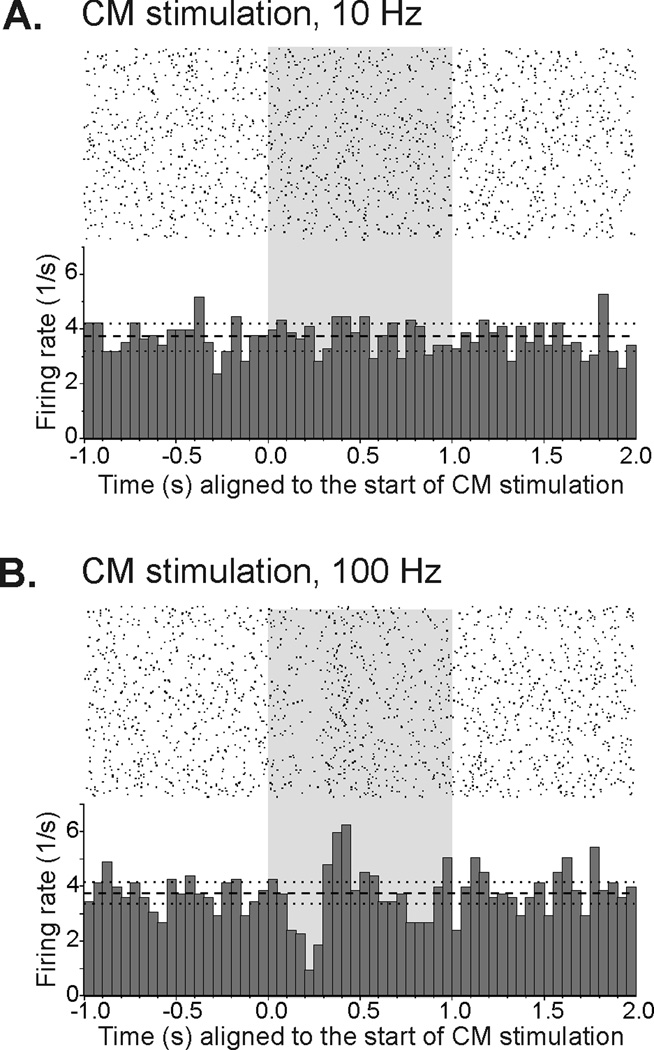
All experiments with multi-pulse stimulation were carried out with 150–175 µA currents. In agreement with the results of the single-pulse experiments, none of the striatal cells tested with stimulation trains showed a discernible change of activity in response to the first pulse of the train. However, significant responses were readily detected in later phases of the stimulation trains (see below). In order to examine whether such responses were dependent on the frequency of stimulation, 16 cells were tested with both, one-second trains of stimulation at 10 Hz and one-second trains of 100 Hz stimulation, delivered in a random sequence. The two stimulation conditions produced qualitatively similar responses in striatal neurons, but the proportion of cells responding to 100 Hz stimulation (15/16) was higher than the proportion responding to 10 Hz stimulation (10/16). The difference between the proportion of neurons responding to 10 or 100 Hz was statistically significant (p = 0.03, Chi-square test). Fig. 1 shows an example of a striatal TAN which failed to respond to 10 Hz stimulation, but showed a significant decrease followed by an increase in firing during the 100 Hz stimulation.
Figure 1.
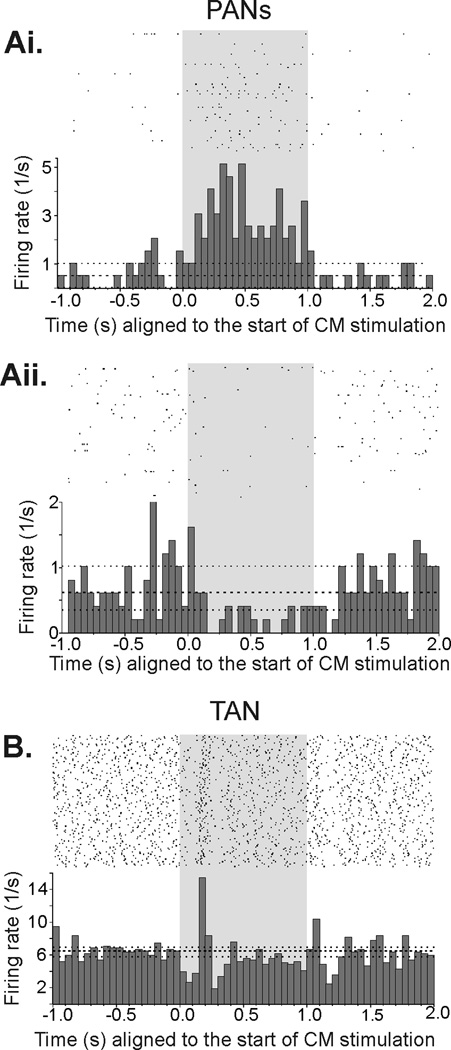
Based on these findings, we used the 100 Hz-stimulation condition for the remainder of the experiments. The responses of 90 striatal neurons were tested in this manner, including 28 PANs and 46 TANs. The remaining 16 cells could not be classified according to our criteria, and were not studied further. Examples of the most common responses of PANs and TANs to CM stimulation are shown in Fig. 2.
Figure 2.
Paired comparison of individual data from the entire one-second stimulation trains with the one-second data segments preceding them showed that PANs responded to CM stimulation with increased firing in 47% of the cases (13/28 cells), while 14% of the recorded neurons (4/28 cells) decreased their activity, and 39% (11/28 cells) did not respond to CM stimulation in this comparison. In contrast, 30% of TANs (14/46 cells) responded with a decrease in activity following CM stimulation, while increased firing was less common (9/46 cells, 20%). Fifty percent of the recorded TANs (23/46 cells) did not respond to CM stimulation in this comparison. As shown in Table 1, the results were generally similar between the two animals, although the proportion of TANs with stimulation-induced increases or decreases in firing was lower in monkey T than in monkey R.
