Abstract
BACKGROUND
The eradication of Helicobacter pylori infection, commonly prevailing in the stomach, has been important since its introduction. Adequate preparations should be made in finding alternatives when faced with first-line treatment failures. Currently, ideal second-line treatments are indistinct and varied among countries as result of different antibiotic resistance patterns. We aimed to evaluate the safety and efficacy of a clarithromycin-containing bismuth-based quadruple regimen as a second-line treatment.
METHODS
Forty-eight H.pylori-positive patients with proven gastric or duodenal ulcers and/or erosions who had previously failed to respond to furazolidone-containing regimens were enrolled. They received pantoprazole (40 mg-bid), amoxicillin (1gr-bid), bismuth subcitrate (240 mg-bid), and clarithromycin (500mg-bid) for 10 days. Eight weeks after treatment, a 14C-urea breath test was performed for the re-evaluation of H. pylori eradication.
RESULTS
Forty-three patients completed the study. H.pylori eradication rates were 79.2% (95% CI=65.00-89.53) and 88.4% (95% CI=74.91-96.11) according to intention-to-treat and per-protocol analyses, respectively. All patients had excellent compliance to treatment and one did not continue therapy because of adverse effects.
CONCLUSION
In developing countries such as Iran, a ten-day clarithromycin-containing bismuth-based quadruple regimen is encouraged as a second-line treatment because of the acceptable rate of eradication and low adverse effects.
Keywords: Helicobacter pylori, Furazolidone, Second-line therapy, Clarithromycin
INTRODUCTION
Eradication of Helicobacter pylori infections has currently become an important concern because it can cause many gastroduodenal disorders.1 The identification of H.pylori as a gastric pathogen has had a significant effect on the gastroenterological practice because many untreatable gastroduodenal disorders have become treatable. Eradication of H.pylori infection has a considerable effect on ulcer healing and can decrease the risk of gastric cancer development.
Only a few antibiotics can be used in vivo to treat the infection despite its susceptibility to several antibiotics in vitro.2 Standard triple therapy is still the first-line therapy; however, when considering the Maastricht IV Consensus Report, it should be avoided in high clarithromycin resistance areas. The options of first-line eradication regimens consist of bismuth-containing quadruple regimen, sequential, concomitant, and hybrid regimens.1 However, adequate preparations should be made in finding alternatives when faced with first-line treatment failures.3 Currently, ideal second-line treatments are indistinct and dissimilar among countries due to different antibiotic resistance patterns.1 Second-line therapies that can be recommended at present in Asia include triple regimens that have not been prescribed for the patient in the past, bismuth-based quadruple regimen, quinolone-based triple regimen, and rifabutin-based triple regimen.4
Current guidelines recommend a bismuth-based quadruple regimen for 7–10 days for second-line therapy, showing eradication rates of 59–95%.5 In the event of initial treatment failure, the Maastricht conferences recommend using treatments of two differing regimens. In other words, a second-line therapy choice depends on what was used first-line; therefore, it should be constituted of various antibiotics based on local antibiotic resistances.6 This strategy has had an eradication rate of 99.5%, given its proper application.7
Third-line therapy should culture-based in order to choose the most effective regimens.1 High antimicrobial resistance and poor patient compliance are the main causes of H.pylori treatment failure. Regarding the different H.pylori prevalence rates and different antimicrobial resistance rates in our country,8 type and dosage of the drugs and duration of treatment should be based on topical review.
According to the results of several studies such as the third Brazilian consensus, furazolidone-containing regimens are recommended as first-line therapies mainly due to their low cost and low resistance.9,10 In Iran, furazolidone, combined with amoxicillin, bismuth, metronidazole, and a PPI(Proton Pump Inhibitor), are prescribed in the form of a quadruple and triple therapy as the first-line therapy with different results based on the dose and duration of treatment.11,12 This study was designed to evaluate the efficacy and safety of the quadruple 10-day clarithromycin containing bismuth-based regimen for H.pylori eradication as a second-line therapy in patients with documented peptic ulcer disease or gastroduodenitis. Those included in the study had received a furazolidone-containing regimen as first-line therapy.
