Abstract
Introduction
Obstructive jaundice as a result of bile duct tumour thrombus (BDTT) is an unusual clinical entity and an uncommon presenting feature of hepatocellular carcinoma (HCC). This study evaluates the outcome of hepatectomy for HCC with obstructive jaundice as a result of BDTT in non-cirrhotic livers.
Methods
Between 1997 and 2012, out of 426 patients with HCC in non-cirrhotic livers, 39 patients with BDTT (Group I n = 39), who underwent a hepatectomy, were analysed and compared with the non-BDTT group (Group II n = 387).
Results
The demographic profile and biochemical parameters between Group I and Group II were compared; apart from the presence of jaundice at presentation and an elevated serum bilirubin, there were no significant differences. Post-operative morbidity and mortality were 11 (28.2%) and 2 (5.1%), respectively, in Group I. There were no differences between the groups with regards to the operative variables and short-term outcomes. The 1-, 3- and 5-year survival rates in Group I were 82%, 48% and 10%, respectively, with a median survival of 28.6 months and were significantly poorer than Group II (90%, 55% and 38%, respectively, with a median survival of 39.2 months).
Conclusion
The mere presence of BDTT in HCC does not indicate an advanced or inoperable lesion. When technically feasible, a formal hepatic resection is the preferred first-line treatment option in these patients.
Introduction
Obstructive jaundice is an uncommon presenting feature of hepatocellular carcinoma (HCC). The incidence of obstructive jaundice in HCC is in the range of 1% to 12%, with a tumour thrombus in the bile duct (BDTT) being one of the causes.1–3 These patients exhibit clinicopathological features which differ from those with parenchymal cholestasis owing to extensive tumour infiltration or advanced cirrhosis.4,5 Even although the prognosis in this type of HCC is poor, it is much better than those with jaundice caused by hepatic insufficiency.4,5 Identification of this group of patients is imperative, because surgical treatment may be beneficial. There is a paucity of studies regarding BDTT in HCC, especially with respect to the outcome of hepatectomy and its impact on prognosis. In this study, we aimed to evaluate the outcome of hepatectomy for HCC in patients with obstructive jaundice as a result of BDTT in a non-cirrhotic liver and compare its morbidity, mortality and long-term prognosis with those who underwent a resection without BDTT.
Materials and methods
From January 1997 to December 2012, 426 patients with a non-fibrolamellar type of HCC in non-cirrhotic livers underwent liver resections at the Institute of Surgical Gastroenterology and Liver Transplantation, Stanley Medical College Hospital, Chennai, India. Among them, 39 patients were diagnosed to have pathologically confirmed (post-operative) BDTT (Group I). These patients were compared with the remaining 387 patients without BDTT (Group II). Their demographic, clinicopathological and investigative (biochemical and radiological) data were noted. Hepatocellular carcinoma with BDTT was classified according to the location of BDTT, as proposed by Ueda et al. (Type 1: involving the second order intrahepatic duct, Type 2: involving the first-order intrahepatic duct, Type 3a: extending to the hepatic confluence, Type 3b: implanted tumour growing in the common hepatic duct (CHD) and Type 4: dislodged BDTT within the CHD) (Fig. 1).6
Figure 1.
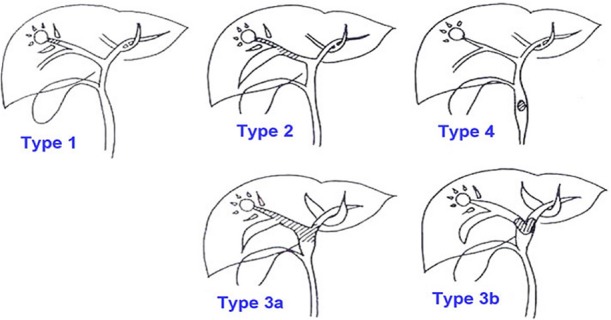
UEDA classification of bile duct thrombi6
Pre-operative workup included liver function tests, viral screen and imaging in the form of abdominal ultrasonography, and abdominal computed tomography (CT) (Fig. 2a–c). Magnetic resonance cholangiopancreatography, when available, was used to evaluate the extent of a BDTT (Fig. 2d). Operative procedures were based on the general condition, tumour status, pre-operative liver function tests, pre-operative diagnosis of the location of the primary tumours, extension of BDTT and the future remnant liver parenchyma. Pre-operative biliary drainage in the form of percutaneous transhepatic biliary drainage was performed selectively when the patient's serum bilirubin was more than 5 mg/dl or if the patient developed cholangitis. Anatomical resection was done for the primary liver tumour. For a free-floating thrombus in the bile duct, a choledochotomy was done and the tumour was extricated. (Fig. 3) In cases of an invaded bile duct, it was resected with the entire tumour, and biliary-enteric anastomosis was performed. The specimen was labelled and sent for histopathological examination. Tumour size, number, surgical margin, classification of thrombus and its size were noted. The operation time was defined as the time period from incision of the skin to closure of the wound. Post-operative morbidity included all the complications related to the hepatic surgery. Peri-operative mortality was defined as either within 30 days of surgery or occurring in hospital. After discharge, patients were followed-up on a 3-monthly basis with alpha fetoprotein (AFP) and ultrasound or CT every 3 months for the first 2 years, beyond which the patient was followed-up on a 6-monthly basis.
