Abstract
Background
The role of hormones in focal nodular hyperplasia (FNH) has been investigated with conflicting results.
Objective
The aim of this study was to evaluate oestrogen and progesterone receptor immunohistochemical expression in FNH and surrounding normal liver (control material).
Methods
Biopsy materials from FNH and control tissue were investigated using an immunostainer. Receptor expression was graded as the proportion score (percentage of nuclear staining) and oestrogen receptor intensity score.
Results
Study material included tissue from 11 resected FNH lesions and two core biopsies in 13 patients (two male). Twelve samples showed oestrogen receptor expression. The percentage of nuclear oestrogen receptor staining was <33% in eight FNH biopsies, 34–66% in two FNH biopsies, and >67% in both core biopsies. The better staining in core biopsies relates to limitations of the staining technique imposed by the fibrous nature of larger resected FNH. Control samples from surrounding tissue were available for nine of the resected specimens and all showed oestrogen receptor expression. Progesterone receptor expression was negligible in FNH and control samples.
Conclusions
By contrast with previous studies, the majority of FNH and surrounding liver in this cohort demonstrated oestrogen receptor nuclear staining. The implications of this for continued oral contraceptive use in women of reproductive age with FNH remain uncertain given the lack of consistent reported growth response to oestrogen stimulation or withdrawal.
Introduction
Focal nodular hyperplasia (FNH) is the most common solid benign liver tumour and is composed of a proliferation of hepatocytes around a central stellate scar. Histologically, FNH resembles focal cirrhosis.1 It is a polyclonal, hyperplastic response to locally disturbed blood flow and thus differs from hepatic adenomas, which are monoclonal tumours.2,3 A study by Paradis et al.2 showed X-chromosome inactivation occurs randomly in a polyclonal tumour and non-randomly in a monoclonal tumour. In all of the 15 FNH studied by these authors, X-chromosome inactivation was random. By contrast, six of seven hepatic adenomas and two hepatocellular carcinomas showed non-random patterns of X-chromosome inactivation consistent with monoclonal tumours.2
The liver is a hormone-sensitive organ and expresses both oestrogen and androgen receptors. Sex hormones have been implicated as drivers in tumour formation. Longterm use of oral contraceptives (OCs) has been shown to induce not only adenomas, but also FNH.4 Baum et al.5 described the association between OC pills (OCPs) and hepatic adenomas in 1973. Liver tumours were relatively rare until the introduction of OCs in the 1960s.6 Edmonson and Steiner7 found only two cases of hepatic adenoma in 48 900 autopsies performed in Los Angeles General Hospital between 1918 and 1954. The incidence of hepatic adenoma decreased after the introduction of modern lower-dose OCPs.4 The role of hormones in FNH is supported by its high female predominance, younger age of onset, and high prevalence amongst individuals using OCPs (50–75% of patients with FNH use OCPs).4,8 Although several reports recommend the cessation of OCPs in FNH,8–10 there are few data to support this recommendation other than the hypothesis that OCs act to accelerate already established tumours.3 However, such recommendations are inconsistent with other reports suggesting that low-dose OCs can be maintained with FNH given that size changes during follow-up are rare and do not relate to OC use and that pregnancy is not associated with FNH changes.11
Hormone receptor immunohistochemical status has been assessed in hepatic adenomas and hepatocellular carcinomas, but less so in FNH. The predominance of FNH in females suggests a hormonal pathophysiological process may play a role in its pathogenesis; however, this has not been clearly proven. Porter et al.12 found that nuclear oestrogen receptors were found in much greater amounts in FNH and adenoma compared with the surrounding liver parenchyma, which suggests the increased sensitivity of these tumours compared with surrounding normal liver. Kubota et al.13 found that neither tumour expressed oestrogen or progesterone receptors in a study of two patients with enlarging FNH and a history of OCP use. Masood et al.14 found no oestrogen receptors in five patients with FNH, one of whom was male. However, variable staining techniques may account for differences in study results.
