Abstract
Aims
To compare the efficacy [low-density lipoprotein cholesterol (LDL-C) lowering] and safety of alirocumab, a fully human monoclonal antibody to proprotein convertase subtilisin/kexin 9, compared with ezetimibe, as add-on therapy to maximally tolerated statin therapy in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia.
Methods and results
COMBO II is a double-blind, double-dummy, active-controlled, parallel-group, 104-week study of alirocumab vs. ezetimibe. Patients (n = 720) with high cardiovascular risk and elevated LDL-C despite maximal doses of statins were enrolled (August 2012–May 2013). This pre-specified analysis was conducted after the last patient completed 52 weeks. Patients were randomized to subcutaneous alirocumab 75 mg every 2 weeks (plus oral placebo) or oral ezetimibe 10 mg daily (plus subcutaneous placebo) on a background of statin therapy. At Week 24, mean ± SE reductions in LDL-C from baseline were 50.6 ± 1.4% for alirocumab vs. 20.7 ± 1.9% for ezetimibe (difference 29.8 ± 2.3%; P < 0.0001); 77.0% of alirocumab and 45.6% of ezetimibe patients achieved LDL-C <1.8 mmol/L (P < 0.0001). Mean achieved LDL-C at Week 24 was 1.3 ± 0.04 mmol/L with alirocumab and 2.1 ± 0.05 mmol/L with ezetimibe, and were maintained to Week 52. Alirocumab was generally well tolerated, with no evidence of an excess of treatment-emergent adverse events.
Conclusion
In patients at high cardiovascular risk with inadequately controlled LDL-C, alirocumab achieved significantly greater reductions in LDL-C compared with ezetimibe, with a similar safety profile.
Trial registration
clinicaltrials.gov Identifier: NCT01644188.
Keywords: Alirocumab, Ezetimibe, Low-Density Lipoprotein Cholesterol, Monoclonal antibody
See page 1146 for the editorial comment on this article (doi:10.1093/eurheartj/ehv056)
Introduction
Hypercholesterolaemia is a major risk factor for the development of atherosclerosis and coronary heart disease (CHD). Reducing low-density lipoprotein cholesterol (LDL-C) with statins lowers the risk of CHD events and all-cause mortality1 and there is a clear relation between the degree of absolute LDL-C lowering and the degree of cardiovascular event reduction.2 Consequently, LDL-C reduction is the primary target to reduce cardiac events.3,4 Comparative data of intensive vs. standard-dose statin treatment suggest that the lower the LDL-C concentration, the greater the benefit in high cardiovascular risk patients.2 The recommended treatment target for LDL-C is <2.6 mmol/L (<100 mg/dL) in patients at high risk and <1.8 mmol/L (<70 mg/dL), or a ≤50% reduction from baseline, in those at very high risk.4–6 Despite more widespread use of intensive statin therapy, a substantial proportion of high-risk hypercholesterolaemic patients do not achieve adequate LDL-C reduction.7,8 While the latest US guidelines emphasize the use of intensive statin therapy, they call for evidence for new lipid-modifying agents to determine the incremental cardiovascular disease event-reduction benefits on top of statin therapy.9 Such therapies include fully human monoclonal antibodies against proprotein convertase subtilisin/kexin 9 (PCSK9), including alirocumab (formerly SAR236553/REGN727) and evolocumab.10,11
Alirocumab reduces LDL-C concentrations by 40–70% in combination with other lipid-lowering therapies (LLT) or as monotherapy.12–14 Guided by these very large reductions, even in combination with concomitant LLT, the COMBO II study (NCT01644188) was designed to test the hypothesis of the superiority of alirocumab vs. ezetimibe in LDL-C reduction in patients at high risk for cardiovascular events and who require additional pharmacological management because their current statin therapy failed to achieve their LDL-C treatment goal. The selection of doses, dosing frequency, and dose-increase approach was based on the LDL-C reduction needed to provide the best achievement of the target LDL-C level at the lowest dose.
Methods
COMBO II is an ongoing double-blind, double-dummy, active-controlled, parallel-group, 104-week study of alirocumab vs. ezetimibe in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins.15 The study was conducted at 126 sites (Europe, Israel, North America, South Africa, South Korea) (Supplementary material online, Text S1), with enrolment from August 2012 to May 2013. Results are presented from a pre-specified analysis, including final efficacy results up to Week 52 and safety data up to the date of the last patient Week 52 visit.
The trial methods have been published.15 The principal study criteria are shown in Supplementary material online, Table S1.15 Patients were to have hypercholesterolaemia and established CHD or CHD risk-equivalents (ischaemic stroke, peripheral artery disease, moderate chronic kidney disease, or diabetes mellitus plus ≥2 additional risk factors), and be treated with a maximally tolerated dose of statin therapy [i.e. rosuvastatin 20/40 mg, atorvastatin 40/80 mg, or simvastatin 80 mg (if on this dose for >1 year)] or on a lower dose provided the reason for doing so was documented. Statin dose had to be stable for ≥4 weeks before the screening visit and use of other LLT was not permitted. At screening, patients with documented cardiovascular disease (CVD) and LDL-C ≥1.8 mmol/L (≥70 mg/dL) or no documented history of CVD but who were at high cardiovascular risk and had LDL-C ≥2.6 mmol/L (≥100 mg/dL) were eligible to participate.