MATERIALS AND METHODS
This study was performed at Imam Khomeini Hospital, Mazandaran University Teaching Hospital in Sari, north Iran. Forty-eight patients with H.pylori positive peptic ulcer disease or gastroduodenitis who had been previously treated with first line furazolidone-containing regimens were enrolled. Omeprazole (20mg-bid), amoxicillin (1gr-bid), and furazolidone (200mg bid or tid) were administered for 10 days. Patients had all failed the above mentioned therapy, and their failure to H.pylori eradication regimen was confirmed by 14 C-urea breath test eight weeks after treatment (by means of urea composition made by Helicap Institute of Isotopes, Budapest, Hungary, and a cartridge made by Heliprobe breath card, Kibion Uppsala, Sweden).
The exclusion criteria were being less than 18 years old, having severe underlying liver, cardiac, pulmonary, or renal diseases, pregnancy, breast-feeding, any kind of malignancy, previous gastric surgery, coagulopathy, having received antibiotics or nonsteroidal anti-inflammatory drugs (NSAIDs) during the previous 4 weeks, and previous history of allergic reactions to any of medications used in this protocol. Demographic and endoscopic information, history of previous upper gastrointestinal bleeding, and smoking habits were also recorded.
All patients received a bismuth-based clarithromycin-containing quadruple therapy (PABC) including pantoprazole (40mg-bid), amoxicillin (1gr-bid), bismuth subcitrate (240mg-bid), and clarithromycin (500mg-bid) for ten days. They were given written instructions and were informed of the treatment modality. Written informed consent was also obtained from all the patients. This study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (code:1077).
During the course of treatment, patients recorded the side effects of the medications each day and were advised to call the doctor in case of severe side effects. After the course of treatment, they were visited and asked about their compliance to treatment, according to residual pill count and side effects of drugs. Their answers were recorded in a specific questionnaire. The severity of side effects were classified as: No side effect, Mild (not interfering with daily activities), Moderate (partially interfering with daily activities), and Severe (abandoning daily activities). Compliance to treatment was considered “excellent” if the patient had used more than 80% of the prescribed drugs, “good” if they had used 71-80%, “moderate” if they had used 60-70%, and “bad” if they had used less than 60%.
Pantoprazole was continued for patients with peptic ulcer disease until 4 weeks after completion of the eradication regimen. Eight weeks after the present treatment course, H.pylorii eradication was reassessed using 14 C-urea breath test (UBT). All patients were advised to discontinue proton-pump inhibitors for at least 2 weeks before the UBT. For the UBT, patients swallowed 37 kBq (1 lCi) of encapsulated 14 C-labled urea composition (Helicap Institute of Isotopes, Budapest, Hungary) with water. After 10 minutes, patients exhaled into a cartridge (Heliprobe breath card, Kibion Uppsala, Sweden) until the indicator of the card changed from orange to yellow. The cards were inserted into a Geiger Muller counter (Heliprobe Analyser, Kibion AB, Uppsala, Sweden), and radioactivity of samples was automatically measured after 250 seconds. Based on radioactivity as count per minute (cpm), counts of more than 50 cpm were considered infected with H.pylori.
Data were analyzed using SPSS software, version 13. For the calculation of the intention-to-treat eradication rate, all the patients who entered the study were considered. To calculate the per-protocol eradication rate, only those who completed the entire protocol with more than 80% compliance (according to the residual pill count) were considered for analysis. Fisher’s exact test was also used as appropriated. p<0.05 were considered statistically significant.
RESULTS
Among the participants, 30 (62.5%) patients were men. The mean±SD age of the participants was 43.75±13.98 years (range: 21-68 years). Seven (14.6%) patients were smokers, and 5 (10.4%) patients had a previous history of upper gastrointestinal bleeding. The demographic and endoscopic findings of the patients are shown in table 1.
Table 1 . The patients demographic and endoscopic findings .
|
PABC
N (%) |
||
| Included by ITT analysis(n) | 48 | |
| Male / female (n) | 30/18 | |
| Mean age ± SD | 43.75±13.98 | |
| Current Smoking | 7(14.6) | |
| History of GIB | 5(10.4) | |
| Endoscopic findings | DU | 27(56.3) |
| GU | 8(16.7) | |
| GE+DE | 13(27.1) | |
| Bulb deformity | 9(18.8) | |
| Number of patients completed the study (PP analysis) | 43 |
PABC=Pantoprazole, Amoxicillin, Bismuth subcitrate, Clarithromycin, ITT=Intention-to-treat, SD=standard deviation, GIB=Gastrointestinal bleeding, DU=Duodenal ulcer, GU=Gastric ulcer, DE=Duodenal erosions, GE=Gastric erosions, PP=per-protocol
Forty-three (97.74%) patients completed the study with excellent compliance. One (2.27%) patient’s regimen was interrupted due to intolerable adverse effects, nausea, and vomiting. Four patients were lost at follow-up; two were lost due to other medical problems, and two failed to follow-up.
