Abstract
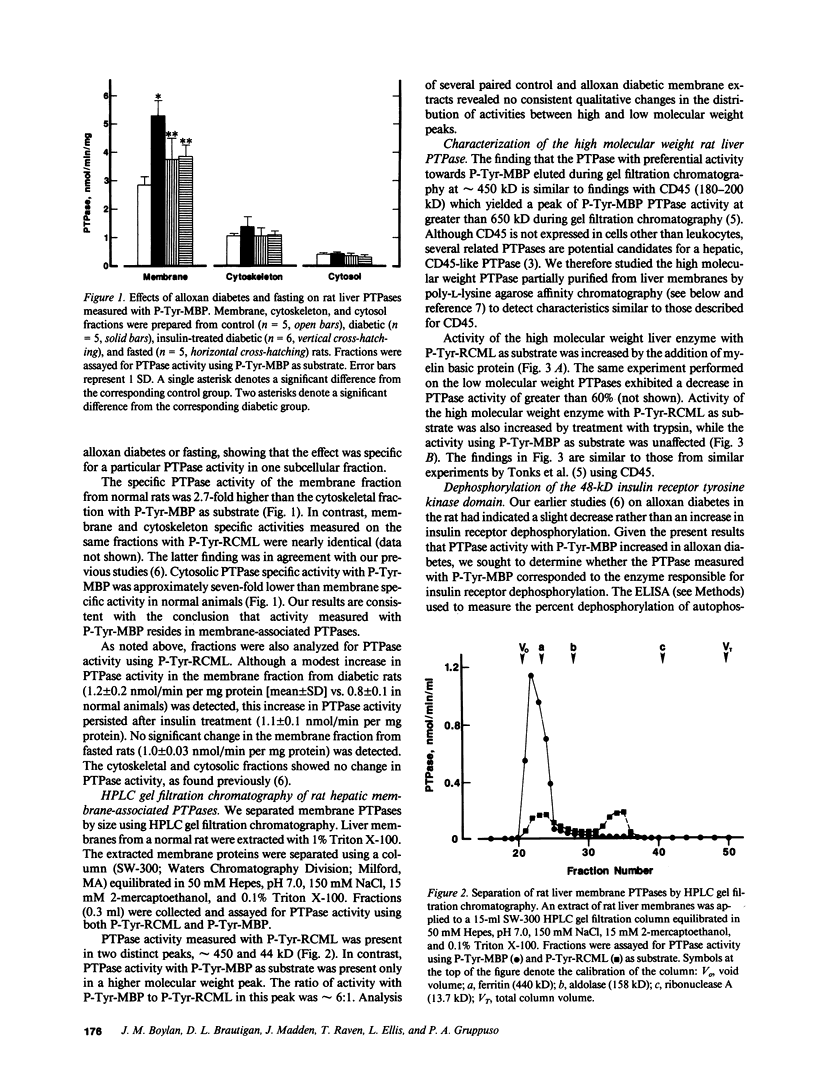
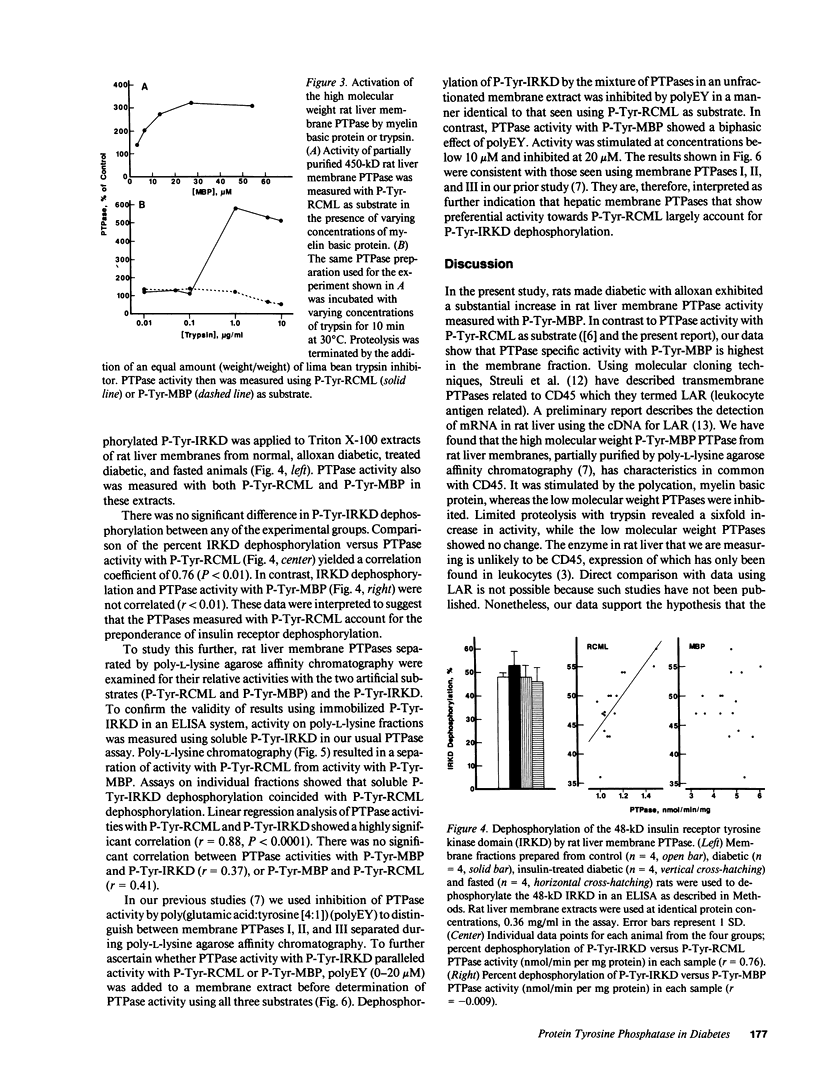
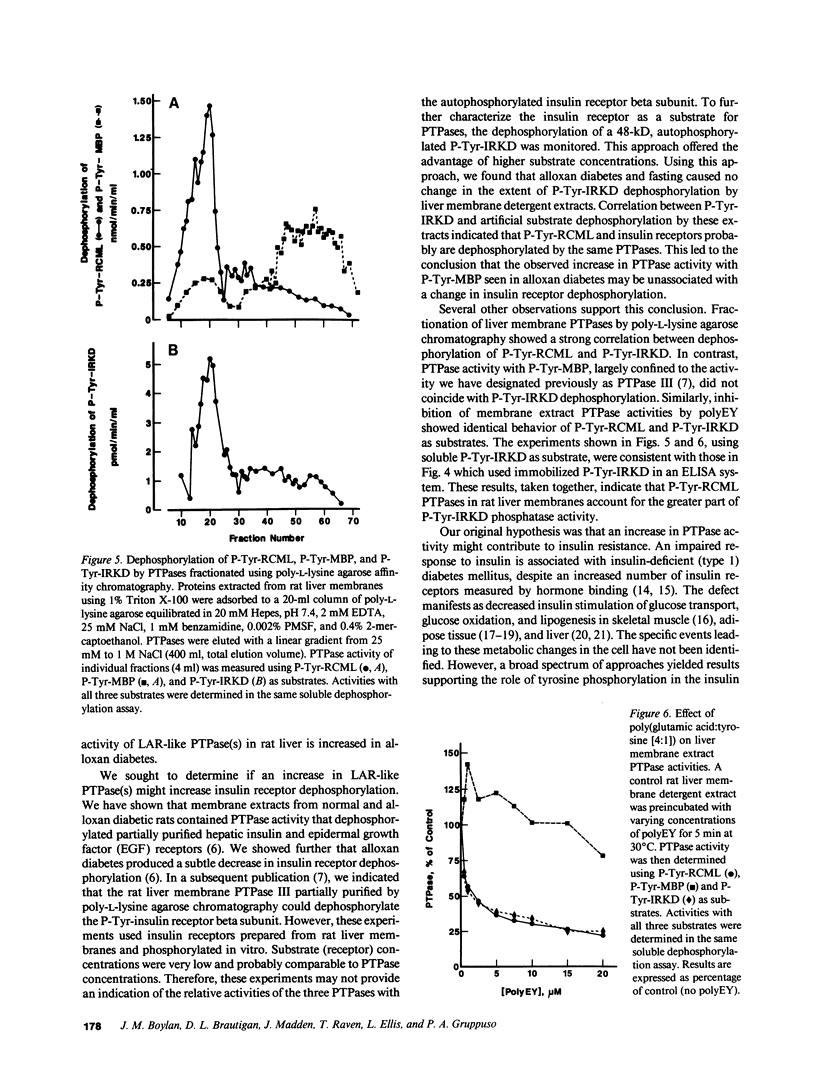
The involvement of tyrosine phosphorylation in insulin action led us to hypothesize that increased activity of protein tyrosine phosphatases (PTPases) might contribute to insulin resistance in alloxan diabetes in the rat. Hepatic PTPase activity was measured using two artificial substrates phosphorylated on tyrosine: reduced, carboxyamidomethylated, and maleylated lysozyme (P-Tyr-RCML) and myelin basic protein (P-Tyr-MBP), as well as an autophosphorylated 48-kD insulin receptor tyrosine kinase domain (P-Tyr-IRKD). Rats that were made alloxan diabetic exhibited a significant increase in hepatic membrane (detergent-soluble) PTPase activity measured with P-Tyr-MBP, without a change in activity measured with P-Tyr-RCML or the P-Tyr-IRKD. The PTPase active with P-Tyr-MBP behaved as a high molecular weight peak during gel filtration chromatography. Characterization of this enzyme indicated it shared properties with CD45, the prototype for a class of transmembrane, receptor-like PTPases. Our results indicate that alloxan diabetes in the rat is associated with an increase in the activity of a large, membrane-associated PTPase which accounts for only a small proportion of insulin receptor tyrosine dephosphorylation. Nonetheless, increased activity of this PTPase may oppose tyrosine kinase-mediated insulin signal transmission, thus contributing to insulin resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blondel O., Simon J., Chevalier B., Portha B. Impaired insulin action but normal insulin receptor activity in diabetic rat liver: effect of vanadate. Am J Physiol. 