The study was performed in accordance with the principles of the Declaration of Helsinki and all applicable amendments by the World Medical Assemblies, and the International Conference on Harmonization Guidelines for Good Clinical Practice. The protocol was approved by the institutional review boards of participating centres. All participants gave written informed consent.
Intervention
Eligible patients entered a screening period of up to 3 weeks before randomization during which they were trained to self-inject using a prefilled pen (autoinjector), vital signs were taken, a 12-lead electrocardiogram was performed, and fasting blood and urine samples were obtained.
LDL-C was calculated using the Friedewald formula.16 For patients whose triglycerides exceeded 4.5 mmol/L, the central laboratory automatically measured LDL-C using the beta-quantification method (Medpace Reference Laboratories; Cincinnati, OH, USA; Leuven, Belgium; Singapore). LDL-C was also measured at Weeks 0 and 24. Other lipid parameters [total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, apolipoprotein B, and lipoprotein a] were measured directly by the central laboratory (Medpace Reference Laboratories).
Eligible patients were randomized to alirocumab or ezetimibe through an interactive voice response system (ALMAC company), using a permuted-block design with a 2:1 allocation ratio. To attain balance between arms for factors that may have influenced treatment response, patients were stratified according to history of myocardial infarction or ischaemic stroke, intensity of statin treatment, and geographic region. After randomization, patients entered a double-blind, double-dummy treatment period lasting 104 weeks. Patients were randomized to either subcutaneous (SC) alirocumab 75 mg (in 1 mL volume) every 2 weeks (Q2W) (plus oral placebo for ezetimibe daily) or 10 mg oral ezetimibe daily (plus placebo SC Q2W for alirocumab) and continued to receive their background statin therapy. The dose in the alirocumab arm (only) was automatically increased, per protocol, at Week 12 to 150 mg Q2W (1 mL volume) if the Week-8 LDL-C value was ≥1.8 mmol/L. Investigators and patients remained blinded to any dose increase. The study is ongoing at the time of writing, and randomized treatment will continue until Week 104, followed by an 8-week post-treatment observational period.
Patients were instructed to remain on a stable diet [National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) therapeutic lifestyle changes diet17 or equivalent] and to maintain the same daily statin dose throughout the study.
Outcome measures
The primary endpoint was percent change in calculated LDL-C from baseline to Week 24, using all LDL-C values from Week 24 regardless of adherence to treatment [intent-to-treat (ITT) approach]. Principal secondary efficacy endpoints included: percent change in calculated LDL-C from baseline to Week 24 (on-treatment analysis), and from baseline to Weeks 12 (ITT/on-treatment analysis) or 52 (ITT analysis); percent change in Apolipoprotein B, non-HDL-C, total cholesterol, lipoprotein a, HDL-C, fasting triglycerides, and Apolipoprotein A-1 from baseline to Week 24 (ITT analysis), and proportion of patients reaching calculated LDL-C <1.8 mmol/L at week 24 (ITT/on-treatment analysis).15
Safety was assessed by analysing adverse-event reports (including adjudicated cardiovascular events and serious adverse events) and laboratory analyses from the time of signed informed consent until the end of the study. This process is described in detail elsewhere.15 Laboratory analyses for all safety parameters, except lipids, were performed by a central laboratory (COVANCE Laboratories; Indianapolis, IN, USA; Geneva, Switzerland). In this analysis, all safety events were analysed through the date of the database lock.
Statistical analyses
We estimated that a sample of 96 participants would have 95% power to detect a difference in mean percent change in LDL-C of 20% at a significance level of 0.05 for a 2-sided test, assuming a common standard deviation of 25% and all 96 patients having an evaluable primary endpoint. However, the sample size was set at 660 (2:1 randomization) to better assess the safety of alirocumab in the context of this study and in the overall integrated safety database of the ODYSSEY program.
The population for the primary efficacy analysis comprised randomized patients with a calculated LDL-C value at baseline and at least one of the planned time-points from Weeks 4 to 24, regardless of treatment adherence (ITT population). The analysis was conducted after the last patient completed the 52-week treatment period. The primary endpoint was analysed using a mixed effect model with a repeated measures (MMRM) approach to account for missing data. All available post-baseline data at planned time-points from Week 4 to 52 regardless of status on- or off-treatment were used in the MMRM for the ITT analysis, with the model used to provide least-squares (LS) mean estimates and comparison between treatment arms of LDL-C reductions at week 24. The models included fixed categorical effects of treatment group, randomization strata, time-point, treatment-by-time-point interaction, and strata-by-time-point interaction, as well as the continuous fixed covariates of baseline LDL-C value and baseline value-by-time-point interaction.
A hierarchical procedure was used to control type I error and to handle multiple secondary endpoint analyses. Because the primary endpoint analysis (ITT) was significant at the 5% alpha level, key secondary efficacy endpoints were tested sequentially.15 LDL-C reduction at Week 24 was analysed ‘on treatment’ in the pre-specified modified ITT (mITT) population (i.e. all patients in the ITT population who had an evaluable primary efficacy endpoint while on treatment, defined as the period between first dose of study treatment up to 21 days after last injection, or 3 days after taking the last capsule, whichever came first). For the on-treatment analysis, all available on-treatment measurements (i.e. up to 21 days after last injection or 3 days after the last capsule, whichever came first) at planned time-points from Weeks 4 to 52 were used in the MMRM. A sensitivity analysis, based on a pattern mixture model, was conducted to evaluate the impact of missing data on the primary endpoint; in this approach, missing calculated LDL-C values during the ‘on-treatment’ period were multiply imputed using a model assuming ‘missing at random’ and missing calculated LDL-C values during the post-treatment period were multiply imputed using random draws from a normal distribution where the mean was equal to subject's own baseline value.