The aim of this study was to evaluate oestrogen and progesterone receptor expression in resected or biopsied FNH and surrounding normal liver tissue that was considered to represent control tissue.
Materials and methods
The study was approved by the institutional ethics committees at the Royal Adelaide Hospital and Flinders Medical Centre. Consecutive patients submitted to resection or core biopsy of FNH from 2001 to 2013 at either of these academic tertiary centres were asked to give consent and were recruited for the study.
Resected or biopsied FNH tissue and available surrounding normal tissue (controls) was stained immunohistochemically for oestrogen and progesterone hormone receptor status using the Ventana BenchMark ULTRA immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA) and the confirm rabbit SP1 clone monoclonal antibody for the oestrogen receptor and the confirm rabbit SP1 clone for the progesterone receptor. Slides were evaluated by two pathologists working independently. Receptor expression was graded according to the proportion score (PS) and intensity score (IS). The PS represents the percentage of nuclei staining for oestrogen and progesterone receptors according to the following categories: 0%; <1%; 1–10%; 11–33%; 34–66%, and >67%. The IS represents receptor staining intensity and is categorized as none, weak, intermediate or strong.
Results
Of 13 patients (median age: 39 years; range: 26–47 years) with FNH, 11 underwent resection of the lesion and two submitted to biopsy (Table1). Resections were undertaken either for symptom control or because atypical imaging characteristics had suggested adenoma. Two patients underwent biopsy of a liver lesion when imaging features were suspicious but not equivocal for FNH. The median diameter of the 11 resected lesions was 18 mm (range: 8–95 mm).
Table 1.
Study population showing age, gender, tumour size and oestrogen receptor (OeR) and progesterone receptor (PR) status
| Patient | Age, years | Gender | Tumour size, mm | Focal Nodular Hyperplasia(FNH) | Surrounding normal liver tissue (controls) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OeR nuclei staining (PS) | OeR intensity score | PRa nuclei staining (PS) | OeR nuclei staining (PS) | OeR intensity score | PRa nuclei staining (PS) | ||||||
| 1 | 35 | F | 8 | 1–10% | Intermediate | 0 | 1–10% | Intermediate | 0 | ||
| 2 | 27b | F | 12 | 1–10% | Weak | 0 | 1–10% | Weak | 0 | ||
| 3 | 40 | F | 21 | 1–10% | Weak | 0 | 1–10% | Weak | 0 | ||
| 4 | 43c | F | 25 | 1–10% | Intermediate | 0 | <1% | Weak | 1–10%a | ||
| 5 | 39 | F | 55 | 1–10% | Intermediate | 0 | 1–10% | Weak | 0 | ||
| 6 | 47 | M | 18 | 11–33% | Intermediate | 0 | 11–33% | Intermediate | 0 | ||
| 7 | 45 | F | 11, 50 | 11–33% | Intermediate | 0 | 11–33% | Intermediate | 0 | ||
| 8 | 42 | F | 95 | 11–33% | Weak | <1%a | 11–33% | Weak | 0 | ||
| 9 | 39 | F | 16, 17, 18 | 34–66% | Intermediate | <1%a | N/A | N/A | N/A | ||
| 10 | 34 | F | 25 | 34–66% | Intermediate | 0 | N/A | N/A | N/A | ||
| 11 | 38 | M | Core biopsy | >67% | Strong | 0 | N/A | N/A | N/A | ||
| 12 | 30d | F | Core biopsy | >67% | Strong | 0 | N/A | N/A | N/A | ||
| 13 | 26c | F | 45 | 0 | None | 0 | 1–10% | Weak | 0 | ||
F, female; M, male; N/A, not available; PCOS, polycystic ovarian syndrome; PS, proportion score.
