Abstract
Background
Delayed gastric emptying (DGE) is a frequent cause of morbidity, prolonged hospital stay and readmission after a pancreaticoduodenectomy (PD). We sought to evaluate predictive peri-operative factors for DGE after a PD.
Methods
Four hundred and sixteen consecutive patients who underwent a PD at our tertiary referral centre were identified. Univariate and multivariate (MV) logistic regression models were used to assess peri-operative factors associated with the development of clinically significant DGE and a post-operative pancreatic fistula (POPF).
Results
DGE occurred in 24% of patients (n = 98) with Grades B and C occurring at 13.5% (n = 55) and 10.5% (n = 43), respectively. Using MV regression, a body mass index (BMI) ≥35 [odds ratio (OR) = 3.19], operating room (OR) length >5.5 h (OR = 2.72) and prophylactic octreotide use (OR = 2.04) were independently associated with an increased risk of DGE. DGE patients had a significantly longer median hospital stay (12 versus 7 days), higher 90-day readmission rates (32% versus 18%) and an increased incidence of a pancreatic fistula (59% versus 27%). When controlling for POPF, only OR length >5.5 h (OR 2.73) remained significantly associated with DGE.
Conclusions
DGE remains a significant cause of morbidity, increased hospital stay and readmission after PD. Our findings suggest patients with a BMI ≥35 or longer OR times have a higher risk of DGE either independently or through the development of POPF. These patients should be considered for possible enteral feeding tube placement along with limited octreotide use to decrease the potential risk and consequences of DGE.
Introduction
After a pancreaticoduodenectomy (PD), delayed gastric emptying (DGE) is one of the most common causes of post-operative morbidity affecting 15–40% of patients.1–5 DGE is strongly correlated with an increased hospital length of stay, cost, readmission and patient dissatisfaction.6–10 New approaches in surgical techniques, medical therapies and post-operative interventions have been successful at reducing post-operative mortality to <3%;2,7,11,12 however, these advances have not significantly reduced the number of patients who continue to suffer from the complications of DGE.
Although there have been several studies investigating the aetiology, associations and complications of DGE, no uniformly accepted definition for DGE after pancreatic surgery existed prior to 2007. In 2007, owing to the growing need for a consensus to evaluate the incidence and risk factors for the development of DGE, the International Study Group of Pancreatic Surgery (ISGPS) developed a standardized definition for classifying the severity of DGE into mild, moderate and severe based on nasogastric tube necessity and time-to-tolerate solid food intake. Moderate (Class B) DGE includes nasogastric tube (NGT) replacement between post-operative day 8 and 14 or an inability to tolerate solid oral intake by post-operative day 14. Severe (Class C) DGE includes nasogastric tube (NGT) replacement after postoperative day 14 or inability to tolerate solid oral intake by postoperative day 21. Even after this definition was accepted, there have been a paucity of studies investigating pre- and intra-operative factors that may help predict DGE in the hope of preventing its development and/or preparing for its consequences.
The objective of this study was to identify pre- and peri-operative factors associated with the development of DGE after a PD at our tertiary referral centre during the last 12 years with the aim of being able to predict those patients at highest risk and therefore develop interventional strategies to prevent DGE before its occurrence.
Patients and methods
Data source and cohort selection
Patients aged 18 years and older who underwent a PD at Vanderbilt University Medical Center between July 2000 and December 2012 were identified from a retrospective pancreatic patient database. Pre-operative variables collected included patient demographics, comorbidities and other patient-specific factors. Intra-operative variables collected included estimated blood loss (EBL), transfusion need, details of the surgical resection and reconstruction, operative time, tumour size and prophylactic octreotide use. DGE grade was classified as per the recommended ISGPS consensus definition as previously discussed. For our study, we defined clinically significant DGE as ISGPS class B or C as previously discussed. Data on the incidence and associations of post-operative pancreatic fistula (POPF) after a PD were also obtained and classified according to the International Study Group on Pancreatic Fistula (ISGPF) recommended guidelines with Grade B and C POPF considered clinically significant.13
Post-operative management
Patients undergoing PD were entered on a clinical pathway for the management of their post-operative course. NGTs placed in the operating room were discontinued on post-operative day 1 if patients were not nauseated and if NGT output was minimal. They were then advanced to clear liquids and upon return of bowel function, advanced to a low-fat diet. Metoclopramide (Reglan) was routinely started on post-operative day 3 if not contraindicated. Erythromycin, however, was not given routinely.
