Abstract
In this study, we investigated the functional role of Akt and JNK signaling cascades in apigenin-induced apoptosis in U937 human leukemia cells and anti-leukemic activity of apigenin in vivo. Apigenin-induced apoptosis by inactivation of Akt with a concomitant activation of JNK, Mcl-1 and Bcl-2 down-regulation, cytochrome c release from mitochondria and activation of caspases. Constitutively active myristolated Akt prevented apigenin-induced JNK, caspases activation, and apoptosis. Conversely, LY294002 and a dominant negative construct of Akt potentiated apigenin-induced apoptosis in leukemia cells. Interruption of JNK pathway showed marked reduction in apigenin-induced caspases activation and apoptosis in leukemia cells. Furthermore, in vivo administration of apigenin resulted in attenuation of tumor growth in U937 xenografts accompanied inactivation of Akt and activation of JNK. Attenuation of tumor growth in U937 xenografts by apigenin raises the possibility that apigenin may have clinical implications and can be further tested for incorporating in leukemia treatment regimens.
Keywords: Apigenin, Apoptosis, Leukemia, Akt, JNK
Introduction
Apigenin is a flavonoid belonging to the flavones structural class and is chemically known as 4’, 5’, 7-trihydroxyflavone (1). It is present in abundance in fruits and vegetables such as oranges, grapefruit, celery, parsley, onions, chamomile, and wheat sprouts (1, 2). It has been reported that apigenin is a potent inhibitor of cell growth and inducer of apoptosis in various cancer cells including breast (3), prostate (4, 5), lung (6), and hematologic (7, 8). Studies have revealed that apigenin induces apoptosis through different cellular signaling transduction pathways such as NFκB (9), p53 (10), MAPK (11), and PI3K/Akt (12, 13).
The PI3K/Akt signaling pathway plays an important role in cell survival and apoptosis. Activation of PI3K through growth receptor kinases leads to phosphorylation of PIP2 at 3’ position at its inositol ring and converts PIP2 to PIP3 at plasma membrane. Successively, PIP3 recruits Akt and PDK1 through their PH domain. Full activation of Akt occurs when it is phosphorylated by PDK1 at Thr308 and by mTORC2 at Ser473 (14). Activated Akt inactivates several proapoptotic factors including BAD, procaspase-9 and forkhead transcription factors (14). Constitutively active Akt has been reported in various types of leukemia (15, 16) and is responsible for uncontrolled proliferation and resistance to apoptosis in leukemia cells, providing, a potential therapeutic target in leukemia.
Jun N-terminal kinases (JNKs) belongs to the super family of MAP kinases which are involved in various cellular processes such as proliferation, differentiation, and apoptosis. JNK can promote apoptosis by different mechanisms. First, activated JNK translocates to the nucleus where it phosphorylate and transactivate c-Jun, which leads to the increased expression of proapoptotic genes such as TNF-alpha, Fas-L and Bak. Second, activated JNK can be translocated to mitochondria where it can phosphorylate Mcl-1 and Bcl-2 to antagonize their anti-apoptotic activity. JNK can also stimulate the release of cytochrome c from mitochondria through Bid-Bax dependent mechanism, which leads to apoptosis (17).
Apigenin exposure to different leukemia cells resulted in selective apoptosis in monocytic and lymphocytic leukemia’s (18). Exposure of human promyelocytic leukemia HL60 cells to apigenin resulted in induction of cell-cycle arrest, caspase-3 and PARP cleavage (7, 19). Another study showed that apigenin-induces apoptosis in primary effusion lymphoma cells via suppression of Akt pathway (20). The relationships between apigenin-induced apoptosis and cell signaling cascades have not yet been examined in depth in human leukemia cells. In this study, we have elucidated the functional role of Akt and JNK pathways in apigenin-induced lethality in leukemia cells. Our results suggest a hierarchical model of apigenin-induced apoptosis in human leukemia cells. In this model, apigenin-induced Akt inactivation represents a primary event resulting in JNK activation, down-regulation of Mcl-1 and Bcl-2, and culminating in caspases activation, and apoptosis. In addition, we have shown that apigenin attenuated tumor formation in U937 xenograft in athymic nude mice, suggesting that apigenin is not only effective in vitro but also in vivo.
Materials and methods
Chemicals
Apigenin was purchased from Sigma (St Louis, MO). LY294002, and SP600125 were from EMD Biosciences (La Jolla, CA). Antibodies against Akt1, phospho-JNK, JNK1 and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Bad, Bax, cytochrome c, and Mcl-1 were from BD Pharmingen (San Diego, CA). Cleaved-caspase-3, cleaved-caspase-7, cleaved-caspase-9, Bcl-XL, phospho-Akt (Ser473), phospho-Bad (Ser136) and Akt kinase assay kit were from Cell Signaling Technology (Beverly, CA). Antibodies against PARP and Bcl-2 were from Biomol (Plymouth Meeting, PA) and DAKO (Carpinteria, CA) respectively.
Cell culture and plasmids transfection
U937, Jurkat and HL-60 human leukemia cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and grown in RPMI 1640 medium with 10% FBS. Normal peripheral blood mononuclear cells (NPBMNC) were obtained from AllCells (Emeryville, CA) and maintained in RPMI 1640 with 10% FBS. Dr. Ruth Craig (Dartmouth Medical School, Hanover, NH) kindly provided U937 cells stably over-expressing Mcl-1 and their empty vector counterpart (pCEP). Authors carried out no further cell line authentication in the last 6 months. Dr. Bing-Hua Jiang (West Virginia University, Morgantown, WV) kindly provided the constitutive active form of Akt (myristolated Akt) and dominant negative Akt mutant (Akt-DN). U937 cells were transfected with m-Akt and Akt-DN using Amaxa Nucleofactor II (Koeln, Germany) as recommended by the manufacturer.
RNA interference and transfection
U937 cells (1.5x106) were transfected with 100 nM JNK1 dsRNAi oligonucleotide with CAAAGAUCCCUGACAAGCAtt (sense) and UGCUUGUCAGGGAUCUUUGgt (antisense) sequence (Ambion, Austin, TX) by using Amaxa Nucleofactor II as recommended by the manufacturer.