Secondary endpoints comprising continuous variables with a normal distribution were analysed using the MMRM model. Those secondary endpoints with a non-normal distribution (lipoprotein a and triglycerides) and the binary (non-continuous) variable secondary endpoints were analysed using a multiple imputation approach for handling of missing values followed by robust regression (for lipoprotein a and triglycerides) or logistic regression (for the binary endpoints).
Safety analyses used a pre-specified cut-off corresponding to the last patient visit at Week 52 and included all data collected between 52 and 104 weeks. Data are reported descriptively based on data from randomized patients who received at least one dose or partial (in the event that <1 mL was injected) dose of study treatment.
The analysis was performed using SAS version 9.2 software (SAS Institute Inc., Cary, NC, USA).
Results
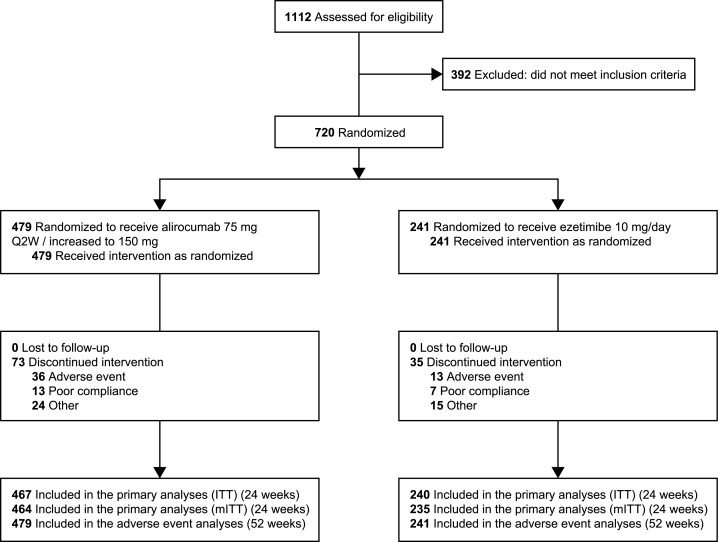
We screened 1112 high cardiovascular risk patients, 720 of whom were eligible and consented to participate (Figure 1). Of these, 479 were randomly assigned to alirocumab and 241 to ezetimibe. The mean ± standard deviation (SD) age was 61.6 ± 9.3 years, 73.6% of participants were men, 90.1% had CHD, and 30.7% had type 2 diabetes mellitus. Mean ± SD body mass index (BMI) was 30.3 ± 5.1 kg/m2 and 113 (n = 46.9%) had a BMI ≥30 kg/m2. The mean ± SD baseline calculated LDL-C concentration was 2.8 ± 0.9 mmol/L; 66.7% (n = 480) were taking atorvastatin 40/80 mg/day or rosuvastatin 20/40 mg/day, and 2.1% (n = 15) were on simvastatin 80 mg. The reasons documented for taking a lower dose of statin are detailed in Supplementary material online, Table S2. Baseline characteristics were balanced between the two groups (Table 1).
Figure 1.
Patient flow chart.
Table 1.
Baseline characteristics (all randomized patients)a
| Characteristic | Alirocumabb (n = 479) | Ezetimibec (n = 241) |
|---|---|---|
| Age (years) | 61.7 ± 9.4 | 61.3 ± 9.2 |
| Men | 360 (75.2) | 170 (70.5) |
| Raced | ||
| White | 404 (84.3) | 206 (85.5) |
| Black or African American | 21 (4.4) | 7 (2.9) |
| Othere | 54 (11.3) | 28 (11.6) |
| Body mass index (kg/m2) | 30.0 ± 5.4 | 30.3 ± 5.1 |
| Cardiovascular history and risk factors | ||
| Any cardiovascular history/risk factor(s) | 477 (99.6) | 241 (100) |
| Coronary heart disease | 437 (91.2) | 212 (88.0) |
| Acute myocardial infarction | 277 (57.8) | 139 (57.7) |
| Silent myocardial infarction | 11 (2.3) | 4 (1.7) |
| Unstable angina | 106 (22.1) | 46 (19.1) |
| Coronary revascularization procedure | 330 (68.9) | 165 (68.5) |
| Other clinically significant CHD | 184 (38.4) | 82 (34.0) |
| CHD associated with ≥1 comorbidity (among hypertension, diabetes or moderate CKD) and/or associated with other CVD (ischaemic stroke, peripheral artery disease) | 366 (76.4) | 178 (73.9) |
| Coronary heart disease risk-equivalent | 151 (31.5) | 72 (29.9) |
| Ischaemic stroke | 40 (8.4) | 20 (8.3) |
| Peripheral artery disease | 24 (5.0) | 11 (4.6) |
| Moderate CKD | 61 (12.7) | 23 (9.5) |
| Diabetes mellitus plus ≥2 additional risk factors | 59 (12.3) | 31 (12.9) |
| ≥2 CHD risk-equivalents or 1 CHD risk-equivalent associated with hypertension or diabetes | 141 (29.4) | 67 (27.8) |