No PR intensity score but weak
No PR intensity score but weak
No PR intensity score but weak
No PR intensity score but weak
Oestrogen receptor expression in FNH
Twelve of the 13 patients demonstrated labelling with the oestrogen receptor antibody. The intensity of oestrogen receptor expression is recorded in Table1. Both core biopsy specimens showed the highest IS and nuclear oestrogen receptor staining.
Oestrogen receptor expression in normal surrounding liver
Surrounding normal liver was available for the assessment of oestrogen receptor expression in control material in nine patients. The proportion of nuclear staining was similar to that in FNH tissue in seven of the nine patients.
Progesterone receptor expression in FNH
Eleven of the 13 FNH patients showed no progesterone receptor expression in FNH specimens.
Progesterone receptor expression in normal surrounding liver
Only one of the nine available control samples demonstrated progesterone receptor expression. The FNH tissue from this patient showed no progesterone receptor expression.
Discussion
In this study, the majority of patients with resected or biopsied FNH demonstrated oestrogen receptor expression. This was also present in control liver tissue in all patients in whom control tissue was available. The proportions of nuclear oestrogen receptor staining were similar in both FNH and normal liver tissue. Only two of nine patients had differential oestrogen receptor expression between the surrounding normal liver and FNH tissue. Progesterone receptor expression was, however, negligible.
The samples showing the strongest nuclear staining for oestrogen receptors were the core biopsies. In addition, many of the excision specimens showed more prominent reactivity in sections obtained from the periphery of the tissue. Formalin penetrates cellular tissue slowly at a rate of 1 mm per hour and therefore core biopsies of 1 mm in diameter will fix rapidly, whereas larger excision specimens will show a similar degree of fixation only at the edge. Given the staining patterns seen in the different specimen types, it seems likely that oestrogen receptor staining is influenced by fixation and that the best results are gained using tissue that fixes rapidly (Figs1–4).
Figure 1.
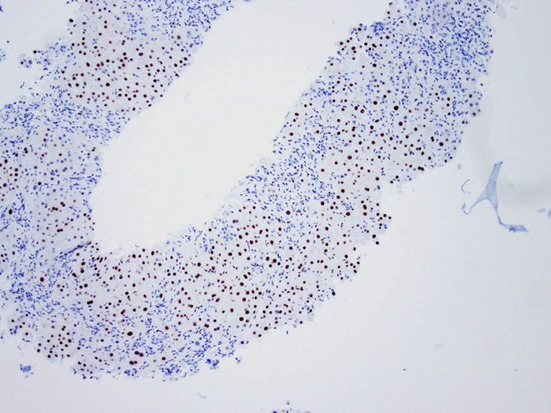
Low-power photomicrograph showing strongly positive oestrogen receptor staining (proportion score: >67%) in a core biopsy of focal nodular hyperplasia (Original magnification ×40)
Figure 4.
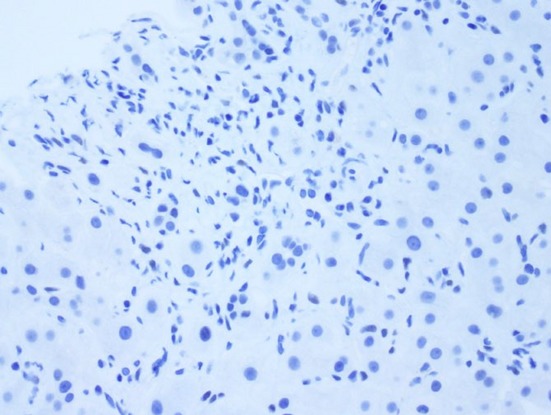
High-power photomicrograph with lack of progesterone receptor staining in a core biopsy of focal nodular hyperplasia (Original magnification ×400)
Figure 2.
