Abstract
Background
Liver surgery for perihilar cholangiocarcinoma (PHC) is associated with high rates of morbidity and mortality.
Objectives
This study investigated the impact of low skeletal muscle mass on short- and longterm outcomes following hepatectomy for PHC.
Methods
Patients included underwent liver surgery for PHC between 1998 and 2013. Total skeletal muscle mass was measured at the level of the third lumbar vertebra using available preoperative computed tomography images. Sex-specific cut-offs for low skeletal muscle mass were determined by optimal stratification.
Results
In 100 patients, low skeletal muscle mass was present in 42 (42.0%) subjects. The rate of postoperative complications (Clavien–Dindo Grade III and higher) was greater in patients with low skeletal muscle mass (66.7% versus 48.3%; multivariable adjusted P = 0.070). Incidences of sepsis (28.6% versus 5.2%) and liver failure (35.7% versus 15.5%) were increased in patients with low skeletal muscle mass. In addition, 90-day mortality was associated with low skeletal muscle mass in univariate analysis (28.6% versus 8.6%; P = 0.009). Median overall survival was shorter in patients with low muscle mass (22.8 months versus 47.5 months; P = 0.014). On multivariable analysis, low skeletal muscle mass remained a significant prognostic factor (hazard ratio 2.02; P = 0.020).
Conclusions
Low skeletal muscle mass has a negative impact on postoperative mortality and overall survival following resection of PHC and should therefore be considered in preoperative risk assessment.
Introduction
Low skeletal muscle mass has been associated with worse outcomes following surgery for malignancies of gastrointestinal origin.1–5 It has been shown to affect postoperative morbidity and mortality in patients with colorectal liver metastases (CRLM),3 hepatocellular carcinoma (HCC)6 and colorectal cancer,4 and to impact on longterm survival following resection of HCC,1,6 pancreatic cancer2 and CRLM.5 In these studies, low or high body mass index (BMI) was not a risk factor for poor outcomes. These results suggest that lower skeletal muscle mass may reflect frailty and may be a highly relevant factor in preoperative risk assessment. Measurement of the total skeletal muscle area at the third lumbar vertebra on computed tomography (CT) images has been widely accepted as a standard method of determining whole-body skeletal muscle mass in the preoperative workup.7–9
Perihilar cholangiocarcinoma (PHC) is a biliary tumour located at the liver hilum, which typically requires a combined extrahepatic bile duct and liver resection in instances of resectable disease. Several studies have shown that an aggressive surgical approach has improved the negative-margin (R0) resection rate and survival.10,11 However, liver resection in PHC is associated with high levels of risk for morbidity and mortality, reported to reach 68% and 14%, respectively.10–13 The identification of potentially reversible risk factors might facilitate the preoperative modification of these risks. The aim of this study was to investigate the impact of low skeletal muscle mass on postoperative morbidity, mortality and overall survival following resection of PHC.
Materials and methods
Data were retrospectively collected from a database including all patients submitted to exploratory laparotomy for PHC between 1998 and 2013 in a single institution (Academic Medical Centre, Amsterdam). Inclusion criteria required patients to have undergone curative-intent major hepatectomy and necessitated the availability of adequate preoperative CT images of the abdomen. The latter are necessary for skeletal muscle mass measurement. Study variables included patient characteristics, laboratory results, tumour characteristics (Bismuth–Corlette classification and histopathological information), details of surgery, complications, and overall survival and recurrence data.
Preoperative measurement of skeletal muscle mass
In all patients, CT scans selected for analysis were performed after adequate preoperative biliary drainage (if indicated) had been achieved as part of the routine preoperative workup. Cross-sectional skeletal muscle surface (cm2) was assessed at the level of the third lumbar vertebra (L3) on two consecutive axial slices with visible vertebral spine, as previously described.14 Plain images were selected by an experienced staff radiologist (CYN) and measurements were obtained using OsiriX Version 5.8 (32-bit; http://www.osirix-viewer.com) by one of the authors (RJSC). Using a Hounsfield units (HU) range of −30 HU to +110 HU to distinguish skeletal muscle tissue, measurements of the psoas, paraspinal, transverse abdominal, internal and external oblique and rectus abdominis muscles were obtained (Fig.1). Cross-sectional areas of the two L3 levels were then averaged and corrected for height to calculate the L3 muscle index expressed in cm2/m2. A previous study has demonstrated very good inter-observer variability.4
Figure 1.