Statistical analysis
Univariate comparisons between patient cohorts were performed using chi-squared or Fisher's exact tests. Forward selection and backward elimination stepwise multivariate (MV) logistic regression models were constructed to estimate the effects of pre- and intra-operative factors on the incidence of DGE. All variables were selected a priori, and variables with P < 0.30 were included as independent factors in the final MV regression model. Univariate associations between the incidence of DGE and length of hospital stay, 90-day readmission and pancreatic fistulas/leaks were also performed. Analyses were performed using STATA 13.1 statistical software (Stata Corporation, College Station, TX, USA).
This study was approved by the Institutional Review Board of the VUMC Human Research Protection Program.
Results
Cohort
In our pancreatic database, a total of 416 patients were identified to have undergone a PD from July 2000 to December 2012 and were included in the cohort. Of these patients, 153 (37%) patients were found to have DGE (all grades). A total of 98 patients (24%) were diagnosed with Grade B (55 patients, 13.2%) or Grade C (43 patients, 10.3%) DGE. For the purposes of this study, we defined these Grade B and C patients as having clinically significant DGE and form the basis of our analysis.
Demographics
Demographic comparisons of those patients having clinically significant DGE versus those who did not are shown in Table 1a. The only factor showing a statistical difference between groups was race with a higher percentage of Caucasians in the DGE group than the non-DGE group (96% versus 87%, P = 0.02). Other factors, including mean age, gender and the incidence of malignancy, did not significantly differ between groups.
Table 1.
(a) Univariate analysis of pre-operative factors on delayed gastric emptying (DGE). (b) Univariate analysis of intra- and post-operative factors on DGE (Grade B or C)
| Variable | No DGE (n = 318) n (%) or mean ± SD | DGE a(n = 98) n (%) or mean ± SD | P-value |
|---|---|---|---|
| (a) | |||
| Demographics | |||
| Mean age (years ± SD) | 61.2 ± 13.7 | 62.2 ± 13.6 | 0.89 |
| Gender | 0.21 | ||
| Male (n) | 152 (48) | 54 (55) | |
| Female (n) | 166 (52) | 44 (45) | |
| Race | 0.02 | ||
| White | 277 (87) | 94 (96) | |
| Black/other | 40 (13) | 4 (4) | |
| Malignancy | 0.11 | ||
| No | 87 (27) | 35 (36) | |
| Yes | 231 (73) | 63 (64) | |
| Mean tumour size (cm ± SD) | 3.0 ± 1.9 | 2.9 ± 1.7 | 0.22 |
| Patient comorbidities | |||
| ASA class | 0.04 | ||
| 2 | 87 (27) | 16 (16) | |
| 3 | 205 (65) | 77 (79) | |
| 4 | 25 (8) | 5 (5) | |
| BMI | 0.02 | ||
| <35 | 295 (93) | 83 (85) | |
| ≥35 | 23 (7) | 15 (15) | |
| DM | 0.63 | ||
| No | 238 (75) | 78 (80) | |
| Yes | 80 (25) | 20 (20) | |
| CAD | 0.40 | ||
| No | 265 (83) | 78 (80) | |
| Yes | 53 (17) | 20 (20) | |
| CHF | 0.41 | ||
| No | 302 (95) | 95 (97) | |
| Yes | 16 (5) | 3 (3) | |
| Cirrhosis | 0.38 | ||
| No | 315 (99) | 96 (98) | |
| Yes | 3 (1) | 2 (2) | |
| Smoking history | 0.20 | ||
| No | 169 (54) | 45 (46) | |
| Yes < 5 pack-years | 53 (17) | 24 (24) | |
| Yes > 5 pack-years | 92 (29) | 29 (30) | |
| Patient characteristics | |||
| Pre-op Biliary Stent | 0.31 | ||
| No | 160 (51) | 55 (57) | |
| Yes | 155 (49) | 42 (43) | |
