Abstract
Background
The clinical perspective on hepatic growth is limited. The goal of the present study was to compare hepatic hypertrophy and the kinetic growth rate(KGR) in patients after the ALPPS (Associating Liver Partition with Portal Vein Ligation for Staged Hepatectomy) procedure, portal vein embolization (PVE) and living donor liver transplantation.
Methods
Volumetry and KGR of the future liver remnant (FLR) were compared from (15) patients undergoing ALPPS, (53) patients undergoing PVE, (90) recipients of living donor liver grafts and (93) donors of living donor liver grafts.
Results
The degree of hypertrophy was significantly greater after ALPPS (84.3 ± 7.8%) than after PVE (36.0 ± 27.2%) (P < 0.001). The KGR was also significantly greater for ALPPS [32.7 ± 13.6 cubic centimetres (cc)/day] (10.8 ± 4.5%/day) compared with PVE (4.4 ± 3.2 cc/day) (0.98 ± 0.75%/day) (P < 0.001). The FLR of living donor donors had the greatest degree of hypertrophy (107.5 ± 39.2%) and was greater than after ALPPS (P = 0.02), PVE (P < 0.001) and in living donor-recipient grafts (P < 0.001). KGR (cc/day) was greater in FLR of living donor donors compared with both ALPPS (P < 0.001) and PVE (P < 0.001). The KGR in patients undergoing ALPPS and living donor liver transplantation had a linear relationship with the size of FLR.
Conclusion
FLR hypertrophy and KGR were greater after ALPPS than PVE. However, the degree of hypertrophy after ALPPS is not unprecedented, as KGR in the FLR from living donor donors is equal to or greater than after ALPPS. The KGR of the FLR in patients after ALPPS and living donor donors correlates directly with the size of the FLR.
Introduction
Hepatic resection provides potentially curative treatment for a variety of primary hepatobiliary malignancies a well as for numerous metastatic malignancies. As more extensive hepatic resections are performed, achieving an adequate functioning future liver remnant (FLR) often remains the rate limiting step.1 Portal vein embolization (PVE) has been repeatedly shown as a reliable technique to induce atrophy of the embolized lobe and compensatory hypertrophy of the FLR, and currently remains the standard for achieving an appropriate FLR before hepatic resection.2 The kinetic growth rate (KGR) of the hepatic FLR after PVE has been reported to average 2.4% per week achieving an increase in FLR of 10% to 46% after 2 to 8 weeks.3
Recently a novel operative approach, the ALPPS procedure (Associating Liver Partition with Portal Vein Ligation for Staged Hepatectomy), has been used to induce hypertrophy of the FLR and expedite stages of hepatic resections. This technique was noteworthy for the apparent accelerated rate and degree of hypertrophy of the FLR.4 In fact, data show that the standardized future liver remnant (sFLR) has grown 40–160% in only 6–9 days after ALPPS.4–12 A single study comparing ALPPS with PVE has demonstrated that the hepatic growth rate was 11 times greater after ALPPS (34.8 cc/day) than after PVE/PVL [3 cubic centimetres (cc)/day].13 Authors have suggested that the rate and degree of hepatic growth after ALPPS is unparalleled.7 The mechanisms of the apparent profound hepatic growth of the FLR after ALPPS are unknown. Previous authors have proposed that closure of the right portal branch (through ligation or embolization) is followed by a reactive perfusion of the ‘deportalized’ liver, from the contralateral one, through the intrahepatic branches and collaterals present between the two lobes.14,15 Indeed partitioning of the liver is the essential difference between ALPPS and PVE, resulting in the division of any communicating branches. Alternatively the deportalized liver after ALPPS may release uniquely circulating inflammatory or growth factors accounting for the accelerated growth.16 It is likely that in all settings, liver growth is multifactorial.
To date, no study has compared the degree and rate of growth of the FLR in patients after PVE and ALPPS with growth after other scenarios involving a hepatic resection. We and others have observed rapid growth of the FLR from donors participating in living donor (LD) liver transplantation.17 The goal of the present study was to compare the degree and rate of hepatic hypertrophy after ALPPS, PVE and LD transplantation to determine whether clinical circumstances associated with major hepatic resections correlated with remnant growth.