| Diabetes mellitus type 1 | 2 (0.4) | 0 |
| Diabetes mellitus type 2 | 145 (30.3) | 76 (31.5) |
| Laboratory values | ||
| HbA1c (%) | 6.05 ± 0.75 | 6.07 ± 0.77 |
| Lipid parameters | ||
| LDL-C (Friedewald formula) (mmol/L) | 2.8 ± 0.9 | 2.7 ± 0.9 |
| Range | 0.6–7.9 | 1.0–6.3 |
| Apolipoprotein B (g/L) | 0.9 ± 0.2 | 0.9 ± 0.2 |
| Total cholesterol (mmol/L) | 4.8 ± 1.1 | 4.8 ± 1.1 |
| Non-HDL-C (mmol/L) | 3.6 ± 1.0 | 3.5 ± 1.0 |
| Lipoprotein a (mmol/L) | 1.0 (0.3, 2.5) | 0.8 (0.3, 2.0) |
| Triglycerides (fasted) (mmol/L) | 1.5 (1.1, 2.2) | 1.6 (1.2, 2.3) |
| HDL-C (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.4 |
| C-reactive protein (nmol/L) | 34.1 ± 74.1 | 34.6 ± 51.1 |
| Statin therapy at randomization | 478 (99.8) | 241 (100) |
| Taking high-intensity statinf | 320 (66.8) | 160 (66.4) |
| Atorvastatin | 237 (49.5) | 118 (49.0) |
| Rosuvastatin | 137 (28.6) | 75 (31.1) |
| Simvastatin | 105 (21.9) | 49 (20.3) |
Data are mean ± SD, n (%), or median (interquartile range) unless otherwise stated. To convert cholesterol measurements to mg/dL, divide by 0.02586; and to convert triglycerides measurements to mg/dL, divide by 0.01129.
CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; HbA1c, glycated haemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SC, subcutaneous; SD, standard deviation; Q2W, every 2 weeks.
aThere were no clinically or statistically significant between-group differences.
bAlirocumab 75 mg SC Q2W with a dose increase to 150 mg Q2W at Week 12 if Week 8 LDL-C was ≥1.8 mmol/L (≥70 mg/dL).
c10 mg/day oral ezetimibe.
dRace was self-reported.
eAsian, American Indian, Alaska Native, Other.
fHigh-intensity statin defined as 40–80 mg/day atorvastatin or 20–40 mg/day rosuvastatin.
The mean ± SD duration of injection exposure was 58.0 ± 18.7 weeks (26.6 ± 8.8 injections) in the alirocumab arm and 57.7 ± 19.0 weeks (26.6 ± 9.0 injections) in the ezetimibe arm. At the time of this analysis, 84.8% of patients in the alirocumab arm and 85.5% in the ezetimibe arm were receiving ongoing treatment (active or placebo); 18.4% (82 patients) of patients in the alirocumab arm had the dose increased at Week 12 to the 150 mg Q2W dosing regimen because their LDL-C at Week 8 was ≥1.8 mmol/L.
Efficacy
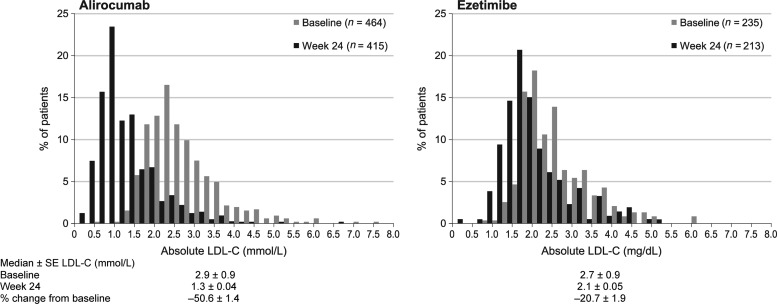
For the primary endpoint, mean ± standard error (SE) reductions in LDL-C from baseline to Week 24 were −50.6 ± 1.4% in the alirocumab arm and −20.7 ± 1.9% in the ezetimibe arm, both on a background of maximally tolerated statin therapy, with a statistically significant difference of the means ± SE between groups of −29.8 (95% CI −34.4 to −25.3, P < 0.0001) (Table 2). The results for the on-treatment analysis (Table 2 and Supplementary material online, Table S3) and the sensitivity analysis (Supplementary material online, Table S4) were consistent with the primary endpoint. The proportion of patients who achieved the target LDL-C of <1.8 mmol/L at Week 24 (ITT analysis) was 77.0% in the alirocumab arm and 45.6% in the ezetimibe arm (P < 0.0001). The distribution of baseline and achieved LDL-C values at 24 weeks is shown in Figure 2. The mean achieved LDL-C at Week 24 was 1.3 ± 0.04 mmol/L with alirocumab and 2.1 ± 0.05 mmol/L with ezetimibe.
Table 2.