Table 1. Responses of TANs and PANs to CM stimulation in monkeys R and T.
Results of paired comparison of recordings made during individual one-second stimulation trains with the respective pre-stimulation control periods (Wilcoxon signed rank test, a criterion of p <0.05 was used for classification; see Methods). The table shows the number and percentages of responding cells.
| Monkey | PANs | TANs | ||||||
|---|---|---|---|---|---|---|---|---|
| Increase | Decrease | No effect | Total number of PANs |
Increase | Decrease | No effect | Total number of TANs |
|
| R | 6 (50%) | 2 (17%) | 4 (33%) | 12 | 7 (39%) | 6 (33%) | 5 (28%) | 18 |
| T | 7 (44%) | 2 (12%) | 7 (44%) | 16 | 2 (7%) | 8 (29%) | 18 (64%) | 28 |
| Combined | 13 (47%) | 4 (14%) | 11 (39%) | 28 | 9 (20%) | 14 (30%) | 23 (50%) | 46 |
It was obvious from inspection of peri-stimulation train histograms that the responses of many striatal neurons were not constant during CM stimulation trains (see, for instance, the example shown in Fig. 2B). To account for brief increases or decreases in firing rate, we carried out an analysis in which we considered an increase or a decrease as significant if it lasted for at least two consecutive 50-ms bins (see Methods). According to this analysis approach, 27/28 PANs and 45/46 TANs responded to the stimulation (Table 2). Among PANs, monophasic increases in firing (i.e., cases in which decreases in firing were not detected during any phase of the stimulation train) were most common, (13/28 cells, 46%, Fig. 2 Ai), but monophasic decreases in firing were also frequently observed (11/28 cells, 39%, Fig. 2 Aii). Combination responses, where increases were followed or preceded by decreases were seen in 3/28 PANs (11%). Among TANs, such combination responses were more commonly recorded (24/46 cells, 52%, Fig. 2B), while monophasic increases or decreases in firing were less common (12/46 cells, 26%, and 9/46 cells, 20%, respectively). For TANs, the proportions of types of responses differed between the two monkeys (Table 2).
Table 2. Responses of TANs and PANs to CM stimulation in monkeys R and T.
The classification into cells showing increases, decreases or combinations of increases and decreases (columns labeled ‘Comb.) is based on the ‘two-bin’ criterion (see Methods). The table shows the number and percentages of responding cells. See table 1 for total n-numbers.
| Monkey | PANs | TANs | ||||||
|---|---|---|---|---|---|---|---|---|
| Increase | Decrease | Comb. | No effect | Increase | Decrease | Comb. | No effect | |
| R | 6 (50%) | 6 (50%) | 0 | 0 | 8 (44%) | 7 (39%) | 3 (17%) | 0 |
| T | 7 (44%) | 5 (31.3%) | 3 (18.8%) | 1 (6.3%) | 4 (14%) | 2 (7%) | 21 (75%) | 1 (4%) |
| Combined | 13 (46%) | 11 (39%) | 3 (11%) | 1 (4%) | 12 (26%) | 9 (20%) | 24 (52%) | 1 (2%) |
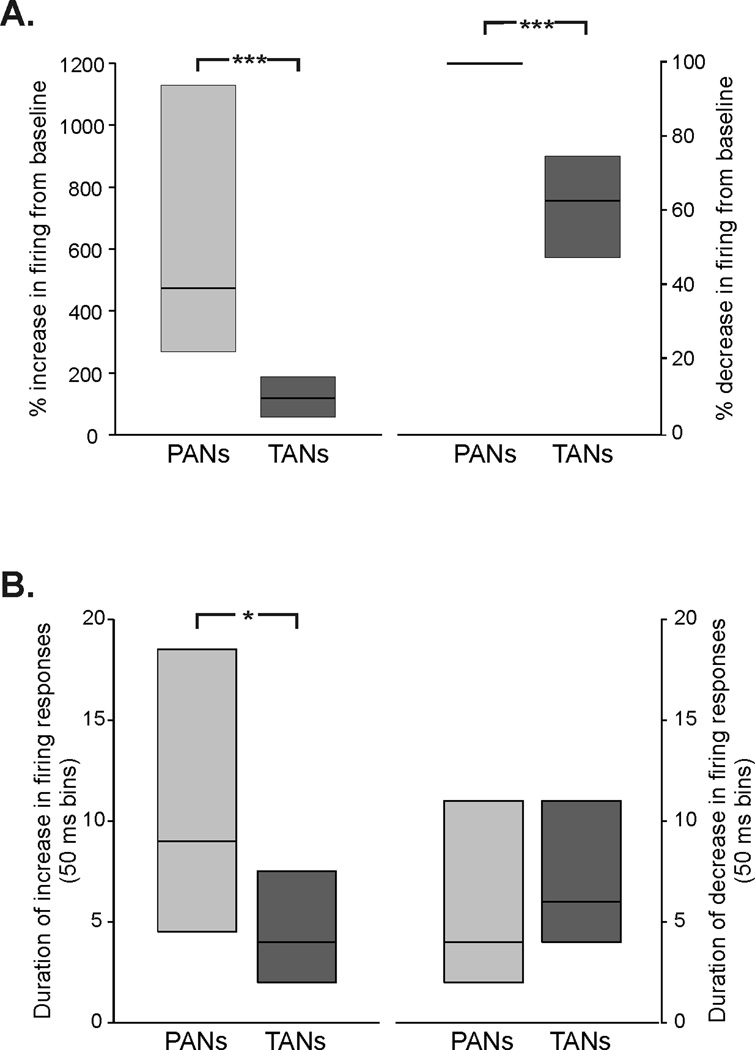
Magnitude and duration of responses
We expressed the magnitude of striatal responses to CM stimulation as the maximal percentage change of the firing rate from the median basal firing rate. We found that increases in firing were larger in magnitude in PANs than in TANs (Fig. 3 A; p < 0.0001, Mann-Whitney test). The magnitude of decreases in firing was variable in TANs, but all PANs with inhibitory responses to CM stimulation were transiently silenced (Fig. 3A). In terms of the duration of such responses, increases in firing lasted longer in PANs than in TANs (Fig. 3B; p = 0.002, Mann-Whitney test ), while the duration of decreases in firing responses did not differ between the two groups of neurons.