| Albumin (gm/dl) < 4.0 | 0.51 | ||
| No | 155 (49) | 44 (45) | |
| Yes | 163 (51) | 54 (55) | |
| Pre-op bilirubin (mg/dl ± SD) | 3.3 ± 5.9 | 2.7 ± 5.2 | 0.15 |
| Pre-op Chemotherapy | 0.25 | ||
| No | 301 (95) | 90 (92) | |
| Yes | 16 (5) | 8 (8) | |
| Pre-op radiation | 0.77 | ||
| No | 306 (97) | 94 (96) | |
| Yes | 11 (3) | 4 (4) | |
| Gastric outlet obstruction | 0.94 | ||
| No | 263 (83) | 81 (83) | |
| Yes | 54 (17) | 17 (17) | |
| Pre-op weight loss | 0.30 | ||
| No | 184 (58) | 51 (52) | |
| Yes | 133 (42) | 47 (48) | |
| (b) | |||
| Whipple reconstruction | 0.06 | ||
| Standard | 125 (39) | 32 (33) | |
| Pylorus-Preserving (PPPD) | 51 (16) | 26 (26) | |
| PPPD + Pyloroplasty | 142 (45) | 40 (41) | |
| Path of reconstruction | 0.41 | ||
| Antecolic | 187 (79) | 53 (73) | |
| Retrocolic | 45 (19) | 19 (26) | |
| Unknown | 5 (2) | 1 (1) | |
| PJ Stent | 0.25 | ||
| No | 164 (52) | 44 (45) | |
| Yes | 154 (48) | 54 (55) | |
| Vascular resection | 0.82 | ||
| No | 263 (83) | 82 (84) | |
| Yes | 55 (17) | 16 (16) | |
| Estimated blood loss (cc) | 632 ± 576 | 718 ± 635 | 0.23 |
| Blood products given | 0.40 | ||
| No | 222 (70) | 64 (65) | |
| Yes | 96 (30) | 34 (35) | |
| Operative time (h) | 0.05 | ||
| <5.5 | 122 (38) | 27 (28) | |
| >5.5 | 196 (62) | 71 (72) | |
| Prophylactic Octreotide | 0.11 | ||
| No | 219 (69) | 59 (60) | |
| Yes | 99 (31) | 39 (40) | |
ISPGS Grade B or C.
ASA, American Society of Anesthesiologists; BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; CHF, congestive heart failure.
Approximately half of the patients were smokers, but this did not differ between groups.
Patient co-morbidities
In regards to patient co-morbidities, patients with DGE had a significantly higher American Society of Anesthesiologists (ASA) class (Table 1a). In addition, a higher percentage of DGE patients had a body mass index (BMI) of greater than or equal to 35 (15% versus 7%, P = 0.02). Although the incidence of diabetes mellitus (DM) and coronary artery disease (CAD) was prevalent in our cohort (20–25% and 17–20%), the incidence did not differ between the groups.
Pre-operative characteristics
As shown in Table 1a, both the DGE and non-DGE patient groups had similar pre-operative patient characteristics including the use of a biliary stent, serum albumin <4.0 gm/dl, the use of neoadjuvant therapy, the incidence of gastric outlet obstruction and pre-operative weight loss. Although the mean serum bilirubin (mg/dl) appeared to be lower in the DGE group (2.7 ± 5.2 versus 3.3 ± 5.9, P = 0.15), this was not statistically significant.
Intra- and post-operative characteristics
Details with regards to intra- and post-operative factors in both groups are shown in Table 1b. Patients with DGE were more likely to have had an operative time over 5.5 h as compared with those without DGE (72% versus 62%, P = 0.05). Patients who received octreotide prophylactically (intra-operative ± post-operative) tended to develop DGE more frequently (40% versus 31%); however, this was not statistically significant in the univariate analysis (P = 0.11). Other factors, including the type of reconstruction, the use of a pancreatico-jejunostomy (PJ) stent, vascular resection, EBL and the use of blood transfusions, were similar in both groups.