Percent change from baseline to week 24 in LDL-C (ITT and on-treatment) and in secondary lipid parameters (ITT)
| All patients on maximally tolerated statin therapya | Alirocumabb | Ezetimibec | Alirocumab vs. ezetimibe |
||
|---|---|---|---|---|---|
| LS mean difference ± SE (%) | 95% CI | P-value | |||
| Primary endpoint: LDL-C | |||||
| ITT | n = 467 | n = 240 | |||
| LS mean ± SE change from baseline (%) | –50.6 ± 1.4 | –20.7 ± 1.9 | –29.8 ± 2.3 | –34.4 to –25.3 | <0.0001 |
| On-treatment | n = 464 | n = 235 | |||
| Baseline LDL-C, mean ± SD (mmol/L) | 2.8 ± 0.9 | 2.7 ± 0.9 | – | – | – |
| Range | 0.6–7.9 | 1.0–6.3 | |||
| LS mean ± SE change from baseline (%) | –52.4 ± 1.3 | –21.8 ± 1.8 | –30.6 ± 2.2 | –34.9 to –26.2 | <0.0001 |
| Secondary lipid parameters (ITT), LS mean ± SE change from baseline (%) | n = 467 | n = 240 | |||
| LDL-C (beta-quantification method)d | –47.7 ± 1.6 | –18.0 ± 2.2 | –29.7 ± 2.7 | –35.0 to –24.4 | <0.0001 |
| LDL-C (baseline to Week 12) | –51.2 ± 1.3 | –21.8 ± 1.8 | –29.4 ± 2.2 | –33.7 to –25.1 | <0.0001 |
| Apolipoprotein B | –40.7 ± 1.1 | –18.3 ± 1.5 | –22.4 ± 1.8 | –26.0 to –18.8 | <0.0001 |
| Non-HDL-C | –42.1 ± 1.2 | –19.2 ± 1.7 | –22.9 ± 2.0 | –26.9 to –18.9 | <0.0001 |
| Total cholesterol | –29.3 ± 0.9 | –14.6 ± 1.2 | –14.7 ± 1.5 | –17.7 to –11.7 | <0.0001 |
| Lipoprotein ae | –27.8 ± 1.4 | –6.1 ± 2.0 | –21.7 ± 2.4 | –26.4 to –17.0 | <0.0001 |
| HDL-C | 8.6 ± 0.8 | 0.5 ± 1.1 | 8.1 ± 1.3 | 5.4 to 10.7 | <0.0001 |
| Triglycerides (fasted)e | –13.0 ± 1.5 | –12.8 ± 2.0 | –0.3 ± 2.5 | –5.1 to 4.6 | 0.91 |
| Apolipoprotein A-1 | 5.0 ± 0.6 | –1.3 ± 0.8 | 6.3 ± 1.0 | 4.3 to 8.3 | <0.0001f |
CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; ITT, intention-to-treat; LDL-C, low-density lipoprotein cholesterol; LS, least squares; Q2W, every 2 weeks; SC, subcutaneous; SE, standard error.
aOne patient was not on maximally tolerated statin therapy.
bAlirocumab 75 mg SC Q2W with a dose increase to 150 mg Q2W at Week 12 if Week 8 LDL-C was ≥1.8 mmol/L (≥70 mg/dL).
c10 mg/day oral ezetimibe.
dSensitivity analysis conducted in 180 patients in the ezetimibe group and 361 patients in the alirocumab group. P-value for descriptive purposes only.
eCombined estimate obtained by combining adjusted means ± SE from robust regression model analyses of the different imputed data sets (multiple imputation).
fP-value for descriptive purposes only (according to the hierarchical analysis, formal analysis was stopped after triglycerides, which were not statistically significant).
Figure 2.
Distribution by 10 mg/dL increments of LDL-C concentration at baseline and at Week 24 in patients on maximally tolerated statinsa and alirocumab or ezetimibe. Comparison between Week 24 vs. baseline is descriptive and exploratory only, as data for all patients were not available at Week 24. aOne patients was not on maximally tolerated statin therapy.
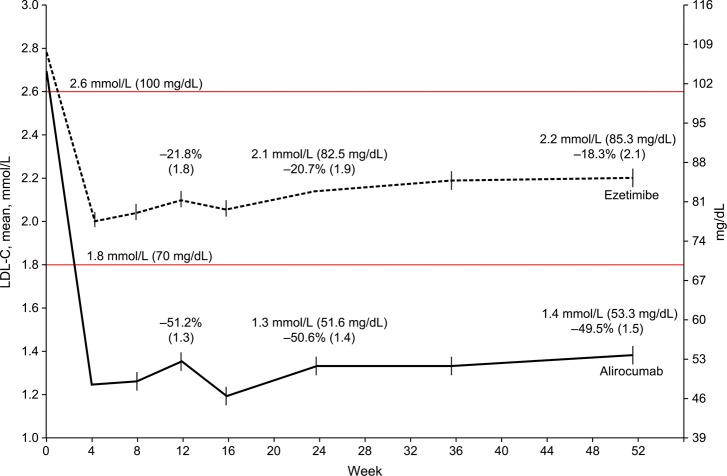
The time-course of changes in LDL-C concentrations in the alirocumab and ezetimibe arms from baseline to 52 weeks is shown in Figure 3. Mean LDL-C concentrations dropped rapidly in the first 4 weeks, but to a greater degree in the alirocumab arm. The reductions achieved by 4 weeks remained largely constant up to 52 weeks.
Figure 3.
LDL-C values achieved vs. study time-points (ITT analysis). Percentages above Weeks 12 and 24 data points indicate LS mean (SE) percent change from baseline. Values above Weeks 24 and 52 indicate achieved LDL-C.
