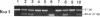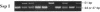Abstract
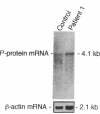
Nonketotic hyperglycinemia (NKH) is an autosomal recessive metabolic disorder caused by the defects in the glycine cleavage system (GCS; EC 2.1.2.10), a multienzyme system that consists of four individual components. NKH is a rare disorder in many countries, but with a very high incidence in northern Finland. To understand the genetic background of this high incidence, we examined the GCS in a typical case of NKH at the molecular level. The activity of P protein, a component of the GCS, was not detected in the lymphoblasts of the patient, while P protein mRNA of a normal size and level was present in the cells. Structural analysis of P protein mRNA from the patient revealed a single nucleotide substitution from G to T in the protein coding region, which resulted in an amino acid alteration from Ser564 to Ile564. No P protein activity was detected when the mutant P protein with this amino acid substitution was expressed in COS 7 cells. The patient was homozygous for this mutation. Furthermore, this mutation was present in 70% (14 of 20) of P protein gene alleles in Finnish patients with NKH, whereas it was not found in 20 alleles of non-Finnish patients. The results suggest that this mutation is responsible for the high incidence of NKH in Finland.
Full text
PDF


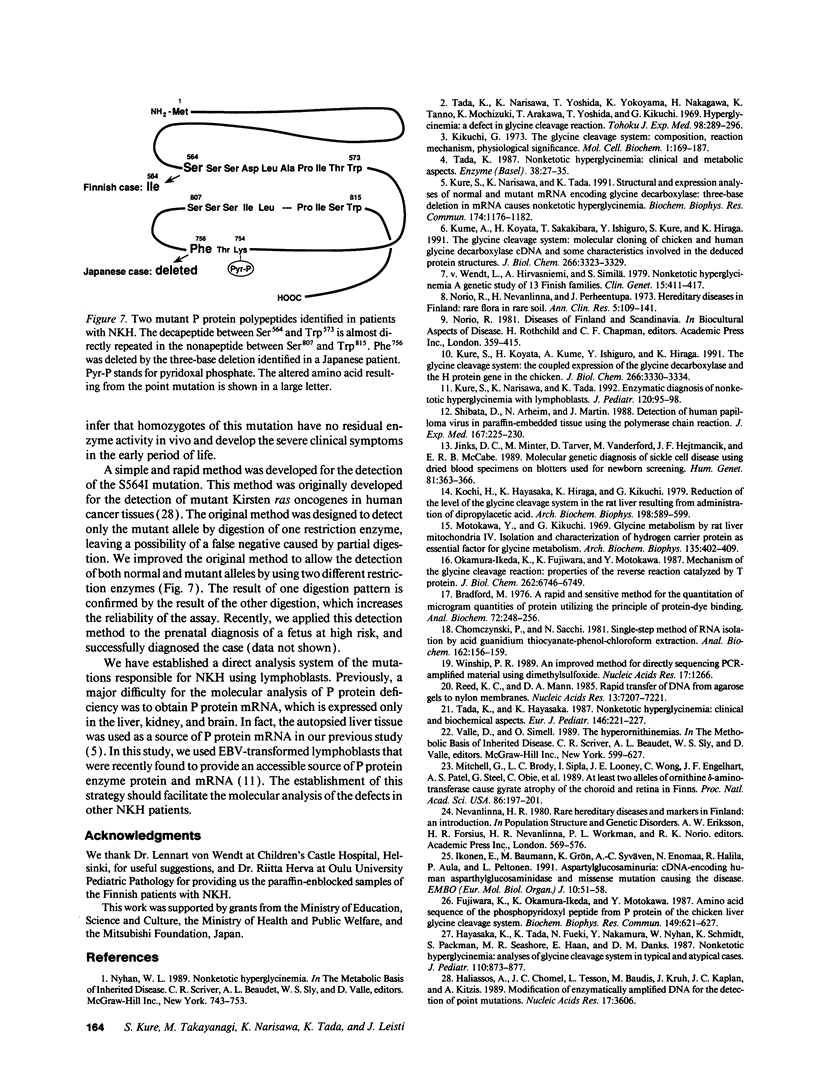
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Okamura-Ikeda K., Motokawa Y. Amino acid sequence of the phosphopyridoxyl peptide from P-protein of the chicken liver glycine cleavage system. Biochem Biophys Res Commun. 1987 Dec 16;149(2):621–627. doi: 10.1016/0006-291x(87)90413-x. [DOI] [PubMed] [Google Scholar]
- Haliassos A., Chomel J. C., Tesson L., Baudis M., Kruh J., Kaplan J. C., Kitzis A. Modification of enzymatically amplified DNA for the detection of point mutations. Nucleic Acids Res. 1989 May 11;17(9):3606–3606. doi: 10.1093/nar/17.9.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka K., Tada K., Fueki N., Nakamura Y., Nyhan W. L., Schmidt K., Packman S., Seashore M. R., Haan E., Danks D. M. Nonketotic hyperglycinemia: analyses of glycine cleavage system in typical and atypical cases. J Pediatr. 1987 Jun;110(6):873–877. doi: 10.1016/s0022-3476(87)80399-2. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Baumann M., Grön K., Syvänen A. C., Enomaa N., Halila R., Aula P., Peltonen L. Aspartylglucosaminuria: cDNA encoding human aspartylglucosaminidase and the missense mutation causing the disease. EMBO J. 1991 Jan;10(1):51–58. doi: 10.1002/j.1460-2075.1991.tb07920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks D. C., Minter M., Tarver D. A., Vanderford M., Hejtmancik J. F., McCabe E. R. Molecular genetic diagnosis of sickle cell disease using dried blood specimens on blotters used for newborn screening. Hum Genet. 1989 Mar;81(4):363–366. doi: 10.1007/BF00283692. [DOI] [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Kochi H., Hayasaka K., Hiraga K., Kikuchi G. Reduction of the level of the glycine cleavage system in the rat liver resulting from administration of dipropylacetic acid: an experimental approach to hyperglycinemia. Arch Biochem Biophys. 1979 Dec;198(2):589–597. doi: 10.1016/0003-9861(79)90535-6. [DOI] [PubMed] [Google Scholar]