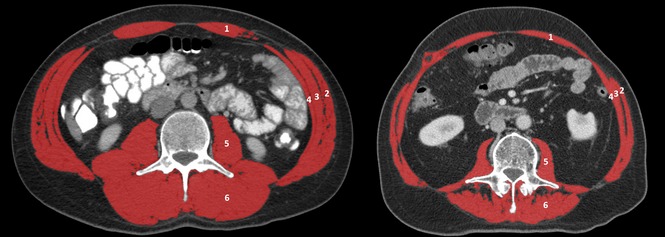
Computed tomography scans at the third lumbar vertebra level of a male patient with normal skeletal muscle mass (left, L3 muscle index 60.04 cm2/m2) and low muscle mass (right, L3 muscle index 39.19 cm2/m2). Skeletal muscle area highlighted in red. 1, rectus abdominis; 2, external oblique; 3, internal oblique; 4, transverse abdominal; 5, psoas; 6, paraspinal.
Weight loss was calculated by subtracting the patient's weight at the time of diagnosis from his or her reported weight prior to illness. The presence of weight loss was then defined as a >2% loss from pre-illness self-reported stable weight.7
Surgical procedures
Prior to surgery, patients underwent endoscopic biliary drainage and/or percutaneous drainage when indicated because of jaundice and dilatation of the bile ducts in the future remnant liver. Preoperative liver function was assessed with CT volumetry and 99mTc-mebrofenin hepatobiliary scintigraphy.15 Patients underwent radical resection of the tumour encompassing hilar resection with en bloc (extended) hemi-hepatectomy including the caudate lobe, excision of the portal vein bifurcation when involved and complete lymphadenectomy of the hepatoduodenal ligament in the majority of cases. For biliary reconstruction, end-to-side anastomoses of the segmental ducts and a Roux-en-Y jejunal loop were constructed.10 Resections were performed by two staff surgeons with extensive hepatobiliary expertise.
Definitions of complications
Postoperative morbidity was defined as any severe complication (i.e. Clavien–Dindo Grade III or higher16) within 30 days after surgery. Overall complications were further stratified into postoperative haemorrhage, anastomotic leakage, intra-abdominal abscess or fluid collections, and acute liver failure, using previously described definitions. Haemorrhage was defined as a drop in haemoglobin level of >3 g/dl after the end of surgery compared with the postoperative baseline level and/or any postoperative transfusion of packed red blood cells for a falling haemoglobin level and/or the need for invasive re-intervention (e.g. embolization or re-laparotomy) to stop bleeding.17 Anastomotic leakage was defined as fluid with an increased bilirubin concentration in the abdominal drain (three times greater than serum bilirubin concentration) on or after postoperative day (PoD) 3 or the need for radiologic intervention because of contrast leakage during percutaneous transhepatic cholangiography (in the event of hepaticojejunostomy leakage)18 or a defect of the intestinal wall at the anastomotic site leading to a communication between the intra- and extraluminal compartments (in the event of enteroenterostomy leakage).19 Sepsis was defined as the presence (probable or documented) of infection together with systemic manifestations of infection.20 Intra-abdominal abscess or fluid collection were defined as the collection of pus or infected fluid inside the abdomen diagnosed by CT and treated by percutaneous drainage or surgery. Acute liver failure was defined as an increasing international normalized ratio (INR) value (or need of clotting factors to maintain normal INR) and increasing plasma bilirubin level on or after PoD 5, impacting clinical management [International Study Group of Liver Surgery (ISGLS) grade B or higher].21 Postoperative mortality was defined as death within 90 days after surgery or within the same hospital admission.
Follow-up
No adjuvant chemotherapy was administered after initial curative resection. Clinical follow-up was performed routinely every 3 months in the first year after surgery and every 6 months throughout the following 5 years. Laboratory tests and follow-up CT scans were performed in the first 6 months to detect early recurrence and later as indicated. The overall survival status of patients discharged from further follow-up was examined by checking the municipal records database.
Statistical analysis
The variable of skeletal muscle mass was dichotomized using optimal stratification to allow for risk classification and the clinical interpretation of effect measures. Optimal stratification is the preferred method for dichotomizing continuous variables, as tertiles, quartiles and means lack sufficient sensitivity to allow the assessment of a variable's true prognostic value.22 Optimal stratification is a statistical method similar to receiver operator curve analysis and is able to find the most significant cut-off for a continuous variable with respect to survival, based on log rank statistics.22 Sex-specific cut-offs were determined for the L3 muscle index, and these cut-offs were subsequently used to categorize patients into low skeletal muscle mass and normal skeletal muscle mass groups.
