Abstract
Background:
The aim of this study was to evaluate the effect on the number of performed biopsies and costs associated with implementing positron emission tomography (PET) and computed tomography (PET/CT) with 16α-[18F]fluoro-17β-oestradiol (FES) or 2-[18F]fluoro-2-deoxy-D-glucose (FDG) as an upfront imaging test for diagnosing metastatic breast cancer (MBC) in comparison with the standard work-up in oestrogen receptor-positive women with symptoms.
Methods:
A published computer simulation model was adapted and validated. Three follow-up strategies were evaluated in a simulated cohort of women with primary breast cancer over a 5-year-time horizon: (1) the standard work-up, (2) upfront FES-PET/CT and (3) upfront FDG-PET/CT. The main outcome was the number of avoided biopsies to assess MBC. The costs for all three strategies were calculated based on the number of imaging tests and biopsies. The incremental cost-effectiveness ratio (ICER) to avoid a biopsy was calculated only based on the costs of initial imaging and staging tests.
Results:
The FES-PET/CT strategy decreased the number of biopsies by 39±9%, while upfront FDG-PET/CT increased the number of biopsies by 38±15% when compared with the standard work-up. Both PET/CT strategies reduced the number of imaging tests and false positives when compared with the standard work-up. The number of false negatives decreased only in the FES-PET/CT strategy. The ICER in the FES-PET/CT strategy per avoided biopsy was 12.1±3.4 thousand Euro. In the FDG-PET/CT strategy, the costs were higher and there were no avoided biopsies as compared with the standard work-up, hence this was an inferior strategy in terms of cost effectiveness.
Conclusions:
The number of performed biopsies was lower in the FES-PET/CT strategy at an ICER of 12.1±3.4 thousand Euro per biopsy avoided, whereas the application of the FDG-PET/CT did not reduce the number of biopsies and was more expensive. Whether the FES-PET/CT strategy has additional benefits for patients in terms of therapy management has to be evaluated in clinical studies.
Keywords: breast neoplasm, neoplasm metastasis, diagnostic imaging, fluorine radioisotopes, fluorodeoxyglucose 18F, costs, cost analysis
In the Netherlands, the crude rate for breast cancer mortality has fallen from 44.9 to 37.8 death cases per 100 000 in the past two decades, and nowadays the 5-year age-adjusted relative survival is 82% (IKNL, 2014). Around 10% of all breast cancer patients will develop metastatic breast cancer (MBC) within 5 years after primary diagnosis (Lord et al, 2012). The incidence of MBC is highest in the first 2 years, slowly decreases till the fifth year and remains relatively constant thereafter (Miller, 2003; Lord et al, 2012). The most common sites for MBC are bone, lung/pleura, liver, lymph nodes and brain (Miller, 2003; Sihto et al, 2011; Lord et al, 2012).
According to the clinical guidelines of the European Society for Medical Oncology and the National Comprehensive Cancer Network (NCCN Guidelines Version 3, 2014), the standard work-up for diagnosing MBC includes conventional imaging with bone X-ray and/or bone scintigraphy, chest X-ray and/or chest computed tomography (CT), liver ultrasound and/or abdominal CT, and magnetic resonance imaging (MRI). Histological biopsies are advised to confirm findings and re-evaluate tumour receptor status of MBC (oestrogen receptor (ER), progesterone receptor, human epidermal growth factor receptor 2 (HER2; Cardoso et al, 2012; NCCN Guidelines Version 3, 2014). However, biopsies can be difficult to obtain owing to the location of the metastasis and results can be inconclusive regarding the receptor status, which hampers optimal treatment (Hammond et al, 2010).
When findings on conventional imaging are equivocal or in locally advanced inoperable breast cancer, positron emission tomography (PET) and CT (PET/CT) with 2-[18F]fluoro-2-deoxy-D-glucose (FDG) is an acceptable option to further evaluate suspected MBC (Grassetto et al, 2011; Cardoso et al, 2012; NCCN Guidelines Version 3, 2014; Cardoso et al, 2014).
Positron emission tomography imaging with the novel tracer 16α-[18F]fluoro-17β-oestradiol (FES) can give insight in tumour ER expression in breast cancer (Peterson et al, 2008), and has high sensitivity for bone (92%) and lung lesions (95% Van Kruchten et al, 2013). FES-PET was found to improve the diagnosis and treatment decision making in patients with a history of ER-positive breast cancer presenting with a clinical dilemma on conventional imaging (Van Kruchten et al, 2012). It should be noted that a negative FES-PET does not rule out tumour, as ER-negative metastases are not visible on FES-PET. The combination of FES-PET and CT may, however, largely overcome this issue by giving insights in tumour lesions on CT that are FES negative. To our knowledge, the effect of upfront PET/CT with the tracers FES and FDG on the number of performed biopsies and associated costs has so far not been examined.
The aim of this study was, therefore, to evaluate the effect on the number of performed biopsies and costs associated with implementing FES or FDG-PET with contrast-enhanced CT as an upfront imaging test for diagnosing ER-positive MBC in comparison with the standard work-up in women presenting with symptoms.
Materials and Methods
A previously published and validated computer simulation model was extended and validated to reflect diagnosing of MBC (Jacobi et al, 2006; Greuter et al, 2010; Lu et al, 2012; De Bock et al, 2013). The model was constructed to simulate the follow-up of women after a diagnosis of initial breast cancer with ER-positive receptor status of the primary tumour. The main outcome was the number of avoided biopsies.
The Medical Ethical Committee of the University Medical Center Groningen concluded that no formal approval for this project was needed, as it was not a medical research study that involved humans. Informed consent from patients was not needed.