Figure 3.
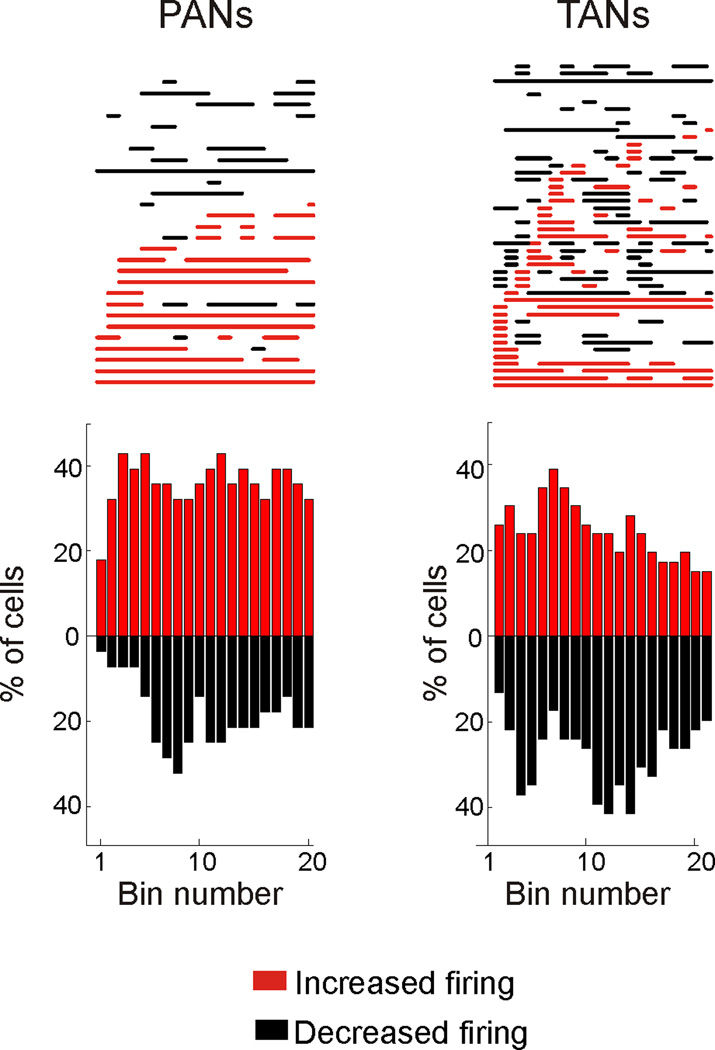
Time course of transient responses
Fig. 4 shows the time course of the responses of PANs and TANs to CM stimulation. During most of the stimulation train (19/20 bins), a larger proportion of PANs responded with increases than decreases in firing. In contrast, during most of the stimulation train (14/20 bins), a larger proportion of TANs showed decreases in firing. When examined across the population of PANs, peaks of increased firing occurred between 100–200 ms, and again around 550 ms after the start of the stimulation train (Fig. 4). These peaks of increased PAN activity coincided with the peaks of decreased firing in TANs (100 ms and 450–500 ms). The maximal proportion of TANs showing an increased firing (39%) was observed 250 ms into the stimulation train.
Figure 4.
Microdialysis
Microdialysis samples were collected before, during and after CM stimulation. The microdialysis probes were placed in the same area within the putamen in which electrophysiologic effects had been observed, and we used stimulation conditions identical to those in the recording experiments.
The baseline GABA level in the striatum, calculated as the mean of the first three samples, was 0.3 ± 0.01 µM (mean ± SEM, n = 6) which is comparable to that reported in previous studies (Hernandez et al., 2003; Takeda et al., 2003; Molchanova et al., 2004). Neostigmine (100 nM) did not change basal GABA levels, but addition of the GABA-A antagonist gabazine (10 µM) increased GABA levels by 25% (0.4 ± 0.01 µM, p = 0.08). CM stimulation did not evoke significant changes in the levels of striatal GABA (not shown).