Univariate analyses for overall morbidity and mortality consisted of Pearson's chi-squared test or Fisher's exact test for categorical variables, and the unpaired t-test or Mann–Whitney U-test for continuous variables. Overall survival, defined as the number of days of survival after hepatectomy, was analysed in univariate analysis using a Kaplan–Meier survival plot, and compared using the log rank test. To further assess the effect of low skeletal muscle mass, multivariable analysis was performed for postoperative morbidity (logistic regression) and overall survival (Cox proportional hazards model). Multivariable analysis for postoperative morbidity was used to adjust for known predictors, which were age, preoperative bilirubin level, hepatectomy side and perioperative blood transfusion.23,24 Multivariable analysis for overall survival was used to adjust for age, sex, American Society of Anesthesiologists (ASA) class and known predictors (resection margin status, lymph node status, tumour differentiation and postoperative morbidity).11,25 In addition, backward variable selection was performed on the full model based on variables with a P-value of <0.05 (variable selection model). Because of the estimated low number of events (<10 events per variable), a multivariable analysis of postoperative mortality was not considered feasible.26 Statistical analysis was performed using R Version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria), and IBM spss Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA). Two-tailed P-values of <0.05 were considered to indicate statistical significance.
Results
A total of 129 patients were identified as having undergone major hepatectomy for PHC during the study period. In 29 patients (22.5%) no adequate preoperative staging CT scan for skeletal muscle mass assessment was available in the radiology information system. In this group of patients, the abdomen had not been scanned through the L3 level, CT scans could not be retrieved from the system or staging had been performed using other imaging modalities such as magnetic resonance imaging. These cases were therefore excluded from further analysis (Table S1, online). Of the 100 remaining patients, 64 (64.0%) were male and 36 (36.0%) were female. Mean ± standard deviation (SD) L3 muscle indices were 47.65 ± 6.38 cm2/m2 in males and 40.74 ± 6.49 cm2/m2 in females (P < 0.001).
Low and normal skeletal muscle mass groups
Sex-specific cut-off values for L3 muscle mass indices were determined at 46.8 cm2/m2 for males and 39.1 cm2/m2 for females. Using these cut-offs, 42 patients (42.0%) were identified as having low skeletal muscle mass. The time interval between muscle mass measurements on CT and surgery did not differ between the low and normal skeletal muscle mass groups (median: 39 days versus 42 days; P = 0.610). The clinicopathological characteristics of patients in both groups are listed in Table1. Lower BMI was observed in patients with low skeletal muscle mass. The majority of patients with low muscle mass were of normal weight. Only four of 42 (9.5%) patients with low muscle mass were defined as cachectic and none of the patients with normal muscle mass fulfilled these criteria.7 Seventeen patients in the cohort (17.0%) were identified as overweight or obese (BMI ≥25 kg/m2) and as having low muscle mass. Other preoperative patient characteristics did not differ between patients with, respectively, low and normal skeletal muscle mass. Furthermore, there were no significant differences between the groups in prognostic tumour-related factors.
Table 1.
Clinicopathological characteristics in patients with low and normal skeletal muscle mass submitted to resection of perihilar cholangiocarcinoma, using cut-off values obtained by optimal stratification
| Normal skeletal muscle mass (n = 58) | Low skeletal muscle mass (n = 42) | P-value | |
|---|---|---|---|
| Age, years, mean ± SD | 62 ± 9 | 61 ± 11 | 0.520a |
| Sex ratio, M:F, n:n | 36:22 | 28:14 | 0.636 |
| BMI, kg/m2, mean ± SD | 26 ± 3 | 24 ± 3 | 0.001a |