The time-course of changes in LDL-C concentrations according to dose-increase status in the alirocumab arm is shown in Supplementary material online, Figure S1.
Percent changes in other lipid measures are shown in Table 2 and Supplementary material online, Table S3. Statistically significant mean ± SE reductions were observed for Apolipoprotein B (22.4 ± 1.8%), lipoprotein a (21.7 ± 2.4%), and non-HDL-C (22.9 ± 2.0%) (all P < 0.0001), and there was an 8.1 ± 1.3% increase in HDL-C at Week 24 in the alirocumab arm compared with ezetimibe (P < 0.0001). Triglycerides were reduced from baseline to Week 24 by 13.0 ± 1.5% in the alirocumab group and by 12.8 ± 2.0% in the ezetimibe group, but the difference between treatment arms was not statistically significant. Apolipoprotein A-1 concentrations increased in the alirocumab group and decreased in the ezetimibe group, but according to the hierarchical analysis rules, formal analysis was stopped following the non-significant difference for triglyceride reduction. C-reactive protein levels did not change over time with alirocumab and were slightly lower with ezetimibe (Supplementary material online, Table S5 and Figure S2).
Alirocumab efficacy vs. ezetimibe was consistent across several subgroups in the ITT population. The results did not differ qualitatively as a function of demographics, region, medical history, baseline total/free PCSK9 concentration, diabetes (personal history), intensity of statin treatment, or baseline lipid values (Supplementary material online, Figure S3).
Safety
Rates of treatment-emergent adverse events (TEAEs) over a mean of 58 ± 19 weeks' follow-up are shown in Table 3. The overall percentages of patients who experienced at least one TEAE were 71.2% in the alirocumab arm and 67.2% in the ezetimibe arm. A TEAE leading to death occurred in 0.4% (n = 2) of patients in the alirocumab arm (both of cardiac origin) and in 1.7% (n = 4) of patients in the ezetimibe arm (two of cardiac origin). Similar percentages of subjects in both groups experienced a serious adverse event (18.8% alirocumab vs. 17.8% ezetimibe). A higher proportion of patients in the alirocumab group experienced TEAEs leading to treatment discontinuation (7.5 vs. 5.4%), with no specific pattern in type of adverse event.
Table 3.
TEAEsa and laboratory parameters (safety population) at 52 weeks
| All patients on maximally tolerated statin therapyb | Alirocumabc (n = 479) | Ezetimibed (n = 241) |
|---|---|---|
| Any TEAE | 341 (71.2) | 162 (67.2) |
| Treatment-emergent SAE | 90 (18.8) | 43 (17.8) |
| TEAE leading to deathe | 2 (0.4) | 4 (1.7) |
| TEAE leading to treatment discontinuation | 36 (7.5) | 13 (5.4) |
| TEAEs occurring in ≥5% of patients in either group or TEAEs of interest | ||
| Accidental overdosef | 30 (6.3) | 16 (6.6) |
| Upper respiratory tract infection | 31 (6.5) | 14 (5.8) |
| Dizziness | 23 (4.8) | 13 (5.4) |
| Myalgia | 21 (4.4) | 12 (5.0) |
| Injection-site reaction | 12 (2.5) | 2 (0.8) |
| Neurocognitive disorder | 4 (0.8) | 3 (1.2) |
| Adjudicated cardiovascular events | 23 (4.8) | 9 (3.7) |
| CHD death (including undetermined cause) | 2 (0.4) | 2 (0.8) |
| Non-fatal myocardial infarction | 12 (2.5) | 3 (1.2) |
| Fatal/non-fatal ischaemic stroke (including stroke not otherwise specified) | 1 (0.2) | 1 (0.4) |
| Unstable angina requiring hospitalization | 1 (0.2) | 0 |
| Congestive heart failure requiring hospitalization | 1 (0.2) | 1 (0.4) |
| Ischaemia-driven coronary revascularization procedure | 16 (3.3) | 4 (1.7) |
| Laboratory parameters | ||
| Alanine aminotransferase >3 × ULN | 8/470 (1.7) | 1/240 (0.4) |
| Creatine kinase >3 × ULN | 13/467 (2.8) | 6/236 (2.5) |
Data are n (%) or n/N (%). CHD, coronary heart disease; Q2W, every 2 weeks; SAE, serious adverse event; SC, subcutaneous; TEAE, treatment-emergent adverse event; ULN, upper limit of normal.
aTEAEs are adverse events that developed or worsened or became serious during the TEAE period [defined as the time from the first dose of double-blind study treatment to the last injection plus 70 days (10 weeks), as the residual effect of alirocumab was expected until 10 weeks after the last injection].
bOne patient was not on maximally tolerated statin therapy.
cAlirocumab 75 mg SC Q2W with a dose increase to 150 mg Q2W at week 12 if week 8 LDL-C was ≥1.8 mmol/L (≥70 mg/dL).
d10 mg/day oral ezetimibe.
eBoth deaths in the alirocumab arm were due to cardiovascular events (cardiac arrest and sudden cardiac death). Of the four deaths in the ezetimibe arm (malignant lung neoplasm, suicide, defect conduction intraventricular plus sudden cardiac death, and sudden death—one patient was counted in two categories), two were due to cardiovascular events.
fAccidental overdose was an event suspected by the investigator or spontaneously notified by the patient (not based on systematic injection/capsule counts) and defined as at least twice the intended dose within the intended therapeutic interval (i.e. ≥2 injections from the double-blind treatment kit administered in <7 calendar days or ≥2 capsules from the double-blind treatment kit were administered within 1 calendar day).