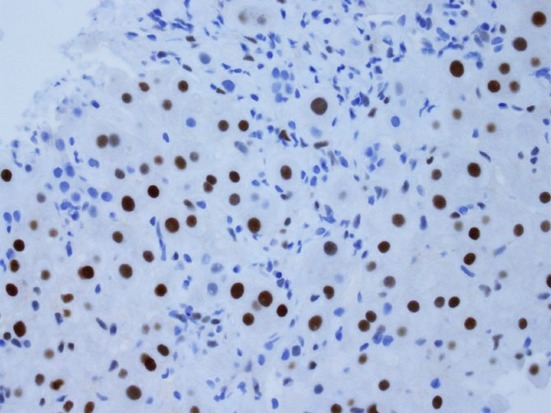
High-power photomicrograph showing strongly positive oestrogen receptor staining (proportion score: >67%) in a core biopsy of focal nodular hyperplasia (Original magnification ×400)
Figure 3.
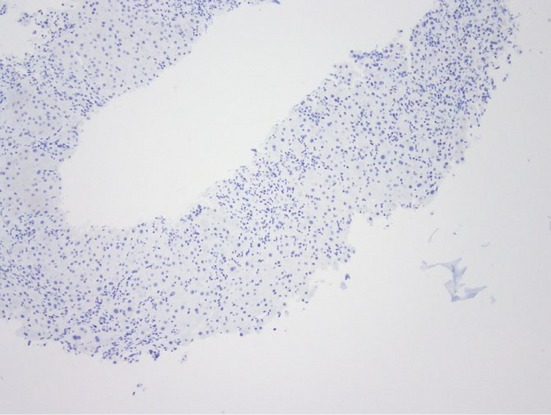
Low-power photomicrograph with lack of progesterone receptor staining in a core biopsy of focal nodular hyperplasia (Original magnification ×40)
Human liver hepatocytes contain oestrogen receptors, which render the liver sensitive to hormonal manipulation. Porter et al.12 studied oestrogen receptors in neoplastic and normal surrounding liver in three hepatic adenomas, one FNH and one normal liver sample. They showed that nuclear oestrogen receptor expression was much greater in adenoma and FNH compared with surrounding normal liver. This relative increase in nuclear oestrogen receptor binding capacity in adenoma and FNH in comparison with normal tissue suggests that FNH and adenomas may be more prone to hormonal manipulation than normal liver tissue. Other studies in seven cases of FNH found no evidence of hormone receptors using immunohistochemical techniques.13,14 The differences in results may relate to differences in staining techniques.
A comprehensive study into the pathogenesis of FNH performed by Wanless describes FNH as an abnormal response to altered blood flow.15 This can occur from anomalous arteries or angiogenesis in response to local vascular factors, such as local venous thrombosis, post-thrombotic arteriovenous shunts and tumour-related angiogenic factors. This process is then enhanced by systemic factors such as OC use, female gender and elevated tumour growth factors.15 The support for the vascular theory of the pathogenesis of FNH lies in several observations.3 In a study of 247 patients, of whom 148 had FNH, a higher incidence of haemangioma was seen in those with FNH compared with those with non-FNH lesions (20% versus 9%).16 A further study of 275 members of a family with hereditary haemorrhagic telangiectasia (HHT) found five members with FNH, with a prevalence of HHT in these patients of 2.9%.17 Interestingly, this is consistent with the overall prevalence of FNH, which is reported to lie between 0.8% and 3.0% based on studies of 95 and 2500 autopsies.18,19 Another observation which led to the suggestion that portal hypertension may contribute to FNH comes from a study of the explanted livers of 130 patients with cirrhosis, in whom the presence of oesophageal varices was associated with the occurrence of FNH-like nodules.20 This association would support the role of alterations in vascularization in the pathogenesis of FNH. Other reports have suggested that although OCP use may not initiate FNH, it can stimulate pre-existing FNH by promoting growth in size and through effects on the local vasculature.9,21 A study that compared 33 users of OCPs with FNH with 15 non-users of OCPs with FNH found a greater degree of vascular alteration, more fibrosis and increased tumour size in OCP users.8,22
Focal nodular hyperplasia is a benign condition that can be accurately diagnosed using techniques such as magnetic resonance imaging (MRI).23 Thus, over the course of more than a decade, in two major tertiary institutions, tumours from only 13 patients were available for testing. Despite the increasing accuracy of MRI in determining the diagnosis of FNH and its benign nature, some patients complain of persistent pain and symptoms caused by the FNH. The other reason for resection or biopsy is that atypical or inconclusive imaging findings may be suspicious for an adenoma or other neoplastic lesion.