Patients and methods
This study was performed with approval of the Mayo Clinic Rochester Institutional Review Boards at the Mayo Clinic College of Medicine, Rochester, Minnesota, USA and Western University, London, Ontario, Canada. Data were abstracted from patient medical records and from a prospectively maintained database on all patients undergoing living donor (LD) liver transplantation, hepatic resection and the ALPPS procedure. Patients belonged to one of four groups: patients who had an ALPPS procedure between April 2012 and November 2013, patients who had PVE and underwent a major hepatic resection or the first part of a staged resection between January 2009–November 2013, LD transplant donors and LD transplant recipients between June 2000 and November 2013. The inclusion dates reflected when the ALPPS procedure was first performed in the aforementioned institutions, as well as when volumetric data were first acquired for the other groups. All patients with available volumetric data were included. As part of our standard protocols, ALPPS and LD transplant (recipients and donors) underwent a CT scan with hepatic volumetry pre-procedure and 7 days post-procedure. CT volumetry was performed in patients undergoing PVE immediately prior to embolization and at 3–6 weeks after a PVE just prior to a major hepatic resection. PVE embolization with segment 4 branches was used when clinically feasible and indicated in patients anticipating segment 4 resection.
The liver volumes were determined by loading the CT images onto a TeraRecon Aquarius workstation (San Mateo, CA, USA). Standardized total liver volume (sTLV) was calculated using the previously published formula: −794.41 + 1267.28 × body surface area (m2).18 The Mosteller formula was used to calculate the body surface area. sFLR was calculated accordingly as FLR/sTLV*100%. The degree of hypertrophy (DH) was defined as the percentage-point difference between the sFLR volume before and after the intervention (PVE, ALPPS and LD donation).19 Kinetic growth rate (KGR) was calculated as both percentage growth per day [DH at the first post-intervention volume assessment (%)/elapsed interval from intervention (days)] as well as cc growth per day (FLR after intervention – FLR prior to intervention/time elapsed).
In those patients that underwent a liver resection, post-operative liver failure was determined. Post-operative liver failure was defined according to the 50-50 criteria [PT < 50% international normalized ratio (INR >1.7) and a serum bilirubin level >2.92 mg/dl on post-operative day 5]20 and by a peak total bilirubin level >7 mg/dl.21 Death from liver failure was calculated at 90 days after surgical resection. In the setting of ALPPS, death from liver failure was calculated at 90 days after the second-stage resection. In patients undergoing a two-staged hepatectomy with PVE, death from liver failure was also calculated at 90 days after the second stage.
Statistical analysis
All statistical analyses were performed using STATA 12 (Stata Corp., College Station, TX, USA). Differences between groups were analysed using the unpaired t-test for continuous variables and by the χ2-test or continuity correction method for categorical variables. Wilcoxon's rank-sum was used for variables that did not display a normal distribution. All statistical tests were two-sided, and differences were considered significant when P < 0.05.
Results
We identified 15 patients who underwent ALPPS procedures, 53 who underwent PVE and major hepatic resection or the first part of a staged resection, 90 who were recipients of a LD graft and 93 who were donors of a LD graft which had complete volumetric data. Three of the LD recipients did not have volumetric data, accounting for the difference in the LD donor and LD recipient numbers. Patient demographics for the four groups are shown in Table 1. There was no significant difference in patient age between the ALPPS (55.9 ± 12.1 years) and PVE (59.5 ± 11.3 years) groups, (P = 0.29), respectively. Similarly, the proportion of male patients did not differ between the ALPPS (73%) and PVE groups (58%) (P = 0.3). The body mass index (BMI) [26.2 ± 4.2 kg/m2 versus 27.9 ± 6.8 kg/m2 (P = 0.36)] and the frequency of diabetes [7% and 8% (P = 0.91)] were similar between the ALPPS and PVE groups, respectively.
Table 1.