Analysis of apoptosis
For Annexin V/propidium iodide (PI) assay, cells were stained with Annexin V-fluorescein isothiocyanate and PI and apoptosis was evaluated by flow cytometry according to manufacturer’s protocol (BD Pharmingen) and described previously (21).
Measurement of Akt kinase activity
U937 cells were seeded in 6-well plate and then treated with apigenin (40 μM) for 24 h. In vitro Akt kinase assay was then used to measure Akt kinase activity as per manufacturer’s instruction.
Western blotting
Western blotting was performed using NuPAGE Bis-Tris electrophoresis system (Invitrogen). For tissue sections, RIPA buffer was added to the sections and homogenized with electric homogenizer. After incubation for 20 minutes on ice, samples were centrifuged for 30 minutes at 12,000 rpm at 4 °C and supernatant was collected as total cell lysate. SDS-PAGE was performed as described previously (21). Blots shown were representative for three separate experiments.
U937 Xenograft Assay
To evaluate the therapeutic effect of apigenin in vivo, xenograft of human U937 cells were utilized. Athymic nude mice (NU/NU, 4 weeks old, The Jackson Laboratory, Bar Harbor, Maine) were housed in a specific pathogen-free room within the animal facilities at the University of Kentucky. Animals were allowed to acclimatize to their new environment for 2 weeks prior to use. All animals were handled according to the Institutional Animal Care and Use (IACUC), University of Kentucky. U937 cells (2X106) were re-suspended in serum-free RPMI 1640 medium with Matrigel basement membrane matrix (BD Biosciences, Bedford, MA) at a 1:1 ratio (total volume 100 μL), then were subcutaneously injected into the flanks of nude mice. Four days after tumor inoculation, mice were randomly divided into three groups (n= 6 in each group) and apigenin (0, 20 and 40 mg/kg body weight) was administered intraperitoneally in 150 μL of DMSO/0.9% physiological saline (1:0.5) daily for five days a week for four weeks. Body weight and tumor mass were measured every 5 days throughout the study. Tumor volumes was determined by a caliper and calculated according to the formula (width2×length)/2. The dose of the apigenin for in vivo study was selected as described previously (22).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 3. For analysis of apoptosis, values were presented as mean + standard deviation (SD). Statistical differences between control and treated groups were determined by Student’s t-test for unpaired observations. Differences were considered statistically significant for value p < 0.05 or p < 0.01.
Results
Apigenin-induced apoptosis, activate caspases, and cleaved PARP in dose- and time-dependent manners in U937 cells
Apigenin-induced a dose-dependent apoptosis in U937 cells. Moderate increase in apoptosis was observed after 12 h and 24 h exposure to apigenin at concentration of 20 μM and marked increase in apoptosis was observed at concentrations ≥ 30 μM (Fig. 1A). Apigenin exposure of U937 cells at concentration of 40 μM also caused apoptosis in a time-dependent manner and a significant increase in apoptosis was observed as early as 6 hours after apigenin exposure (Fig. 1B).
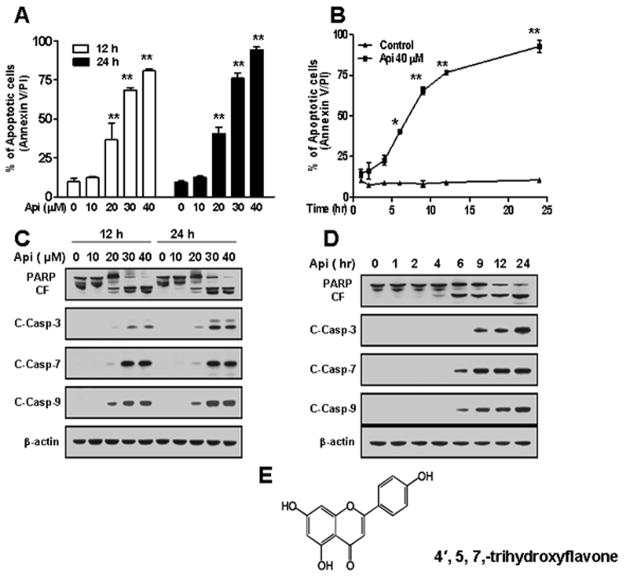
Fig. 1.
Effects of apigenin on apoptosis, caspases activation, and PARP cleavage in U937 cells. (A) U937 cells were treated without/with various concentrations of apigenin for 12 h and 24 h. (B) U937 cells were treated without/with 40 μM apigenin for indicated times. Cells were stained with Annexin V/ PI, and apoptosis was determined using flow cytometry as described in Materials and methods. The values obtained from Annexin V assays represent the means ± SD for three separate experiments. * or ** Values for cells exposed to apigenin were significantly increased compared to values in control cells by Student’s t-test; p < 0.05 or p < 0.01. (C–D) U937 cells were exposed to apigenin in dose and time-dependent manners. Total cellular extracts were subjected to Western blotting using indicated antibodies. (E) Structure of apigenin (4′, 5, 7,-trihydroxyflavone).
Western blotting revealed that apigenin-induced apoptosis in a caspase-dependent manner. Exposure of U937 cells at indicated concentrations of apigenin for 12 h and 24 h activates caspases-3, 7 and 9, and cleaved PARP (Fig. 1C). In addition, a time-course study of U937 cells exposed to 40 μM apigenin showed marked increase in activation of caspases-3, 7 and 9, and of PARP cleavage (Fig. 1D). Fig. 1E showed structure of apigenin. Together, these results indicate that apigenin induces apoptosis in dose- and time-dependent manners in U937 cells.
Exposure of U937 cells to apigenin resulted in down-regulation of Bcl-2 and Mcl-1
Further, we evaluated the expression of various members of Bcl-2 family of proteins. A dose- and time-dependent exposure of U937 cells to apigenin showed cleavage of Bcl-2 and down-regulation of Mcl-1 (Figs. 2A and 2B). No changes in Bcl-XL, and Bax expression were observed in U937 cells in dose as well as time-dependent manners (Figs. 2A and 2B). These results suggested that exposure of leukemia cells to apigenin resulted in cleavage or down- regulation of anti-apoptotic members of Bcl-2 family members such as Bcl-2 and Mcl-1.