- Kume A., Koyata H., Sakakibara T., Ishiguro Y., Kure S., Hiraga K. The glycine cleavage system. Molecular cloning of the chicken and human glycine decarboxylase cDNAs and some characteristics involved in the deduced protein structures. J Biol Chem. 1991 Feb 15;266(5):3323–3329. [PubMed] [Google Scholar]
- Kure S., Koyata H., Kume A., Ishiguro Y., Hiraga K. The glycine cleavage system. The coupled expression of the glycine decarboxylase gene and the H-protein gene in the chicken. J Biol Chem. 1991 Feb 15;266(5):3330–3334. [PubMed] [Google Scholar]
- Kure S., Narisawa K., Tada K. Enzymatic diagnosis of nonketotic hyperglycinemia with lymphoblasts. J Pediatr. 1992 Jan;120(1):95–98. doi: 10.1016/s0022-3476(05)80610-9. [DOI] [PubMed] [Google Scholar]
- Kure S., Narisawa K., Tada K. Structural and expression analyses of normal and mutant mRNA encoding glycine decarboxylase: three-base deletion in mRNA causes nonketotic hyperglycinemia. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1176–1182. doi: 10.1016/0006-291x(91)91545-n. [DOI] [PubMed] [Google Scholar]
- Mitchell G. A., Brody L. C., Sipila I., Looney J. E., Wong C., Engelhardt J. F., Patel A. S., Steel G., Obie C., Kaiser-Kupfer M. At least two mutant alleles of ornithine delta-aminotransferase cause gyrate atrophy of the choroid and retina in Finns. Proc Natl Acad Sci U S A. 1989 Jan;86(1):197–201. doi: 10.1073/pnas.86.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism by rat liver mitochondria. IV. Isolation and characterization of hydrogen carrier protein, an essential factor for glycine metabolism. Arch Biochem Biophys. 1969 Dec;135(1):402–409. doi: 10.1016/0003-9861(69)90556-6. [DOI] [PubMed] [Google Scholar]
- Norio R., Nevanlinna H. R., Perheentupa J. Hereditary diseases in Finland; rare flora in rare soul. Ann Clin Res. 1973 Jun;5(3):109–141. [PubMed] [Google Scholar]
- Okamura-Ikeda K., Fujiwara K., Motokawa Y. Mechanism of the glycine cleavage reaction. Properties of the reverse reaction catalyzed by T-protein. J Biol Chem. 1987 May 15;262(14):6746–6749. [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K., Hayasaka K. Non-ketotic hyperglycinaemia: clinical and biochemical aspects. Eur J Pediatr. 1987 May;146(3):221–227. doi: 10.1007/BF00716464. [DOI] [PubMed] [Google Scholar]
- Tada K., Narisawa K., Yoshida T., Konno T., Yokoyama Y. Hyperglycinemia: a defect in glycine cleavage reaction. Tohoku J Exp Med. 1969 Jul;98(3):289–296. doi: 10.1620/tjem.98.289. [DOI] [PubMed] [Google Scholar]
- Tada K. Nonketotic hyperglycinemia: clinical and metabolic aspects. Enzyme. 1987;38(1-4):27–35. doi: 10.1159/000469187. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wendt L., Hirvasniemi A., Similä S. Nonketotic hyperglycinemia. A genetic study of 13 Finnish families. Clin Genet. 1979 May;15(5):411–417. doi: 10.1111/j.1399-0004.1979.tb01773.x. [DOI] [PubMed] [Google Scholar]