Patient demographics
| ALPPS (n = 15) | PVE (n = 53) | LD (recip) (n = 90) | LD (donor) (n = 93) | P value (ALPPS versus PVE) | |
|---|---|---|---|---|---|
| Age (years) | 55.9 ± 12.1 | 59.5 ± 11.3 | 50.1 ± 12.8 | 38.0 ± 9.2 | 0.29 |
| Gender(male) | 11 (73%) | 31 (58%) | 56 (62%) | 41 (44%) | 0.3 |
| BMI | 26.2 ± 4.2 | 27.9 ± 6.8 | 27.6 ± 5.4 | 25.6 ± 3.5 | 0.36 |
| Diabetes | 1 (7%) | 4 (8%) | 12 (13%) | 1 (1%) | 0.91 |
| Diagnosis | |||||
| CRLM | 14 (93%) | 37 (70%) | NA | NA | 0.06 |
| Cholangiocarcinoma | 0 | 7 (13%) | NA | NA | 0.14 |
| HCC | 0 | 2 (4%) | NA | NA | 0.45 |
| Other | 1 (7%) | 7 (13%) | NA | NA | 0.49 |
| Transplant Indication | |||||
| Cholangiocarcinoma | NA | NA | 36 (40%) | NA | NA |
| PSC | NA | NA | 20 (22%) | NA | NA |
| Hepatitis C | NA | NA | 5 (6%) | NA | NA |
| NASH | NA | NA | 4 (4%) | NA | NA |
| Other | NA | NA | 25 (28%) | NA | NA |
| Chemotherapy | 14 (93%) | 40 (75%) | NA | NA | 0.13 |
| Failure to complete planned resection | 0 | 11 (21%) | NA | NA | 0.05 |
| 50/50 Criteria | 2 (13%) | 12 (29%)a | NA | 13 (14%) | 0.24 |
| Peak bilirubin >7 mg/dl | 2 (13%) | 4 (10%)a | NA | 3 (3%) | 0.68 |
| 90 day mortality | 0 (0%) | 2 (5%)a | 3 (3%) | 0(0%) | 0.39 |
BMI, body mass index; HCC, hepatocellular carcinoma; LD, living donor; NASH, non-alcoholic steatohepatitis; PSC, primary sclerosing cholangitis; PVE, portal vein embolization.
= of 42 patients that completed the planned resection.
The mean age in the LD donor group was 38.0 ± 9.2 years, which was lower than the ALPPS (P < 0.001), PVE (P < 0.001) and LD recipient (P < 0.001) groups. The LD donor group also had a lower proportion of male patients (44%) than the ALPPS group (P = 0.4). The BMI was not significantly different from the ALPPS group (P = 0.55) but was lower than the PVE (P = 0.008) and LD recipient groups (P = 0.003). Only one patient had diabetes (1%) which did not differ from the ALPPS group (P = 0.14) but was significantly lower than the PVE (P = 0.04) and LD recipient groups (P < 0.001).
Hepatic metastases from colorectal cancer were the predominant diagnoses for both patients in the ALPPS group (93%) and in the PVE group (70%) which was not statistically significant. One patient in the ALPPS group had a gastrointestinal stromal tumour (GIST). Other diagnoses in the PVE group included hilar cholangiocarcionoma (n = 3), intrahepatic cholangiocarcinoma (n = 3), hepatocellular carcinoma (n = 2), GIST (n = 2), a metatstatic gastrointestinal neuroendocrine tumour (n = 4). gallbladder cancer (n = 1), metastatic adrenocortical carcinoma (n = 1) and metastatic sarcoma (n = 1). None of the patients in the ALPPS group had underlying cirrhosis. One of the patients with HCC in the PVE group had mild cirrhosis, Childs–Pugh A5. All patients with CRLM in both groups had received neoadjuvant chemotherapy as well as three patients in the PVE group with intrahepatic cholangiocarcinoma. All patients in the ALPPS group completed their planned resections compared with 79% of patients in the PVE group. Reasons for not completing the intended resection in the PVE group included disease progression (n = 8) and inadequate hypertrophy (n = 3). The indication for transplantation in the LD recipient group included: cholangiocarcinoma in the setting of primary sclerosing cholangitis (PSC) (n = 20), de novo hilar cholangiocarcinoma (n = 16), PSC (n = 20), primary biliary cirrhosis (n = 9), hepatitis C (n = 5), NASH (n = 4), metastatic neuroendocrine (n = 4), hepatocellular carcinoma (n = 4) and other (n = 8).