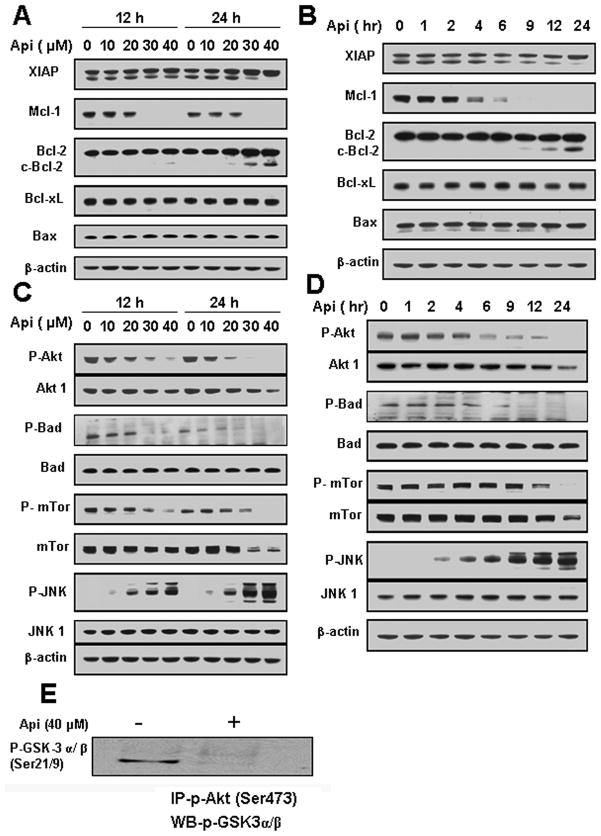
Fig. 2.
Effects of apigenin on Bcl-2 family proteins and stress-induced signaling. (A and C) U937 cells were treated with the indicated concentrations of apigenin for 12 h and 24 h. (B and D) U937 cell were treated with 40 μM apigenin for indicated times. Total cellular extracts were subjected to Western blotting using indicated antibodies. (E) U937 cells were treated with 40 μM apigenin for 24 h. Akt kinase activity was determined. Phospho-Akt was immunoprecipitated using immobilized phospho-Akt antibody and subjected to Western blotting using antibody against P-GSK-3 α/ β (Ser21/9).
Exposure of U937 cells to apigenin resulted in inactivation of Akt, and pronounced increase in JNK activation
Next, we examined the effects of apigenin on cell survival and stress-induced signaling pathways. A dose-dependent study showed that apigenin dephosphorylate Akt at Ser473 and its downstream targets mTOR (Ser2448) and Bad (Ser136) at concentrations of ≥ 30 μM. Total Akt1 and mTOR levels were also decreased (Fig. 2C). In addition, we observed that JNK phosphorylation levels increased concomitantly with a decrease in Akt phosphorylation in dose-dependent manner while JNK1 levels remained unchanged (Fig. 2C).
A time-course study showed that exposure of U937 cells to 40 μM apigenin resulted in dephosphorylation of Akt as early as 6 h after drug exposure and a concomitant increase in JNK phosphorylation, which reached maximum level at 24 h (Fig. 2D).
Exposure of U937 cells to apigenin for 24 h reduced kinase activity of Akt as shown by decreased phosphorylation of GSK-3α/β (Ser21/9) (Fig. 2E). Collectively, these results suggest that inactivation of Akt with a concomitant activation of JNK may play an important role in apigenin-induced apoptosis.
Apigenin-induced apoptosis in leukemia cells via caspase-independent inactivation of Akt and activation of JNK
To determine whether inactivation of Akt and activation of JNK were secondary to caspase activation, we treated U937 cells with 40 μM apigenin in the presence or absence of the broad-spectrum caspase inhibitor Z-VAD-FMK at 20 μM. Exposure of Z-VAD-FMK to U937 cells attenuated apigenin-induced apoptosis (Supplementary Fig. 1A), PARP cleavage, and caspases activation (Supplementary Fig. 1B). In addition, Z-VAD-FMK failed to inactivate Akt, activate JNK and down-regulate the expression of Mcl-1 (Supplementary Figs. 1C and 1D). Interestingly, Z-VAD-FMK inhibited apigenin-induced cleavage of Bcl-2 (Supplementary Fig. 1D), suggesting that the cleavage of Bcl-2 was caspase-dependent. Together these findings, suggest that apigenin-induced inactivation of Akt, activation of JNK and Mcl-1 down-regulation were caspase-independent.
Apigenin-induced apoptosis in leukemia cells via mitochondrial-dependent mechanism
It has been reported that exposure of leukemia cells to apigenin resulted in mitochondrial injury, release of cytochrome c into cytosol, and caspases activation (7). We investigated apigenin-induced mitochondrial alterations in U937 cells by DiOC6 staining. As shown in Supplementary Figs. 2A and 2B, exposure of cells to apigenin in dose- and time-dependent manners increased the number of cells with low mitochondrial trans-membrane potential as compared to control. In addition, apigenin-induced release of cytochrome c from mitochondria to cytosol in dose- and time-dependent manners (Supplementary Figs. 2C and 2D). Such findings indicate that apigenin-induced mitochondrial injury in leukemia cells that leads to cytochrome c release, caspase activation, and apoptosis.