Structure of the model
The structure of the simulation model and its parameters are presented in Figures 1, 2, 3 and Tables 1 and 2. Every year a woman might die, might develop symptoms and MBC or might develop symptoms suggestive for MBC without actual disease. The model first evaluated whether a woman would die, using age and general death rate. In case of death, the woman exited the loop. Otherwise, the presence of MBC was simulated based on the risk to develop MBC over time and distributed over the four locations. If MBC was present, the woman developed symptoms at the specific location(s) of the MBC(s). However, if no MBC was present, symptoms suggestive for MBC could still be present depending on the risk to develop such symptoms per year (Table 1). In the absence of symptoms, the woman's age was increased by 1 year and the loop was repeated. If presenting with symptoms, with or without underlying MBC, the woman was subjected to a diagnostic strategy. When actual MBC was diagnosed (as confirmed by a positive biopsy or a positive FES-PET/CT) the woman exited the simulation. If the woman was not diagnosed with MBC, either due to false symptoms or false-negative test results, her age was increased and she re-entered the loop.
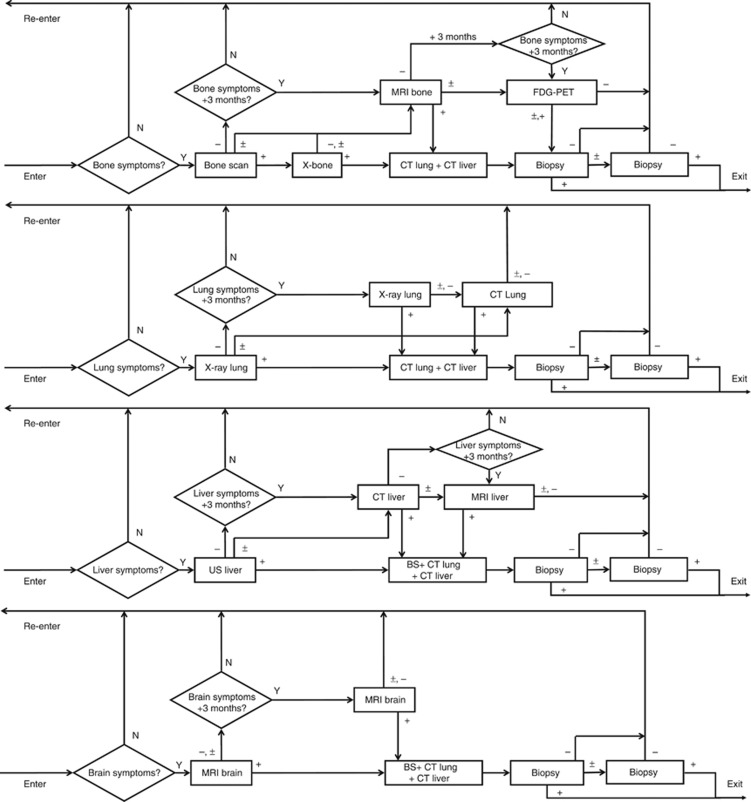
Figure 1.
Structure of the MBCSIM simulation model for the standard work-up. Women enter the simulation with bone, lung, liver and/or brain symptoms. The triangles indicate questions with output indicators Y=yes and N=no. The squares indicate imaging tests with output indicators, + denotes positive, − denotes negative and ± denotes inconclusive.
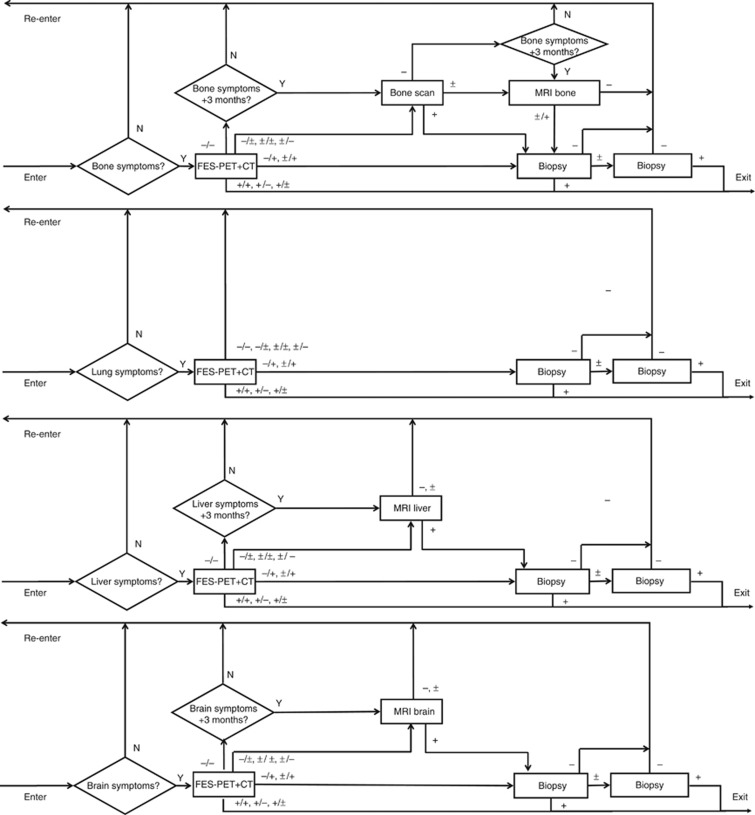
Figure 2.