Table 2 summarizes volumetric measurements of sFLR before and after ALPPS, PVE and after LD liver transplantation in both donors and recipients. Growth of the sFLR is shown in Fig. 1. The degree of hypertrophy (DH) was significantly greater after ALPPS (84.3 ± 7.8%) than after PVE (36.0 ± 27.2%) (P < 0.001) and LD recipients (49.2 ± 26.0%) (P = 0.002). The FLR of LD donors had the greatest DH (107.5 ± 39.2%) and significantly exceeded that after ALPPS (P = 0.02), PVE (P < 0.001) and LD recipients (P < 0.001).
Table 2.
Future liver remnant growth
| Procedure | FLR – pre (cc) | sFLR – pre (%) | FLR – post (cc) | sFLR – Post (%) | Time interval (days) | DH (%) | KGR (cc/d) | KGR (%/d) |
|---|---|---|---|---|---|---|---|---|
| ALPPS (n = 15) | 312.9 ± 84.7 | 20.1 ± 3.8 | 566.8 ± 147.6 | 36.1 ± 6.4 | 7.8 ± 1.1 | 84.3 ± 7.8 | 32.7 ± 13.6 | 10.8 ± 4.5 |
| PVE (n = 53) | 524.9 ± 219.5 | 31.4 ± 13.7 | 686.2 ± 250.8 | 41.0 ± 15.3 | 39.9 ± 14.2 | 36.0 ± 27.2 | 4.4 ± 3.2 | 0.98 ± 0.79 |
| LD (recip) (n = 90) | 968.2 ± 243.7 | 58.8 ± 11.7 | 1404.6 ± 279.8 | 86.1 ± 17.1 | 7.2 ± 1.0 | 49.2 ± 26.0 | 60.4 ± 28.9 | 6.8 ± 3.7 |
| LD (donor) (n = 93) | 479.9 ± 208.1 | 28.8 ± 9.4 | 946.1 ± 237.8 | 56.9 ± 9.2 | 6.9 ± 0.60 | 107.5 ± 39.2 | 67.9 ± 22.5 | 15.7 ± 5.9 |
cc, cubic centimetres; DH, degree hypertrophy; FLR, future liver remnant; KGH, kinetic growth rate; LD, living donor; PVE, portal vein embolization; sFLR, standardized future liver remnant.
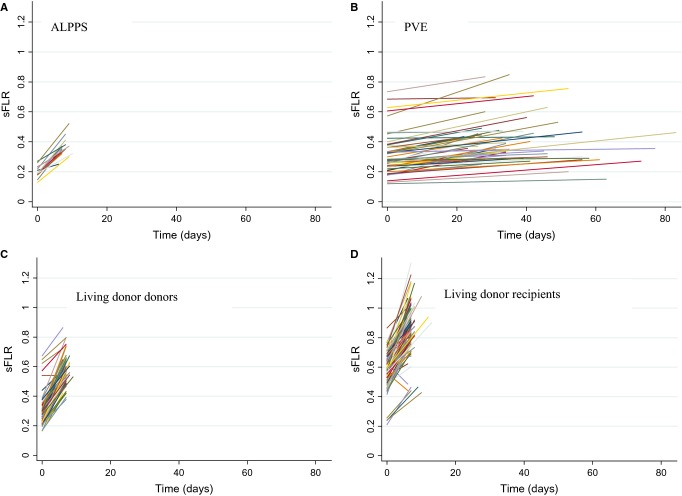
Figure 1.
Extrapolation of kinetic growth of standardized future liver remnant (sFLR) volumes determined by volumetry prior to and after intervention in the four groups: a) ALPPS, b) portal vein embolization (PVE), c) living donor donors and d) living-donor recipients
The KGR was significantly greater after ALPPS (32.7 ± 13.6cc/day) (10.8 ± 4.5%/day) than after PVE (4.4 ± 3.2cc/day) (0.98 ± 0.75%/day) (P < 0.001). KGR in cc/day was greater for LD donors compared with both ALPPS (P < 0.001) and PVE (P < 0.001). KGR in %/day was greater than ALPPS (P = 0.003), PVE (P < 0.001) and living-donor recipients (P < 0.001). The KGR in cc/day was similar after LD donors and LD recipients (P = 0.051). KGR in (%/day) and initial sFLR volume was plotted for all patients (Fig. 2). An inverse correlation between KGR (%/day) and sFLR was suggested. KGR (%/day) decreased with increasing volume of the initial sFLR (P < 0.001). There was a 1.6%/day decrease in KGR for every 0.1 (10%) increase in initial sFLR volume. A similar KGR%/day was seen for ALPPS, LD recipients and LD donors with a similar initial sFLR volume. Patients undergoing PVE had the least KGR regardless of the initial sFLR volume. There was no sFLR minimum size cutoff below which hypertrophy was impaired. KGR was similar for all patients undergoing PVE that was significantly less than all other groups. The relationship of KGR (cc/day increase) and initial sFLR volume was also plotted for ALPPS, LD recipients and LD donors. A trend of 1.81cc/day decrease in KGR for every 0.1 (10%) increase in the size of the initial sFLR was shown (Fig. 3).