H.pylori eradication rates were 79.2% (95% CI=65.00-89.53) and 88.4% (95% CI=74.91-96.11) according to an intention-to-treat and per-protocol analysis, respectively (flowchart 1).
Flowchart 1 .
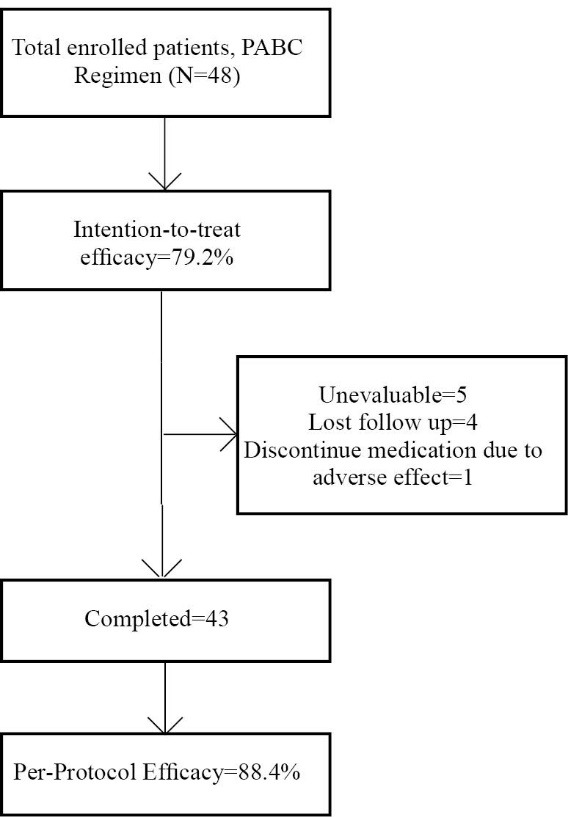
Method of follow-up and treatment efficacy
None of the base-line demographic variables or endoscopic findings was associated with UBT results, according to Fisher’s exact test.
Twenty-two (51.2%) patients complained of adverse effects after therapy (table 2), the most common being bitter taste (43.8%). Side effects were mild in 17 (39.5%) patients, moderate in 4(9.3%), and severe in 1(2.3%). Adverse reactions mostly occurred during the first five days of treatment in 12 of the 22 patients(table 3).
Table 2 . Side effects of therapy reported by the patients .
| Side effects | Frequency N (%) |
| Bad taste | 21(43.8) |
| Nausea | 9(18.8) |
| Abdominal pain | 8(16.7) |
| Dry Mouth | 5(10.4) |
| Diarrhea | 4(8.3) |
| Dizziness | 4(8.3) |
| Burning sensation of tongue | 3(6.3) |
| Weakness | 2(4.2) |
| Anorexia | 2(4.2) |
| Vomiting | 2(4.2) |
| Constipation | 1(2.1) |
| Headache | 1(2.1) |
Table 3 . Prevalence of mild, moderate, and severe drug adverse effects .