Interestingly, both male patients in this study showed oestrogen receptor expression in their FNH. Luciani et al.24 studied clinical and imaging findings in 18 men with FNH and compared them with those in 216 women with FNH. They found that men were diagnosed at an older age than women, that the mean size of FNH was smaller in men than in women and that surgery was performed far more frequently in men than in women (72% versus 17%). These differences may relate to a larger proportion of atypical MRI findings and the greater risk for hepatocellular carcinoma in men.24 There is conflicting evidence to support the hormonal basis to the pathophysiology of FNH, but, interestingly, there is evidence linking an increased risk for FNH with prolonged use of OCs.25 An epidemiologic study of 23 women with FNH and 94 control subjects found contraceptive use was reported in 83% of those with FNH and 59% of controls, and identified a significant trend in increased risk for FNH with increased duration of OCP use [odds ratios (ORs): 1.62 in those using OCPs for <3 years and 4.48 in those using OCPs for >3 years in comparison with that in women who had never used OCPs].26 Heinemann et al.27 performed two parallel case–control studies in a total of 51 patients with hepatocellular adenomas and 143 with FNH treated at 15 German liver centres between 1990 and 1997 and a comparison group of 240 population controls. They found a significant association between FNH and OCP use, but not between hepatic adenoma and the use of modern contraceptives. In subjects with <10 years of OCP use, the ORs for developing hepatocellular adenoma and FNH were 0.96 [95% confidence interval (CI) 0.25–3.39] and 1.52 (95% CI 0.63–3.62), respectively. In subjects with >10 years of OCP use, the ORs were 1.78 (95% CI 0.50–6.34) for hepatic adenoma and 2.45 (95% CI 1.03–5.82) for FNH.27
D'Halluin et al.28 followed 44 women with FNH, with a median tumour size of 56 mm. The tumour remained stable in 19, decreased in 13 and increased in 12 subjects. However, of the 21/37 women who stopped using OCPs at diagnosis, the lesion remained stable in 11, decreased in seven and increased in three. Although D'Halluin et al.28 concluded that hormonal status had little to do with the disease, it appeared at first glance that tumour stability and regression were proportionately greater in women who stopped using OCPs (18/21, 86%) than in the remaining women (14/23, 61%) (P = 0.09). These findings suggest that in women found to have FNH while taking OCPs, the cessation of OCPs may be potentially beneficial, but evidence of such benefit would require a dedicated and appropriately powered study.
In the most important study to dispute the role of OCPs in the development of FNH, Mathieu et al.29 studied 216 women. The authors found that neither size nor number of lesions at baseline related to OCP use, although the vast majority of their patients were OCP users (n = 188, 87%). They followed 136 women for a mean of 23 months (range: 6 months to 9 years), of whom 37% were lost from follow-up. Of the 89 women who stopped OCPs, FNH lesions decreased in two women and increased in one. Of the 26 who continued on low-dose OCPs, one demonstrated the disappearance of FNH. The 14 non-OCP users and 7 progestogen-only users had no change in the size of their FNH lesions. The authors concluded that size changes during follow-up were not influenced by OCP use. This was further supported by follow-up in 12 patients in this cohort who became pregnant and submitted to MRI at 2–14 months (mean: 4.3 months) after delivery, which demonstrated no change in the FNH.
