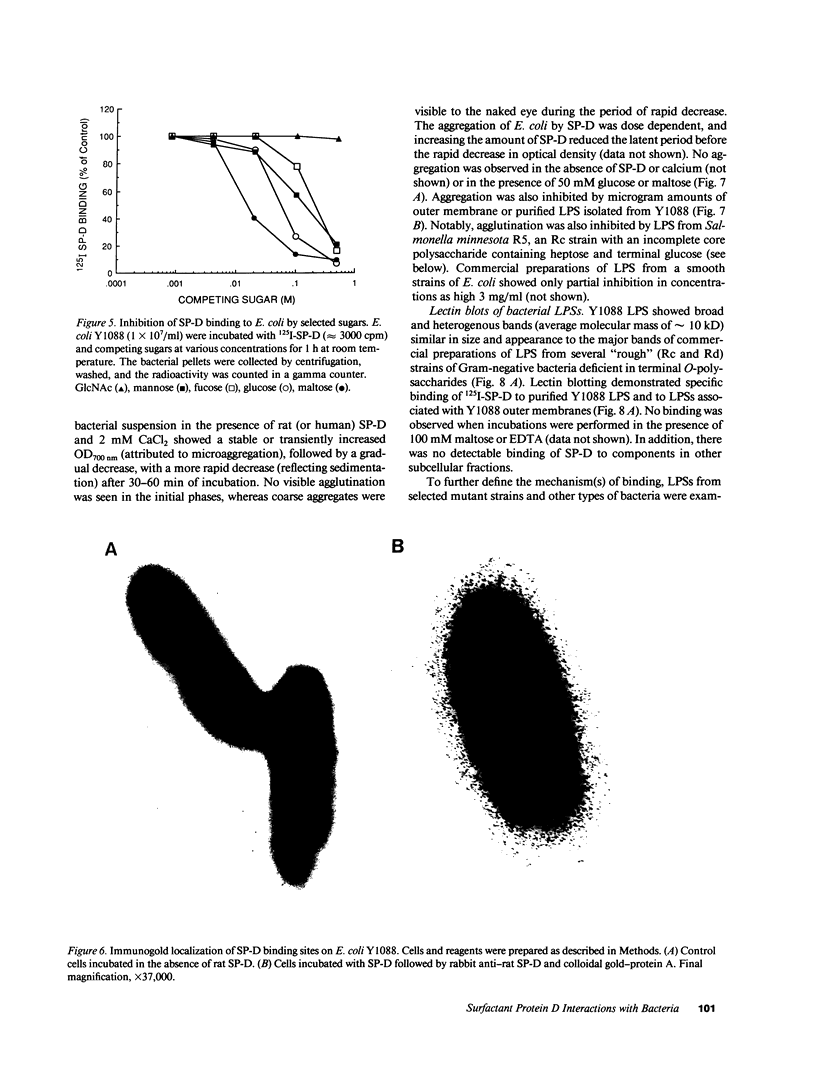
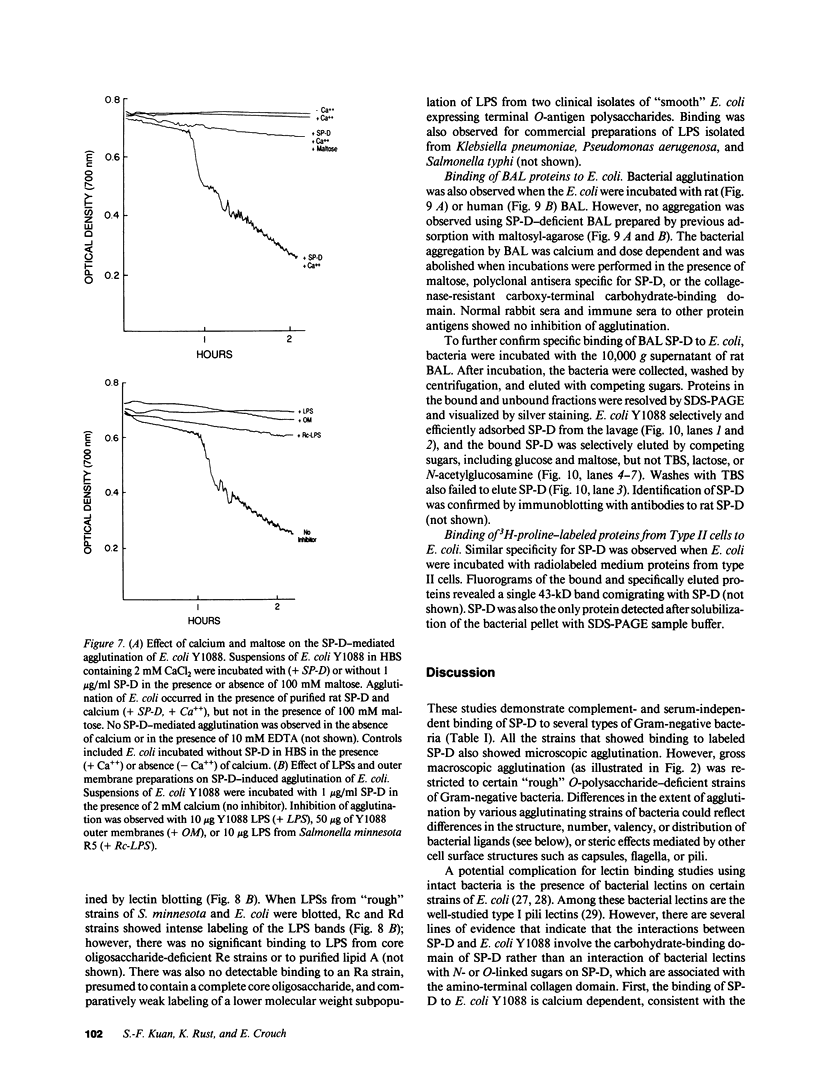
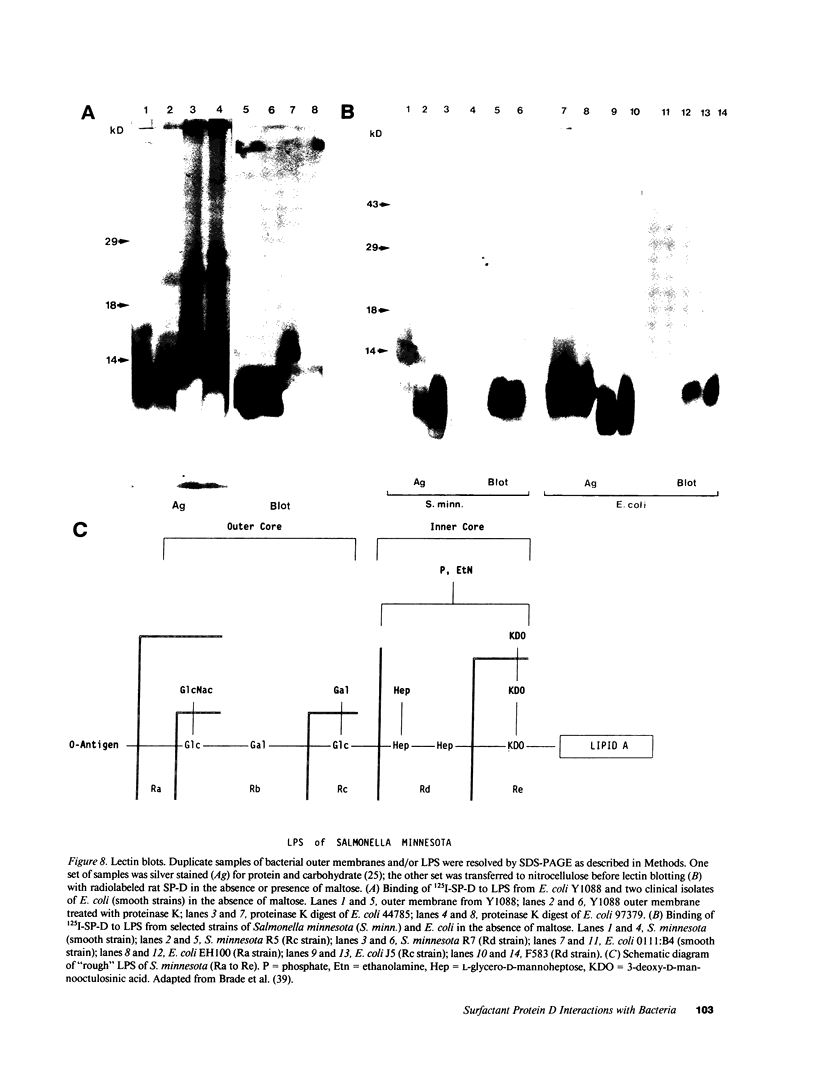
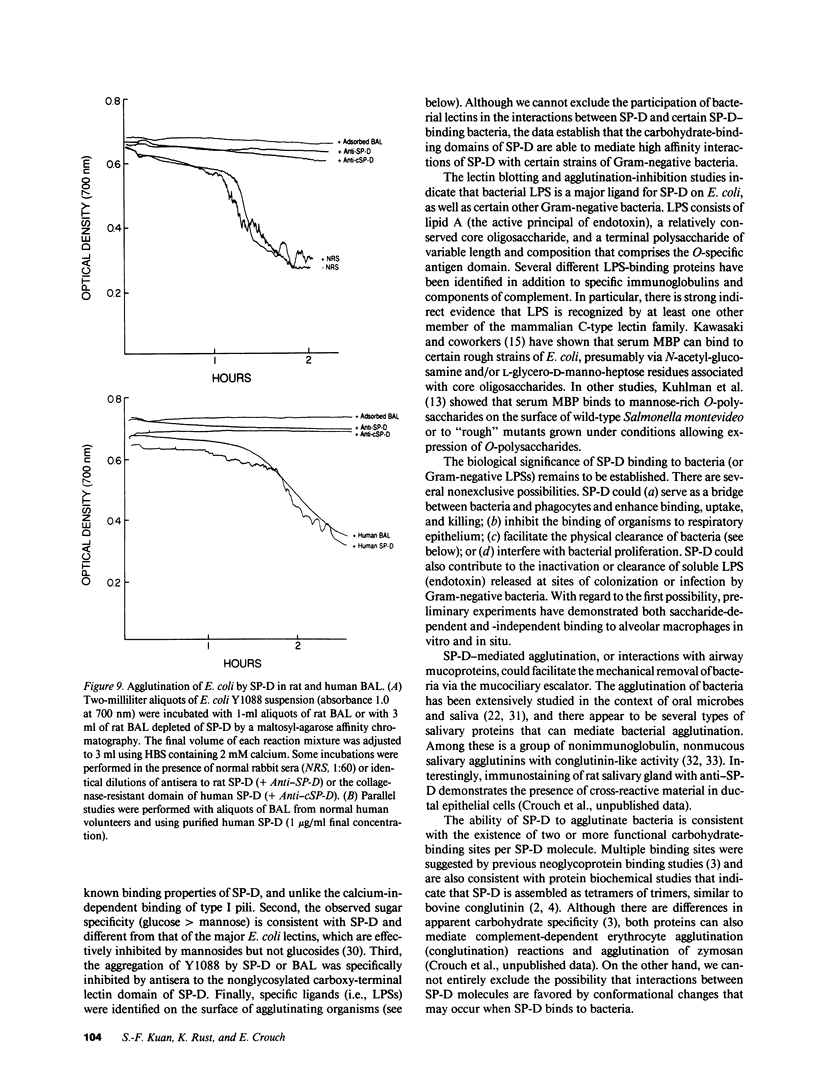
Abstract
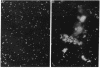
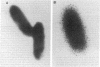
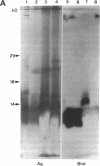
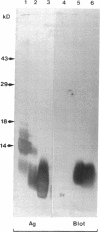
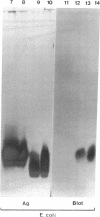
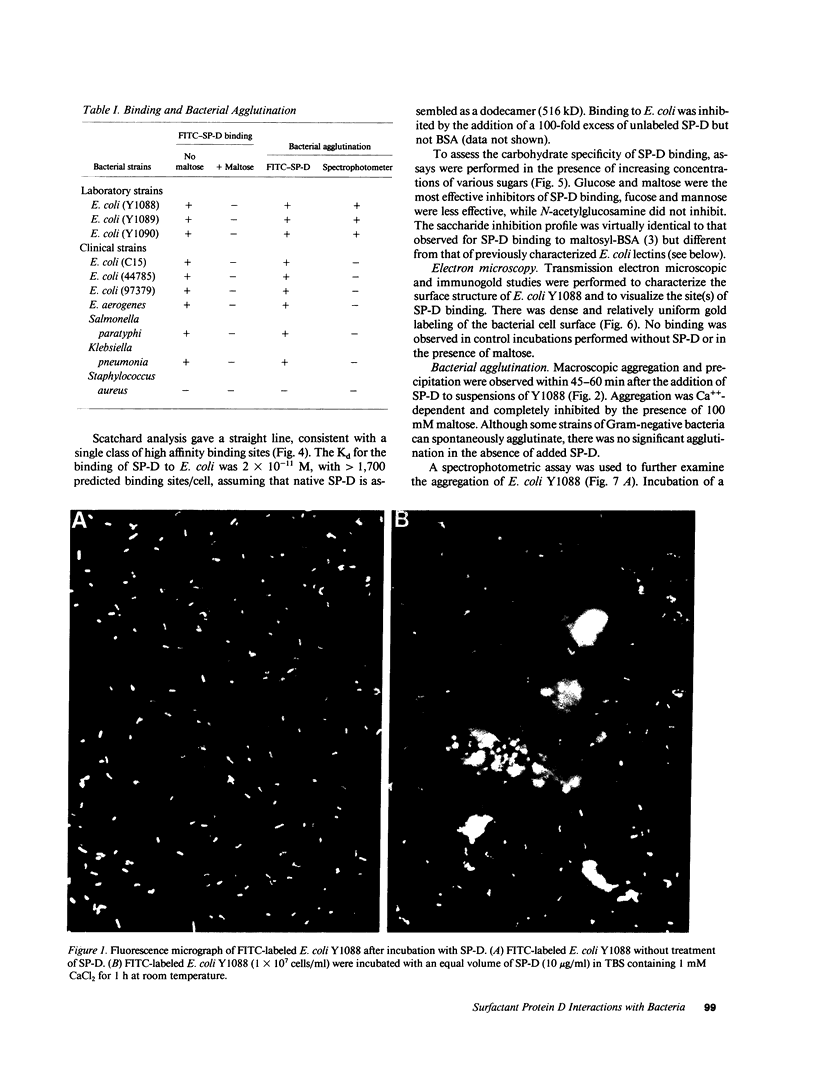
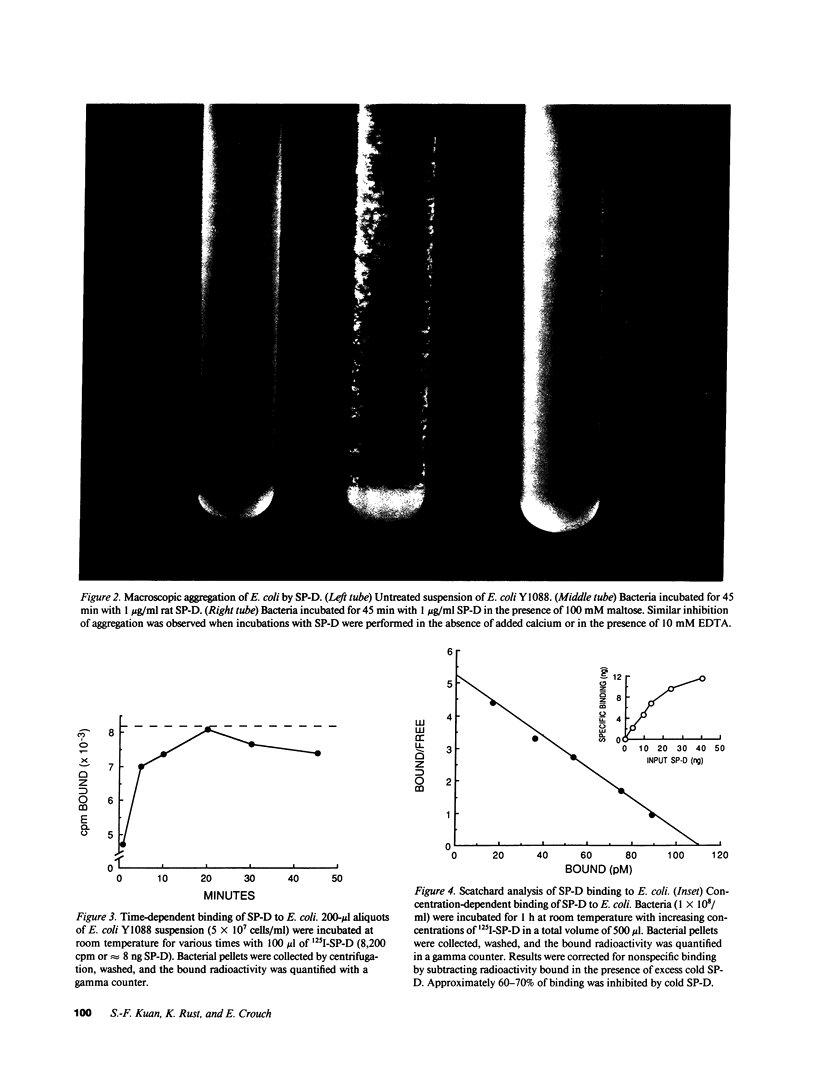
Surfactant protein D (SP-D) is a collagenous glycoprotein that is secreted into the pulmonary airspaces by alveolar type II and nonciliated bronchiolar cells. SP-D exhibits Ca(++)-dependent carbohydrate binding in vitro and is structurally related to the collagenous C-type lectins, including serum conglutinin, serum mannose-binding proteins, and surfactant protein A. Preliminary studies showed calcium- and saccharide-dependent binding of fluorescein-conjugated or radioiodinated SP-D to a variety of microorganisms, including Gram-negative bacteria and fungi. A laboratory strain of Escherichia coli (Y1088) was chosen to further examine the mechanism(s) of binding. Binding of SP-D to Y1088 was time dependent, saturable, and inhibited by cold SP-D or competing saccharides; Scatchard analysis gave a Kd of 2 x 10(-11) M. At higher concentrations, SP-D