Figure 2.
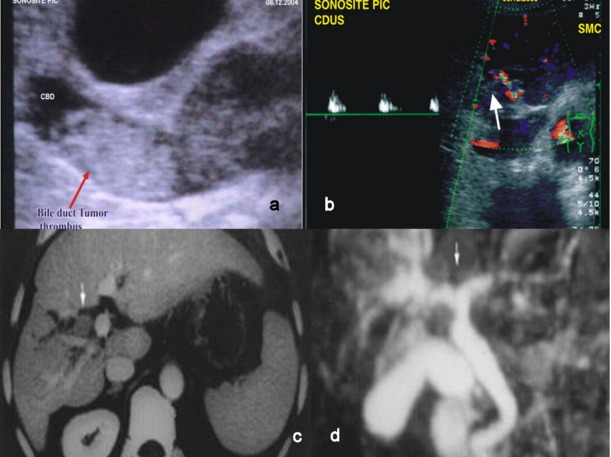
Pre-operative imaging of bile duct tumour thombus (BDTT) in hepatocellular carcinoma (HCC). (a) Ultrasonography of BDTT (arrow). (b) Colour Doppler of BDTT (arrow). (c) Computed tomography (CT) showing BDTT. (d) Magnetic resonance cholangiopancreatography showing BDTT
Figure 3.
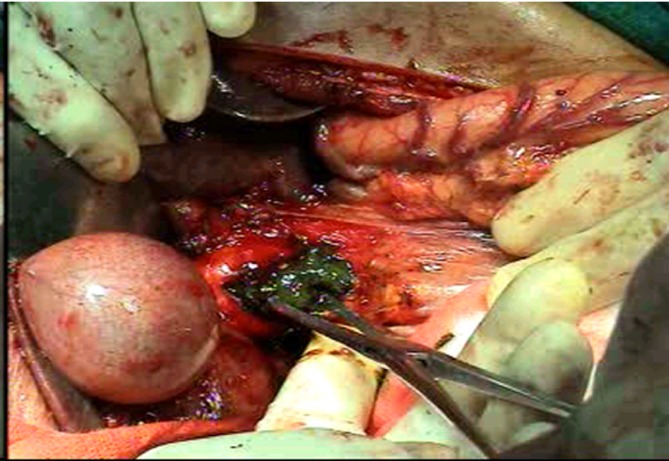
Intra-operative bile duct tumour thombus (BDTT) within the lumen of the common bile duct
Statistical analysis
Data were expressed as the mean ± SD for numerical variables or percentages for nominal variables. Mann-Whitney U test or t-tests were used to compare numerical variables, and the Chi-Square test or Fisher's exact test was carried out to compare nominal variables. Overall survival rates were estimated using the Kaplan–Meier method and compared using the log-rank test. Statistical analysis was performed using the SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
The demographic profile of the patients in BDTT and non-BDTT groups were compared with regards to their age, gender, viral marker status, Child–Pugh status and jaundice at presentation (Table 1). In total, 18 patients with BDTT (46.1%) presented with jaundice on admission, which was significantly higher than the non-BDTT group (1.5%). In the non-BDTT group, jaundice was as a result of the presence of a centrally located tumour with direct compression over the biliary radicals or haemobilia. There were no other significant differences between the two groups. On comparison of biochemical parameters between the two groups (Table 2), the serum bilirubin levels were significantly higher in the BDTT group. There were no other differences in biochemical parameters noted between the groups.
Table 1.