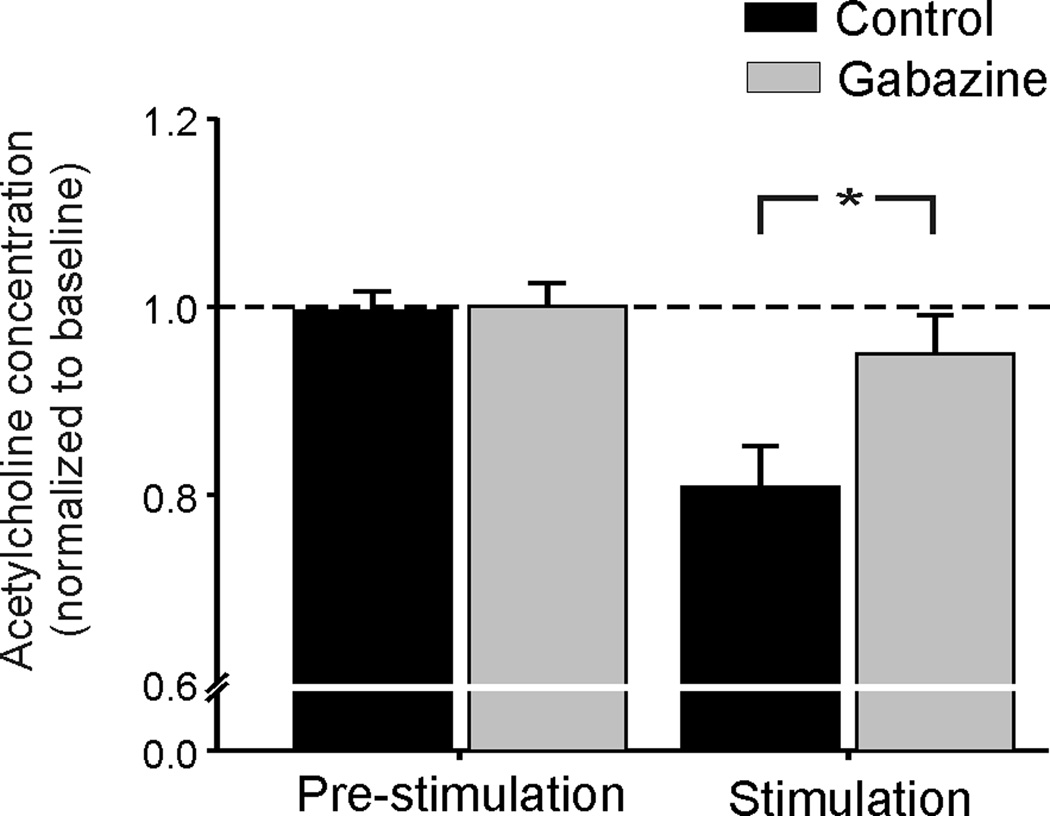
In agreement with earlier studies, the basal acetylcholine level, 2.03 ± 0.12 nM (n = 5), was increased to 28.8 ± 5.4 nM (n = 5) following addition of 100 nM neostigmine to the striatal perfusion medium (DeBoer & Westerink, 1994; Ragozzino & Choi, 2004; Zackheim & Abercrombie, 2005). CM stimulation reduced the level of ACh in samples 4 and 5. In the absence of neostigmine, the average of samples #4 and #5 was reduced to 88.5 ± 2.8% of the baseline. In the presence of 100 nM neostigmine, the reduction was 81 ± 5.6% from the baseline (Fig. 5). This inhibitory effect of CM stimulation on acetylcholine levels was abolished in the presence of gabazine 10 µM (p = 0.022, Mann-Whitney test, Fig. 5).
Figure 5.
Histology
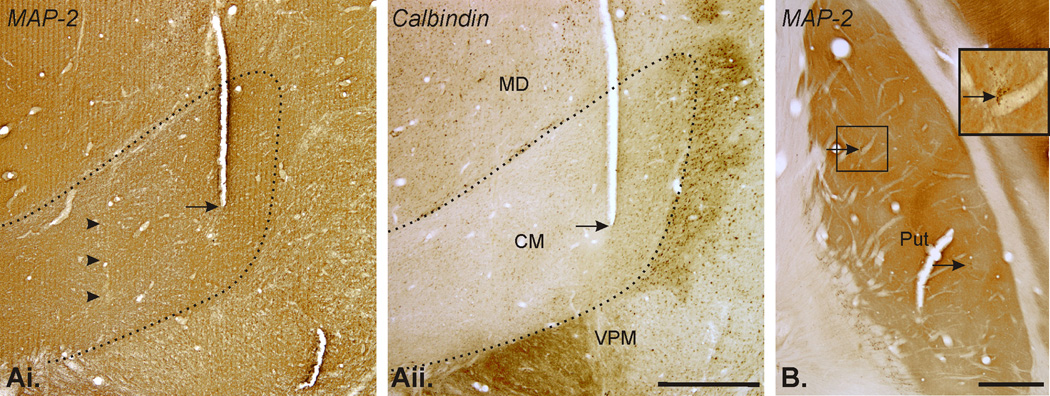
At the end of the experiments the placement of the stimulation electrode in CM was confirmed histologically in both monkeys. In calbindin- and MAP-2- stained coronal sections, the tracks used for the repeated placement of the stimulation electrode were clearly visible (Fig. 6 Aii, arrows), as were microelectrode penetration tracks (Fig. 6 Ai, arrowheads). In both cases, the stimulation electrode tracks were located in the anterior two thirds of CM. The placement of the recording electrodes and microdialysis probes in the putamen was confirmed histologically. Fig. 6 B shows examples of microdialysis probes tracks in the post-commissural putamen.
Figure 6.
DISCUSSION
Our findings demonstrate that most striatal projection neurons and cholinergic interneurons respond to prolonged trains of electrical stimuli in CM, but not to single pulse stimulation of the nucleus. The responses varied greatly in polarity, onset times and duration.
Technical considerations
Electrical stimulation with parameters chosen in this study are likely to have affected predominately axons within the stimulated area (Ranck, 1975). Some of the responses recorded in the striatum may have resulted from recruitment of thalamostriatal fibers that originated outside of CM. However, most nuclei in the vicinity of CM do not have significant projections to the postcommissural putamen (Smith & Parent, 1986), making it unlikely that they contributed significantly to the responses. Furthermore, modeling studies (Ranck, 1975; Gimsa et al., 2005a; Gimsa et al., 2005b) have suggested that the current spread generated by the stimulations did not extend beyond a ∼0.5 mm radius from the stimulating electrode. Based on the location of the electrodes in the CM nucleus and the low stimulation currents, it is, therefore likely that the stimulation effects in our study are not due to current spread to neighboring thalamic nuclei.