| Weight loss, n (%) | 36 (62.1%) | 23 (54.8%) | 0.463 |
| Jaundice, n (%) | 39 (67.2%) | 31 (73.8%) | 0.367 |
| Diabetes, n (%) | 6 (10.3%) | 4 (9.5%) | 0.893 |
| CA19-9, kU/l, median (range) | 176 (1–1363) | 98 (16–482) | 0.424b |
| Albumin, g/l, mean ± SD | 39 ± 5 | 37 ± 6 | 0.081a |
| CRP, mg/l, median (range) | 11 (2–269) | 17 (1–300) | 0.276b |
| Haemoglobin, mmol/l, mean ± SD | 8.2 ± 0.9 | 8.0 ± 0.9 | 0.228a |
| Total bilirubin, μmol/l, median (range) | 10 (4–48) | 18 (4–57) | 0.069b |
| Platelet count, ×109/l, mean ± SD | 321 ± 105 | 325 ± 96 | 0.864a |
| Preoperative cholangitis, n (%) | 20 (34.5%) | 20 (50.0%) | 0.125 |
| Tumour size, cm, median (range) | 2.6 (0.9–12.5) | 2.6 (1.3–7.0) | 0.978b |
| Tumour classification, n (%) | |||
| BC 1 | 1 (1.7%) | 0 | 0.844 |
| BC 2 | 4 (6.9%) | 2 (4.8%) | |
| BC 3a | 19 (32.8%) | 18 (42.9%) | |
| BC 3b | 14 (24.1%) | 9 (21.4%) | |
| BC 4 | 12 (20.7%) | 9 (21.4%) | |
| Left or right hepatic duct | 8 (13.8%) | 4 (9.5%) | |
| ASA class, n (%) | |||
| 0–2 | 51 (87.9%) | 36 (85.7%) | 0.745 |
| 3, 4 | 7 (12.1%) | 6 (14.3%) | |
| Left:right (extended) hemi-hepatectomy ratioc, n:n | 28:29 | 20:21 | 0.973 |
| TNM stage, n (%) | |||
| 0–II | 24 (41.4%) | 19 (45.2%) | 0.700 |
| III, IV | 34 (58.6%) | 23 (54.8%) | |
| N1 status, n (%) | 13 (22.4%) | 12 (28.6%) | 0.483 |
| R0 resection, n (%) | 40 (69.0%) | 32 (76.2%) | 0.427 |
| Perineural invasion, n (%) | 43 (74.1%) | 29 (69.0%) | 0.666 |
| Moderate/poor differentiation, n (%) | 38 (65.5%) | 29 (69.0%) | 0.845 |
| Papillary tumour type, n (%) | 6 (10.3%) | 4 (9.5%) | 0.893 |
Unpaired t-test.
Mann–Whitney U-test.
One patient in the normal muscle mass group and one in the low muscle mass group underwent a central resection.
Statistical tests were performed using the chi-squared test or Fisher's exact test or as indicated otherwise.
ASA, American Society of Anesthesiologists; BC, Bismuth–Corlette; BMI, body mass index; CA 19-9, cancer antigen 19-9; CRP, C-reactive protein; N1, positive lymph node; R0, negative margin; SD, standard deviation; TNM, tumour–node–metastasis (stage defined by 7th edition of American Joint Committee on Cancer).
Postoperative morbidity
Overall complications (Clavien–Dindo Grade III and higher) in the 30-day postoperative period or during the hospital stay showed a trend towards a higher incidence in patients with low skeletal muscle mass (n = 28, 66.7%) compared with patients with normal muscle mass (n = 28, 48.3%) (P = 0.067). An overview of postoperative complications is shown in Table2. Incidences of sepsis (or septic shock) and liver failure (ISGLS grade B or higher) differed between the patient groups. A total of 15 patients developed sepsis (or septic shock) in the postoperative period; these included three patients (5.2%) in the normal muscle mass group and 12 patients (28.6%) in the low muscle mass group (P = 0.002). Twenty-four patients developed liver failure (ISGLS grade B or higher), including nine patients (15.5%) in the normal skeletal muscle mass group and 15 (35.7%) in the low muscle mass group (P = 0.020). On multivariable analysis, low skeletal muscle mass showed an association with postoperative morbidity of borderline significance (P = 0.070) (Table3). Because of the low number of events, multivariable analysis for the risk factors of sepsis and liver failure was not considered to be feasible.
Table 2.
Postoperative complications (Clavien–Dindo Grade III and higher) in the low and normal skeletal muscle mass groups
| Normal skeletal muscle mass (n = 58) | Low skeletal muscle mass (n = 42) | P-value | |
|---|---|---|---|
| Any complication Clavien–Dindo Grade III+ | 28 (48.3%) | 28 (66.7%) | 0.067 |
| Sepsis | 3 (5.2%) | 12 (28.6%) | 0.002 |
| Acute liver failure (ISGLS grade B+) | 9 (15.5%) | 15 (35.7%) | 0.020 |
| Haemorrhage | 6 (10.3%) | 2 (4.8%) | 0.462 |
| Anastomotic leakage | 11 (19.0%) | 8 (19.0%) | 0.992 |
| Abscess/fluid collection | 13 (22.4%) | 12 (28.6%) | 0.483 |
Statistical tests were performed using the chi-squared test or Fisher's exact test.