Demographic profile
| BDTT n = 39 | Non BDTT n = 387 | P value | |
|---|---|---|---|
| Age (years) | 52.1 ± 10.9 | 50.9 ± 18.8 | NS |
| Males/Females n |
28/11 | 278/109 | NS |
| HBV positive n (%) |
7 (17.9%) | 61 (15.7%) | NS |
| HCV positive n (%) |
2 (5.1%) | 6 (1.5%) | NS |
| Jaundice at presentation n (%) | 18 (46.1%) | 5 (1.3%) | P < 0.05 |
| Child–Pugh Class A/B (%) | 27 (69.3)/12 (30.7) | 271 (70.1)/116 (28.9) | NS |
HBV, hepatitis B virus; HBC, hepatitis C virus.
Table 2.
Biochemical parameters
| BDTT n = 39 | Non BDTT n = 387 | P-value | |
|---|---|---|---|
| S. bilirubin (mg/dl) | 6.1 ± 5.1 | 1.2 ± 0.7 | P < 0.05 |
| S. AST (IU/l) | 88.9 ± 39 | 69.2 ± 31 | NS |
| S. alkaline phosphatase (IU/l) | 522 ± 248 | 284 ± 166 | NS |
| S. albumin (g/dl) | 4.1 ± 3.1 | 4.0 ± 1.4 | NS |
| AFP (> 400 ng/ml) | 28 (71.7%) | 281 (72.6%) | NS |
BDTT, bile duct tumour thombus; AST, aspartate aminotransferase; AFP, alpha fetoprotein; NS, not significant.
Sixteen patients underwent a right hepatectomy with a tumour thrombectomy via a choledochotomy. An extended right hepatectomy with extra hepatic bile duct excision was the next most common procedure (n = 10). A left hepatectomy was performed in 9 patients whereas 2 patients each underwent an extended left hepatectomy and left lateral segmentectomy (Table 3). There were no differences in the average blood loss and operative times between the two groups. (Table 4).
Table 3.
Operative procedure
| Operative procedure | n = 39 |
|---|---|
| Right hepatectomy with thrombectomy through choledochotomy and T tube drainage | 16 |
| Extended right hepatectomy with extra hepatic bile duct excision | 10 |
| Left hepatectomy | 9 |
| Extended left hepatectomy | 2 |
| Left Lateral Segmentectomy | 2 |
Table 4.
Operative variables
| BDTT n = 39 | Non BDTT n = 387 | P-value | |
|---|---|---|---|
| Duration of surgery (min) | 480 ± 45 | 465 ± 55 | NS |
| Blood loss (ml) | 390 ± 45 | 375 ± 65 | NS |
BDTT, bile duct tumour thombus; NS, not significant.
The tumour characteristics (number, size, tumour thrombus length and Ueda classification based on pathology) of the resected specimen are summarized in Table 5. Post-operative morbidity in the BDTT group was 28.2% (n = 11) which included bile leak in four patients. Two patients died as a result of post-hepatectomy liver failure (mortality 5.1%). There was no significant difference in the morbidity, length of hospital stay, mortality and margin positivity between the groups (Table 6). The median follow-up for the patients was 64 months (12–144). The median survival and overall 1-, 3- and 5-year survival were significantly poorer in the BDTT group (Table 7). Kaplan–Meier curves for overall survival of the two groups were plotted, and are shown in Fig. 4.
Table 5.
Tumour characteristics
| Tumour | |
|---|---|
| Number | 2 ± 1 |
| Size (cm) | 5.6 ± 3.2 |
| Thrombus length (cm) | 5.5 ± 2.5 |
| UEDA classification | |
| I | 4 |
| II | 8 |
| IIIa | 17 |
| IIIb | 2 |
| IV | 8 |
Table 6.
Short-term outcomes
| BDTT n = 39 | Non BDTT n = 387 | P value | |
|---|---|---|---|
| Morbidity (%) | 11 (28.2) | 105 (27.1) | NS |
| Length of hospital stay (Days) | 17.6 ± 6.0 | 16.1 ± 5.1 | NS |
| Mortality (%) | 2 (5.1) | 15 (3.9) | NS |
| Positive Margin (%) | 3 (7.7) | 27 (6.9) | NS |
BDTT, bile duct tumour thombus; NS, not significant.
Table 7.
Long-term survival
| Survival | BDTT n = 39 | Non BDTT n = 387 | P-value |
|---|---|---|---|
| Median survival (months) | 28.6 | 39.2 | P < 0.05 |
| Overall survival (%) | |||
| 1 year | 82 | 90 | P < 0.05 |
| 3 years | 48 | 55 | P < 0.05 |
| 5 years | 10 | 38 | P < 0.05 |
BDTT, bile duct tumour thombus; NS, not significant.
