Abstract
The objective of this retrospective study was to evaluate complications in the management of refractory status epilepticus (RSE) treated with benzodiazepine and pentobarbital infusions. Of 28 children with RSE, eleven (39%) were treated with a pentobarbital infusion after failure to control RSE with a benzodiazepine infusion; while17 children (61%) required only a benzodiazepine infusion. The mean maximum pentobarbital infusion dosage was 5.2 ± 1.8 mg/kg/h. Twenty-five patients received a continuous midazolam infusion with an average dosage of 0.41 ± 0.43 mg/kg/h. The median length of stay was longer for the pentobarbital group. Children requiring pentobarbital therapy were more likely to develop hypotension, require inotropic support, need intubation, mechanical ventilation, peripheral nutrition, and blood products; furthermore, they were more likely to develop hypertension and movement disorder after or during weaning. In conclusion, children with RSE who required pentobarbital therapy had a longer hospital stay with more complications.
Keywords: Child, pediatric intensive care unit, status epilepticus, seizures
Introduction
Refractory status epilepticus (RSE) is defined as seizures that are nonresponsive to adequate initial anti-epileptic therapy and require a continuous infusion of an antiepileptic medication or administration of inhalation anesthetic agent to stop the seizure activity.[1] RSE is associated with a high morbidity and mortality.[2] The care of children with RSE is largely based on extrapolations of limited evidence derived from adult literature and supplemented with case reports and case series in children. Midazolam and pentobarbital infusions are used in the treatment of RSE.[3] The use of a pentobarbital infusion in the management of RSE has shown to be associated with a high morbidity and mortality. The current study describes a single center experience of management of RSE with the use of midazolam and pentobarbital.
Methods
In this retrospective cohort study, children (age: 0-21 years) admitted to a tertiary-care pediatric intensive care unit with RSE, over a 3-year period, were identified and included. The study was approved by the institutional review board. Demographic and clinical data were collected from the hospital administrative database and electronic patient healthcare records. All children who continued to have either clinical or electrical seizure activity despite initial treatment with lorazepam, diazepam, phenytoin, and phenobarbital and required a continuous anti-epileptic medication infusion to control their seizure activity were included in the study. Children who had recurrent seizures and required a continuous infusion of anti-epileptic medication were included. Patients who were admitted with status epilepticus and did not require a continuous infusion to control their seizures were excluded from the study. Patients who were transferred from an outside institution, intubated and on a benzodiazepine infusion, and whose seizures had terminated were also excluded. All children who receive pentobarbital were monitored with a continuous electroencephalogram (EEG) recording.
Outcome of children who required continuous pentobarbital infusion to control RSE were compared with those who were controlled with continuous benzodiazepine infusion alone. Continuous data were analyzed with the Mann-Whitney U test, and binary data were analyzed using Fisher exact test. Differences were considered statistically significant at P < 0.05.
Results
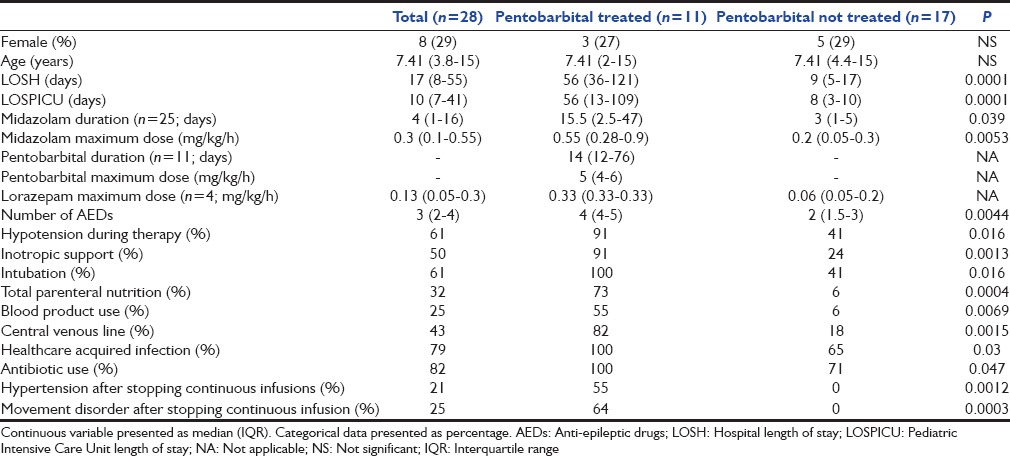
Of 28 admissions, 4 (14%) were admitted with new onset of seizures and all others had a known seizure disorder and were taking multiple antiepileptic medications. The descriptive and analytic data are presented in Table 1. All children in this cohort received continuous infusion of a benzodiazepine (midazolam = 25; lorazepam = 4; both = 1) for seizure control. Eleven patients (39%) required pentobarbital infusion in addition to benzodiazepines to control seizures while others (61%) received only a benzodiazepine infusion. The mean maximum pentobarbital infusion dosage was 5.2 ± 1.8 mg/kg/h with a median duration of treatment of 14 (interquartile range [IQR]: 12-76) days. Twenty-five patients received a continuous midazolam infusion with a mean maximum dosage of 0.41 ± 0.43 mg/kg/h for a median duration of treatment of 4 (IQR: 1-16) days. Only one child died who did not receive pentobarbital and the death was not related to seizure management.