1990 Mar;258(3 Pt 1):E459–E467. doi: 10.1152/ajpendo.1990.258.3.E459. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli M. F., Tonks N. K., Diltz C. D., Weiel J. E., Fischer E. H., Krebs E. G. Microinjection of a protein-tyrosine-phosphatase inhibits insulin action in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5514–5518. doi: 10.1073/pnas.87.14.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Levitan A., Cobb M. H., Ramos P. Efficient expression in insect cells of a soluble, active human insulin receptor protein-tyrosine kinase domain by use of a baculovirus vector. J Virol. 1988 May;62(5):1634–1639. doi: 10.1128/jvi.62.5.1634-1639.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Gruppuso P. A., Boylan J. M., Posner B. I., Faure R., Brautigan D. L. Hepatic protein phosphotyrosine phosphatase. Dephosphorylation of insulin and epidermal growth factor receptors in normal and alloxan diabetic rats. J Clin Invest. 1990 Jun;85(6):1754–1760. doi: 10.1172/JCI114632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppuso P. A., Boylan J. M., Smiley B. L., Fallon R. J., Brautigan D. L. Hepatic protein tyrosine phosphatases in the rat. Biochem J. 1991 Mar 1;274(Pt 2):361–367. doi: 10.1042/bj2740361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft D. E. Studies of the metabolism of isolated livers of normal and alloxan-diabetic rats perfused with insulin. Diabetes. 1968 May;17(5):244–250. doi: 10.2337/diab.17.5.244. [DOI] [PubMed] [Google Scholar]