Structure of the MBCSIM simulation model for the FES-PET/CT strategy. Women enter the simulation with bone, lung, liver and/or brain symptoms. The triangles indicate questions with output indicators Y=yes and N=no. The squares indicate imaging tests with output indicators, + denotes positive, − denotes negative and ± denotes inconclusive. In case of multimodality imaging (i.e., FES-PET and CT), each individual modality can have these three outcomes, leading to nine possible outcomes for the combined modality (i.e., FES-PET/CT).
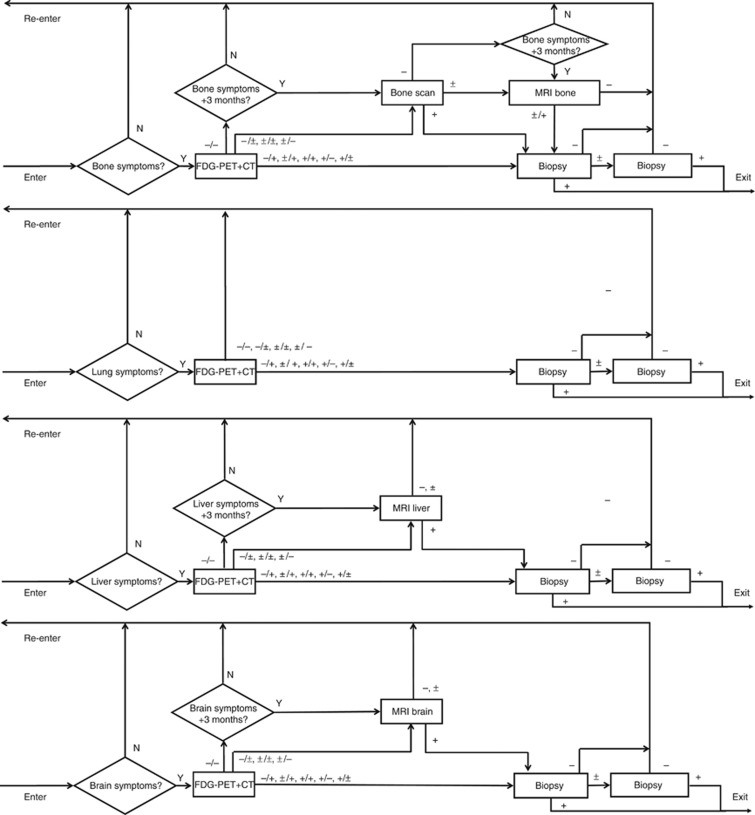
Figure 3.
Structure of the MBCSIM simulation model for the FDG-PET/CT strategy. Women enter the simulation with bone, lung, liver and/or brain symptoms. The triangles indicate questions with output indicators Y=yes and N=no. The squares indicate imaging tests with output indicators, + denotes positive, − denotes negative and ± denotes inconclusive. In case of multimodality imaging (i.e., FDG-PET and CT), each individual modality can have these three outcomes, leading to nine possible outcomes for the combined modality (i.e., FDG-PET/CT).
Table 1. Parameters of the simulation model.
| Baseline estimate (%) | Minimum estimate (%) | Maximum estimate (%) | Reference | |
|---|---|---|---|---|
|
Risk to develop MBC | ||||
| 1st Year | 1.2 | 1.0 | 1.5 | Lord et al, 2012 |
| 2nd Year | 2.9 | 2.5 | 3.3 | |
| 3rd Year | 2.7 | 2.3 | 3.1 | |
| 4th Year | 1.9 | 1.6 | 2.3 | |
| 5th Year | 1.6 | 1.3 | 1.9 | |
|
Distribution of location of MBC | ||||
| Bone | 46 | 25 | 61 | Lord et al, 2012 |
| Lung | 40 | 26 | 60 | |
| Brain | 13 | 7 | 28 | |
| Liver | 32 | 24 | 49 | |
|
Probability of symptoms suggestive for MBC | ||||
| Bone | 10.0 | 8.1 | 11.8 | Roorda et al, 2012; Christensen et al, 2012 |
| Lung | 3.0 | 2.3 | 3.4 | |
| Brain | 2.0 | 1.8 | 2.7 | |
| Liver | 1.0 | 0.8 | 1.1 | |
| Probability of inconclusive biopsy | 20 | 10 | 30 | Expert opinion |
| Probability of inconclusive imaging | 10 | 5 | 15 | Expert opinion |
Abbreviation: MBC=metastatic breast cancer.
Table 2. Sensitivity and specificity percentages and costs of imaging tests based on location of the metastatic breast cancer.