Figure 2.
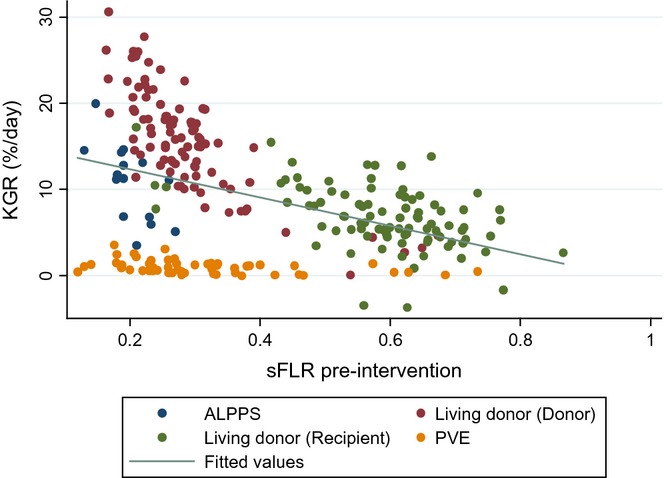
The kinetic growth rate (KGR) (%/day) plotted versus pre-intervention standardized future liver remnant (sFLR) size in the ALPPS, portal vein embolization (PVE), living donor donor and living donor recipient groups. Inverse linear relationship between pre-intervention sFLR volume and KGR (P < 0.001)
Figure 3.
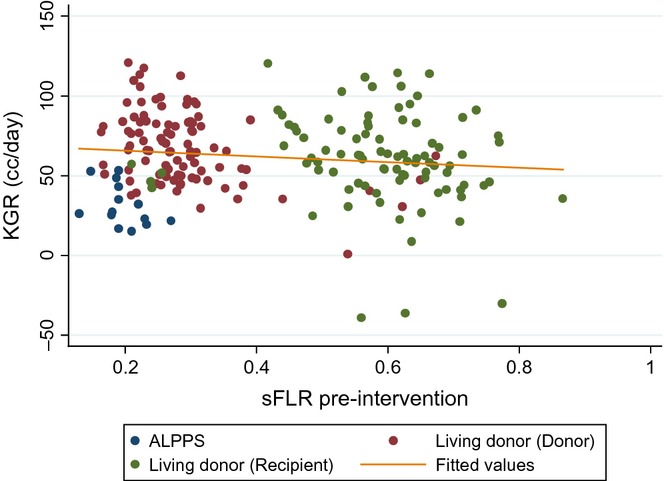
Kinetic growth rate (cc/day) plotted versus pre-intervention standardized future liver remnant (sFLR) volume in the ALPPS, living donor donor and living donor recipient groups
Separate multivariate linear regressions were performed examining DH, KGR (cc/day) and KGR (%/day). Analysis was adjusted for pre-intervention sFLR and patient age. For all three growth measures [DH, KGR (cc/day and KGR (%/day)], LD donors had a significantly greater growth (P < 0.001) than ALPPS, whereas ALPPS has greater growth than PVE (P < 0.001).
A subgroup analysis was performed on all LD donors. There was no difference by gender in DH, KGR (cc/day increase) or KGR (%/day increase) after adjustment for the size of the initial sFLR. A statistically significant decrease in KGR (cc/day) was seen with increasing age even after adjusting for the size of the initial sFLR (P = 0.002). The rate of decrease was 7.14cc/day in KGR for every 10-years increase in LD donor age. For DH and KGR (%/day increase) trends of decreasing growth with increasing age were seen. However, these were not statistically significant; P = 0.09, P = 0.07, respectively.