There are data to support the suggestion that pregnancy does not increase the risk for significant growth or complications from FNH30–32 and that pregnancy need not be discouraged in these patients. In a study conducted by Rifai et al.30 in 20 patients with FNH monitored during pregnancy, three experienced tumour growth, seven showed a stable tumour size and 10 showed some degree of involution. In a study by Weimann et al.,32 conducted in 10 pregnant women, three of whom were pregnant twice, only one patient developed right upper quadrant pain in the course of the pregnancy and none showed an increase in tumour size. However, occasional case reports have described an increase in the size of FNH and heightened symptoms during pregnancy.33,34
Although FNH is traditionally associated with a very low to no incidence of complications, scattered reports suggest that complications of FNH such as rupture and bleeding do occur, although much less frequently than in the context of hepatic adenoma.35–39 Albeit that the majority of patients with FNH are asymptomatic and are managed conservatively with observation, patients who develop abdominal pain, marked tumour enlargement or have atypical imaging findings may require surgery.40,41 On follow-up, up to 13% of patients who are under surveillance for FNH go on to develop protracted symptoms.42 Hence, it is imperative that the surgeon who diagnoses the patient with FNH is able to give sound advice on OCP use. Several contemporary reports recommend the cessation of OCPs in patients with FNH.25,41
Conclusions
The present study demonstrates that the majority of FNH lesions and surrounding liver parenchyma express oestrogen receptors. This has not been demonstrated in previous studies. The current findings add additional uncertainty to the ongoing debate of the role of cessation of OCPs in women with FNH, thereby supporting the case for further study of this issue.
Conflicts of interest
None declared.
References
- Mailette De Buy Wenniger L, Terpstra V, Beuers U. Focal nodular hyperplasia and hepatic adenoma: epidemiology and pathology. Dig Surg. 2010;27:24–31. doi: 10.1159/000268404. [DOI] [PubMed] [Google Scholar]
- Paradis V, Laurent A, Flejou JF, Vidaud M, Bedossa P. Evidence of the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology. 1997;26:891–895. doi: 10.1002/hep.510260414. [DOI] [PubMed] [Google Scholar]
- Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–1200. doi: 10.1002/hep.1840050622. [DOI] [PubMed] [Google Scholar]
- Giannitrapani L, Soresi M, La Spada E, Cervello M, D'Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann N Y Acad Sci. 2006;1089:228–236. doi: 10.1196/annals.1386.044. [DOI] [PubMed] [Google Scholar]
- Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926–929. doi: 10.1016/s0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- Li G, Su Q, Zhang W, Li A, Zhu S, Feng Y. Liver cell adenoma: a case report with clonal analysis and literature review. World J Gastroenterol. 2006;12:2125–2129. doi: 10.3748/wjg.v12.i13.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonson HA, Steiner PE. Primary carcinoma of the liver a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Pain JA, Gimson AES, Williams R, Howard ER. Focal nodular hyperplasia of the liver: results of treatment and options in management. Gut. 1991;32:524–527. doi: 10.1136/gut.32.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Nikiforov YE, Moulton JS, Lowy AM. Multiple focal nodular hyperplasia of the liver in a 21-year-old woman. J Gastrointest Surg. 2004;8:591–595. doi: 10.1016/j.gassur.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Pulvirenti E, Toto A, Di Carlo I. An update on indications for treatment of solid hepatic neoplasms in noncirrhotic liver. Future Oncol. 2010;6:1243–1250. doi: 10.2217/fon.10.85. [DOI] [PubMed] [Google Scholar]