ISGLS, International Study group of Liver Surgery.
Table 3.
Univariate and multivariable analysis for risk factors of postoperative morbidity
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Morbidity rate, n (%) | P-value | OR (95% CI) | P-value | |
| Low skeletal muscle mass | ||||
| No | 28/58 (48.3%) | 0.067 | 1 (reference) | 0.070 |
| Yes | 28/42 (66.7%) | 2.36 (0.93–5.96) | ||
| Age | N/A | 1.01 (0.97–1.06) | 0.592 | |
| Preoperative bilirubin | N/A | 1.00 (0.97–1.06) | 0.978 | |
| Hepatectomy sidea | ||||
| Left | 25/48 (52.1%) | 0.556 | 1 (reference) | 0.720 |
| Right | 29/50 (58.0%) | |||
| Perioperative blood transfusion | ||||
| No | 16/38 (42.1%) | 0.028 | 1 (reference) | 0.061 |
| Yes | 40/62 (64.5%) | 2.70 (0.96–7.62) | ||
Two patients underwent a central liver resection.
Univariate analysis was performed using the chi-squared test or Fisher's exact test.
95% CI, 95% confidence interval; N/A, not applicable; OR, odds ratio.
Postoperative mortality
Seventeen patients (17.0%) in the selected cohort died within the 90-day postoperative period or during the index hospital stay. Ninety-day postoperative mortality was higher among patients with low skeletal muscle mass (28.6% versus 8.6%; P = 0.009). Other factors associated with postoperative death in univariate analysis were age of >65 years (P = 0.022) and perioperative blood transfusion (P < 0.001) (Table4). Because of the low number of events, multivariable analysis was not considered possible. Among patients with low muscle mass, those who died within the 90-day postoperative period were older (median age: 67 years versus 61 years; P = 0.029) and had higher rates of preoperative cholangitis (83.3% versus 35.7%; P = 0.014) and perioperative blood transfusion (100% versus 63.3%; P = 0.018). Further, a trend towards more extended hepatectomies (66.7% versus 37.7%; P = 0.098) was noted in these patients.
Table 4.
Univariate analysis for risk factors for 90-day/in-hospital mortality
| Mortality, n (%) | P-value | |
|---|---|---|
| Low skeletal muscle mass | ||
| No | 5/58 (8.6%) | 0.009 |
| Yes | 12/42 (28.6%) | |
| Age >65 years | ||
| No | 6/60 (10.0%) | 0.022 |
| Yes | 11/40 (27.5%) | |
| ASA class | ||
| 0–2 | 14/87 (16.1%) | 0.532 |
| 3, 4 | 3/13 (23.1%) | |
| Hepatectomy side | ||
| Left | 5/48 (10.4%) | 0.076 |
| Right | 12/50 (24.0%) | |
| Extended hemi-hepatectomya | ||
| No | 6/48 (12.5%) | 0.250 |
| Yes | 11/52 (21.2%) | |
| Perioperative blood transfusion | ||
| No | 0/38 | <0.001 |
| Yes | 16/62 (25.8%) | |
Two patients underwent a central liver resection.
Statistical tests were performed using the chi-squared test or Fisher's exact test.
ASA, American Society of Anesthesiologists.
Survival
Median follow-up among patients who were alive at follow-up was 28 months (range: 2–122 months). The median overall survival of the total cohort was 36.7 months. Overall survival was shorter in the low skeletal muscle mass group in univariate analysis (22.8 months versus 47.5 months; P = 0.014) (Fig.2). Corresponding estimated 5-year survival rates in the low and normal skeletal muscle mass groups were 20.3% and 36.2%, respectively. Disease recurrence was noted in 34 patients (34.0%). Median disease-free survival did not differ between patients with, respectively, low and normal skeletal muscle mass (43.3 months versus 39.8 months; P = 0.748).
Figure 2.