CM stimulation could affect striatal activity through routes other than the CM-striatal projection, specifically by activating corticostriatal pathways, either through orthodromic activation of the cerebral cortex or through antidromic invasion of corticofugal fibers. There is no practical method to distinguish definitively between thalamo- and corticostriatal contributions to the responses of striatal cells to CM stimulation. However, there is a strong anatomical basis for assuming that the effects of CM stimulation in our experiments were largely mediated via the CM-striatal pathway. First, in contrast to the sparse and divergent CM projections to the cerebral cortex (Deschenes et al., 1996; Kincaid et al., 1998; Parent & Parent, 2005), the CM-striatal projection is massive, ending in dense clusters of terminals in the striatum (Sadikot et al., 1992a; Sidibe & Smith, 1996; Parent & Parent, 2005). Furthermore, most CM-cortex projections (Royce, 1983; Sadikot et al., 1992a; Deschenes et al., 1995; Parent & Hazrati, 1995; Deschenes et al., 1996; Smith et al., 2004; Parent & Parent, 2005) originate in a lateral crescent of CM (an area not targeted by our stimulation electrodes), so that orthodromic activation of corticostriatal fibers is not likely. Finally, while corticostriatal projections send collaterals to CM in cats and rats (Levesque et al., 1996), this pathway has not been observed in monkeys (Parent & Parent, 2006b; a), so that activation of putamenal neurons by antidromic stimulation of a cortex-CM projection appears less likely in primates. Taken together, these arguments suggest that the effects of CM stimulation in our experiments were largely mediated via activation of CM-striatal pathways.
It is also important to realize that the parameters of stimulation used in our studies, do not reflect the physiological (non-bursty) pattern of firing of CM neurons at rest, although the frequency and duration of the stimulation trains mimic some of the known burst firing properties of CM neurons (Matsumoto et al., 2001). As in all in vivo stimulation studies, it is likely that the electrical stimulation of CM resulted in a non-physiologic level of synchronization of thalamostriatal fibers.
Excitatory responses
Given the anatomic evidence for a massive glutamatergic CM/PF projection to the striatum, we initially predicted that many striatal neurons would show short-latency excitatory responses to the electrical CM stimulation, as had been identified in previous intracellular recording studies in anesthetized rats (Wilson et al., 1983; Wilson et al., 1990) and in more recent brain slice recording experiments (Ding et al., 2008). In our experiments, however, such short-latency responses were not detected. These discrepancies are likely methodological in nature, as the stimulation currents that were used in the rodent studies were substantially higher and the stimulation sites were different. Furthermore, at least some of the short latency responses recorded in rodents consisted of EPSPs (which are not detectable with the methods used in our study) rather than spikes. Single EPSPs generated by thalamic inputs may simply not be sufficient to produce action potentials in the recipient striatal cells. Summation of several closely spaced EPSPs may be needed to produce a spiking response in these neurons (e.g., Onn et al., 1994) which could then be detectable with the extracellular electrophysiologic recording methods. This response pattern would render striatal neurons particularly sensitive to burst inputs from CM.
The anatomical structure of the thalamostriatal system also provides clues to explain why the expected short-latency responses did not occur in our study. In this regard, it is interesting to contrast the thalamostriatal and the corticostriatal system. Single pulse stimulation of the latter, with parameters similar to those used in this study, elicits short latency responses in the striatum that are readily detectable in PANs or TANs in awake monkeys (Nambu et al., 2002). The cortical afferents that mediate such responses distribute diffuse termination fields that involve large striatal areas, providing axonal varicosities to a large number of striatal cells, thereby increase the likelihood of detecting short-latency responses. CM axons, on the other hand, give rise to dense clusters of terminals concentrated in discrete portions of the striatum (Parent & Parent, 2005; 2006b). Under these circumstances, short-latency excitations may only be detectable with precise matching of stimulation and recording sites which is difficult to accomplish in primate in vivo recordings.
Most of the excitatory responses in our study were transient in nature, i.e., the response terminated before the end of the stimulation train. The transient character of these responses is in line with recent in vitro data from mice brain slices demonstrating short-term plasticity at thalamostriatal synapses (Ding et al., 2008). These authors showed that the striatal responses to electrical stimulation of thalamostriatal fibers are reduced by repetitive stimulation (in their case at 50 Hz). Interestingly, these authors also found that stimulation of corticostriatal inputs results in facilitation of responses instead. The paucity of sustained excitation of striatal cells during the thalamic stimulation in our experiments argues therefore against the possibility that trans-cortical transmission of the thalamic stimulation contributed strongly to the excitatory responses recorded in our study. Additional factors intrinsic to the striatum that may have acted to truncate the excitatory responses include the delayed activation of local inhibitory inputs to the cells under study (see below).
Inhibitory responses
Considering the fact that the CM-striatal projection is glutamatergic, the proportion of striatal TANs and PANs responding to CM stimulation with a decrease in firing was surprisingly high. Such inhibitory responses were particularly common among TANs. Decreases in firing are obviously not explainable by direct (monosynaptic) actions of CM inputs to the recorded neurons, but are likely to result from the activation of intrastriatal inhibitory circuits.
PANs receive inhibitory GABAergic inputs from other PANs, and from interneurons (Tepper et al., 2004). Inputs from neighboring PANs are considered to be fairly weak (see discussions in ref. Tepper et al., 2004), while inputs from GABAergic interneurons (mainly parvalbumin-positive interneurons which form a rich synaptic connectivity network with PANs) may have stronger inhibitory effects onto PANs (Koos & Tepper, 1999). Parvalbumin-positive interneurons receive direct thalamic inputs from CM (Sidibe & Smith, 1999). Activation of cholinergic interneurons may also have resulted in inhibition of PANs, via an indirect mechanism. It has been documented that acetylcholine depolarizes fast-spiking GABAergic interneurons, thus increasing inhibitory control over MSNs (Koos & Tepper, 2002). Suggestive of this possibility, we found that excitatory TAN responses peaked around 250 ms into the stimulation trains, slightly earlier than the time period during which inhibitory effects in PANs peaked.