| Severity of side effects | N(%) |
| None | 21(48.8) |
| Mild | 17(39.5) |
| Moderate | 4(9.3) |
| Severe | 1(2.3) |
| Drug withdrawal due to severe side effects | 1 |
DISCUSSION
Standard triple therapy is still the first-line therapy; however, when considering the Maastricht IV Consensus Report, it should be avoided in high clarithromycin resistance areas. Options of first-line eradication regimens contain bismuth-based quadruple regimen, sequential, concomitant, and hybrid regimens.1 Quinolone-based triple regimen may be a good alternative as first-line therapy in areas of clarithromycin resistance of >15–20% and quinolone resistance of <10%.1 In developing countries such as Iran, furazolidone has also been used in first-line eradication regimens due to its low cost, drug resistance, and high efficacy. It serves as an alternative for clarithromycin or metronidazole, which have shown an increase in resistance in Iran.11 Meanwhile, adequate preparations should be made in finding alternatives when faced with first-line treatment failures.3 Currently, ideal second-line treatments are indistinct and usually differ from country to country due to different antibiotic resistance patterns.1 For example, the prevalence of clarithromycin resistant strains was 29.3%, 18.9%, and 11.1% in the United States, Asia, and Europe, respectively.13 There appears to be an increasing rate of resistance to clarithromycin and metronidazole in parts of Asia, leading to reduced efficacy of PPI-based triple therapy.4
Primary resistance to antibiotics is about 26.7% for metronidazole, 11.2% for amoxicillin, 17.2% for clarithromycin, 5.9% for tetracycline, and 16.2% for levofloxacin across Asia.13 In Iran, the resistance rates of H.pylori to clarithromycin, amoxicillin, and furazolidone are 7.3%, 7.3%, and 4.5%, respectively. Additionally, the resistance to metronidazole is 55.6% and 38.1% to tetracycline.14,15
The main causes of H.pylori treatment failure are high antimicrobial resistance and poor patient compliance; however, other factors including a high bacterial load and low gastric PH must also be considered.5 Antibiotic resistance to different antimicrobials is considered the major reason for H.pylori treatment failure when considering the extremely high rate of H.pylori infection (more than 80%) in Iran.16 However, there is debate whether antibiotic susceptibility testing should be implemented when first-line eradication fails. This is because even though such testing has been shown to enhance eradication rates, the test’s high price implies that its availability will not be consistent. Generally, this measure is considered unnecessary for most patients and may delay the treatment for patients with H.pylori-related peptic ulcer disease.17,18 Since our patients had peptic ulcer disease, we initiated second-line therapy without performing culture. This was carried out according to the primary antibiotic resistance patterns in Iran.14,15
In the eradication of H.pylori, it is wise to keep in mind the high incidence of treatment-related side effects, such as dysgensia with a metallic and/or bad taste, diarrhea, nausea, and epigastric discomfort, due to the alteration of the intestinal microbiota as a result of antibiotic use.19 These adverse effects can decrease patient compliance. The ideal substitute regimen should contain a short-term and simple drug combination including susceptible agents, assuring good compliance and low toxicity. The regimens used in our study have low side effects similarly to those of other studies.5 Nowadays safe and effective empirical protocols are of great need.18
Stomach acidity might affect the stabilization of acid-labile antibiotics, such as clarithromycin.1 It is still doubtful whether genotyping of CYP2C19 should be performed prior to starting second-line therapy. The Second Asia-Pacific consensus does not recommend it because of the cost and low availability.4
A number of regimens that overcome bacterial resistance to standard antibiotics and have lesser adverse effects were assessed in recent years. Second-generation fluoroquinolones (levofloxacin, moxifloxacin)-based regimens have been assessed as either first-line or rescue therapies.14 Moxifloxacin-based triple regimens displayed higher eradication rates.20 Another study reported that moxifloxacin-based triple therapy was an equally or more effective and a more tolerable second-line eradication protocol for H.pylori when compared with conventional bismuth-based quadruple therapy.21
Similar efficacy has been shown by levofloxacin-based triple therapy, which has a lower rate of adverse effects compared with bismuth-based regimens. Nevertheless, increasing resistance towards levofloxacin should be considered.18,22 At present, levofloxacin is very expensive in Iran; therefore, only a few studies have been able to evaluate its effects on the eradication of H.pylori in this country.14
The synergistic effects of bismuth salt are exerted by destroying bacteria similar to an antibiotic; thus, we included this agent in the second-line regimen to increase the effects of other antibiotics.5
Current guidelines recommend a bismuth-based quadruple regimen for 7–10 days for the second-line therapy, showing eradication rates of 59–95%.5 In the event of failure in the initial treatment, the Maastricht conferences have recommended the use of treatments consisting of two different regimens designed such that a second-line therapy choice depending on what was used as a first-line; therefore, it should constitute various antibiotics. Such recommendations are built upon a knowledge that acquired bacterial resistance to metronidazole or clarithromycin is chiefly from the previous treatment failure.23 It is fundamentally known that when first-line therapy is unsuccessful, the same antibiotics should not be re-administered. Studies have reported that amoxicillin can be “reused” as the resistance rates are small across the globe.17
Success rates of 99.5% in eradication have been reported by means of using this strategy when accurately applied in H.pylori-positive patients.7 An eradication rate of 90% is the least accepted rate of eradication in second-line therapy.24 In our previous study of first-line treatment, the intention-to-treat and per-protocol eradication rates were 76.19% and 81.63% in group OAF-400mg and 80.95% and 89.47% in group OAF-600mg, respectively.12 In another study that we conducted on first-line treatment, the intention-to-treat and per-protocol eradication rates were 84.6% and 90.5% for OAF-600 mg, respectively.11
It was according to this concept that we switched furazolidone, with a low primary resistance rate, to clarithromycin and used pantoprazole 40 mg bid instead of omeprazole 20 mg bid. Bismuth was also added to the rescue regimen in this study and obtained 79.2% and 88.4% intention-to-treat and per-protocol eradication rates, respectively.