- Mathieu D, Kobeiter H, Cherqui D, Rahmouni A, Dhumeaux D. Oral contraceptive intake with focal nodular hyperplasia of the liver. Lancet. 1998;352:1679–1680. doi: 10.1016/S0140-6736(05)61451-1. [DOI] [PubMed] [Google Scholar]
- Porter LE, Elm MS, Van Thiel DH, Eagon PK. Hepatic estrogen receptor in human liver disease. Gastroenterology. 1987;92:735–745. doi: 10.1016/0016-5085(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Kubota T, Shimuzu K, Sonoyama T, Ikeda E, Kurioka H, Ouchi T, et al. Enlarged focal nodular hyperplasia of the liver under the influence of oral contraceptives. Hepatogastroenterology. 2001;48:1736–1739. [PubMed] [Google Scholar]
- Masood S, West AB, Barwick KW. Expression of hormone receptors in benign hepatic tumors. An immunocytochemical study. Arch Pathol Lab Med. 1992;116:1355–1359. [PubMed] [Google Scholar]
- Wanless IR. The pathogenesis of focal nodular hyperplasia of the liver. J Gastroenterol Hepatol. 2004;19(Suppl. 7):342–343. [Google Scholar]
- Vilgrain V, Uzan F, Brancatelli G, Federle MP, Zappa M, Menu Y. Prevalence of hepatic haemoangioma in patients with focal nodular hyperplasia: MRI analysis. Radiology. 2003;229:75–79. doi: 10.1148/radiol.2291021284. [DOI] [PubMed] [Google Scholar]
- Buscarini E, Danesino C, Plauchu H, de Fazio C, Oliveri C, Brambilla G, et al. High prevalence of hepatic focal nodular hyperplasia in subjects with hereditary haemorrhagic telangiectasia. Ultrasound Med Biol. 2004;30:1089–1097. doi: 10.1016/j.ultrasmedbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Karhunen PJ. Benign hepatic tumours and tumour-like conditions in men. J Clin Pathol. 1986;39:183–188. doi: 10.1136/jcp.39.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787–797. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Cassiman D, Verslype C, Maleux G, Van Hees D, Pirenne J, et al. Clinicopathological features of focal nodular hyperplasia-like nodules in 130 cirrhotic explant livers. Am J Gastroenterol. 2006;101:2341–2346. doi: 10.1111/j.1572-0241.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Moesner J, Baunsgaard P, Starklint H, Thommesen N. Focal nodular hyperplasia of the liver. Acta Pathol Microbiol Scand. 1977;85a:113–121. [PubMed] [Google Scholar]
- Nime P, Pickren JW, Vana J, Aronoff BL, Baker HW, Murphy GP. The histology of liver tumours in oral contraceptive users during a national survey by the American College of Surgeons. Cancer. 1979;44:1481–1489. doi: 10.1002/1097-0142(197910)44:4<1481::aid-cncr2820440443>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cherqui D, Rahmouni A, Charlotte F, Boulahdour H, Meignan M, Fagniez P, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological and pathological correlations. Hepatology. 1995;22:1674–1681. [PubMed] [Google Scholar]
- Luciani A, Kobeiter H, Maison P, Cherqui D, Zafrani ES, Dhumeaux D, et al. Focal nodular hyperplasia of the liver in men: is presentation the same in men and women? Gut. 2002;50:877–880. doi: 10.1136/gut.50.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm CB, Ng K, Lockie P, Samra JS, Hugh TJ. Focal nodular hyperplasia – a review of myths and truths. J Gastrointest Surg. 2011;15:2275–2283. doi: 10.1007/s11605-011-1680-x. [DOI] [PubMed] [Google Scholar]
- Scalori A, Tavani A, Gallus S, La Vecchia C, Colombo M. Oral contraceptives and the risk of focal nodular hyperplasia of the liver: a case–control study. Am J Obstet Gynecol. 2002;186:195–197. doi: 10.1067/mob.2002.120277. [DOI] [PubMed] [Google Scholar]