Apigenin-induced similar effects in other leukemia cells but not in normal peripheral blood mononuclear cells (NPBMNC)
To assess whether apigenin-induced effects are restricted to monocytic (U937) leukemia cells, we conducted similar studies in T-cell lymphoblastic leukemia cells (Jurkat) and acute promyelocytic leukemia (HL60). The cells also showed apoptotic effects on apigenin exposure but were less sensitive than U937 cells (Supplementary Fig. 3A). Moreover, apigenin showed no significant apoptotic effect on NPBMNC at concentration of 40 μM (Supplementary Fig. 3B). Supplementary Fig. 3C revealed that in HL60 cells there was very little or no PARP cleavage or caspase-3 activation or expression of phospho-Akt (Ser473). These observations suggest that HL60 cells were less sensitive to apigenin-induced apoptosis at the same experimental condition. In addition, Mcl-1 expression was down-regulated in all three cell lines. To elucidate the mechanism by which the NPBMNC did not undergo apoptosis we performed western blotting and the results show that treatment of NPBMNC with apigenin at various concentrations neither cause cleavage of PARP nor dephosphorylation of Akt, Mcl-1 down-regulation and activation of JNK (Supplementary Fig. 3D). Collectively, these results show that apigenin-induced apoptosis in several leukemia cells but not in NPBMNC.
Inactivation of Akt is responsible for apigenin-induced JNK, and caspases activation and apoptosis
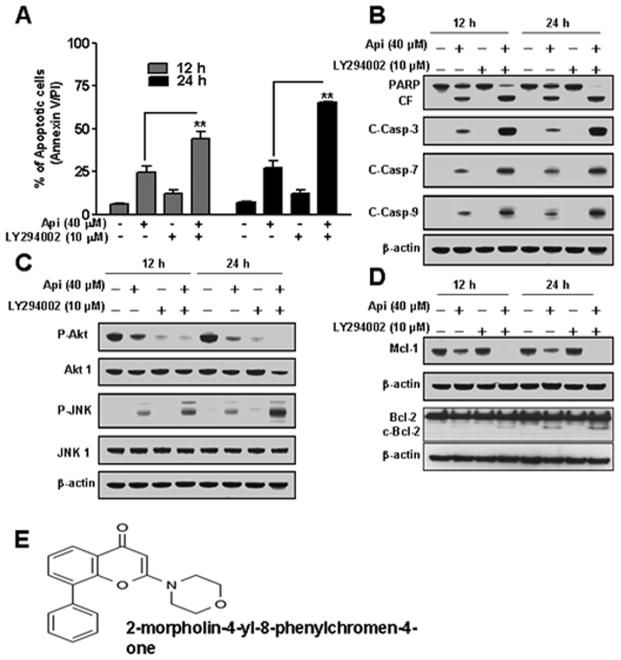
Results in Fig. 2 show that inactivation of Akt may play an important role in apigenin-induced apoptosis. To test this hypothesis, we pretreated U937 cells with PI3K inhibitor LY294002 (10 μM) for 1 h, followed by exposure to apigenin (20 μM) for 12 h and 24 h. As shown in Fig. 3A, pretreatment of cells with LY294002 and then to apigenin resulted in a sharp increase in apoptosis as compared to apigenin alone. Fig. 3B revealed that pretreatment with LY294002 and then apigenin exposure resulted in a pronounced increase in activation of caspases-3, 7, 9, and PARP cleavage. In addition, LY294002 potentiates apigenin-induced Akt inactivation, phosphorylation of JNK, Bcl-2 cleavage and Mcl-1 down-regulation. However, LY294002 showed no effect on total Akt1 and JNK1 levels (Figs. 3C and 3D). Fig. 3E showed structure of LY294002.
Fig. 3.
Effects of LY294002 on apigenin-induced apoptosis in U937 cells. (A) U937 cells were pretreated with 10 μM of LY294002 for 1 h, followed by the addition of 20 μM of apigenin for 12 h and 24 h. Cells were stained with Annexin V/PI, and apoptosis was determined using flow cytometry. The values obtained from Annexin V assays represent the means ± SD for three separate experiments. **Values for cells treated with apigenin and LY294002 in combination were significantly increased as compared to apigenin alone by Student’s t-test; p < 0.01. (B–D) Total cellular extracts were subjected to Western blotting using indicated antibodies. (E) Structure of LY294002 (2-morpholin-4-yl-8-phenylchromen-4-one) (38).
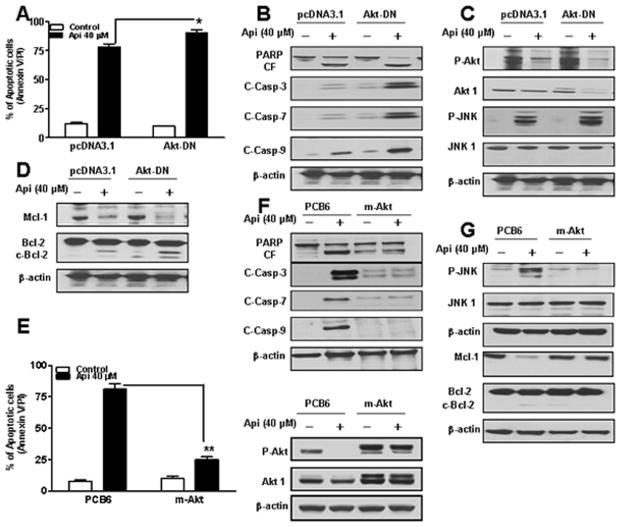
To assess the functional significance of Akt inactivation in apigenin-induced apoptosis, we employed Akt-DN construct. As shown in Fig. 4A, Akt-DN expressing cells were more sensitive to apigenin-induced apoptosis as compared to pcDNA3.1 vector control cells. Western blotting revealed that Akt-DN construct potentiates apigenin-induced activation of caspases-3, 7, 9, and PARP cleavage as compared to vector control (Fig. 4B). Consistent with these findings, Akt-DN construct potentiates apigenin-induced Akt inactivation and JNK activation with no change in the levels of total JNK1 (Fig. 4C). In addition, apigenin-induced Bcl-2 cleavage, and Mcl-1 down-regulation were enhanced in Akt-DN expressing cells (Fig. 4D). Over-expression of Akt by a constitutively active m-Akt prevented apigenin-induced apoptosis in U937 cells as compared to vector control (Fig. 4E). In addition, there was a marked increase in levels of total Akt1 and ability of apigenin to dephosphorylate Akt was inhibited in cells expressing m-Akt (Fig. 4E western). Apigenin-induced activation of caspases-3, 7, 9, and PARP cleavage were drastically reduced in U937 cells expressing m-Akt (Fig. 4F). Interestingly, the ability of apigenin to induce JNK activation was abrogated in cells expressing m-Akt (Fig. 4G). Furthermore, over-expression of Akt essentially abolished apigenin-induced Bcl-2 cleavage and Mcl-1 down-regulation (Fig. 4G). Together, these findings indicate that inactivation of Akt plays a critical role in apigenin-induced apoptosis and that this event lies upstream of Mcl-1 and Bcl-2 down-regulation and JNK activation.