Table 1.
Demographic, resource utilization, and complications of children with refractory status epilepticus were treated with and without pentobarbital
Discussion
All patients in our study received a benzodiazepine infusion as an initial anticonvulsant continuous therapy. Complications and resource utilization were less frequent in children whose seizures were controlled with only a benzodiazepine infusion. A meta-analysis of 111 children concluded that midazolam was as effective as other coma-inducing medications and involved a lower mortality (zero with midazolam),[4] however, Morrison noted that breakthrough seizure activity is common with midazolam.[5] A standardized protocol for escalation or weaning of anti-epileptic infusion therapy was not utilized in our cohort. Pentobarbital infusion was titrated to achieve a burst suppression pattern during continuous video-EEG monitoring; the required degree of burst suppression (number of burst patterns per unit time) was not standardized. An aggressive step-wise approach to maximize benzodiazepine prior to initiating a pentobarbital infusion may have prevented some patients from requiring pentobarbital, thus limiting their complications. Developing a protocolized treatment plan similar to Abend et al.[6] may have been beneficial in limiting the side effects that we noted in our patients.
Recent guidelines for the management of status epilepticus do not recommend preference to any one drug among midazolam, propofol, and pentobarbital for the management of RSE.[7] In a meta-analysis, pentobarbital treatment was associated with a lower incidence of short-term treatment failure, fewer breakthrough seizures, and decreased need for additional anti-epileptic medications, but was associated with a significantly higher frequency of hypotension as seen in our study.[3]
Studies of pentobarbital therapy for RSE in children have reported an efficacy of 74-100%.[8,9,10] The loading dose of pentobarbital is generally 5 mg/kg administered over an hour, followed by an infusion at a rate of 1 mg/kg/h, which can be increased as needed to 3 mg/kg/h. Continuous blood pressure monitoring is important because hypotension may occur with dose escalation. In our cohort, a loading pentobarbital bolus was used inconsistently, often requiring a higher continuous dose to achieve a therapeutic effect.
Our study noted two unexpected complications associated with prolonged pentobarbital treatment: Movement disorders that developed during weaning or after discontinuation of pentobarbital infusion therapy and hypertension, which required medical therapy. These two complications have not been previously described during pentobarbital treatment.
We report a lowest mortality in a cohort of children with RSE. Low mortality in our cohort may be due to lower percent of new onset RSE. Previously healthy children with encephalitis and presenting with RSE had a poor neurologic outcome. However, children with a known seizure disorder presenting with RSE had a low morality and their neurologic status did not worsen in spite of prolonged therapy with benzodiazepines with or without pentobarbital.
Conclusion
Patients who required pentobarbital to control RSE had a longer hospital stay with more complications. The mortality rate was low (3.5%) in children presenting with RSE. Whether aggressive escalation of midazolam therapy will reduce the use of pentobarbital and reduce complications warrants a prospective examination.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: Frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–10. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Holtkamp M, Othman J, Buchheim K, Meierkord H. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry. 2005;76:534–9. doi: 10.1136/jnnp.2004.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: A systematic review. Epilepsia. 2002;43:146–53. doi: 10.1046/j.1528-1157.2002.28501.x. [DOI] [PubMed] [Google Scholar]
- 4.Hubert P, Parain D, Vallée L. Management of convulsive status epilepticus in infants and children. Rev Neurol (Paris) 2009;165:390–7. doi: 10.1016/j.neurol.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Morrison G, Gibbons E, Whitehouse WP. High-dose midazolam therapy for refractory status epilepticus in children. Intensive Care Med. 2006;32:2070–6. doi: 10.1007/s00134-006-0362-8. [DOI] [PubMed] [Google Scholar]
- 6.Abend NS, Huh JW, Helfaer MA, Dlugos DJ. Anticonvulsant medications in the pediatric emergency room and intensive care unit. Pediatr Emerg Care. 2008;24:705–18. doi: 10.1097/PEC.0b013e318188fcac. [DOI] [PubMed] [Google Scholar]
- 7.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert DL, Gartside PS, Glauser TA. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: A meta-analysis. J Child Neurol. 1999;14:602–9. doi: 10.1177/088307389901400909. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Lee DY, Kim JS. Neurologic outcomes of pediatric epileptic patients with pentobarbital coma. Pediatr Neurol. 2001;25:217–20. doi: 10.1016/s0887-8994(01)00311-3. [DOI] [PubMed] [Google Scholar]
- 10.Holmes GL, Riviello JJ., Jr Midazolam and pentobarbital for refractory status epilepticus. Pediatr Neurol. 1999;20:259–64. doi: 10.1016/s0887-8994(98)00155-6. [DOI] [PubMed] [Google Scholar]