also caused Ca(++)-dependent agglutination of Y1088 that was inhibited by alpha-glucosyl-containing saccharides, antisera to the carbohydrate-binding domain of SP-D, or Y1088 LPS. Lectin blots showed specific binding of 125I-SP-D to Y1088 LPS, as well as LPS from other several strains of enteric Gram-negative bacteria. Immunogold studies demonstrated strong and uniform surface labeling of the bacteria. Rat and human bronchoalveolar lavage (BAL) caused Ca(++)-dependent agglutination of E. coli that was dose dependent and inhibited by competing saccharides or anti-SP-D. SP-D was selectively and efficiently adsorbed from rat BAL by incubation with E. coli, and incubation of E. coli with radiolabeled rat type II cell medium revealed that SP-D is the major E. coli-binding protein secreted by freshly isolated cells in culture. We suggest that SP-D plays important roles in the lung's defense against Gram-negative bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Sun D., Dale J. B., Beachey E. H. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988 Dec 15;336(6200):682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- Babu J. P., Abraham S. N., Dabbous M. K., Beachey E. H. Interaction of a 60-kilodalton D-mannose-containing salivary glycoprotein with type 1 fimbriae of Escherichia coli. Infect Immun. 1986 Oct;54(1):104–108. doi: 10.1128/iai.54.1.104-108.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Crouch E., Persson A., Chang D., Parghi D. Surfactant protein D. Increased accumulation in silica-induced pulmonary lipoproteinosis. Am J Pathol. 1991 Oct;139(4):765–776. [PMC free article] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Lachmann P. J. Bovine conglutinin is a collagen-like protein. Biochemistry. 1984 May 8;23(10):2139–2144. doi: 10.1021/bi00305a006. [DOI] [PubMed] [Google Scholar]
- Dougan G., Dowd G., Kehoe M. Organization of K88ac-encoded polypeptides in the Escherichia coli cell envelope: use of minicells and outer membrane protein mutants for studying assembly of pili. J Bacteriol. 1983 Jan;153(1):364–370. doi: 10.1128/jb.153.1.364-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Eggert F. M. The nature of secretory agglutinins and aggregating factors. I. Secretory conglutinin-like factor, secretory bacterial aggregating factors and secretory IgA antibody in human saliva and amniotic fluid. Int Arch Allergy Appl Immunol. 1980;61(2):192–202. doi: 10.1159/000232433. [DOI] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Day L. E., Herman G. A. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J Exp Med. 1988 Mar 1;167(3):1034–1046. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomsgaard A., Conrad R. S., Galanos C., Shand G. H., Høiby N. Comparative immunochemistry of lipopolysaccharides from typable and polyagglutinable Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis. J Clin Microbiol. 1988 May;26(5):821–826. doi: 10.1128/jcm.26.5.821-826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Christiansen P., Thiel S., Svehag S. E., Dessau R., Svendsen P., Andersen O., Laursen S. B., Jensenius J. C. In vivo and in vitro antibacterial activity of conglutinin, a mammalian plasma lectin. Scand J Immunol. 1990 Apr;31(4):453–460. doi: 10.1111/j.1365-3083.1990.tb02792.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf U., Möller B., Stemerowicz R., Lobeck H., Rodloff A., Freudenberg M., Galanos C., Huhn D. Relation between Escherichia coli R(rough)-forms in gut, lipid A in liver, and primary biliary cirrhosis. Lancet. 1989 Dec 16;2(8677):1419–1422. doi: 10.1016/s0140-6736(89)92034-5. [DOI] [PubMed] [Google Scholar]
- INGRAM D. G., BARNUM D. A. FLUCTUATIONS IN THE LEVEL OF CONGLUTININ IN BOVINE SERUM. Can Vet J. 1965 Jul;6(7):162–169. [PMC free article] [PubMed] [Google Scholar]
- Johnson D. B. The stability of immobilized enzymes. Biochem Soc Trans. 1979 Feb;7(1):7–10. doi: 10.1042/bst0070007. [DOI] [PubMed] [Google Scholar]