A recent study on a fourteen-day OABC regimen showed the same drug dosage as our study except for omeprazole (20 mg bid) as the second-line therapy.5 In their study, clarithromycin was used instead of metronidazole. The intention-to-treat and per-protocol rates were 64.5% and 74.7%, respectively. It seems that the use of clarithromycin instead of metronidazole, with a high primary resistance rate, lowered the eradication rate of the above mentioned study’s second-line treatment.
However, culture and antibiotic susceptibility tests appear to be useful in the selection of a third-line therapy since empirical therapies have displayed sub-optimal results.7,17 Microbial culture is, conversely, pricey, invasive, and only available in a few specific and/or research centers.7
Among the limitations of our study was that an evaluation of antibiotic resistance and an investigation of its relation to eradication rates did not take place. In clinical practice, however, there remains controversy regarding the efficacy of susceptibility testing before second-line therapy.
The H.pylori culture rate is <60% with rate variation in different institutions and various regions. Since this was a study carried out in a clinical setting, we assumed that by performing susceptibility testing, the results of the study would have been less applicable to what happens at an everyday gastroenterology outpatient clinic. Furthermore, in vitro drug-resistance cannot always predict in-vivo non-response to treatment.1,5,14
The second limitation of this study was the use of a single laboratory test to evaluate eradication, which could have formed false positive results. However, with the known sensitivity and specificity of the 14C-urea breath test, the risk of overestimation was at an acceptable range.21
The third limitation of the study was its small sample size.
A clarithromycin containing bismuth-based regimen had good efficacy with an 88.4% per-protocol eradication rate. The regimen had little side effects, and we recommend it as a second-line therapy, especially if furazolidone containing treatments fail.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Mokhtare M , Agah S, Fakheri H , Hosseini V, Rezaei Hemami M , Ghafoori SMS. Efficacy of Clarithromycin Containing Bismuth–Based Regimen as a Second-Line Therapy in Helicobacter Pylori Eradication. Middle East J Dig Dis 2015;7:75-81.
References
- 1.Tzung-Shiun Wu, Huang-Ming Hu, Fu-Chen Kuo, Kuo C-H. Eradication of Helicobacter pylori infection. Kaohsiung J Med Sci. 2014;30:167–72. doi: 10.1016/j.kjms.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monique M Gerrits, Arnoud HM van Vliet, Ernst J Kuipers, Kusters JG. Helicobacter pyloriand antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6:699–709. doi: 10.1016/S1473-3099(06)70627-2. [DOI] [PubMed] [Google Scholar]
- 3.Gisbert JP. Rescue Therapy for Helicobacter pylori Infection 2012. Gastroenterol Res Pract. 2012;2012:974594. doi: 10.1155/2012/974594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ. et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 5.Minakari M, Davarpanah Jazi AH, Shavakhi A, Moghareabed N, Fatahi F. A Randomized Controlled Trial :Efficacy and Safety of Azithromycin, Ofloxacin,Bismuth, and Omeprazole Compared With Amoxicillin,Clarithromycin, Bismuth , and Omeprazole as Second-Line Therapy in Patients With Helocobacter pylori Infection. Helicobacter. 2010;15:154–9. doi: 10.1111/j.1523-5378.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi M, Bizzarri B, Manzali E, Fugazza Al, Gismondi P, de’Angelis GL. Which Treatment in Helicobacter pylori Infection? Clin Exp Pharmacol. 2013:3–4. [Google Scholar]
- 7.Roccarina D, Franceschi F, Zocco MA, Garcovich M, Gasbarrini G, Gasbarrini A. Different Antibiotic No Culture Eradicating (DANCE) strategy: An easy way to manage H pylori eradication. Dig Liver Dis. 2012;44:889–92. doi: 10.1016/j.dld.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi M, Doroud D, Mohajerani N, Massarrat S. Helicobacter pylori antibiotic resistance in Iran. World J Gastroenterol. 2005;11:6009–13. doi: 10.3748/wjg.v11.i38.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coelho LG, Maguinilk I, Zaterka S, Parente JM, do Carmo Friche Passos M , Moraes-Filho JP. 3rd Brazilian Consensus on Helicobacter pylori. Arq Gastroenterol. 2013;50 doi: 10.1590/S0004-28032013005000001. [DOI] [PubMed] [Google Scholar]