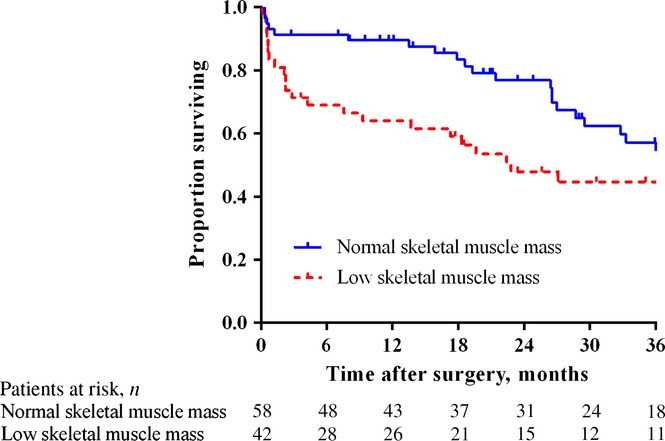
Overall survival after resection of perihilar carcinoma in patients with low (n = 42) and normal (n = 58) skeletal muscle mass
After adjustment for potential and known predictors of survival in multivariable analysis, low skeletal muscle mass remained independently associated with poor overall survival [adjusted hazard ratio (HR) 2.02, 95% confidence interval (CI) 1.12–3.65; P = 0.020] (Table5). When postoperative deaths were excluded, low muscle mass was still an independent predictor of worse survival (HR 2.02, 95% CI 1.04–3.92; P = 0.037). The combination of low muscle mass and overweight/obesity was not independently associated with poor survival.
Table 5.
Univariate and multivariable analysis of clinicopathological factors and overall survival
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Full modela | Variable selectionb | |||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.00 (0.97–1.03) | 0.870 | 1.00 (0.97–1.03) | 0.771 | – | – |
| Male sex | 1.70 (0.97–2.98) | 0.065 | 1.41 (0.76–2.63) | 0.281 | – | – |
| ASA class | 0.94 (0.58–1.50) | 0.783 | 0.98 (0.55–1.73) | 0.933 | – | – |
| Low skeletal muscle mass | 1.90 (1.13–3.19) | 0.015 | 2.02 (1.12–3.65) | 0.020 | 2.01 (1.14–3.54) | 0.016 |
| R1 status | 1.89 (1.07–3.33) | 0.028 | 1.88 (0.96–3.67) | 0.064 | 2.06 (1.08–3.91) | 0.028 |
| N1 status | 1.82 (1.03–3.22) | 0.040 | 1.77 (0.95–3.30) | 0.072 | 1.83 (0.99–3.39) | 0.056 |
| Moderate/poor differentiation | 2.08 (1.03–4.20) | 0.041 | 2.43 (1.20–4.93) | 0.014 | 2.40 (1.19–4.84) | 0.015 |
| Postoperative complications | 1.70 (1.00–2.87) | 0.049 | 1.47 (0.78–2.78) | 0.234 | 1.42 (0.77–2.62) | 0.261 |
Cox proportional hazards regression analysis for all factors.
Cox proportional hazards regression analysis after backward variable selection.
95% CI, 95% confidence interval; ASA, American Society of Anesthesiologists; BMI, body mass index; HR, hazard ratio; N1, positive lymph node; R1, positive margin; TNM, tumour–node–metastasis.
Discussion
The present study shows that low skeletal muscle mass, as defined by a cholangiocarcinoma-specific cut-off, was present in 42.0% of patients with resectable PHC. Low skeletal muscle mass was associated with postoperative sepsis and liver failure, 90-day mortality and overall survival after major liver resection.
Low skeletal muscle mass has been recognized as a predictor of postoperative morbidity and mortality in liver transplantation, as well as in several other gastrointestinal cancers, including HCC,1,6 CRLM3,5 and pancreatic carcinoma.2 Previous studies have related low muscle mass to an increased rate of postoperative infectious complications, including sepsis and wound infections.27–29 Reisinger et al. assessed the combined effect of low skeletal muscle mass and a lower nutritional and frailty status, and confirmed an associated risk for sepsis.4 High expression of proinflammatory cytokines [e.g. interleukin-1β (IL-1β) and IL-6] in low skeletal muscle mass and cancer cachexia may induce this proinflammatory state and may increase the risk for infectious complications.30 Septic complications are of particular relevance in patients with PHC, as concomitant biliary obstruction predisposes patients to cholangitis and secondary infections. Similarly to studies in colorectal cancer and liver transplantation for benign disease, the present authors observed a higher rate of septic complications (28.6% in patients with low muscle mass versus 5.2% in patients with normal muscle mass) after liver resection for PHC. An association between lower muscle mass and infectious complications was further suggested by a higher rate of preoperative cholangitis of borderline significance. Nonetheless, the temporal sequence of this association remains uncertain: cholangitis may have caused muscle wasting, or patients with lower muscle mass may be at higher risk for preoperative cholangitis. Furthermore, a higher rate of postoperative liver failure was observed in the low muscle mass group (35.7% versus 15.5%). Overall, skeletal muscle mass showed an association with morbidity of only borderline significance, which may be partly explained by a lack of sufficient statistical power. Skeletal muscle mass showed a significant association with postoperative 90-day mortality in univariate analysis, although the statistical power of the present data may be insufficient to confirm this result in multivariable analysis. Nonetheless, these results warrant the assessment of low skeletal muscle mass as a risk factor prior to liver surgery in PHC.