- Kawasaki N., Kawasaki T., Yamashina I. A serum lectin (mannan-binding protein) has complement-dependent bactericidal activity. J Biochem. 1989 Sep;106(3):483–489. doi: 10.1093/oxfordjournals.jbchem.a122878. [DOI] [PubMed] [Google Scholar]
- Kuhlman M., Joiner K., Ezekowitz R. A. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989 May 1;169(5):1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. M., Leiby K. R., Allar J., Paris K., Lerch B., Okarma T. B. Primary structure of bovine conglutinin, a member of the C-type animal lectin family. J Biol Chem. 1991 Feb 15;266(5):2715–2723. [PubMed] [Google Scholar]
- McCallus D. E., Norcross N. L. Antibody specific for Escherichia coli J5 cross-reacts to various degrees with an Escherichia coli clinical isolate grown for different lengths of time. Infect Immun. 1987 May;55(5):1042–1046. doi: 10.1128/iai.55.5.1042-1046.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Sharon N. Adhesins as lectins: specificity and role in infection. Curr Top Microbiol Immunol. 1990;151:91–113. doi: 10.1007/978-3-642-74703-8_5. [DOI] [PubMed] [Google Scholar]
- Ohta M., Okada M., Yamashina I., Kawasaki T. The mechanism of carbohydrate-mediated complement activation by the serum mannan-binding protein. J Biol Chem. 1990 Feb 5;265(4):1980–1984. [PubMed] [Google Scholar]