CM-stimulation related reductions in TAN activity are likely mediated via GABAergic transmission in the striatum. This is supported by our finding that CM stimulation induced a decrease in striatal acetylcholine which was reversed by blocking GABA-A receptors in the striatum (see also Anderson et al., 1993; DeBoer & Westerink, 1994), in agreement with recent rodent studies in which chemical activation of PF produced a GABAergic inhibition of striatal acetylcholine release (Zackheim & Abercrombie, 2005). The rodent literature suggests that axon collaterals of direct pathway MSNs are the primary sources of intrinsic GABAergic inputs to cholinergic interneurons (Bolam et al., 1986; Martone et al., 1992). We observed that the peak of excitatory PAN responses occurred close to the peak of reductions in firing in TANs, suggesting that the excitation of PANs may indeed be the main source of TAN inhibition following CM stimulation. In addition, a recent study suggested that striatal cholinergic interneurons are reciprocally connected through GABAergic interneurons in rats, thus providing another mechanism to mediate inhibition among cholinergic interneurons (Sullivan et al., 2008).
Responses including excitatory and inhibitory components
CM stimulation frequently produced mixed responses in striatal neurons, consisting of transient increases and decreases in activity. Such responses were particularly common in TANs. This response pattern may have been the result of successive, and potentially overlapping, inhibitory and excitatory effects of CM stimulation, mediated via the interactions between striatal output and interneurons and perhaps further modified by the short-term adaptive responses of striatal neurons (Ding et al., 2008). The existence of mixed response patterns betrays intrastriatal processing of the thalamostriatal inputs which effectively acts to translate the thalamic burst input into complex patterns of responses of striatal output neurons. Such response patterns may also occur under physiologic conditions: For instance, it is possible that the tri-phasic responses of TANs in reward situations (Apicella, 2002) may occur as the result of intrastriatal processing of burst inputs from CM (Matsumoto et al., 2001).
Role of CM input in striatal physiology and in movement disorders
Through its influence upon striatal firing, CM activity may alter striatal output under pathological conditions. For instance, in parkinsonism, the basal ganglia outflow from GPi to CM is likely to be increased, and abnormally patterned and synchronized (discussed in DeLong & Wichmann, 2007; Galvan & Wichmann, 2008). Such changes in CM activity may secondarily influence the CM-striatal interaction. An unknown factor in the consideration of the role of altered CM activity in parkinsonism is whether the concomitant degeneration of CM neurons in Parkinson’s disease also influences the effects of CM activation (but see ref. Xuereb et al., 1991; Freyaldenhoven et al., 1997; Henderson et al., 2000a; b; Henderson et al., 2005; Aymerich et al., 2006).
Deep brain stimulation (DBS) of CM has recently been introduced as a promising method to alter striatal activity through modulation of the thalamostriatal system. CM/PF-DBS appears to be beneficial in patients with the Tourette syndrome (Visser-Vandewalle et al., 2004; Houeto et al., 2005; Swerdlow & Sutherland, 2005; Perlmutter & Mink, 2006; Skidmore et al., 2006; Visser-Vandewalle et al., 2006), and Parkinson’s disease (Caparros-Lefebvre et al., 1999a; Caparros-Lefebvre et al., 1999b; Krauss et al., 2002; Mazzone et al., 2006). These treatments are currently done on an empiric basis, but our results indicate that local CM stimulation has indeed strong effects on striatal activity that may shape basal ganglia output. Our study also demonstrates that such effects are likely regionally specific, and that stimulation will result in prominent changes in striatal firing patterns rather than simple activation or inactivation of striatal activity.
ACKNOWLEDGEMENTS
These studies were supported by a grant from the Tourette Syndrome Association (YS) and by the base grant to the Yerkes National Primate Research Center (NIH/NCRR RR-00165). We thank Xing Hu, Susan Jenkins and Yuxian Ma for technical assistance.
ABBREVIATIONS
- CM
Centromedian nucleus of the thalamus
- GABA
γ-amino-butyric acid
- MSN
Medium sized spiny neuron
- PF
Parafascicular nucleus of the thalamus
- PAN
Phasically active neuron
- TAN
Tonically active neuron
REFERENCES
- Anderson JJ, Kuo S, Chase TN, Engber TM. GABAA and GABAB receptors differentially regulate striatal acetylcholine release in vivo. Neurosci Lett. 1993;160:126–130. doi: 10.1016/0304-3940(93)90395-2. [DOI] [PubMed] [Google Scholar]
- Anonymous. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci. 2002;16:2017–2026. doi: 10.1046/j.1460-9568.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- Apicella P, Legallet E, Trouche E. Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Exp Brain Res. 1997;116:456–466. doi: 10.1007/pl00005773. [DOI] [PubMed] [Google Scholar]
- Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL. Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci. 2006;23:2099–2108. doi: 10.1111/j.1460-9568.2006.04741.x. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Chang HT, Kitai ST. Morphological and physiological properties of neostriatal neurons: an intracellular horseradish peroxidase study in the rat. Neuroscience. 1982;7:179–191. doi: 10.1016/0306-4522(82)90159-2. [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Fujii N, Kojima J, Graybiel AM. A network representation of response probability in the striatum. Neuron. 2002;33:973–982. doi: 10.1016/s0896-6273(02)00627-x. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Ingham CA, Izzo PN, Levey AI, Rye DB, Smith AD, Wainer BH. Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Res. 1986;397:279–289. doi: 10.1016/0006-8993(86)90629-3. [DOI] [PubMed] [Google Scholar]