Post-Operative liver failure was assessed by two widely used criteria. In the ALPPS group, two patients (13%) had liver failure using both the 50/50 criteria and peak bilirubin >7 mg/dl. There was no association between KGR and liver failure in this group. There were no patient deaths within 90 days. In the PVE group, 12 (29%) of the 42 patients that underwent a liver resection had liver failure by the 50/50 criteria. Patients with a KGR of greater than 2%/week had a higher likelihood of liver failure (67%) compared with those not meeting the definition (18%) (P = 0.002). Only four patients in the PVE group (10%) had liver failure based on a bilirubin >7 mg/dl. Liver failure did not correlate with KGR [>2% (11%) versus <2%/week (9%)], (P = 0.85). There were two patient deaths within 90 days in the PVE group. There was no association between KGR and 90-day mortality in the PVE group. In the LD donor group, 13 (14%) patient met the definition of liver failure using the 50/50 criteria whereas 3 (3%) patients met the criteria using and peak bilirubin >7 mg/dl, although no donors required a liver transplantation, and all regained normal liver function by 30 days post-resection. There was no association between KGR and liver failure in this group. No patient deaths within 90 days occurred. In the LD-recipient group, owing to abnormal bilirubin and INR levels in many patients prior to surgery, the definitions of liver failure were not used. Three (3%) patient deaths occurred in this group within 90 days. None of these deaths were related to liver insufficiency.
Discussion
Issues related to the size of the FLR have become increasing relevant in hepatic surgery as the envelope of resectability with larger and more complex hepatic resections continues to expand. PVE has reliably and successfully increased the volume of the FLR. It remains the standard pre-operative intervention to induce hypertrophy of the FLR for more extensive hepatic resections.18 More recently the ALPPS procedure has also led to increases in the FLR but the growth rate of ALPPS (34.8 cc/day) compared with PVE/PVL (3 cc/day) was 11 times greater.13 Those findings suggested that the ALPPS procedure represents an unparalleled rate and degree of hypertrophy.7 Our study sought to revisit the issue of FLR growth after ALPPS and PVE and provide additional perspectives on FLR growth associated with LD liver transplantation in which rapid hypertrophy has been observed.17 The main findings of our study were that we confirmed that the significant degree and rate of growth of the FLR associated with ALPPS compared with PVE. Importantly, we also showed that marked FLR growth is not unique to ALPPS and, in fact, were exceeded by that seen in the FLR of LD donors.
Although our study did demonstrate that the degree and rate of hepatic growth with ALPPS was greater than PVE, the impression that such growth is unprecedented or unique was dispelled. Previous authors have shown the vast majority of liver regeneration occurs within the first week after major hepatic resections.22,23 Indeed, after a right hepatectomy the FLR volume has been shown to increase by 64%.24 Even more strikingly we have shown a 107.5% increase in FLR volume in LD donors at 1 week, which represented a significantly greater FLR growth than that in the ALPPS group. The perspective presented by these findings is novel, as most studies investigating liver hypertrophy report volumetry of the remnant based on CT imaging at 1 month or later after the initial procedure.25 Such an interval assessment precludes accurate capture of the early growth kinetics of the FLR, which is not linear. Importantly both the ALPPS and LD groups had CT scans performed at 7 days allowing direct comparison of KGR over this period. When comparing KGR in terms of %/day, LD donors had the greatest rate followed by ALPPS then LD recipients and then PVE. LD donors possess an optimal situation for liver hypertrophy given their young age, low comorbidities and lack of hepatotoxicity from chemotherapy; compared with patients undergoing PVE or ALPPS. It is therefore still impressive that patients undergoing ALPPS, who had a level of underlying hepatotoxicity, had hyperaphy and KGR comparable to LD donors.