There was no imbalance in TEAEs at the system organ class level (Supplementary material online, Table S6). The most common TEAEs (occurring in ≥5% of patients from either treatment arm) were upper respiratory tract infection, accidental overdose, dizziness, and myalgia (Table 3). Adjudicated cardiovascular events were infrequent, occurring in 4.8% (n = 23) of the alirocumab group vs. 3.7% (n = 9) in the ezetimibe group. Treatment-emergent local injection site reactions occurred in 2.5% of patients in the alirocumab arm vs. 0.8% for ezetimibe/placebo injections (Table 3). Reactions were of mild intensity, except for one of moderate intensity, and none were serious; two events led to discontinuation in the alirocumab group. Few neurocognitive events took place in either group (Table 3). Abnormalities in laboratory measurements were uncommon and occurred at similar rates in both groups. Exceptions were the incidence of elevated alanine aminotransaminase, which was more frequent in the alirocumab group, and impaired glucose control, which was less frequent in the alirocumab group (Table 3 and Supplementary material online, Table S6).
One-hundred and five (22.8% of 460) patients in the alirocumab arm and none in the ezetimibe arm had two consecutive LDL-C values <0.65 mmol/L during the treatment period. Rates of TEAEs in this group were similar to those in the ezetimibe group, with the exception of nasopharyngitis, which was more frequent in the alirocumab group (Supplementary material online, Table S6).
Discussion
In this active-controlled, double-blind trial, alirocumab demonstrated superior efficacy in reducing LDL-C concentrations compared with ezetimibe in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia over and above that seen with maximally tolerated doses of potent statins. A 50.6% reduction in LDL-C was achieved with alirocumab, with a mean difference vs. ezetimibe of 29.8%. This degree of efficacy translated to mean (SE) on-treatment LDL-C values of 1.3 ± 0.04 and 2.1 ± 0.05 mmol/L at Week 24, respectively, and a greater proportion of participants reached the treatment target of <1.8 mmol/L [or the even lower potential target of <1.3 mmol/L (<50 mg/dL)]. These results were consistent across various patient subgroups. No safety concerns were apparent in this ongoing study. The substantial difference between arms in LDL-C lowering after 24 weeks was sustained through follow-up to 52 weeks. With the recent preliminary data from the IMPROVE IT trial just presented,18 the control arm of this study could potentially now be considered the appropriate reference for any new therapy.
In current practice, 45–60% of patients on LLT7,8,19 fail to achieve the LDL-C goal (<1.8 mmol/L) per NCEP ATP III6 or European guidelines.3,4 The proportion is even lower (∼18%) for those on non-statin therapies.19 Even in randomized trials using high-dose statins with high treatment adherence, >40% of patients fail to achieve the target, leaving them at substantially increased risk of a major cardiovascular event.20,21 Initial data from the IMPROVE IT trial18 suggest that further lowering of LDL-C with the non-statin agent ezetimibe reduces cardiovascular events, but this is being studied in several large outcomes trials with other agents, including with alirocumab.22,23 The data presented here suggest that addition of alirocumab to a treatment regimen with maximally tolerated statins will provide substantial lowering of LDL-C so that many more patients can achieve LDL-C goals than by adding ezetimibe. Furthermore, the maximum LDL-C response to a PCSK9 inhibitor is greater with combination therapy, as in COMBO II, vs. monotherapy (i.e. with no background lipid-lowering therapies),12 indicating a possible additive effect, or synergy, with these two classes of drugs, as also suggested in studies involving evolocumab.11
The COMBO II study included a strategy of individualized goal attainment, with a pre-planned dose-increase in patients who failed to reach the LDL-C target by Week 8. We hypothesized that most patients would gain substantial lipid lowering (∼50%) even with the starting dose, and this proved correct. Approximately 80% of patients treated with alirocumab did not require a dose increase. Of note, the 18% alirocumab-treated patients who required a dose increase had much higher mean baseline LDL-C values vs. patients who did not require an increase. The dose increase at 12 weeks led to an additional mean reduction of 10.5% in LDL-C. Furthermore, the absolute reduction in LDL-C by Week 24 was slightly greater in the dose-increase group (1.6 vs. 1.5 mmol/L).
Alirocumab was generally well tolerated, with no evidence of an excess of TEAEs, serious adverse events, or deaths in this ongoing study. Injection site reactions occurred more frequently in the alirocumab arm; these were mild in intensity in all but one case with moderate intensity. The rate of adjudicated cardiovascular events was slightly higher with alirocumab (4.8%) vs. ezetimibe (3.7%). Cardiovascular outcomes will be assessed in an ongoing study (http://clinicaltrials.gov/show/NCT01663402)22 and in a pooled analysis from overall ODYSSEY program. This study was limited to high cardiovascular risk patients with inadequately controlled hypercholesterolaemia, but will complement the range of data emerging from the ODYSSEY program. Further research is needed to evaluate the efficacy of alirocumab in different racial groups. While the primary endpoint in this study was LDL-C reduction at 24 weeks, the study will continue up to 104 weeks to maximize available safety data and generate information on the durability of alirocumab lipid-lowering effects.15
Conclusions
In this population of high cardiovascular risk patients with inadequately controlled LDL-C on maximally tolerated doses of potent statins, alirocumab produced significantly greater reductions in LDL-C vs. ezetimibe using a dose-increase approach, with a comparable safety profile.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Sanofi and Regeneron Pharmaceuticals, Inc. and is registered on ClinicalTrial.gov ID: NCT01644188.