Peri-operative factors associated with DGE
All variables were added into the model sequentially using forward selection stepwise multivariable regression modelling. Those variables with P < 0.30 by MV regression were used in the final model. Variables included in the final model included malignancy, albumin < 4.0, smoking, race, operating room (OR) time >5.5 h, prophylactic octreotide use and a BMI ≥35. Of these, a BMI ≥35 [OR = 3.19; 95% confidence interval (CI) 1.24–8.18], operating room length >5.5 h (OR = 2.72; 95% CI 1.37–5.39) and prophylactic octreotide use (OR = 2.04; 95% CI 1.09–3.80) were independently associated with an increased risk of DGE as shown in Fig. 1. None of the other variables tested were associated with an increased risk of DGE.
Figure 1.
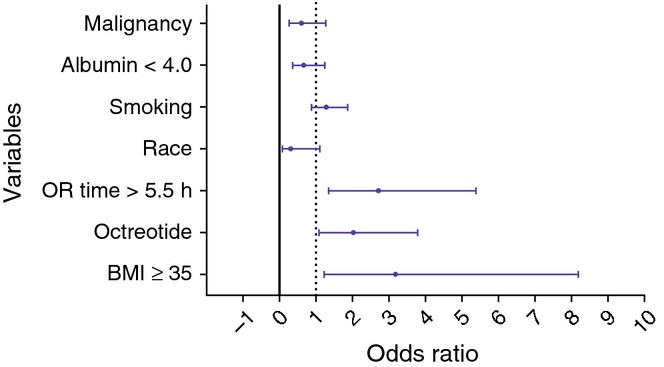
Forest plot, multivariate logistic regression analysis of factors associated with delayed gastric emptying. OR, operating room; BMI, body mass index
DGE and other associated complications
Patients with DGE had a significantly higher rate of post-operative pancreatic fistulae (POPF) as compared with those without DGE (59% versus 27% P < 0.001). This difference was particularly pronounced when looking at Grade B and Grade C POPF (52% versus 11%, P < 0.001) as shown in Fig. 2. Patients with clinically significant DGE also had a significantly longer median hospital stay [12 days interquartile range (IQR) = 6–17] versus 7 days (IQR = 3–8), P < 0.001] and a higher rate of 90-day readmission (32% versus 18%, P = 0.004) as shown in Fig. 3.
Figure 2.
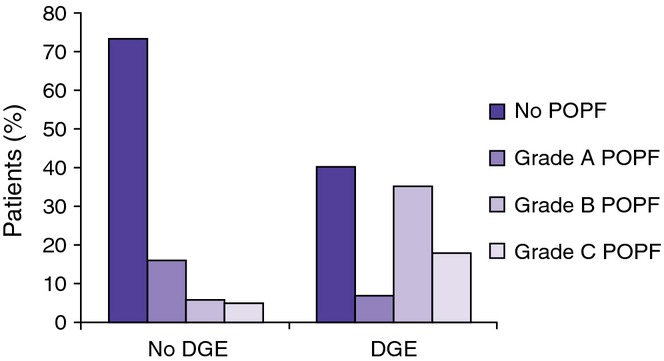
Patients with delayed gastric emptying (DGE) had a significantly higher rate of post-operative pancreatic fistulae (POPF) as compared with those without DGE (59% versus 27% P < 0.001). This was most pronounced when looking at clinically significant (Grade B and C) POPF with an incidence of 52% in patients with DGE versus those without DGE (11%, P < 0.0001)
Figure 3.
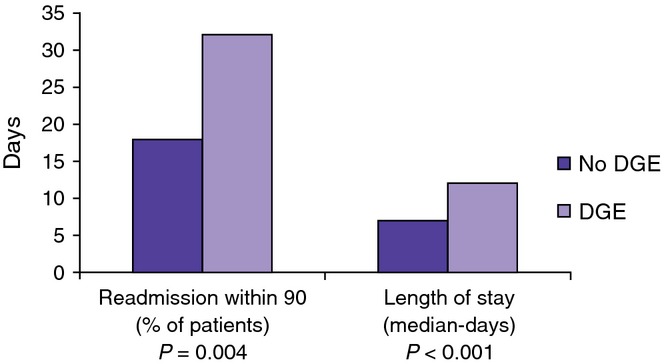
Patients with clinically significant delayed gastric emptying (DGE) had a significantly longer median hospital length of stay(LOS) [12 days (IQR=6–17) versus 7 days (IQR=3–8), P < 0.001] and a higher rate of 90-day readmission (32% versus 18%, P = 0.004)
Post-operative pancreatic fistula
As there was a very strong relationship between the development of DGE and POPF, we investigated the possible role of POPF as a confounder and possible causal factor in the development of DGE. When the development of POPF was added to the forward stepwise MV regression model for the development of DGE, the only factors that were significantly associated with DGE included operating length length >5.5 h (OR 2.73, 95% CI 1.32–5.64) and POPF (OR 10.4, 95% CI 5.1–21.4). When controlling for POPF, therefore, a BMI ≥35 and the use of octreotide were no longer associated with the development of DGE (data not shown).