Fig. 4.
Determination of functional significance of Akt in apigenin-induced apoptosis. (A) U937 cells were transfected with an empty vector (pcDNA3.1) and Akt-DN as described in Materials and methods. Akt-DN expressing cells were treated without/with 40 μM of apigenin for 24 h, after which apoptosis was analyzed using Annexin V/PI assay. *Values for Akt-DN cells treated with apigenin were significantly increased compared to those for pcDNA3.1 cells by Student’s t-test; p < 0.05. (B-D) Total cellular extracts were subjected to Western blotting using indicated antibodies. (E) U937 cells were transfected with an empty vector (PCB6) and constitutively active myristolated Akt (m-Akt). m-Akt and PCB6 expressing cells were treated without/with 40 μM of apigenin for 24 h, after which apoptosis and P-Akt, and Akt1 expressions were analyzed. **Values for m-Akt cells treated with apigenin were significantly decreased compared to those for PCB6 cells by Student’s t-test; p < 0.01. (F-G) Total cellular extracts were subjected to Western blotting using indicated antibodies.
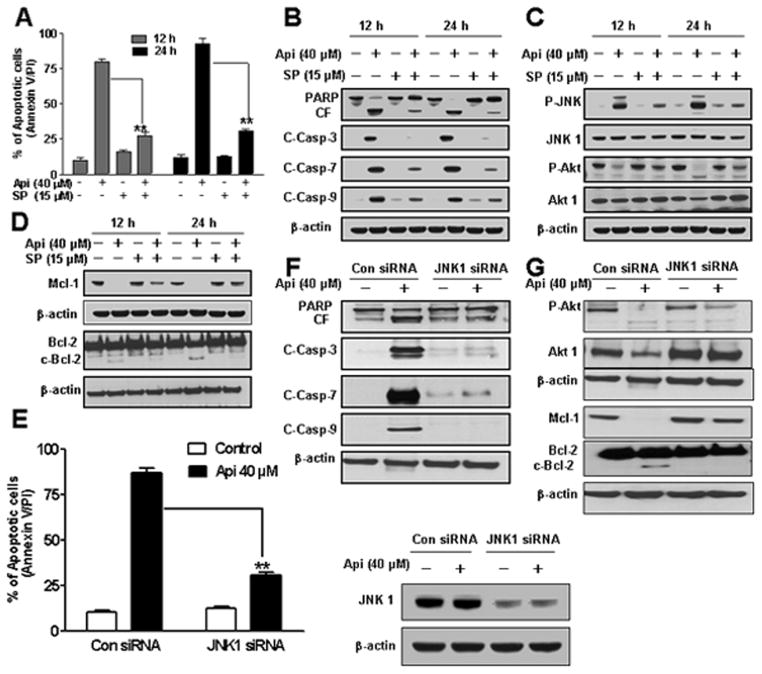
Activation of JNK played an important role in apigenin-induced caspase activation, and apoptosis
To dissect the possible functional significance of JNK activation in apigenin-induced apoptosis, we employed both pharmacologic and genetic approaches. Pretreatment of U937 cells with JNK inhibitor SP600125 (15 μM) for 1 h diminished apigenin-induced apoptosis (Fig. 5A). Fig. 5B revealed that pretreatment with SP600125 blocked apigenin-induced caspase-3, 7 and 9, activation and PARP cleavage. In addition, SP600125 reduced apigenin-induced phosphorylation of JNK and showed no effect on phosphorylation of Akt, total Akt1 or JNK1 levels (Fig. 5C). Furthermore, interruption of JNK by SP600125 inhibited apigenin-induced Bcl-2 cleavage and Mcl-1 down-regulation (Fig. 5D).
Fig. 5.
Effects of pharmacological and genetic inhibition of JNK on apigenin-induced apoptosis. U937 cells were pretreated with 15 μM SP600125 for 1 h followed by the addition of 40 μM of apigenin for 12 h and 24 h. (A) Cells were stained with Annexin V/PI and apoptosis was determined using flow cytometry. The values obtained from Annexin V assays represent the means ± SD for three separate experiments. **Values for cells treated with apigenin and SP600125 were significantly less than those obtained for cells treated with apigenin alone by Student’s t-test; p < 0.01. (B–D) Total cellular extracts were subjected to Western blotting using indicated antibodies. (E) U937 cells were transfected with JNK1 siRNA oligonucleotides or control siRNA and allowed to grow for 24 h, after which cells were treated with 40 μM of apigenin for another 24 h. Apoptosis was determined using the Annexin V/PI assay and JNK1 expression by western. **Values for cells treated with apigenin after transfection with JNK1 siRNA oligonucleotides were significantly decreased compared to those for control cells treated with apigenin alone by Student’s t-test; p < 0.01. (F–G) Total cellular extracts were subjected to Western blotting using indicated antibodies.
To confirm the role of JNK in apigenin-induced apoptosis, siRNA of JNK1 was employed. Apigenin-induced apoptosis was sharply reduced in JNK1 siRNA transfected cells and that JNK1 levels were reduced to one-third (approximately) as compared to control siRNA (Fig. 5E). As shown in Fig. 5F, JNK1 siRNA transfected cells diminished apigenin-induced caspases-3, 7 and 9, activation and PARP cleavage. Furthermore, JNK1 siRNA cells showed no change in phosphorylation of Akt and total Akt1 levels (Fig. 5G). Apigenin-induced Bcl-2 cleavage and Mcl-1 down-regulation were inhibited in JNK1 siRNA transfected cells (Fig. 5G). Collectively, these results indicate that apigenin-induced JNK activation played an important functional role in apoptosis and that the activation of JNK occurred downstream of Akt inactivation.