- Heinemann LA, Weimann A, Gerken G, Thiel C, Schlaud M, DoMinh T. Modern oral contraceptive use and benign liver tumors: the German Benign Liver Tumor Case-Control Study. Eur J Contracept Reprod Health Care. 1998;3:194–200. doi: 10.3109/13625189809167253. [DOI] [PubMed] [Google Scholar]
- D'Halluin V, Vilgrain V, Pelletier G, Rocher L, Belghiti J, Erlinger S, et al. Natural history of focal nodular hyperplasia. A retrospective study of 44 cases. Gastroenterol Clin Biol. 2001;25:1008–1010. [PubMed] [Google Scholar]
- Mathieu D, Kobeitzer H, Maison P, Rahmouni A, Cherqui D, Zafrani ES, et al. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology. 2000;118:560–564. doi: 10.1016/s0016-5085(00)70262-9. [DOI] [PubMed] [Google Scholar]
- Rifai K, Mix H, Krusche S, Potthoff A, Manns MP, Gebel MJ. No evidence of substantial growth progression or complications of large focal nodular hyperplasia during pregnancy. Scand J Gastroenterol. 2013;48:88–92. doi: 10.3109/00365521.2012.737361. [DOI] [PubMed] [Google Scholar]
- Cobey FC, Salem RR. A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg. 2004;187:181–191. doi: 10.1016/j.amjsurg.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Weimann A, Mössinger M, Fronhoff K, Nadalin S, Raab R. Pregnancy in women with observed focal nodular hyperplasia of the liver. Lancet. 1998;351:1251–1252. doi: 10.1016/S0140-6736(05)79318-1. [DOI] [PubMed] [Google Scholar]
- Byrnes V, Cárdenas A, Afdhal N, Hanto D. Symptomatic focal nodular hyperplasia during pregnancy: a case report. Ann Hepatol. 2004;3:35–37. [PubMed] [Google Scholar]
- Reddy KR, Kligerman S, Levi J, Livingstone A, Molina E, Franceschi D, et al. Benign and solid tumours of the liver: relationship to sex, age, size of tumours and outcome. Am Surg. 2001;67:173–178. [PubMed] [Google Scholar]
- Becker YT, Raiford DS, Webb L, Wright JK, Chapman WC, Pinson CW. Rupture and haemorrhage of hepatic focal nodular hyperplasia. Am Surg. 1995;61:210–214. [PubMed] [Google Scholar]
- Chang SKY, Chung YFA, Thng CH, Loo HW. Focal nodular hyperplasia presenting as acute abdomen. Singapore Med J. 2005;46:90–92. [PubMed] [Google Scholar]
- Koch N, Gintzburger D, Seelentag W, Denys A, Gillet M, Halkic N. Rupture of hepatic focal nodular hyperplasia. About two cases. Ann Chir. 2006;131:279–282. doi: 10.1016/j.anchir.2005.07.007. . [In French.] [DOI] [PubMed] [Google Scholar]
- Hardwigsen J, Pons J, Veit V, Garcia S, Le Treut YP. A life threatening complication of focal nodular hyperplasia. J Hepatol. 2001;35:310–312. doi: 10.1016/s0168-8278(01)00096-4. [DOI] [PubMed] [Google Scholar]
- Rahili A, Cai J, Trastour C, Juwid A, Benchimol D, Zheng M, et al. Spontaneous rupture and haemorrhage of hepatic focal nodular hyperplasia in lobus caudatus. J Hepatobiliary Pancreat Surg. 2005;12:138–142. doi: 10.1007/s00534-004-0936-1. [DOI] [PubMed] [Google Scholar]
- Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Yong F, et al. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808–813. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- Christiano A, Dietrich A, Spina JC, Ardiles V, de Santibañes E. Focal nodular hyperplasia and hepatic adenoma: current diagnosis and management. Updates Surg. 2014;66:9–21. doi: 10.1007/s13304-013-0222-3. [DOI] [PubMed] [Google Scholar]
- Perrakis A, Demir R, Müller V, Mulsow J, Aydin U, Alibek S, et al. Management of the focal nodular hyperplasia of the liver: evaluation of the surgical treatment comparing with observation only. Am J Surg. 2012;204:689–696. doi: 10.1016/j.amjsurg.2012.02.006. [DOI] [PubMed] [Google Scholar]