- Persson A., Chang D., Crouch E. Surfactant protein D is a divalent cation-dependent carbohydrate-binding protein. J Biol Chem. 1990 Apr 5;265(10):5755–5760. [PubMed] [Google Scholar]
- Persson A., Chang D., Rust K., Moxley M., Longmore W., Crouch E. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry. 1989 Jul 25;28(15):6361–6367. doi: 10.1021/bi00441a031. [DOI] [PubMed] [Google Scholar]
- Persson A., Rust K., Chang D., Moxley M., Longmore W., Crouch E. CP4: a pneumocyte-derived collagenous surfactant-associated protein. Evidence for heterogeneity of collagenous surfactant proteins. Biochemistry. 1988 Nov 15;27(23):8576–8584. doi: 10.1021/bi00423a011. [DOI] [PubMed] [Google Scholar]
- Rankin J. A. Pulmonary immunology. Clin Chest Med. 1988 Sep;9(3):387–393. [PubMed] [Google Scholar]
- Rundegren J. Calcium-dependent salivary agglutinin with reactivity to various oral bacterial species. Infect Immun. 1986 Jul;53(1):173–178. doi: 10.1128/iai.53.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust K., Grosso L., Zhang V., Chang D., Persson A., Longmore W., Cai G. Z., Crouch E. Human surfactant protein D: SP-D contains a C-type lectin carbohydrate recognition domain. Arch Biochem Biophys. 1991 Oct;290(1):116–126. doi: 10.1016/0003-9861(91)90597-c. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Robinson S. L., Borchelt J., Wright J. R. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989 Aug 15;264(23):13923–13928. [PubMed] [Google Scholar]
- Thiel S., Reid K. B. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989 Jun 19;250(1):78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- Thurston J. R., Kehrli M. E., Jr, Driftmier K., Deyoe B. L. Detection of conglutinin in bovine serum by complement-dependent agglutination of Escherichia coli. Vet Immunol Immunopathol. 1989 Jun;21(2):207–212. doi: 10.1016/0165-2427(89)90068-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Weiss J., Hutzler M., Kao L. Environmental modulation of lipopolysaccharide chain length alters the sensitivity of Escherichia coli to the neutrophil bactericidal/permeability-increasing protein. Infect Immun. 1986 Feb;51(2):594–599. doi: 10.1128/iai.51.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K. Genetics of adhesive fimbriae of intestinal Escherichia coli. Curr Top Microbiol Immunol. 1990;151:29–53. doi: 10.1007/978-3-642-74703-8_2. [DOI] [PubMed] [Google Scholar]
- van Iwaarden F., Welmers B., Verhoef J., Haagsman H. P., van Golde L. M. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990 Jan;2(1):91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]