Apigenin-induced Mcl-1 down-regulation proceeds via transcriptional and proteasome- dependent mechanisms
Results from Fig.2 indicate that down-regulation of Mcl-1 upon apigenin treatment is tightly connected to Akt dephosphorylation and JNK activation. Therefore, to elucidate the mechanism underlying Mcl-1 down-regulation by apigenin, we utilized quantitative RT-PCR analysis. As shown in Supplementary Figs. 4A and 4B, exposure of U937 cells to apigenin resulted in significant decrease in Mcl-1 mRNA levels in dose- and time-dependent manners. Inhibition of transcription by exposing cells to actinomycin D (5 μg/ml) in the presence or absence of apigenin failed to reduce apigenin-mediated Mcl-1 down-regulation (data not shown).
To further delineate the mechanism by which apigenin diminishes Mcl-1 expression in U937 cells, we exposed the cells to apigenin 40 μM for various intervals in the presence or absence of the proteasome inhibitor MG132 (10 μM). As shown in Supplementary Fig. 4C, MG132 essentially blocked the down-regulation of Mcl-1. In addition, co-administration of protein synthesis inhibitor cyclohexamide (20 μM) hastened the rate of Mcl-1 down-regulation (Supplementary Fig. 4D). Together, these findings suggest that apigenin not only blocks Mcl-1 transcription but also degrade Mcl-1 via proteasome dependent mechanism.
Over-expression of Mcl-1 substantially diminished apigenin-induced apoptosis, caspases activation and PARP cleavage in U937 cells
To asses the functional significance of Mcl-1 in apigenin-induced apoptosis, we utilized U937 cells over-expressing Mcl-1. As shown in Supplementary Fig. 5A, over-expression of Mcl-1 substantially diminished the apigenin-induced apoptosis whereas empty vector control (pCEP) cells were as sensitive as parental cells. Treatment with apigenin diminished Mcl-1 expression in pCEP cells but failed to down-regulate Mcl-1 in over-expressing cells (Supplementary Fig. 5B). Supplementary Fig. 5A revealed that apigenin failed to activate caspases-3, 9 and cleave PARP in Mcl-1 over-expressing cells (Supplementary Fig. 5C). In addition, apigenin-induced cytochrome c release from mitochondria was diminished in Mcl-1 over-expressing cells (Supplementary Fig. 5D). These findings demonstrate that apigenin-induced down-regulation of Mcl-1 is an essential event in apigenin-induced apoptosis in U937 cells.
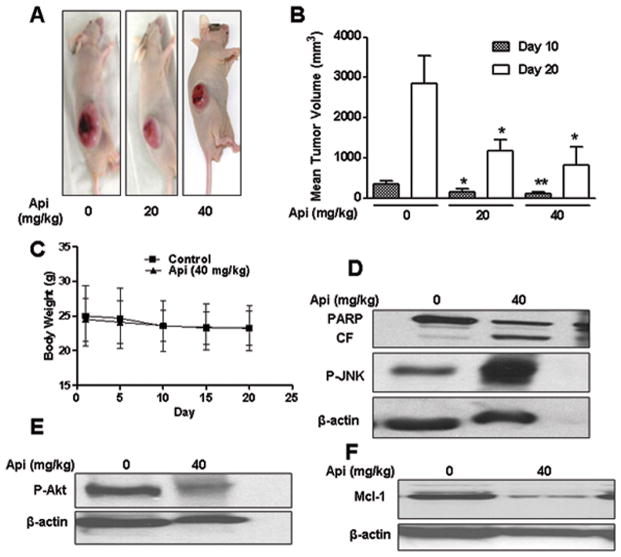
Apigenin inhibited tumor formation in xenografts of U937 human leukemia cells
Based on the in vitro studies described in above sections, we extend our studies to test the anti-leukemic activity of apigenin in vivo in U937 human leukemia xenografts. Athymic nude mice were inoculated with U937 cells subcutaneously, after which mice were injected with vehicle or apigenin (20 and 40 mg/kg intraperitoneally) daily for five days a week for four weeks as described in Materials and methods. As shown in Figs. 6A and 6B, treatment of mice with 20 and 40 mg/kg apigenin resulted in 58.4 % and 71 % inhibition of tumor growth as compared to control group on day 20. In addition, no statistically significant change in body weight was observed in control and apigenin treated animals (Fig. 6C), indicating that apigenin was not toxic. Figs. 6D and 6E revealed that apigenin (40 mg/kg) dephosphorylated Akt and activate JNK as compared to vehicle in tissue sections. PARP cleavage, an indicator of apoptosis, was also increased in treatment group (Fig. 6D). Down-regulation in Mcl-1 levels in apigenin treated mice was consistent with in vitro finding (Fig. 6F). Collectively, these findings suggest that inactivation of Akt and activation of JNK signaling contributes to apigenin-induced apoptosis not only in vitro but also in vivo.
Fig. 6.
In vivo anti-leukemic activity of apigenin in U937 xenografts. 18 athymic nude mice were inoculated with U937 cells (2×106 cells/mouse, subcutaneously) and randomly divided into three groups (6/group) for treatment with vehicle/apigenin as described in Materials and methods. (A) Representative animals from each group as indicated with tumor. (B) Average tumor volume in vehicle control mice and mice treated with 20 and 40 mg/kg apigenin on days 10 and 20. Data are means ± SD (n =18; 6 mice/group with tumors implanted on right flank of each mouse). * or ** Values p < 0.05 or p < 0.01 significantly different compared with vehicle control by Student’s t-test. (C) Body weight changes of mice during 20 days of treatment. (D–F) Tissue sections subjected to western blotting using indicated antibodies.
Discussion
Previous studies have shown that apigenin-induced apoptosis in several types of cancer cells such as breast, prostate, lung, and hematologic (3–8). Presently, no information is available regarding the functional importance of Akt and JNK pathways in apigenin-induced lethality in leukemia cells. The results in the present study indicate that exposure of apigenin to leukemia cells leads to mitochondrial injury, caspases activation, and apoptosis. In addition, our results provide the mechanistic information of how apigenin exerts its proapoptotic effects in leukemia cells i.e. by inactivation of Akt and activation of JNK.