In agreement with these results, when a stepwise MV regression modelling for the development of DGE was constructed in those patients without a clinically significant leak (n = 318), the only significant factors associated with the development of DGE was a operative time >5.5 h (OR 3.73, 95% CI 1.39–9.95, data not shown).
Discussion
This compilation of 416 patients from a single institution allows for a thorough analysis of patients after a PD and risk factors for the development of DGE according to the recent ISGPS definition. The overall incidence of DGE in this study was 37%, with 24% having Grades B or C DGE, consistent with other reports.1–3,5 This study, unlike most prior, classifies the severity of DGE based on the ISGPS definition. The present study also revealed significant independent pre- and peri-operative risk factors for developing clinically significant DGE, including a BMI ≥35 (OR 3.19), operative time >5.5 h (OR 2.72) and the prophylactic use of octreotide (OR 2.04). Similar to the prior series, DGE was also associated with a significantly longer median hospital stay, a higher 90-day readmission rate and an increased incidence of POPF.6,7,9,10 When investigating possible confounders for the association of these factors with the development of DGE, we found that the development of a POPF was the most significant factor in predicting DGE.
The pathogenesis of DGE is still largely unclear. Current hypotheses include pylorospasm secondary to denervation of the vagus nerves, ischaemia or congestion secondary to vascular compromise, and acute changes in plasma gastrointestinal hormone (specifically motilin) levels.14 In addition, prior reports show conflicting results on pre-operative variables that may contribute to DGE, including age, gender, race and gastric outlet obstruction.5,10,15–17 In the present study, however, these factors did not appear to play a role. Operative technique has in many studies been suggested to play a role in the development of DGE. The standard Whipple procedure versus the pylorus-preserving pancreaticoduodenectomy,3,18–22 antecolic versus retrocolic gastric/duodenal reconstruction,4,15,23 pancreaticogastrostomy versus pancreaticojejunostomy reconstruction,24,25 PJ stent placement26,27 and portal vein resection28 have been evaluated in many studies. However, the results are mixed and many of the studies are small cohorts with retrospective analyses that lack consistency in the definition of DGE. In the present study, operative technique including preservation of the pylorus, antecolic versus retrocolic reconstruction as well as vascular resection or the use of a PJ stent were not associated with differences in the incidence of DGE.
Comorbidities including DM, CAD, congestive heart failure (CHF), cirrhosis and smoking history did not show a statistically significant correlation with the development of DGE and were consistent with prior studies.1,3,5,10 The present study revealed that patients with severe obesity or greater, defined by a BMI of 35 or higher, was strongly associated with an increased development of DGE by multivariate analysis. There have been studies investigating the relationship between obesity and gastric emptying in both non-operative29,30 and operative patients;1,10,17,31 however, the present study is, to our knowledge, the first to show a relationship between BMI and DGE after a PD. As a BMI ≥35 was also associated with a significant risk of developing a POPF, we sought to investigate whether obesity directly led to an increase incidence of DGE or indirectly by increasing POPF. In the MV analysis of patients without a POPF as well as the MV analysis with POPF included in the model we found that a BMI ≥35 was not associated with the development of DGE, suggesting that the increase in DGE was actually secondary to a POPF and not as a result of an increased BMI. Nevertheless, as the development of a POPF is not known pre- or intra-operatively, it would still be important to counsel these patients that they may be at a higher risk of DGE (as well as a POPF) after a PD.