| Bone | Lung | Liver | Brain | Costs in ɛ (NZA, 2014) | Reference | |
|---|---|---|---|---|---|---|
|
Sensitivity | ||||||
| Bone scintigraphy | 80 (67–93) | NA | NA | NA | 135 | (Houssami and Costelloe, 2012) |
| X-ray | 61 (57–66) | 74 (67–80) | NA | NA | 47 | (Mahner et al, 2008, National Lung Screening Trial Research Team, 2013) |
| Magnetic resonance imaging | 94 (92–96) | NA | 81 (76–86) | 96 (84–99) | 251a/199b | (Weber et al, 2006; Costelloe et al, 2009; Floriani et al, 2010) |
| Ultrasound | NA | NA | 63 (25–87) | NA | 52 | (Floriani et al, 2010) |
| Computed tomography | 67 (48–80) | 94 (91–96) | 97 (89–100) | 86 (71–93) | 199 | (Ellika et al, 2007; Mahner et al, 2008, National Lung Screening Trial Research Team, 2013, Sadigh et al, 2014) |
| FES-PETc | 91 (88–100) | 90 (82–97) | 20 (10–30) | 46 (40–52) | 1.505 | Expert opinion |
| FDG-PET | 90 (88–96) | 90 (88–96) | 90 (88–96) | 50 (40–60) | 1.505 | (Radan et al, 2006; Hahn et al, 2011;Evangelista et al, 2012; Murakami et al, 2012), Expert opinion |
|
Specificity | ||||||
| Bone scintigraphy | 86 (68–100) | NA | NA | NA | (Houssami and Costelloe, 2012) | |
| X-ray | 100 (99–100) | 91 (91–92) | NA | NA | (Mahner et al, 2008, National Lung Screening Trial Research Team, 2013) | |
| Magnetic resonance imaging | 89 (76–100) | NA | 97 (94–99) | 62 (48–76) | (Weber et al, 2006; Costelloe et al, 2009; Floriani et al, 2010) | |
| Ultrasound | NA | NA | 98 (96–100) | NA | (Floriani et al, 2010) | |
| Computed tomography | 95 (80–95) | 90 (90–100) | 76 (68–84) | 100 (92–100) | (Mahner et al, 2008, Hendriks et al, 2013; National Lung Screening Trial Research Team, 2013, Sadigh et al, 2014) | |
| FES-PETc | 99 (98–100) | 99 (98–100) | 99 (98–100) | 99 (98–100) | Expert opinion | |
| FDG-PET | 81 (71–95) | 81 (71–95) | 81 (71–95) | 50 (40–60) | (Hahn et al, 2011; Radan et al, 2006; Evangelista et al, 2012; Murakami et al, 2012), Expert opinion, Expert opinion | |
Abbreviations: ER=oestrogen receptor; FDG=2-[18F]fluoro-2-deoxy-D-glucose; FES=16α-[18F]fluoro-17β-oestradiol; MRI=magnetic resonance imaging; NA=not applicable; PET=positron emission tomography.
Listed are estimated averages (95% confidence interval).
Price per bone MRI.
Price per brain/liver MRI.
The sensitivity and specificity values presented are relevant for ER-positive patients only.
Strategies for detecting MBC
Three diagnostic strategies for detecting symptomatic MBC were compared (Figures 1, 2, 3). The standard work-up was based on the Dutch clinical guidelines and included the recommended tests, that is, X-ray, bone scintigraphy, CT, ultrasound, MRI and biopsies (Mammacarcinoom Landelijke richtlijn, Version 2.0, 2014; Figure 1).
In the FES-PET/CT strategy, whole-body FES-PET/CT replaced the conventional imaging tests as upfront diagnostic for symptomatic MBC. In case of positive FES-PET/CT, no biopsy procedures were performed. When the FES-PET/CT was negative and there were no longer (at 3 months) symptoms suggestive for MBC, the patients exited the loop without further testing. In case of negative or inconclusive FES-PET/CT and persistent MBC symptoms, conventional imaging and biopsies were added to exclude the option of ER-negative MBC (Figure 2).
In the FDG-PET/CT strategy, patients followed the same route and diagnostic sequence as in the FES-PET/CT, but whole-body FDG-PET/CT was the upfront diagnostic test. As the FDG-PET/CT was not informative of the MBC receptor status, biopsies still had to be performed in case of positive FDG-PET/CT (Figure 3).
The parameters of the model
The model incorporated seven parameters (Table 1) with distinctive baseline, minimum and maximum estimates derived from literature. The risk to develop MBC and the incidence distribution was calculated from a study including 6644 patients diagnosed with breast cancer in the period 2001–2002 and followed till the end of 2007 (Lord et al, 2012). The yearly risk to develop symptoms suggestive for MBC and its distribution over the four locations was based on the observed referral rate to diagnostic services in primary care cancer survivors, which could be considered a good proxy for the incidence of such symptoms in this population (Roorda et al, 2012; Christensen et al, 2012). The death rate model was based on the cumulative death rates at ages up to 100 years for the Dutch cohort born in 1970 (Lu et al, 2012; De Bock et al, 2013). The model contained the mean sensitivity and specificity of the imaging modalities independent of age (Table 2; Radan et al, 2006; Weber et al, 2006; Ellika et al, 2007; Mahner et al, 2008; Costelloe et al, 2009; Floriani et al, 2010; Hahn et al, 2011; Evangelista et al, 2012; Houssami and Costelloe, 2012; Murakami et al, 2012; Hendriks et al, 2013; National Lung Screening Trial Research Team, 2013; Sadigh et al, 2014). The specificity of FES-PET was based on earlier studies that compared FES-uptake with tumour biopsies, with either qualitative assessment of FES-uptake or quantitative assessment with a maximum standardised uptake value (SUVmax) threshold of ⩾1.0 (Van Kruchten et al, 2013). The inconclusive rates of imaging tests and biopsies were based on expert opinion.
The model validation
To validate the model, a cohort of all female patients diagnosed and treated with ER-positive primary breast cancer in the period 2000–2002 at our hospital was identified (n=108) and followed for 5 years.
The simulation
To evaluate the strategies for detecting MBC, a cohort of women treated for primary breast cancer was simulated. The ages of breast cancer diagnosis in this cohort were derived from a cohort of 5073 women diagnosed with and treated for breast cancer between January 1989 and January 2003 in four hospitals in the Netherlands (one academic hospital, one large teaching hospital and two non-teaching hospitals). The women were representative for the Dutch breast cancer population with respect to their age at diagnosis (61.1±14.4 years; Lu et al, 2012).