- Häring H., Obermaier-Kusser B. Insulin receptor kinase defects in insulin-resistant tissues and their role in the pathogenesis of NIDDM. Diabetes Metab Rev. 1989 Aug;5(5):431–441. doi: 10.1002/dmr.5610050502. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effects of streptozotocin-induced diabetes on insulin binding, glucose transport, and intracellular glucose metabolism in isolated rat adipocytes. Diabetes. 1979 Feb;28(2):87–95. doi: 10.2337/diab.28.2.87. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Loten E. G., Assimacopoulos-Jeannet F., Forgue M. E., Freychet P., Jeanrenaud B. Effect of fasting and streptozotocin in the obese-hyperglycemic (ob/ob) mouse. Apparent lack of a direct relationship between insulin binding and insulin effects. Diabetes. 1977 Jun;26(6):582–590. doi: 10.2337/diab.26.6.582. [DOI] [PubMed] [Google Scholar]
- Madden J. A., Bird M. I., Man Y., Raven T., Myles D. D. Two nonradioactive assays for phosphotyrosine phosphatases with activity toward the insulin receptor. Anal Biochem. 1991 Dec;199(2):210–215. doi: 10.1016/0003-2697(91)90091-7. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Shriner C. L., Brautigan D. L. Cytosolic protein phosphotyrosine phosphatases from rabbit kidney. Purification of two distinct enzymes that bind to Zn2+-iminodiacetate agarose. J Biol Chem. 1984 Sep 25;259(18):11383–11390. [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. CD45, an integral membrane protein tyrosine phosphatase. Characterization of enzyme activity. J Biol Chem. 1990 Jun 25;265(18):10674–10680. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]