We also sought to determine whether KGR (% volume increase/day) was related to FLR volume. When KGR (%/day) was plotted against the volume of the FLR, the correlation showed a clear linear trend of increasing KGR with a smaller sFLR regardless of the procedure. These findings support the noteworthy observation that FLR growth after ALPPS is marked, but not unique. The term ‘unprecedented’ growth when describing the hypertrophy seen with ALPPS should, therefore, be abandoned. In fact, FLR growth after ALPPS reflects a response that is expected based on the volume of the FLR after an extended hepatectomy. Although FLR growth after ALPPS and in LD donors is seemingly spectacular, it actually reflects a perspective from volumetry obtained only 7 days after an extended hepatic resection,which is not usually obtained at such a brief post-operative interval and early non-linear hepatic growth kinetics. We have also demonstrated that both the rate and extent of FLR growth was significantly less after PVE. Interestingly the PVE group was the only group herein in which a parenchymal transection was not performed. Preliminary animal studies have suggested that a parenchymal transection may play a key role in the release of inflammatory or growth mediators.16 The validity of this theory remains unproven. It is also unclear whether the parenchymal splitting or simply trauma related to the surgery plays a greater role in instigating liver regeneration. The findings of a lower KGR after PVE also reflect the delayed timing of volumetry and non-linear hepatic growth kinetics. Importantly, hepatic growth after resection or PVE slows over time. KGR, in part, addresses this issue and identifies impaired growth capacity in some patients.20 Both the rate and volume of FLR growth are important clinically and decreases in either aspect of hepatic growth increases the risk of hepatic failure.
The growth characteristic of the FLR in LD donors, although remarkable, is not unexpected. These patients were highly selected to exclude any underlying hepatic disease, and all exceeded minimal volumetric criteria of the planned FLR (≥ 30%). All LD donors had a normal performance status and were significantly younger in age than the other groups and, therefore, the potential for optimal hepatic regeneration was expected. This was shown in our subgroup analysis of this group that demonstrated a decrease of 7.14cc/day in KGR for every 10-year increase in the LD donor age. Conversely, patients in the ALPPS and PVE groups frequently had an impaired performance status and had undergone pre-operative chemotherapy and therefore their livers probably had some degree of injury. What direct effect this may have had on hepatic regeneration is not yet clear.26 It is interesting to note that the ALPPS patients demonstrated a higher degree of hypertrophy than the LD-recipient group, who obviously had more comorbidities than the LD donor group. In addition, LD recipients received post-operative immunosuppression that may have effects on liver regeneration. Clinical factors such as biliary tract infection, cholestasis and degree of fibrosis or cirrhosis may adversely affect hepatic regeneration as well. Thus, the KGR herein may not be widely applicable to other patient cohorts.
Some authors have suggested that major liver resections should be avoided in patients with low a KGR because these patients have a strongly increased risk of post-operative liver failure.18 Indeed these authors suggested that a KGR of < 2% per week (0.29%/day) was associated with a greater rate of hepatic insufficiency. We did not find a correlation between hepatic insufficiency and KGR in the ALPPS group, although all the patients had a KGR >2% per week. Whether the process of liver regeneration varies in different clinical settings is unknown and cannot be determined by this study. We did, however, find that a KGR of < 2% per week (0.29%/day) was associated with a greater rate of hepatic insufficiency using the 50/50 criteria in the PVE group. Interestingly others have shown that in patients with insufficient FLR growth after PVE salvage the ALPPS approach has provided adequate growth for selected patients.27 In the present study, two patients with insufficient FLR growth after PVE underwent an ALPPS resection without post-operative hepatic insufficiency and subsequent FLR growth.
In conclusion, we have demonstrated a greater FLR volume and KGR after the ALPPS procedure than after PVE, although most patients had adequate FLR growth regardless. We have also demonstrated that FLR growth after ALPPS is not unprecedented. Indeed similar or even greater KGR has been seen in FLR of LD livers. The KGR of patients undergoing both the ALPPS procedure and LD liver transplantation appears inversely related to the volume of the sFLR. The importance of the present study is, therefore not to provide a pro or con argument for ALPPS, but simply to put the liver hypertrophy and KGR seen with this novel procedure into context.
Contributions
Participated in research design: Kris Croome, David Nagorney; participated in the writing of the paper: Kris Croome, David Nagorney; participated in the performance of the research: Kris Croome, David Nagorney, Maile Parker, Roberto Hernandez-Alejandro, Julie Heimbach, Charles Rosen; participated in data analysis: Kris Croome, David Nagorney.
Conflicts of interest
None of the authors have any conflict of interest to disclose.
The manuscript did not receive any funding.