The only operative factor in the current study that was significantly associated with the development of DGE was operative time, as defined by time from incision to closure of the wound, of greater than 5.5 h. This was also the only factor to show significance for the development of DGE when POPF was added to the model as well as in patients who do not develop a POPF. Longer OR times than 5.5 h did not further increase the risk of DGE (data not shown). Presumably, increased complexity of the operation contributed to an increased OR time; however, other factors associated with increased complexity including EBL, the need for blood transfusions, as well as vascular resections were not associated with the development of DGE as suggested in some prior studies.28,32 Prior studies have evaluated operative time for an association with DGE; however, this is the first study to suggest that operative time is independently associated with a risk DGE after PD.10,22
The final factor shown to be associated with DGE in the current study was the use of prophylactic octreotide (Sandostatin). Octreotide is a somatostatin analogue shown to inhibit growth hormone, insulin and glucagon. It has been reported that even a subcutaneous administration of a single dose of octreotide can induce a marked delay in the gastric emptying of healthy individuals.33 Some prior studies have shown that octreotide use does not have a correlation with DGE,34,35 and a single study consisting of a small cohort of patients (n = 23) showed a much higher incidence of DGE with the use of prophylactic somatostatin (91% versus 25%).36,37 Similar to the case of BMI, however, the use of a prophylactic octreotide was significantly associated with the incidence of a POPF. As we only use a prophylactic octreotide in those patients considered to be a higher risk of a leak (including those with small pancreatic ducts and a soft pancreatic texture), this association is not surprising. It is interesting to note, however, that in the absence of a POPF, the use of octreotide did not increase the risk of DGE.
Post-operative complications have also been implicated as a leading factor in the development of DGE after a PD. Intra-abdominal complications including POPF, intra-abdominal abscess, post-operative sepsis, the need for reoperation, pancreatitis and pancreatic fibrosis have been associated with the development of DGE.1,10,16,17,19 Some studies have even suggested that DGE does not occur in the absence of other post-operative complications.3 Data from thus study are in agreement with these findings as POPF was the most significant factor associated with DGE. Measures to try to reduce POPF may in fact, therefore, reduce the incidence of DGE. Additional studies investigating a possible causal relationship between POPF and DGE are warranted, however, before making final conclusions.
As with all retrospective studies, our study has several limitations including incomplete documentation, interpretation bias, and difficulty establishing cause and effect. In addition, our study is limited in that it is a single-institutional study with a relatively small sample size, causing our analysis potentially to lack sufficient power to detect some significant factors associated with DGE. In addition, despite the utilization of clinical pathways, variability in the management of patients after a PD most certainly exist and may account for some of the observed differences.
In conclusion, our study confirms that despite improved surgical mortality after a PD, DGE remains a significant clinical problem. DGE is associated with significant other sequelae including significant increases in hospital length of stay and readmission rates compared with those who did not develop DGE. These associated sequelae may be as a direct result of DGE or as a result of associated complications of DGE including POPF, particularly as the development of POPF is one of the strongest predictors of the development of DGE. Indeed patients with the highest risk of pancreatico-jejunostomy leak may be those with a higher BMI, longer OR times, and in which prophylactic octreotide is used, are also at the highest risk of DGE (as well as POPF). Strategies to combat DGE after PD should also therefore focus on reducing the formation of POPF. In these patients deemed to be at higher risk of DGE as well as POPF, enteral feeding access (i.e. intra-operatively placed nasojejunal post-anastomotic feeding tubes or jejunostomy tubes) should be considered. Additional studies validating our findings are also warranted, particularly in a prospective manner and with stratification of the presence or absence of POPF, in order to further elucidate the causes and mechanisms of DGE to allow for potential interventions to decrease this major source of morbidity following pancreaticoduodenectomy.
Conflicts of interest
None declared.