Sensitivity analysis
A sensitivity analysis was performed to test the effect of the uncertainty regarding input estimates on model output. A normal distribution of all parameters was assumed with minimum and maximum estimates equal to the 95% confidence interval around the baseline. For each parameter, a random value was obtained from the normal distribution. The simulations for the three scenarios were performed with 10 data sets for 5073 women and for 20 simulation rounds. For the validation, the simulation data was scaled to represent the outcomes for 108 women. All outcomes were presented in terms of mean and s.d. (Tables 3 and 4).
Table 3. Validation of the MBCSIM model in 108 ER-positive breast cancer patients for a follow-up period of 5 years.
| Validation database | Simulation (mean±s.d.) | |
|---|---|---|
|
Initial imaging tests | ||
| 116 | 104.0±6.5 | |
| BS and X-bone | 53 | 52.2±3.8 |
| MRI bone | 8 | 15.3±2.2 |
| FDG-PET/CT | 3 | 1.2±0.3 |
| X-ray lung | 31 | 16.9±0.8 |
| CT lung/liver | 7 | 2.0±0.4 |
| Ultrasound liver | 7 | 7.0±0.3 |
| MRI liver | 0 | 0.2±0.1 |
| MRI brain | 7 | 9.2±2.5 |
| FES-PET/CT | 0 | 0.0±0.0 |
|
Staging tests | ||
| 13 | 17.9±1.3 | |
| CT lung+CT liver | 5 | 11.3±1.0 |
| BS+CT lung+CT liver | 8 | 6.6±0.9 |
|
Biopsy tests | ||
| 18 | 20.3±1.5 | |
| Bone | 6 | 7.4±1.0 |
| Lung | 10 | 5.6±0.5 |
| Liver | 0 | 3.3±0.2 |
| Brain | 2 | 4.0±1.0 |
|
MBCs found | ||
| 14 | 10.2±1.0 | |
| Bone | 8 | 3.7±0.6 |
| Lung | 3 | 2.7±0.3 |
| Liver | 2 | 2.6±0.2 |
| Brain | 1 | 1.2±0.3 |
Abbreviations: BS, bone scan; CT=computed tomography; ER=oestrogen receptor; FDG=2-[18F]fluoro-2-deoxy-D-glucose; FES=16α-[18F]fluoro-17β-oestradiol; MBC=metastatic breast cancer; MBCSIM, metastatic breast cancer simulation; MRI=magnetic resonance imaging; PET=positron emission tomography.
Table 4. Simulation outcomes for the standard work-up, FES-PET/CT and FDG-PET/CT strategies for a follow-up period of 5 years with 10 sets of data for 5073 women for 20 simulation rounds.
| Standard | FES-PET/CT | FDG-PET/CT | |
|---|---|---|---|
| Work-up (mean±s.d.) | Strategy (mean±s.d.) | Strategy (mean±s.d.) | |
| Initial imaging tests | 963.19±60.0 | 1030.5±39.5 | 493.5±28.7 |
| Staging tests | 165.4±12.5 | 0.0±0.0 | 0.0±0.0 |
| Biopsy tests | 188.4±14.3 | 114.7±14.7 | 260.4±21.5 |
| MBCs found | 94.7±8.9 | 107.0±10.5 | 91.2±8.8 |
| False-positive tests | 103.9±24.4 | 43.1±8.0 | 76.4±14.8 |
| Total costs × ɛ1000 | 232±15 | 1124±43 | 652±35 |
| Additional costs × ɛ1000 | NA | 892±45 | 420±38 |
| Saved biopsies | NA | 73.7±20.5 | (-71.9±14.8) |
| ICER × ɛ1000 | NA | 12.1±3.4 | (-5.8±2.2)a |
Abbreviations: CT=computed tomography; ER=oestrogen receptor; FDG=2-[18F]fluoro-2-deoxy-D-glucose; FES=16α-[18F]fluoro-17β-oestradiol; ICER=incremental cost-effectiveness ratio; MBC=metastatic breast cancer; MRI=magnetic resonance imaging; PET=positron emission tomography.
The ICER of the FDG-PET/CT over the standard work-up per avoided biopsy was negative as the costs were higher and there were no avoided biopsies (i.e., the health effect was negative).
Costs
Cost parameters included the unit prices of the tests based on tariffs (Table 2; NZA, 2014). The price level of 2013 was applied and valued in Euros (ɛ). Only direct costs related to diagnosing MBC were considered. Discounting was not applied.
Economic analysis of model outcomes
The total costs for the strategies were computed by summing the costs of all performed tests. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the additional costs of the alternative strategies resulting from initial imaging and staging tests by the number of avoided biopsies.
Results
Validation of the model
The simulation model reproduced sufficiently the number of initial tests and biopsies, whose actual observed values were in the models' confidence intervals (104.0±6.5 vs 116 and 20.3±1.5 vs 18). The model slightly overestimated the staging tests (17.9±1.3 vs 13) and underestimated the MBCs (10.2±1.0 vs 14; Table 3).
Clinical effects
The replacement of the standard testing by FES-PET/CT decreased the total number of biopsies by 39±9% as compared with the standard work-up, whereas the FDG-PET/CT increased the number of biopsies by 38±15% in comparison with standard care.
The number of false-negative results decreased only in the FES-PET/CT strategy by 13±15% compared with the standard work-up and by 17±16% compared with the FDG-PET/CT strategy.
The application of FES-PET/CT decreased the number of false positives by 58±12% compared with the standard work-up and by 44±15% compared with the FDG-PET/CT strategy, while in the FDG-PET/CT the false positives decreased by 26±22% compared with the standard work-up.