- 10.Riahizadeh S, Malekzadeh R, Agah S, Zendehdel N, Sotoudehmanesh R, Ebrahimi-Dariani N. et al. Sequential metronidazole-furazolidone or clarithromycin-furazolidone compared to clarithromycin-based quadruple regimens for the eradication of Helicobacter pylori in peptic ulcer disease: a double-blind randomized controlled trial. Helicobacter. 2010;15:497–504. doi: 10.1111/j.1523-5378.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 11.Mokhtare M, Hosseini V, Tirgar Fakheri Hafez H, Maleki I, Taghvaei T, Valizadeh SM. et al. Comparison of Quadruple and Triple Furazolidone Containing Regimens on Eradication of Helicobacter Pylori. Med J Islamic Republic Iran. 2014;19 [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini V, Mokhtare M, Gholami M, Taghvaei T, Maleki I, Valizadeh M. et al. A Comparison between Moderate- and High-dose Furazolidone in Triple Regimens for Helicobacterpylori Eradication in Iran. Middle East J Dig Dis. 2014;6:195–202. [PMC free article] [PubMed] [Google Scholar]
- 13.De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C. et al. Worldwide H pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409–14. [PubMed] [Google Scholar]
- 14.Fakheri H, Bari Z, Sardarian H. A modified bismuth-based quadruple therapy containing a short course of furazolidone for Helicobacter pylori eradication after sequential therapy failure. Helicobacter. 2012;17:264–8. doi: 10.1111/j.1523-5378.2012.00946.x. [DOI] [PubMed] [Google Scholar]
- 15.Siavoshi F, Saniee P, Latifi-Navid S, Massarrat S, Sheykholeslami A. Increase in resistance rates of H pylori isolates to metronidazole and tetracycline--comparison of three 3-year studies. Arch Iran Med. 2010;13:177–87. [PubMed] [Google Scholar]
- 16.Khademi F, Faghri J, Poursina F, Esfahani BN, Moghim S, Fazeli H. et al. Resistance pattern of Helicobacter pylori strains to clarithromycin, metronidazole, and amoxicillin in Isfahan, Iran. J Res Med Sci. 2013;18:1056–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Song M, Ang TL. Second and third line treatment options forHelicobacter pylori eradication. World J Gastroenterol. 2014;20:1517–28. doi: 10.3748/wjg.v20.i6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisbert JP, Morena F. Systematic review and meta-analysis:levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35–44. doi: 10.1111/j.1365-2036.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 19.Manfredi M, Bizzarri B, Sacchero RI, Maccari S, Calabrese L, Fabbian F. et al. Helicobacter pylori infection in clinical practice: probiotics and a combination of probiotics + lactoferrin improve compliance, but not eradication, in sequential therapy. Helicobacter. 2012;17:254–63. doi: 10.1111/j.1523-5378.2012.00944.x. [DOI] [PubMed] [Google Scholar]
- 20.Bago P, Vcev A, Tomic M, Rozankovic M, Marusić M, Bago J. High eradication rate of H Pylori with moxifloxacin-based treatment: a randomized controlled trial. Wien Klin Wochenschr. 2007;119:372–8. doi: 10.1007/s00508-007-0807-2. [DOI] [PubMed] [Google Scholar]
- 21.Bago J, Pevec B, Tomić M, Marusić M, Bakula V, Bago P. Second-line treatment for Helicobacter pylori infection based on moxifloxacin triple therapy: a randomized controlled trial. Wien Klin Wochenschr. 2009;121:47–52. doi: 10.1007/s00508-008-1122-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuo CH, Hu HM, Kuo FC, Hsu PI, Chen A, Yu FJ. et al. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. J Antimicrob Chemother. 2009;63:1017–24. doi: 10.1093/jac/dkp034. [DOI] [PubMed] [Google Scholar]
- 23.Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007;56:1502. doi: 10.1136/gut.2007.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham DY, Calvet X. Guide regarding choice of second-line therapy to obtain a high cumulative cure rate. Helicobacter. 2012;17:243–5. doi: 10.1111/j.1523-5378.2012.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