References
- Parmar AD, Sheffield KM, Vargas GM, Pitt HA, Kilbane EM, Hall BL, et al. Factors associated with delayed gastric emptying after pancreaticoduodenectomy. HPB. 2013;15:763–772. doi: 10.1111/hpb.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. ; discussion 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann O, Markus PM, Ghadimi MB, Becker H. Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas. 2004;28:69–74. doi: 10.1097/00006676-200401000-00011. [DOI] [PubMed] [Google Scholar]
- Eshuis WJ, van Eijck CH, Gerhards MF, Coene PP, de Hingh IH, Karsten TM, et al. Antecolic versus retrocolic route of the gastroenteric anastomosis after pancreatoduodenectomy: a randomized controlled trial. Ann Surg. 2014;259:45–51. doi: 10.1097/SLA.0b013e3182a6f529. [DOI] [PubMed] [Google Scholar]
- Atema JJ, Eshuis WJ, Busch OR, van Gulik TM, Gouma DJ. Association of preoperative symptoms of gastric outlet obstruction with delayed gastric emptying after pancreatoduodenectomy. Surgery. 2013;154:583–588. doi: 10.1016/j.surg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Tan WJ, Kow AW, Liau KH. Moving towards the New International Study Group for Pancreatic Surgery (ISGPS) definitions in pancreaticoduodenectomy: a comparison between the old and new. HPB. 2011;13:566–572. doi: 10.1111/j.1477-2574.2011.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong ZV, Ferrone CR, Thayer SP, Wargo JA, Sahora K, Seefeld KJ, et al. Understanding hospital readmissions after pancreaticoduodenectomy: can we prevent them?: a 10-year contemporary experience with 1,173 patients at the massachusetts general hospital. J Gastrointest Surg. 2014;18:137–144. doi: 10.1007/s11605-013-2336-9. [DOI] [PubMed] [Google Scholar]
- Enestvedt CK, Diggs BS, Cassera MA, Hammill C, Hansen PD, Wolf RF. Complications nearly double the cost of care after pancreaticoduodenectomy. Am J Surg. 2012;204:332–338. doi: 10.1016/j.amjsurg.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Ahmad SA, Edwards MJ, Sutton JM, Grewal SS, Hanseman DJ, Maithel SK, et al. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. 2012;256:529–537. doi: 10.1097/SLA.0b013e318265ef0b. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Yamamoto Y, Hata S, Nara S, Esaki M, Sano T, et al. Analysis of risk factors for delayed gastric emptying (DGE) after 387 pancreaticoduodenectomies with usage of 70 stapled reconstructions. J Gastrointest Surg. 2011;15:1789–1797. doi: 10.1007/s11605-011-1498-6. [DOI] [PubMed] [Google Scholar]
- Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg. 1987;206:358–365. doi: 10.1097/00000658-198709000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi C, Falconi M, Salvia R, Mascetta G, Molinari E, Pederzoli P. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453–457. doi: 10.1159/000050193. ; discussion 8. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kim DK, Hindenburg AA, Sharma SK, Suk CH, Gress FG, Staszewski H, et al. Is pylorospasm a cause of delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? Ann Surg Oncol. 2005;12:222–227. doi: 10.1245/ASO.2005.03.078. [DOI] [PubMed] [Google Scholar]
- Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Muller MW, et al. Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg. 2005;140:1094–1099. doi: 10.1001/archsurg.140.11.1094. [DOI] [PubMed] [Google Scholar]
- Fabre JM, Burgel JS, Navarro F, Boccarat G, Lemoine C, Domergue J. Delayed gastric emptying after pancreaticoduodenectomy and pancreaticogastrostomy. Eur J Surg. 1999;165:560–565. doi: 10.1080/110241599750006460. [DOI] [PubMed] [Google Scholar]
- Lermite E, Pessaux P, Brehant O, Teyssedou C, Pelletier I, Etienne S, et al. Risk factors of pancreatic fistula and delayed gastric emptying after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2007;204:588–596. doi: 10.1016/j.jamcollsurg.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Klinkenbijl JH, van der Schelling GP, Hop WC, van Pel R, Bruining HA, Jeekel J. The advantages of pylorus-preserving pancreatoduodenectomy in malignant disease of the pancreas and periampullary region. Ann Surg. 1992;216:142–145. doi: 10.1097/00000658-199208000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berge Henegouwen MI, van Gulik TM, DeWit LT, Allema JH, Rauws EA, Obertop H, et al. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 1997;185:373–379. doi: 10.1016/s1072-7515(97)00078-1. [DOI] [PubMed] [Google Scholar]