Since phosphorylation by upstream kinases is required for complete activation of Akt (14), it is not surprising that various protein phosphatases dephosphorylate Akt. Activation of Akt generally involves PTEN inactivation and results in attenuation of apoptosis (23). In our study, apigenin exposure resulted in a dephosphorylation of Akt. Thus, it would be enticing to relate this observation to PTEN activation. However, the absence of wild-type PTEN in U937 cells does not support this possibility (24). In addition, Akt can be dephosphorylated by phosphatases such as PP2A and PHLPP (25, 26). It is likely that apigenin increases the activity or expression of these phosphatases that in turn dephosphorylate Akt. Infact, our unpublished results shown that apigenin enhanced the expression of PP2A in U937 cells. Additional mechanistic studies are required to demonstrate the role of these phosphatases in apigenin-induced Akt dephosphorylation in leukemia cells. It is possible that apigenin, through a mechanism not known yet, blocks the actions of PI3K. The findings that LY29004 augmented apigenin-induced inactivation of Akt, caspases activation, and PARP cleavage were in agreement with this hypothesis.
Akt is a well-known substrate of caspases. For instance, cleavage of Akt1 was caspase dependent in U937 and Jurkat cells exposed to ultraviolet light, etoposide and Fas ligation (27). The results shown in the present study demonstrated that pretreatment of leukemia cells with caspase inhibitor Z-VAD-FMK failed to prevent inactivation of Akt, suggesting that inactivation of Akt by apigenin was caspase-independent.
Results from the present study suggest that Akt inactivation by apigenin plays a functional role in apigenin-induced lethality in human leukemia cells. First, pretreatment with the LY29004 followed by treatment with apigenin enhanced apigenin-induced caspases activation and apoptosis. Second, U937 cells expressing Akt-DN enhanced apigenin-induced lethality as compared to vector control. Last, over-expression of Akt by a constitutively active m-Akt prevented apigenin-induced caspases activation and apoptosis. In addition, we have shown that apigenin down-regulated Akt kinase activity, suggesting its reduced ability to phosphorylate downstream targets.
Furthermore, we elucidated the functional role of JNK pathway in apigenin-induced apoptosis in leukemia cells. We have shown a dose- and time-dependent correlation between Akt inactivation and JNK activation. Inhibition of JNK by SP600125 and JNK1 siRNA had no effect on Akt, suggesting that Akt lies upstream of JNK. Constitutively active m-Akt prevented apigenin-induced JNK activation, indicating that one of the mechanisms by which Akt pathway protects leukemia cells from apigenin-induced lethality is due to the inhibition of JNK. Conversely, inhibition of Akt pathway by LY29004 and Akt-DN potentiated apigenin-induced JNK activation and apoptosis. The following evidences can explain the correlation between Akt inactivation and JNK activation. It has been shown that Akt suppresses the JNK pathway by phosphorylating and negatively regulating ASK-1 (apoptosis signal-regulating kinase-1), MLK3 (mixed-lineage protein kinase), and MKK4/SEK1 (mitogen-activated protein/ERK kinase) (28, 29, 30). In addition, Akt suppress JNK activation by directly interacting with JNK-interacting protein (JIP) and thus preventing the recruitment of upstream kinases to JNK (31).
JNK can induce apoptosis by phosphorylating and inhibiting anti-apoptotic function of Bcl-2 and Mcl-1 (32, 33). In this study, we found that apigenin down-regulate Mcl-1 and induced Bcl-2 cleavage. We have also shown that inhibition of JNK decreased apigenin-induced Mcl-1 down-regulation and Bcl-2 cleavage, suggesting that these anti-apoptotic proteins lie downstream of JNK. Collectively, these findings demonstrated an important functional role of JNK pathway in apigenin-induced apoptosis.
Notably, apigenin exposure resulted in down-regulation of Mcl-1, an anti-apoptotic protein that may play an important role in apoptosis in malignant hematopoietic cells (34). Over-expression of Akt inhibited apigenin-induced Mcl-1 down-regulation, suggesting that Mcl-1 down-regulation is significant for apigenin-induced apoptosis. This finding was supported by the evidence that Mcl-1 is upregulated by PI3K/Akt pathway through a transcription factor complex CREB (35). In addition, we have demonstrated that apigenin inhibited Mcl-1 transcription and over-expression of Mcl-1 substantially diminished apigenin-induced apoptosis, caspases activation, PARP cleavage and mitochondrial injury (cytochrome c release). Together, these findings suggest that apigenin-induced down-regulation of Mcl-1 plays an essential role in apigenin-induced apoptosis in leukemia cells.
Apigenin has been shown to inhibit tumor growth of prostate, ovarian and lung xenografts by inducing apoptosis (36, 37). The present study shows that apigenin exhibits significant inhibitory effects on the growth of U937 leukemia tumor xenograft. We found an increase in phospho-JNK expression, PARP cleavage, dephosphorylation of Akt (Ser473) and Mcl-1 down-regulation in the apigenin treated group compared with the vehicle group, providing the apoptotic evidence in apigenin treated U937 xenograft mice. The expression levels of phospho-Akt, phospho-JNK, PARP cleavage and Mcl-1 in tissue sections of U937 xenograft tumor were closely correlated with the reduction of U937 tumor xenografts.
In summary, the results obtained from the present study provide an evidence that Akt and JNK signaling pathways are potential targets for apigenin-induced apoptosis in leukemia cells and in vivo. Further efforts are required to understand the mechanism by which apigenin inactivates Akt and induces apoptosis in human leukemia cells and U937 tumor xenografts, which could provide a reasonable evidence to incorporate apigenin in leukemia treatment regimens.