Economic analysis
The total costs for the FES-PET/CT strategy were higher as compared with the standard work-up and the FDG-PET/CT strategy, although the total number of performed tests decreased in both PET/CT strategies as compared with the standard work-up (FES-PET/CT: 9±6% and FDG-PET/CT: 56±3%). The ICER to avoid an additional biopsy in the FES-PET/CT over the standard work-up was calculated at 12.1±3.4 thousand Euros. The FDG-PET/CT did not avoid biopsies and the costs were higher compared with the standard work-up, and it was a dominated strategy (Table 4).
Discussion
The aim of this study was to evaluate the effect on the number of performed biopsies and costs associated with implementing PET/CT with FES or FDG as an upfront imaging test for diagnosing ER-positive MBC as compared with the standard work-up in women with symptoms. A previously applied and validated simulation model was expanded and revalidated for this study. The model proved to be sufficiently valid for reproducing the cohort results, which made it suitable for performing this economic evaluation. The PET/CT strategies yielded higher additional costs to diagnose MBC, although the total number of tests decreased as compared with the standard work-up. The number of false positives decreased in both PET/CT strategies. Biopsies were avoided and false negatives were decreased only in the FES-PET/CT strategy.
Novel diagnostic tests and therapies not only improved the survival rate among cancer patients in the last decades but also increased health-care expenditures (RIVM report, 2012), so prior evaluation of costs and effects is warranted. Combined PET/ contrast-enhanced CT was suggested to provide a noninvasive evaluation of MBC in a single procedure (Pennant et al, 2010). This study showed that the PET/CT strategies decreased the number of imaging tests and thus could reduce the associated hospital visits, time off work and time to diagnosis.
The FES-PET/CT strategy decreased the number of false-negative diagnoses that could avoid potential delay in treatment and relief of symptoms. In addition, the FES-PET/CT strategy decreased the number of obtained biopsies and thus the number of painful and invasive episodes for patients. The sensitivity of FES-PET/CT is limited by several factors, including background uptake, ER expression levels in the tumour and the size of the tumour relative to the resolution of the PET camera. In our study, patients with initially ER-positive breast cancer underwent FES-PET only in case they presented with symptoms suggestive for metastatic recurrence. Modern PET cameras have a spatial resolution that can be as high as 2–4 mm, provided that the lesion has sufficient ER expression and is not obscured by the uptake of the organ it is located in (i.e., liver). As it is unlikely that a lesion smaller than the spatial resolution of the PET camera would cause actual symptoms, it seems unlikely that resolution limitations of PET has a significant impact on its sensitivity in this study.
PET/CT strategies reduced the number of false-positive imaging tests and the associated distress on patients. A false positive could as well generate additional diagnostic costs in terms of further imaging and biopsy testing to elucidate metastatic status or even unnecessary treatment. Furthermore, FES-PET/CT may guide therapeutic decision making (Van Kruchten et al, 2012) as it provided insights in ER status and potential heterogeneity herein (Mortimer et al, 2001; Linden et al, 2006; Van Kruchten et al, 2012), and thus could reduce costs resulting from ineffective therapy.
A recent economic evaluation concluded that PET/CT was more effective in terms of generated quality-adjusted life years (QALYs) for true positives, false positives and true negatives than the standard work-up for diagnosing MBC, and the ICER per QALY was 29.700 pounds (∼37.868 Euros) in a UK setting (Auguste et al, 2011). In our study, both PET/CT strategies generated less false positives and the FES-PET/CT generated less false negatives, but the ICER was calculated per avoided biopsy (ɛ12.100±3.400).
Although the simulation model reproduced the data in the validation cohort sufficiently, some differences were observed in the number of diagnosed bone MBCs and staging tests. A possible explanation might be that the cohort described by Lord et al, 2012 included patients irrespective of their ER status. ER-positive primary breast cancers showed tendency to give more often a first relapse to the bone; however, the reported numbers (35–68%) were not significantly different than the estimates in the simulation (Sihto et al, 2011). Other potential explanation could be the variation between clinical guidelines and self-reported actual practice that was partly owing to the time gap between developing the model and the validation cohort follow-up.
A limitation of this study was that the main evaluated outcome was avoided biopsies and other outcomes such as QALY and survival were not studied. Our study showed that FES-PET/CT could potentially have a positive impact on quality of life by decreasing the delay in relief of MBC symptoms and the negative effect on patients' health resulting from a biopsy or false positive. FES-PET/CT could provide more information regarding metastatic recurrence than conventional approach (Mortimer et al, 2001; Linden et al, 2006; Van Kruchten et al, 2012), and might have added value in therapy selection, individualised treatment and follow-up resulting in increase of survival. However, the impact of FES-PET/CT on treatment selection cannot yet be expressed in life years saved or QALYs, as the data for such an estimate are lacking.
Only ER-positive patients were considered, in which FES-PET/CT performs with a higher sensitivity. The ER status of the primary tumour and its probability to change over time should be further investigated, as it could have impact on proper patient and treatment selection and thus affect costs. A limitation of the FES-PET/CT strategy was that when a biopsy was avoided, no information on the status of other tumour receptors (i.e., HER2 status) was obtained. As HER2 status of the metastatic disease is of clinical relevance for the application of targeted therapy, the HER2 expression could be determined by PET imaging with 89Zr-trastuzumab (Gaykema et al, 2014), which in contrast to biopsy would give information about the HER2 status in all lesions in the patient. Further study will have to point out whether adding this marker would be cost effective particularly from a clinical perspective. Another limitation was the follow-up period that was simulated for only 5 years owing to the lack of data regarding metastatic recurrence after that period.