Figure 4.
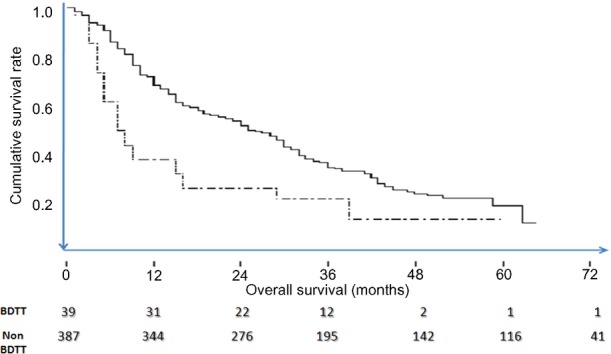
Kaplan–Meier curves comparing the cumulative survivals of patients with hepatocellular carcinoma with bile duct tumour thombus (BDTT) (dotted line) versus without BDTT (bold line)
Discussion
Since its first description in 1949, icteric hepatomas have remained an enigmatic subset of HCC.7 Their diagnosis, ideal management and outcomes after a hepatectomy have been an area of speculation and controversy. The incidence of BDTT in HCC varies from 0.8% to 12.5%.2,3 In our series, the incidence of BDTT among non-cirrhotic livers with HCC was 9.1%. The outcome in patients who receive palliative treatment is uniformly dismal, with a mean survival time of less than 5 months.8–11 For HCC patients with BDTT, surgical resection is the only option for curative treatment, increasing the chance of survival.
As HCC with BDTT is often accompanied by obstructive jaundice, it is difficult to assess the hepatic functional reserve before determining treatment. However, liver function does recover if jaundice is relieved by biliary decompression. This permits an assessment of hepatic functional reserve and safe liver resection.1,5,12 Theories regarding the pathogenesis of BDTT include a rupture of the HCC into the common bile duct, or an invasion of the intrahepatic bile duct system by the tumour. The presence of concurrent haemobilia can accelerate the obstructive jaundice, causing patients to become icteric within a few days.1,5,13 BDTT in the proximal bile duct system may be expanding in nature and loosely attached to the bile duct epithelium, hence enabling a tumour thrombectomy through choledochotomy, avoiding the need for resection of the bile duct.5,14–16 In our patients, pre-operative biliary decompression was done on a selective basis, percutaneous transhepatic biliary drainage (PTBD) was the modality of choice and there were no PTBD-related complications.
Venous invasion is a well-established prognostic indicator in HCC.5,15,16 In contrast, whether BDTT has a significant impact on the prognosis of HCC remains controversial, and there is no consensus regarding its efficacy as an indicator of poor prognosis. Shiomi et al. and Satoh et al. found no significant differences in survival between patients with BDTT and those without BDTT.1,13 On the other hand, recent studies including ours, have observed significantly poorer survival outcomes in patients with BDTT after surgery.2,10–12,17
The results of univariate survival analysis from a recent Korean multicentre study showed that the maximal tumour size, bile duct resection and surgical curability were significant risk factors for survival, and surgical curability was a significant risk factor for recurrence. However, multivariate analysis did not reveal any independent risk factors.5 Experience with liver transplantation in patients with BDTT is limited. Reports from Korea have suggested liver transplantation may be an effective treatment in cirrhotic patients with HCC-BDTT.18
Conclusion
HCC with BDTT, particularly with obstructive jaundice as the main presenting clinical feature, is uncommon. The prognosis of this type of HCC is generally dismal, but is better than those who have not undergone a resection. Jaundice is not necessarily a harbinger of advanced disease and a contraindication for surgery. When appropriately selected and carried out safely, surgery can result in long-term survival. A deeper understanding and active attitude to treatment of this type of disease are the keys to further improving survival of these patients. When technically feasible, curative surgery is recommended as the first-line treatment for icteric hepatomas.
Conflicts of interest and source of funding
The above doctors have no conflicts of interest or financial ties to disclose.