Supplementary Material
Acknowledgments
Grant Support: NIH Grant RO1 ES015375 (X.Shi)
References
- 1.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 2.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 3.Choi EJ, Kim GH. Apigenin induces apoptosis through a mitochondria/caspase pathway in human breast cancer MDA-MB-453 cells. J Clin Biochem Nutr. 2009;44:260–265. doi: 10.3164/jcbn.08-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla S, Mishra A, Fu P, MacLennan GT, Resnick MI, Gupta S. Up-regulation of insulin-like growth factor binding protein-3 by apigenin leads to growth inhibition and apoptosis of 22Rv1 xenograft in athymic nude mice. FASEB J. 2005;19:2042–2044. doi: 10.1096/fj.05-3740fje. [DOI] [PubMed] [Google Scholar]
- 5.Morrissey C, O’Neill A, Spengler B, Christoffel V, Fitzpatrick JM, Watson RW. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63:131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 6.Li ZD, Liu LZ, Shi X, Fang J, Jiang BH. Benzo[a]pyrene-3, 6-dione inhibited VEGF expression through inducing HIF-1 alpha degradation. Biochem Biophys Res Commun. 2007;357:517–523. doi: 10.1016/j.bbrc.2007.03.178. [DOI] [PubMed] [Google Scholar]
- 7.Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of capsase-9 and caspase-3 in leukemia HL-60 cells. Eur J Cancer. 1999;35:1517–1525. [PubMed] [Google Scholar]
- 8.Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteosome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Afaq F, Mukhtar H. Involvement of NF-κB, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- 10.Zheng PW, Chiang LC, Lin CC. Apigenin-induced apoptosis through p53- dependent pathway in human cervical carcinoma cells. Life Sci. 2005;76:1367–1379. doi: 10.1016/j.lfs.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Shin GC, Kim C, Lee JM, Cho WS, Lee SG, Jeong M, et al. Apigenin-induced apoptosis is mediated by reactive oxygen species and activation of ERK1/2 in rheumatoid fibroblast-like synoviocytes. Chem Biol Interact. 2009;182:29–36. doi: 10.1016/j.cbi.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Zhang L, Bertucci AM, Pope RM, Datta SK. Apigenin, a dietary flavonoid, sensitizes human T cells for activation-induced cell death by inhibiting PKB/Akt and NF-κB activation pathway. Immunol Lett. 2008;121:74–83. doi: 10.1016/j.imlet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through Proteosomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via PI3K/Akt-dependent pathway. J Biol Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 14.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Hideshima T, Catley L, Raje N, Chauhan D, Podar K, Mitsiades C, et al. Inhibition of Akt induces significant downregulation of surviving and cytotoxicity in human multiple myeloma cells. Brit J Hematol. 2007;138:783–791. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- 16.Shehata M, Schnabl S, Demirtas D, Hilgarth M, Hubmann R, Ponath E, et al. Reconstitution of PTEN activity by CK2 inhibitors and interference with PI3K/Akt cascade counteract the anti-apoptotic effect of human stromal cells in CLL. Blood. 2010;116:2513–2521. doi: 10.1182/blood-2009-10-248054. [DOI] [PubMed] [Google Scholar]
- 17.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem Pharmacol. 2006;72:681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Ruela-de-Sousa RR, Fuhler GM, Blom N, Ferreira CV, Aoyama H, Peppelenbosch MP. Cytotoxicity of apigenin on leukemia cell line: implications for prevention and therapy. Cell death and Disease. 2010;1:e19. doi: 10.1038/cddis.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain AR, Khan AS, Ahmed SO, Ahmed M, Plataniast LC, Al-Kuraya KS, et al. Apigenin induces apoptosis via downregulation of S-phase kimase-associated protein 2-mediated induction of p27Kip1 in primary effusion lymphoma cells. Cell Proliferation. 2010;43:170–183. doi: 10.1111/j.1365-2184.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao N, Kramer L, Rahmani M, Dent P, Grant S. The Three-Substituted Indolinone Cyclin-Dependent Kinase 2 Inhibitor 3-[1-(3H-Imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516) Kills Human Leukemia Cells via Down-Regulation of Mcl-1 through a Transcriptional Mechanism. Mol. Pharmacol. 2006;70:645–655. doi: 10.1124/mol.106.024505. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Can Res. 2007;9(6):R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu TC, Lin PM, Chang JG, Lee JP, Chen TP, Lin SF. Mutation analysis of PTEN/MMAC1 in acute myeloid leukemia. Am J Hematol. 2000;63:170–175. doi: 10.1002/(sici)1096-8652(200004)63:4<170::aid-ajh2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt promotes apoptosis and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Widmann C, Gibson S, Johnson GL. Caspase-dependent Cleavage of Signaling Proteins during Apoptosis. J Biol Chem. 1998;273:7141–7147. doi: 10.1074/jbc.273.12.7141. [DOI] [PubMed] [Google Scholar]
- 28.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthwal MK, Sathyanarayana P, Kundu CN, Rana B, Pradeep A, Sharma C, et al. Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J Biol Chem. 2003;278:3897–3902. doi: 10.1074/jbc.M211598200. [DOI] [PubMed] [Google Scholar]
- 30.Park HS, Kim MS, Huh SH, Park J, Chung J, Kang SS, et al. Akt (protein kinase B) negatively regulates SEK1 by means of protein phosphorylation. J Biol Chem. 2002;277:2573–2578. doi: 10.1074/jbc.M110299200. [DOI] [PubMed] [Google Scholar]
- 31.Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, et al. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, et al. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730–43734. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 34.Bae J, Donigian JR, Hsueh AJ. Tankyrase 1 interacts with Mcl-1 proteins and inhibits their regulation of apoptosis. J Biol Chem. 2003;278:5195–5204. doi: 10.1074/jbc.M201988200. [DOI] [PubMed] [Google Scholar]
- 35.Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu XW, Meng D, Fang J. Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis. 2008;29:2369–2376. doi: 10.1093/carcin/bgn244. [DOI] [PubMed] [Google Scholar]
- 37.Kaur P, Shukla S, Gupta S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis. 2008;29:2210–2217. doi: 10.1093/carcin/bgn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlahos CJ, Matter WF, Hui KY, Brown RF. A Specific Inhibitor of Phosphatidylinositol-3-Kinase,2-(4-Morpholinyl)-8-phenyl-4H-l-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.