Conclusions
The application of upfront PET/CT with FES and FDG in MBC patients comes at additional costs. The number of performed biopsies was lower in the FES-PET/CT strategy at an ICER of 12.1±3.4 thousand Euro, whereas the FDG-PET/CT did not reduce the number of performed biopsies and was more expensive. Whether the FES-PET/CT strategy has additional benefits for patients in terms of therapy management has to be evaluated in clinical studies.
As FES-PET/CT could guide treatment selection and thus potentially affect cost effectiveness by avoiding ineffective therapies, its impact on treatment decision making in first line MBC should be further addressed.
Acknowledgments
We would like to thank Sarah J Lord for providing valuable unpublished data regarding distribution of location of MBC, which was used in estimating the input parameters of the simulation model. This research was performed within the framework of the Center for Translational Molecular Medicine, project MAMMOTH (grant 03O-201).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Auguste P, Barton P, Hyde C, Roberts TE. An economic evaluation of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess. 2011;15 (18:1–54. doi: 10.3310/hta15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, ESMO Guidelines Working Group Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012. pp. vii11–vii19. [DOI] [PubMed]
- Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL, Cardoso MJ, Cufer T, Saghir NE, Fallowfield L, Fenech D, Francis P, Gelmon K, Giordano SH, Gligorov J, Goldhirsch A, Harbeck N, Houssami N, Hudis C, Kaufman B, Krop I, Kyriakides S, Lin UN, Mayer M, Merjaver SD, Nordström EB, Pagani O, Partridge A, Penault-Llorca F, Piccart MJ, Rugo H, Sledge G, Thomssen C, van't Veer L, Vorobiof D, Vrieling C, West N, Xu B, Winer E. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25:1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KG, Fenger-Grøn M, Flarup KR, Vedsted P. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis – a population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res. 2012;12:224. doi: 10.1186/1472-6963-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costelloe CM, Rohren EM, Madewell JE, Hamaoka T, Theriault RL, Yu TK, Lewis VO, Ma J, Stafford RJ, Tari AM, Hortobagyi GN, Ueno NT. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–614. doi: 10.1016/S1470-2045(09)70088-9. [DOI] [PubMed] [Google Scholar]
- De Bock GH, Vermeulen KM, Jansen L, Oosterwijk JC, Siesling S, Dorrius MD, Feenstra T, Houssami N, Greuter MJW. Which screening strategy should be offered to women with BRCA1 or BRCA2 mutations? A simulation of comparative cost-effectiveness. Br J Cancer. 2013;108:1579–1586. doi: 10.1038/bjc.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellika SK, Jain R, Patel SC, Scarpace L, Schultz LR, Rock JP, Mikkelsen T. Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. Am J Neuroradiol. 2007;28:1981–1987. doi: 10.3174/ajnr.A0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista L, Panunzio A, Polverosi R, Ferretti A, Chondrogiannis S, Pomerri F, Rubello D, Muzzio PC. Early bone marrow metastasis detection: the additional value of FDG-PET/CT vs. CT imaging. Biomed Pharmacother. 2012;66:448–453. doi: 10.1016/j.biopha.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–31. doi: 10.1002/jmri.22010. [DOI] [PubMed] [Google Scholar]
- Gaykema SBM, Schröder CP, Vitfell-Rasmussen J, Chua S, Oude Munnink TH, Brouwers AH, Bongaerts AHH, Akimov M, Fernandez-Ibarra C, Lub-de Hooge MN, de Vries EGE, Swanton C, Banerji U. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014;20:3945–3954. doi: 10.1158/1078-0432.CCR-14-0491. [DOI] [PubMed] [Google Scholar]
- Grassetto G, Fornasiero A, Otello D, Bonciarelli G, Rossi E, Nashimben O, Minicozzi AM, Crepaldi G, Pasini F, Facci E, Mandoliti G, Marzola MC, Al-Nahhas A, Rubello D. 18F-FDG-PET/CT in patients with breast cancer and rising Ca 15-3 with negative conventional imaging: a multicentre study. Eur J Radiol. 2011;80:828–833. doi: 10.1016/j.ejrad.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Greuter MJW, Jansen-Van der Weide MC, Jacobi CE, Oosterwijk JC, Jansen L, Oudkerk M, de Bock GH. The validation of a simulation model incorporating radiation risk for mammography breast cancer screening in women with a hereditary-increased breast cancer risk. Eur J Cancer. 2010;46:495–504. doi: 10.1016/j.ejca.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Hahn S, Heusner T, Kümmel S, Köninger A, Nagarajah J, Müller S, Boy C, Forsting M, Bockisch A, Antoch G, Stahl A. Comparison of FDG-PET/CT and bone scintigraphy for detection of bone metastases in breast cancer. Acta Radiol. 2011;52:1009–1014. doi: 10.1258/ar.2011.100507. [DOI] [PubMed] [Google Scholar]
- Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, EEm Torlakovic, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssami N, Costelloe CM. Imaging bone metastases in breast cancer: evidence on comparative test accuracy. Ann Oncol. 2012;23:834–843. doi: 10.1093/annonc/mdr397. [DOI] [PubMed] [Google Scholar]
- Hendriks LEL, Bootsma GP, de Ruysscher DKM, Scheppers NAM, Hofman PAM, Brans BT, Dingemans AMC. Screening for brain metastases in patients with stage III non-small cell lung cancer: is there additive value of magnetic resonance imaging above a contrast-enhanced computed tomography of the brain. Lung Cancer. 2013;80:293–297. doi: 10.1016/j.lungcan.2013.02.006. [DOI] [PubMed] [Google Scholar]
- IKNL 2014. Available at http://www.cijfersoverkanker.nl (accessed 6 October 2014).