References
- Shiomi M, Kamiya J, Nagino M, Uesaka K, Sano T, Hayakawa N, et al. Hepatocellular carcinoma with biliary tumor thrombi: aggressive operative approach after appropriate preoperative management. Surgery. 2001;129:692–698. doi: 10.1067/msy.2001.113889. [DOI] [PubMed] [Google Scholar]
- Meng KW, Dong M, Zhang WG, Huang QX. Clinical characteristics and surgical prognosis of hepatocellular carcinoma with bile duct invasion. Gastroenterol Res Pract. 2014;2014 doi: 10.1155/2014/604971. :ID 604971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9:385–391. doi: 10.3748/wjg.v9.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LX, Ma ZC, Wu ZQ, Fan J, Zhou XD, Sun HC, et al. Diagnosis and surgical treatments of hepatocellular carcinoma with tumor thrombosis in bile duct: experience of 34 patients. World J Gastroenterol. 2004;10:1397–1401. doi: 10.3748/wjg.v10.i10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DB, Hwang S, Wang HJ, Yun SS, Kim KS, Lee YJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg. 2013;37:443–451. doi: 10.1007/s00268-012-1845-0. [DOI] [PubMed] [Google Scholar]
- Ueda M, Takeuchi T, Takayasu T, Takahashi K, Okamoto S, Tanaka A. Classification and surgical treatment of hepatocellular carcinoma (HCC) with bile duct thrombi. Hepatogastroenterology. 1994;41:349–354. [PubMed] [Google Scholar]
- Mallory TB, Castleman B, Parris EE. Case records of the Massachusetts General Hospital. N Engl J Med. 1947;237:673–676. [Google Scholar]
- Lau WY, Leung KL, Leung TW, Liew CT, Chan MS, Yu SC, et al. A logical approach to hepatocellular carcinoma presenting with jaundice. Ann Surg. 1997;225:281–285. doi: 10.1097/00000658-199703000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SY, Wang JW, Liu YB, Cai XJ, Deng GL, Xu B, et al. Surgical intervention for obstructive jaundice due to biliary tumor thrombus in hepatocellular carcinoma. World J Surg. 2004;28:43–46. doi: 10.1007/s00268-003-7079-4. [DOI] [PubMed] [Google Scholar]
- Yu XH, Xu LB, Liu C, Zhang R, Wang J. Clinicopathological characteristics of 20 cases of hepatocellular carcinoma with bile duct tumor thrombi. Dig Dis Sci. 2011;56:252–259. doi: 10.1007/s10620-010-1256-8. [DOI] [PubMed] [Google Scholar]
- Shao W, Sui C, Liu Z, Yang J, Zhou Y. Surgical outcome of hepatocellular carcinoma patients with biliary tumor thrombi. World J Surg Oncol. 2011;9:2–5. doi: 10.1186/1477-7819-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CN, Jan YY, Lee WC, Chen MF. Hepatic resection for hepatocellular carcinoma with obstructive jaundice due to biliary tumor thrombi. World J Surg. 2004;28:471–475. doi: 10.1007/s00268-004-7185-y. [DOI] [PubMed] [Google Scholar]
- Satoh S, Ikai I, Honda G, Okabe H, Takeyama O, Yamamoto Y, et al. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi. Surgery. 2000;128:779–783. doi: 10.1067/msy.2000.108659. [DOI] [PubMed] [Google Scholar]
- Huang JF, Wang LY, Lin ZY, Chen SC, Hsieh MY, Chuang WL. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:190–195. doi: 10.1046/j.1440-1746.2002.02677.x. [DOI] [PubMed] [Google Scholar]
- Esaki M, Shimada K, Sano T, Sakamoto Y, Kosuge T, Ojima H. Surgical results for hepatocellular carcinoma with bile duct invasion: a clinicopathologic comparison between macroscopic and microscopic tumor thrombus. J Surg Oncol. 2005;90:226–232. doi: 10.1002/jso.20260. [DOI] [PubMed] [Google Scholar]
- Liu QY, Lai DM, Liu C, Zhang L, Zhang WD, Li HG. A special recurrent pattern in small hepatocellular carcinoma after treatment: bile duct tumor thrombus formation. World J Gastroenterol. 2011;17:4817–4824. doi: 10.3748/wjg.v17.i43.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenaga N, Chijiiwa K, Otani K, Ohuchida J, Uchiyama S, Kondo K. Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion. J Gastrointest Surg. 2009;13:492–497. doi: 10.1007/s11605-008-0751-0. [DOI] [PubMed] [Google Scholar]
- Lee KW, Park JW, Park JB, Kim SJ, Choi SH, Heo JS, et al. Liver transplantation for hepatocellular carcinoma with bile duct thrombi. Transplant Proc. 2006;38:2093–2094. doi: 10.1016/j.transproceed.2006.06.034. [DOI] [PubMed] [Google Scholar]