Conflict of interest: C.P.C. reports personal fees from Sanofi, personal fees from Regeneron Pharmaceuticals, Inc., during the conduct of the study; grants from Accumetrics, grants from Arisaph, grants from Astra Zeneca, grants from Boehringer-Ingelheim, personal fees from CSL Behring, personal fees from Essentialis, grants and personal fees from GlaxoSmithKline, grants from Janssen, grants and personal fees from Merck, grants and personal fees from Takeda, personal fees from Lipimedix, personal fees from BMS, personal fees from Pfizer, outside the submitted work. B.C. reports personal fees from Sanofi/Regeneron Pharmaceuticals, Inc., personal fees from Amgen, outside the submitted work. D.B. reports grants from Sanofi/Regeneron Pharmaceuticals, Inc., non-financial support from Sanofi/Regeneron Pharmaceuticals, Inc., during the conduct of the study; personal fees from Amgen, personal fees from Sanofi/Regeneron Pharmaceuticals, Inc., personal fees from Aegerion, personal fees from Astra Zeneca, personal fees from MSD, personal fees from Pfizer, personal fees from Servier, personal fees from Unilever, grants from Amgen, grants from Sanofi/Regeneron Pharmaceuticals, Inc., grants from Eli Lilly, grants from Novartis, grants from Aegerion, outside the submitted work. J.M.M. reports grants from Sanofi, grants from Regeneron Pharmaceuticals, Inc., outside the submitted work. C.L. is an employee and stockholder of Sanofi. R.P. is an employee and stockholder of Regeneron Pharmaceuticals, Inc. U.C. is an employee and stockholder of Sanofi. H.M.C. reports grants, personal fees and non-financial support from Sanofi/Regeneron Pharmaceuticals, Inc., during the conduct of the study; grants, personal fees, non-financial support, and other from Pfizer Inc., grants, personal fees, and non-financial support from Sanofi Aventis, grants and non-financial support from Novartis Pharmaceuticals, grants and other from Eli Lilly & Company, grants and other from Roche Pharmaceuticals, grants from Boehringer Ingelheim, grants from AstraZeneca LP, grants and other from Regeneron Pharmaceuticals, Inc., outside the submitted work.
Acknowledgements
Writing support was provided by Sophie Rushton-Smith, PhD, and was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
References
- 1.Cholesterol Treatment Trialists C Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists (CTT) Collaboration Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 4.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr., Stone NJ, National Heart L, Blood I, American College of Cardiology F, American Heart A. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 7.Banegas JR, Lopez-Garcia E, Dallongeville J, Guallar E, Halcox JP, Borghi C, Masso-Gonzalez EL, Jimenez FJ, Perk J, Steg PG, De Backer G, Rodriguez-Artalejo F. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J 2011;32:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermans MP, Castro Cabezas M, Strandberg T, Ferrieres J, Feely J, Elisaf M, Michel G, Sansoy V. Centralized Pan-European survey on the under-treatment of hypercholesterolaemia (CEPHEUS): overall findings from eight countries. Curr Med Res Opin 2010;26:445–454. [DOI] [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl. 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 10.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA, Investigators D. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 11.Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R, Investigators L-. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 12.Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara-Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: Results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol 2014;176:55–61. [DOI] [PubMed] [Google Scholar]
- 13.McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344–2353. [DOI] [PubMed] [Google Scholar]
- 14.Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891–1900. [DOI] [PubMed] [Google Scholar]
- 15.Colhoun HM, Robinson JG, Farnier M, Cariou B, Blom D, Kereiakes DJ, Lorenzato C, Pordy R, Chaudhari U. Efficacy and safety of alirocumab, a fully human PCSK9 monoclonal antibody, in high cardiovascular risk patients with poorly controlled hypercholesterolemia on maximally tolerated doses of statins: rationale and design of the ODYSSEY COMBO I and II trials. BMC Cardiovasc Disord 2014;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421. [PubMed] [Google Scholar]
- 18.Cannon CP. IMPROVE IT trial. In: American Heart Association Scientific Sessions 2014. Chicago, IL, USA, 2014. [Google Scholar]
- 19.Karalis DG, Victor B, Ahedor L, Liu L. Use of Lipid-Lowering Medications and the Likelihood of Achieving Optimal LDL-Cholesterol Goals in Coronary Artery Disease Patients. Cholesterol 2012;2012:861924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM, Jr, Ridker PM, Grundy SM, Kastelein JJ. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E, Pravastatin or Atorvastatin E, Infection Therapy-Thrombolysis in Myocardial Infarction I. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–28. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GS, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby J-F, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: Rationale and design of the ODYSSEY Outcomes trial. Am Heart J 2014:1–8.e1. [DOI] [PubMed] [Google Scholar]
- 23.Cannon CP, Giugliano RP, Blazing MA, Harrington RA, Peterson JL, Sisk CM, Strony J, Musliner TA, McCabe CH, Veltri E, Braunwald E, Califf RM, Investigators I-I. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J 2008;156:826–832. [DOI] [PubMed] [Google Scholar]