The negative impact of low skeletal muscle mass on survival following surgery has also been shown in other hepatobiliary diseases, including HCC.1,6 The present study found an effect on survival after liver resection for PHC that was comparable with the effects of resection margin status, lymph node status and tumour differentiation grade. The majority of patients with lower muscle mass did not meet the defined criteria for cancer cachexia, but were simply individuals who had demonstrated lower skeletal muscle mass prior to surgery. Interestingly, even in the absence of severe cancer-related wasting, CT assessment of skeletal muscle mass showed prognostic value. These results underscore the importance of this patient-related factor.
Interest in the assessment of frailty in surgery patients has increased in recent years. Preoperative frailty scores to evaluate the general condition of patients have been developed and have shown associations with postoperative outcomes.31–33 These scores, however, are mainly based on patient comorbidities and rarely involve nutritional status, although decreased nutritional intake has been shown to have significant negative impact on postoperative mortality.4 Some scores include surrogates of skeletal muscle mass (anthropometry, weight loss), but many require additional measurements in patients. However, as almost 50% of patients with low muscle mass may not have any weight loss, whether this factor is an adequate surrogate marker of skeletal muscle mass should be questioned. So far, no skeletal muscle mass index has been included in frailty scores. On the basis of the aforementioned studies and the present results, the current authors propose that skeletal muscle mass should be considered a factor in such scores. Of course, whether the inclusion of CT-based muscle measurements is as discriminative as current practice protocols remains to be ascertained. Although some studies have used dual-energy X-ray absorptiometry to determine muscle mass depletion, the quantification of muscle mass at L3 on CT images is straightforward and easily reproducible, and CT scans are usually available in all patients scheduled for surgery.7,9,34
The question of whether skeletal muscle mass can be increased preoperatively remains. A recent study on outcomes after liver transplantation in patients with low muscle mass observed a significant improvement in overall survival with perioperative nutritional therapy, although skeletal muscle mass itself was not increased.35 Other studies have focused on inhibiting catabolic pathways that promote muscle wasting. In patients with advanced cholangiocarcinoma, the administration of selumetinib, which is an inhibitor of mitogen-activated protein/extracellular signal-regulated kinase and IL-6, led to significant muscle gain in the majority of patients, but no survival benefit was observed.36 It has been suggested that regular exercise prevents frailty and improves skeletal muscle mass and physical function in older patients,37 but further studies are needed to assess the effects of preoperative training on muscle mass and survival. In general, modulating skeletal muscle mass seems possible and may therefore represent a promising strategy towards improving postoperative outcomes and even lowering health care costs.38
The present study has several limitations. Firstly, the exclusion from analysis of patients without adequate CT scans has presumably resulted in some degree of selection bias, although the comparison of demographics and complications between included and excluded patients revealed only a clinically significant lower number of R0 resections in the latter group (Table S1). The process of selecting study subjects from a total cohort of patients with PHC undergoing hepatectomy led to a smaller sample size and lower number of events. The consequent statistical uncertainty may have particularly influenced the analysis of postoperative morbidity, which showed associations with the analysed predictors of only borderline significance. Moreover, the even lower number of events in the analysis of postoperative mortality prevented the performance of multivariable analysis for that endpoint. Secondly, the study was designed to use a disease-specific cut-off for PHC, as others have done previously for various diseases.5,8 Previously established cut-offs were found to show an inaccurate fit that would lead to inaccurate effect measurement. This observation may be explained by the inclusion in the present cohort of less overweight patients. Established cut-offs may not be applicable to all cancer populations as a result of clinicopathological differences and the present authors therefore propose that the optimal stratification method be used to investigate a relationship between skeletal muscle mass and postoperative outcome in such instances.
Thirdly, a relatively high 90-day mortality rate (17.0%) was observed in the preselected cohort (especially in individuals with low muscle mass), given that previous results established by the present group had shown postoperative mortality of 7–10%. The latter series, however, also included patients undergoing only local resection of the extrahepatic bile duct.10 The high mortality rate (28.6%) within the low muscle mass group may have resulted from higher age, the presence of preoperative cholangitis and more extensive resections in these diseased patients.