- Jacobi CE, Nagelkerke NJ, Van Houwelingen JH, De Bock GH. Breast cancer screening, outside the population-screening program, of women from breast cancer families without proven BRCA1/BRCA2 mutations: a simulation study. Cancer Epidemiol Biomarkers Prev. 2006;15:429–436. doi: 10.1158/1055-9965.EPI-05-0223. [DOI] [PubMed] [Google Scholar]
- Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, Petra PH, Peterson LM, Schubert EK, Dunnwald LK, Krohn KA, Mankoff DA. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- Lord SJ, Marinovich ML, Patterson JA, Wilcken N, Kiely BE, Gebski V, Crossing S, Roder DM, Gattellari M, Houssami N. Incidence of metastatic breast cancer in an Australian population-based cohort of women with non-metastatic breast cancer at diagnosis. Med J Aust. 2012;196:688–692. doi: 10.5694/mja12.10026. [DOI] [PubMed] [Google Scholar]
- Lu W, Greuter MJW, Schaapveld M, Vermeulen KM, Wiggers T, de Bock GH. Safety and cost-effectiveness of shortening hospital follow-up after breast cancer treatment. Br J Surgery. 2012;99:1227–1233. doi: 10.1002/bjs.8850. [DOI] [PubMed] [Google Scholar]
- Mahner S, Schirrmacher S, Brenner W, Jenicke L, Habermann CR, Avril N, Dose-Schwarz J. Comparison between positron emission tomography using 2-[fluorine-18]fluoro-2-deoxy-D-glucose, conventional imaging and computed tomography for staging of breast cancer. Ann Oncol. 2008;19:1249–1254. doi: 10.1093/annonc/mdn057. [DOI] [PubMed] [Google Scholar]
- Mammacarcinoom Landelijke richtlijn, Version 2.0 2014. Available at http://www.iknl.nl (accessed 9 January 2014).
- Miller KD.2003Breast cancer: in Handbook of Advanced Cancer CareIn MJ Fisch, E Bruera, (eds)vol. 500pp 150–161Cambridge University Press: Cambridge, UK [Google Scholar]
- Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;11:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- Murakami R, Kumita S, Yoshida T, Ishihara K, Kiriyama T, Hakozaki K, Yanagihara K, Lida S, Tsuchiya S. FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta Radiol. 2012;53:12–16. doi: 10.1258/ar.2011.110245. [DOI] [PubMed] [Google Scholar]
- National Lung Screening Trial Research Team Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN Guidelines Version 3 2014. Available at http://www.NCCN.com (accessed 25 April 2014).
- NZA 2014. Available at http://www.nza.nl (accessed 17 June 2014).
- Pennant M, Takwoingi Y, Pennant L, Davenport C, Fry-Smith A, Eisinga A, Andronis L, Arvanitis T, Deeks J, Hyde C. A systematic review of positron emission tomography (PET) and positron emission tomography/ computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess. 2010;14:1–103. doi: 10.3310/hta14500. [DOI] [PubMed] [Google Scholar]
- Peterson LM, Mankoff DA, Lawton T, Yagle K, Schubert EK, Stekhova S, Gown A, Link JM, Tewson T, Krohn KA. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49:367–374. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- Radan L, Ben-Haim S, Bar-Shalom R, Guralnik L, Israel O. The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer. 2006;107:2545–2551. doi: 10.1002/cncr.22292. [DOI] [PubMed] [Google Scholar]
- RIVM report 2012. Available at http://www.gezondheidszorgbalans.nl/kosten/zorguitgaven/totale-zorguitgaven/ (accessed January 2013).
- Roorda C, de Bock GH, van der Veen WJ, Lindeman A, Jansen L, van der Meer K. Role of the general practitioner during the active breast cancer treatment phase: an analysis of health care use. Support Care Cancer. 2012;20:705–714. doi: 10.1007/s00520-011-1133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadigh G, Applegate KE, Baumgarten DA. Comparative accuracy of intravenous contrast-enhanced CT versus noncontrast CT plus intravenous contrast-enhanced CT in the detection and characterization of patients with hypervascular liver metastases: a critically appraised topic. Acad Radiol. 2014;21:113–125. doi: 10.1016/j.acra.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Sihto H, Lundin J, Lundin M, Lehtimäki T, Ristimäki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T, Isola J, Heikkilä P, Joensuu H. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 2011;13:R87. doi: 10.1186/bcr2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kruchten M, de Vries EGE, Brown M, de Vries EFJ, Glaudemans AWJM, Dierckx RAJO, Schröder CP, Hospers GAP. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14:e465–e475. doi: 10.1016/S1470-2045(13)70292-4. [DOI] [PubMed] [Google Scholar]
- Van Kruchten M, Glaudemans AW, De Vries EF, Beets-Tan RG, Schröder CP, Dierckx RA, de Vries EG, Hospers GAP. PET imaging of estrogen receptors as diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53:182–191. doi: 10.2967/jnumed.111.092734. [DOI] [PubMed] [Google Scholar]
- Weber MA, Zoubaa S, Schlieter M, Jüttler E, Huttner HB, Geletneky K, Ittrich C, Lichy MP, Kroll A, Debus J, Giesel FL, Hartmann M, Essig M. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology. 2006;66:1899–1906. doi: 10.1212/01.wnl.0000219767.49705.9c. [DOI] [PubMed] [Google Scholar]