References
- Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–681. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009;197:686–690. doi: 10.1016/j.amjsurg.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, Croome KP. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 2015;157:194–201. doi: 10.1016/j.surg.2014.08.041. [DOI] [PubMed] [Google Scholar]
- Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814–821. doi: 10.1007/s11605-012-2092-2. [DOI] [PubMed] [Google Scholar]
- Sala S, Ardiles V, Ulla M, Alvarez F, Pekolj J, de Santibañes E. Our initial experience with ALPPS technique: encouraging results. Updates Surg. 2012;64:167–172. doi: 10.1007/s13304-012-0175-y. [DOI] [PubMed] [Google Scholar]
- de Santibanes E, Alvarez FA, Ardiles V. How to avoid postoperative liver failure: a novel method. World J Surg. 2012;36:125–128. doi: 10.1007/s00268-011-1331-0. [DOI] [PubMed] [Google Scholar]
- Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388–394. doi: 10.1002/bjs.8955. [DOI] [PubMed] [Google Scholar]
- Torres OJ, Fernandes ED, Oliveira CV, Lima CX, Waechter FL, Moraes-Junior JM, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig. 2013;26:40–43. doi: 10.1590/s0102-67202013000100009. [DOI] [PubMed] [Google Scholar]
- Li J, Girotti P, Königsrainer I, Ladurner R, Königsrainer A, Nadalin S. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg. 2013;17:956–961. doi: 10.1007/s11605-012-2132-y. [DOI] [PubMed] [Google Scholar]
- de Santibanes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the ‘ALPPS’ approach. Ann Surg. 2012;255:415–417. doi: 10.1097/SLA.0b013e318248577d. [DOI] [PubMed] [Google Scholar]
- Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510–1519. doi: 10.1007/s00268-014-2513-3. [DOI] [PubMed] [Google Scholar]
- Wilms C, Mueller L, Lenk C, Wittkugel O, Helmke K, Krupski-Berdien G, et al. Comparative study of portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant using a mini-pig model. Ann Surg. 2008;247:825–834. doi: 10.1097/SLA.0b013e31816a9d7c. [DOI] [PubMed] [Google Scholar]
- van Lienden KP, Hoekstra LT, Bennink RJ, van Gulik TM. Intrahepatic left to right portoportal venous collateral vascular formation in patients undergoing right portal vein ligation. Cardiovasc Intervent Radiol. 2013;36:1572–1579. doi: 10.1007/s00270-013-0591-5. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Limani P, Melloul E, Lesurtel M, Humar B, Graf R, et al. 2014. From humans back to mice. Systemic triggering Of unprecedented liver regeneration in alpps. European Surgical Association: Annual meeting, Athens, Greece. [DOI] [PubMed]
- Nadalin S, Testa G, Malagó M, Beste M, Frilling A, Schroeder T, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl. 2004;10:1024–1029. doi: 10.1002/lt.20182. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201–209. doi: 10.1016/j.jamcollsurg.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50–50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Kele PG, de Boer M, van der Jagt EJ, Lisman T, Porte RJ. Early hepatic regeneration index and completeness of regeneration at 6 months after partial hepatectomy. Br J Surg. 2012;99:1113–1119. doi: 10.1002/bjs.8807. [DOI] [PubMed] [Google Scholar]
- Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, et al. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5–10. doi: 10.1097/01.TP.0000079064.08263.8E. [DOI] [PubMed] [Google Scholar]
- Zappa M, Dondero F, Sibert A, Vullierme MP, Belghiti J, Vilgrain V. Liver regeneration at day 7 after right hepatectomy: global and segmental volumetric analysis by using CT. Radiology. 2009;252:426–432. doi: 10.1148/radiol.2523080922. [DOI] [PubMed] [Google Scholar]
- Corrêa D, Schwartz L, Jarnagin WR, Tuorto S, DeMatteo R, D'Angelica MI, et al. Kinetics of liver volume changes in the first year after portal vein embolization. Arch Surg. 2010;145:351–354. doi: 10.1001/archsurg.2010.42. [DOI] [PubMed] [Google Scholar]
- Kele PG, van der Jagt EJ, Gouw AS, Lisman T, Porte RJ, de Boer MT. The impact of hepatic steatosis on liver regeneration after partial hepatectomy. Liver Int. 2013;33:469–475. doi: 10.1111/liv.12089. [DOI] [PubMed] [Google Scholar]
- Tschuor C, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion – an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39:1230–1235. doi: 10.1016/j.ejso.2013.08.009. [DOI] [PubMed] [Google Scholar]