- Tran KT, Smeenk HG, van Eijck CH, Kazemier G, Hop WC, Greve JW, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240:738–745. doi: 10.1097/01.sla.0000143248.71964.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevas KI, Avgerinos C, Manes C, Lytras D, Dervenis C. Delayed gastric emptying is associated with pylorus-preserving but not classical Whipple pancreaticoduodenectomy: a review of the literature and critical reappraisal of the implicated pathomechanism. World J Gastroenterol. 2006;12:5951–5958. doi: 10.3748/wjg.v12.i37.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Wu HS, Chen XL, Wang CY, Gou SM, Xiao J, et al. Pylorus-preserving versus pylorus-resecting pancreaticoduodenectomy for periampullary and pancreatic carcinoma: a meta-analysis. PLoS ONE. 2014;9:e90316. doi: 10.1371/journal.pone.0090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshuis WJ, van Dalen JW, Busch OR, van Gulik TM, Gouma DJ. Route of gastroenteric reconstruction in pancreatoduodenectomy and delayed gastric emptying. HPB. 2012;14:54–59. doi: 10.1111/j.1477-2574.2011.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner UF, Sick O, Olschewski M, Adam U, Hopt UT, Keck T. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg. 2012;16:1686–1695. doi: 10.1007/s11605-012-1940-4. [DOI] [PubMed] [Google Scholar]
- Makni A, Bedioui H, Jouini M, Chebbi F, Ksantini R, Fetirich F, et al. Pancreaticojejunostomy vs. pancreaticogastrostomy following pancreaticoduodenectomy: results of comparative study. Minerva Chir. 2011;66:295–302. [PubMed] [Google Scholar]
- Pessaux P, Sauvanet A, Mariette C, Paye F, Muscari F, Cunha AS, et al. External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Ann Surg. 2011;253:879–885. doi: 10.1097/SLA.0b013e31821219af. [DOI] [PubMed] [Google Scholar]
- Hong S, Wang H, Yang S, Yang K. External stent versus no stent for pancreaticojejunostomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg. 2013;17:1516–1525. doi: 10.1007/s11605-013-2187-4. [DOI] [PubMed] [Google Scholar]
- Ravikumar R, Sabin C, Abu Hilal M, Bramhall S, White S, Wigmore S, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg. 2014;218:401–411. doi: 10.1016/j.jamcollsurg.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Hellmig S, Von Schoning F, Gadow C, Katsoulis S, Hedderich J, Folsch UR, et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol. 2006;21:1832–1838. doi: 10.1111/j.1440-1746.2006.04449.x. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Leahy FE, McGowan AA, Bluck LJ, Coward WA, Jebb SA. Delayed gastric emptying in the obese: an assessment using the non-invasive (13)C-octanoic acid breath test. Diabetes Obes Metab. 2004;6:264–270. doi: 10.1111/j.1462-8902.2004.0344.x. [DOI] [PubMed] [Google Scholar]
- House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–278. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- Ross A, Mohammed S, Vanburen G, Silberfein EJ, Artinyan A, Hodges SE, et al. An assessment of the necessity of transfusion during pancreatoduodenectomy. Surgery. 2013;154:504–511. doi: 10.1016/j.surg.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Maes BD, Ghoos YF, Geypens BJ, Hiele MI, Rutgeerts PJ. Influence of octreotide on the gastric emptying of solids and liquids in normal healthy subjects. Aliment Pharmacol Ther. 1995;9:11–18. doi: 10.1111/j.1365-2036.1995.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Zhang Q, Han S, Yu Z, Zheng M, Zhou M, et al. Efficacy of somatostatin and its analogues in prevention of postoperative complications after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Pancreas. 2008;36:18–25. doi: 10.1097/mpa.0b013e3181343f5d. [DOI] [PubMed] [Google Scholar]
- Lowy AM, Lee JE, Pisters PW, Davidson BS, Fenoglio CJ, Stanford P, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg. 1997;226:632–641. doi: 10.1097/00000658-199711000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan YS, Sy ED, Tsai ML, Tang LY, Li PS, Lin PW. Effects of somatostatin prophylaxis after pylorus-preserving pancreaticoduodenectomy: increased delayed gastric emptying and reduced plasma motilin. World J Surg. 2005;29:1319–1324. doi: 10.1007/s00268-005-7943-5. [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. 2013;(4) doi: 10.1002/14651858.CD008370.pub3. CD008370. [DOI] [PMC free article] [PubMed] [Google Scholar]