- Caparros-Lefebvre D, Blond S, Feltin MP, Pollak P, Benabid AL. Improvement of levodopa induced dyskinesias by thalamic deep brain stimulation is related to slight variation in electrode placement: possible involvement of the centre median and parafascicularis complex. J Neurol Neurosurg Psychiatry. 1999a;67:308–314. doi: 10.1136/jnnp.67.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparros-Lefebvre D, Pollak P, Feltin MP, Blond S, Benabid AL. [The effect of thalamic stimulation on levodopa induced dyskinesias--evaluation of a new target: the center parafascicular median] Rev Neurol (Paris) 1999b;155:543–550. [PubMed] [Google Scholar]
- Damsma G, Biessels PT, Westerink BH, De Vries JB, Horn AS. Differential effects of 4-aminopyridine and 2,4-diaminopyridine on the in vivo release of acetylcholine and dopamine in freely moving rats measured by intrastriatal dialysis. Eur J Pharmacol. 1988;145:15–20. doi: 10.1016/0014-2999(88)90343-3. [DOI] [PubMed] [Google Scholar]
- Damsma G, Flentge F. Liquid chromatography with electrochemical detection for the determination of choline and acetylcholine in plasma and red blood cells. Failure to detect acetylcholine in blood of humans and mice. J Chromatogr. 1988;428:1–8. doi: 10.1016/s0378-4347(00)83884-0. [DOI] [PubMed] [Google Scholar]
- DeBoer P, Westerink BH. GABAergic modulation of striatal cholinergic interneurons: an in vivo microdialysis study. J Neurochem. 1994;62:70–75. doi: 10.1046/j.1471-4159.1994.62010070.x. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Parent A. Two different types of thalamic fibers innervate the rat striatum. Brain Res. 1995;701:288–292. doi: 10.1016/0006-8993(95)01124-3. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Pasik P, Pasik T. A Golgi study of neuronal types in the neostriatum of monkeys. Brain Res. 1976;114:245–256. doi: 10.1016/0006-8993(76)90669-7. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and Thalamostriatal Synapses Have Distinctive Properties. J. Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyaldenhoven TE, Ali SF, Schmued LC. Systemic administration of MPTP induces thalamic neuronal degeneration in mice. Brain Res. 1997;759:9–17. doi: 10.1016/s0006-8993(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Galvan A, Charara A, Pare JF, Levey AI, Smith Y. Differential subcellular and subsynaptic distribution of GABA(A) and GABA(B) receptors in the monkey subthalamic nucleus. Neuroscience. 2004;127:709–721. doi: 10.1016/j.neuroscience.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa J, Habel B, Schreiber U, Rienen U, Strauss U, Gimsa U. Choosing electrodes for deep brain stimulation experiments-electrochemical considerations. J Neurosci Methods. 2005a;142:251–265. doi: 10.1016/j.jneumeth.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gimsa U, Schreiber U, Habel B, Flehr J, van Rienen U, Gimsa J. Matching geometry and stimulation parameters of electrodes for deep brain stimulation experiments-Numerical considerations. J Neurosci Methods. 2005b doi: 10.1016/j.jneumeth.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Ann Neurol. 2000a;47:345–352. [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain. 2000b;123(Pt 7):1410–1421. doi: 10.1093/brain/123.7.1410. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Schleimer SB, Allbutt H, Dabholkar V, Abela D, Jovic J, Quinlivan M. Behavioural effects of parafascicular thalamic lesions in an animal model of parkinsonism. Behav Brain Res. 2005;162:222–232. doi: 10.1016/j.bbr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Hernandez LF, Segovia G, Mora F. Effects of activation of NMDA and AMPA glutamate receptors on the extracellular concentrations of dopamine, acetylcholine, and GABA in striatum of the awake rat: a microdialysis study. Neurochem Res. 2003;28:1819–1827. doi: 10.1023/a:1026115607216. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol. 1989;61:780–798. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, Welter ML, Navarro S, Pelissolo A, Damier P, Pidoux B, Dormont D, Cornu P, Agid Y. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76:992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Hendry SH. Differential Calcium Binding Protein Immunoreactivity Distinguishes Classes of Relay Neurons in Monkey Thalamic Nuclei. Eur J Neurosci. 1989;1:222–246. doi: 10.1111/j.1460-9568.1989.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kimura M. The role of primate putamen neurons in the association of sensory stimuli with movement. Neurosci.Res. 1986;3:436–443. doi: 10.1016/0168-0102(86)90035-0. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss JK, Pohle T, Weigel R, Burgunder JM. Deep brain stimulation of the centre median-parafascicular complex in patients with movement disorders. J Neurol Neurosurg Psychiatry. 2002;72:546–548. doi: 10.1136/jnnp.72.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- Lacey CJ, Bolam JP, Magill PJ. Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J Neurosci. 2007;27:4374–4384. doi: 10.1523/JNEUROSCI.5519-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontalcortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Levesque M, Gagnon S, Parent A, Deschenes Axonal arborizations of corticostriatal and corticothalamic fibers arising from the second somatosensory area in the rat. Cerebral Cortex. 1996;6:759–770. doi: 10.1093/cercor/6.6.759. [DOI] [PubMed] [Google Scholar]
- Martone ME, Armstrong DM, Young SJ, Groves PM. Ultrastructural examination of enkephalin and substance P input to cholinergic neurons within the rat neostriatum. Brain Res. 1992;594:253–262. doi: 10.1016/0006-8993(92)91132-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Stocchi F, Galati S, Insola A, Altibrandi MG, Modugno N, Tropepi D, Brusa L, Stefani A. Bilateral implantation of centromedian-parafascicularis complex and GPi: a new combination of unconventional targets for deep brain stimulation in severe Parkinson disease. Neuromodulation. 2006;9:221–228. doi: 10.1111/j.1525-1403.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Wouterlood FG. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol. 1990;296:204–221. doi: 10.1002/cne.902960203. [DOI] [PubMed] [Google Scholar]