Finally, as biliary drainage has been shown to increase nutritional status, it may also indirectly influence preoperative skeletal muscle mass.39 Standard measurement of skeletal muscle mass status on post-drainage CT scans ensured the standardization of the current analysis. However, the effect of biliary drainage on skeletal muscle mass was not measured because adequate pre-drainage scans were not available.
To conclude, the present study shows that low skeletal muscle mass has a negative impact on short- and longterm outcomes after hepatectomy for PHC. Therefore, measurement of skeletal muscle mass should be considered in the preoperative risk assessment of patients with PHC. Preoperative amplification of skeletal muscle mass in patients with low muscle mass, using nutritional intervention and/or exercise, potentially improves postoperative outcomes.
Acknowledgments
The authors would like to thank Dr S. van Dieren, Clinical Research Unit, Amsterdam Medical Centre, for statistical advice.
Conflicts of interest
None declared.
Supporting Information
Table S1. Comparison of clinicopathological characteristics and complications after resection of perihilar cholangiocarcinoma in patients included in (n = 100) and excluded from (n = 29) the present study.
References
- Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. doi: 10.1111/j.1477-2574.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewe KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000743. PMID: 24950264 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Wang Z, Gallagher DS, Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- van Gulik TM, Kloek JJ, Ruys AT, Busch OR, van Tienhoven GJ, Lameris JS, et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol. 2011;37:65–71. doi: 10.1016/j.ejso.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]
- Gomez D, Patel PB, Lacasia-Purroy C, Byrne C, Sturgess RP, Palmer D, et al. Impact of specialized multi-disciplinary approach and an integrated pathway on outcomes in hilar cholangiocarcinoma. Eur J Surg Oncol. 2014;40:77–84. doi: 10.1016/j.ejso.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford) 2005;7:259–262. doi: 10.1080/13651820500373010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello SA, Lodewick TM, van Dam RM, Reisinger KW, van den Broek MA, von Meyenfeldt MF, et al. Sarcopenia negatively affects preoperative total functional liver volume in patients undergoing liver resection. HPB (Oxford) 2013;15:165–169. doi: 10.1111/j.1477-2574.2012.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf W, van Lienden KP, Dinant S, Roelofs JJ, Busch OR, Gouma DJ, et al. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14:369–378. doi: 10.1007/s11605-009-1085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB (Oxford) 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Williams BA, Mandrekar JA, Mandrekar SJ, Cha SS. Furth AF. 2006. , & Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. Mayo Foundation, Technical Report Series 79: Rochester, MN.
- Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100:274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, House MG, Pitt HA, Nakeeb A, Howard TJ, Zyromski NJ, et al. Post-operative morbidity results in decreased long-term survival after resection for hilar cholangiocarcinoma. HPB (Oxford) 2011;13:139–147. doi: 10.1111/j.1477-2574.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401–407. doi: 10.1002/lt.23811. [DOI] [PubMed] [Google Scholar]
- Scheede-Bergdahl C, Watt HL, Trutschnigg B, Kilgour RD, Haggarty A, Lucar E, et al. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? Clin Nutr. 2012;31:85–88. doi: 10.1016/j.clnu.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Kim SW, Han HS, Jung HW, Kim KI, Hwang DW, Kang SB, et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149:633–640. doi: 10.1001/jamasurg.2014.241. [DOI] [PubMed] [Google Scholar]
- Revenig LM, Canter DJ, Taylor MD, Tai C, Sweeney JF, Sarmiento JM, et al. Too frail for surgery? Initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg. 2013;217:665–670. doi: 10.1016/j.jamcollsurg.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183:40–46. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3:269–275. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549–1556. doi: 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer. 2012;106:1583–1586. doi: 10.1038/bjc.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Marzetti E, Martone AM, Bernabei R, Onder G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:25–31. doi: 10.1097/MCO.0000000000000018. [DOI] [PubMed] [Google Scholar]
- Sheetz KH, Waits SA, Terjimanian MN, Sullivan J, Campbell DA, Wang SC, et al. Cost of major surgery in the sarcopenic patient. J Am Coll Surg. 2013;217:813–818. doi: 10.1016/j.jamcollsurg.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouma DJ, Roughneen PT, Kumar S, Moody FG, Rowlands BJ. Changes in nutritional status associated with obstructive jaundice and biliary drainage in rats. Am J Clin Nutr. 1986;44:362–369. doi: 10.1093/ajcn/44.3.362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of clinicopathological characteristics and complications after resection of perihilar cholangiocarcinoma in patients included in (n = 100) and excluded from (n = 29) the present study.