- Minamimoto T, Hori Y, Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science. 2005;308:1798–1801. doi: 10.1126/science.1109154. [DOI] [PubMed] [Google Scholar]
- Minamimoto T, Kimura M. Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophysiol. 2002;87:3090–3101. doi: 10.1152/jn.2002.87.6.3090. [DOI] [PubMed] [Google Scholar]
- Molchanova S, Koobi P, Oja SS, Saransaari P. Interstitial concentrations of amino acids in the rat striatum during global forebrain ischemia and potassium-evoked spreading depression. Neurochem Res. 2004;29:1519–1527. doi: 10.1023/b:nere.0000029564.98905.5c. [DOI] [PubMed] [Google Scholar]
- Moor E, Schirm E, Jacso J, Westerink BH. Effects of neostigmine and atropine on basal and handling-induced acetylcholine output from ventral hippocampus. Neuroscience. 1998;82:819–825. doi: 10.1016/s0306-4522(97)00331-x. [DOI] [PubMed] [Google Scholar]
- Nambu A, Kaneda K, Tokuno H, Takada M. Organization of Corticostriatal Motor Inputs in Monkey Putamen. J Neurophysiol. 2002;88:1830–1842. doi: 10.1152/jn.2002.88.4.1830. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol. 2005;481:127–144. doi: 10.1002/cne.20348. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Relationship between axonal collateralization and neuronal degeneration in basal ganglia. J Neural Transm Suppl. 2006a:85–88. doi: 10.1007/978-3-211-45295-0_14. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J Comp Neurol. 2006b;496:202–213. doi: 10.1002/cne.20925. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TPS, Cowan WM. A study of thalamo-striate relations in the monkey. Brain. 1956;79:364–390. doi: 10.1093/brain/79.2.364. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB. Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Rea K, Cremers TI, Westerink BH. HPLC conditions are critical for the detection of GABA by microdialysis. J Neurochem. 2005;94:672–679. doi: 10.1111/j.1471-4159.2005.03218.x. [DOI] [PubMed] [Google Scholar]
- Royce GJ. Single thalamic neurons which project to both the rostral cortex and caudate nucleus studied with the fluorescent double labeling method. Exp Neurol. 1983;79:773–784. doi: 10.1016/0014-4886(83)90041-9. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992a;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Smith Y, Bolam JP. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol. 1992b;320:228–242. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- Sidibe M, Bevan MD, Bolam JP, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: I. Topography and synaptic organization of the pallidothalamic projection. J Comp Neurol. 1997;382:323–347. [PubMed] [Google Scholar]
- Sidibe M, Pare JF, Smith Y. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J Comp Neurol. 2002;447:286–299. doi: 10.1002/cne.10247. [DOI] [PubMed] [Google Scholar]
- Sidibe M, Smith Y. Differential synaptic innervation of striatofugal neurones projecting to the internal or external segments of the globus pallidus by thalamic afferents in the squirrel monkey. J Comp Neurol. 1996;365:445–465. doi: 10.1002/(SICI)1096-9861(19960212)365:3<445::AID-CNE8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sidibe M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89:1189–1208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- Skidmore FM, Rodriguez RL, Fernandez HH, Goodman WK, Foote KD, Okun MS. Lessons learned in deep brain stimulation for movement and neuropsychiatric disorders. CNS Spectr. 2006;11:521–536. doi: 10.1017/s1092852900013559. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus) Neuroscience. 1986;18:347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Chen H, Morikawa H. Recurrent Inhibitory Network among Striatal Cholinergic Interneurons. J. Neurosci. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Sutherland AN. Using animal models to develop therapeutics for Tourette Syndrome. Pharmacol Ther. 2005;108:281–293. doi: 10.1016/j.pharmthera.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Takeda A, Sotogaku N, Oku N. Influence of manganese on the release of neurotransmitters in rat striatum. Brain Res. 2003;965:279–282. doi: 10.1016/s0006-8993(02)04157-4. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Visser-Vandewalle V, Ackermans L, van der Linden C, Temel Y, Tijssen MA, Schruers KR, Nederveen P, Kleijer M, Boon P, Weber W, Cath D. Deep brain stimulation in Gilles de la Tourette’s syndrome. Neurosurgery. 2006;58:E590. doi: 10.1227/01.NEU.0000207959.53198.D6. [DOI] [PubMed] [Google Scholar]
- Visser-Vandewalle V, Temel Y, van der Linden C, Ackermans L, Beuls E. Deep brain stimulation in movement disorders. The applications reconsidered. Acta Neurol Belg. 2004;104:33–36. [PubMed] [Google Scholar]
- Vogt C, Vogt O. Thalamusstudien I-III: I. Zur Einführung. II. Homogenität and Grenzgestaltung der Grisea des Thalamus. III. Das Griseum centrale (Centrum medianum Luys) J Psychol Neurol. 1941;50:32–154. [Google Scholar]
- Wichmann T. A digital averaging method for removal of stimulus artifacts in neurophysiologic experiments. J Neurosci Methods. 2000;98:57–62. doi: 10.1016/s0165-0270(00)00190-4. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Origins of post synaptic potentials evoked in spiny neostriatal projection neurons by thalamic stimulation in the rat. Exp Brain Res. 1983;51:217–226. doi: 10.1007/BF00237197. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuereb JH, Perry RH, Candy JM, Perry EK, Marshall E, Bonham JR. Nerve cell loss in the thalamus in Alzheimer’s disease and Parkinson’s disease. Brain. 1991;114:1363–1379. [PubMed] [Google Scholar]
- Zackheim J, Abercrombie ED. Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience. 2005;131:423–436. doi: 10.1016/j.neuroscience.2004.11.006. [DOI] [PubMed] [Google Scholar]