Abstract
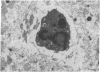
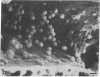
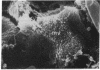
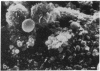
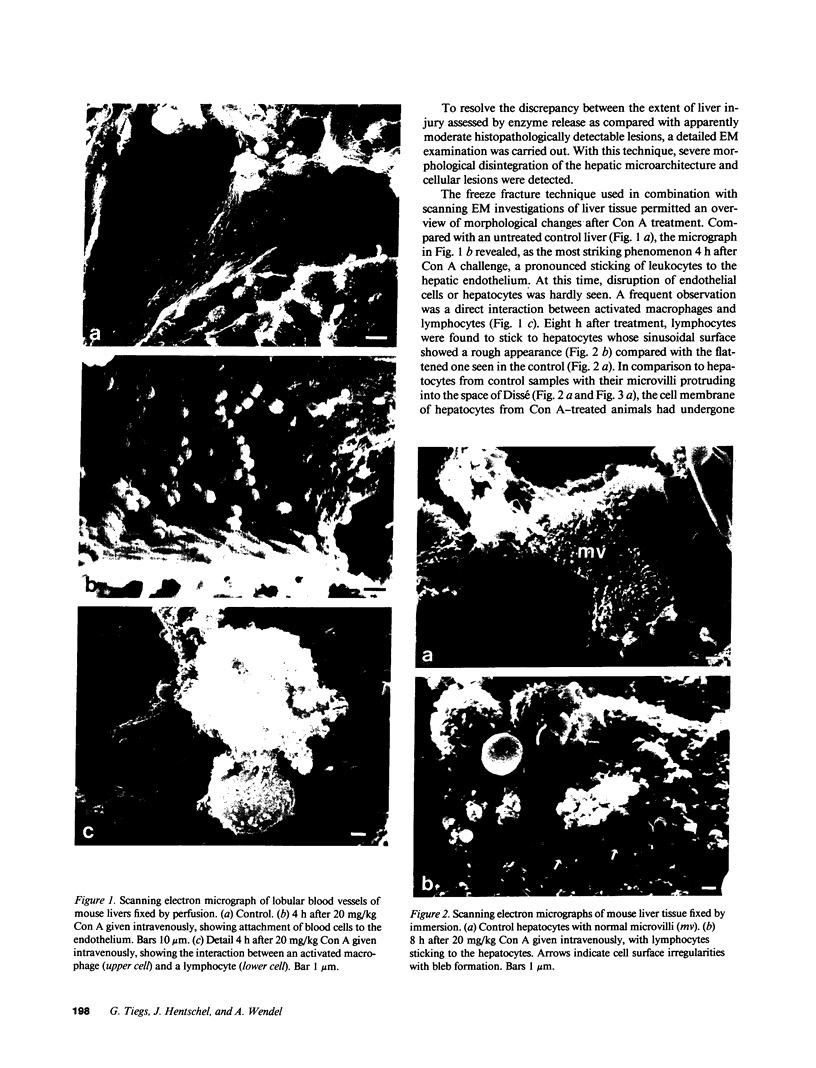
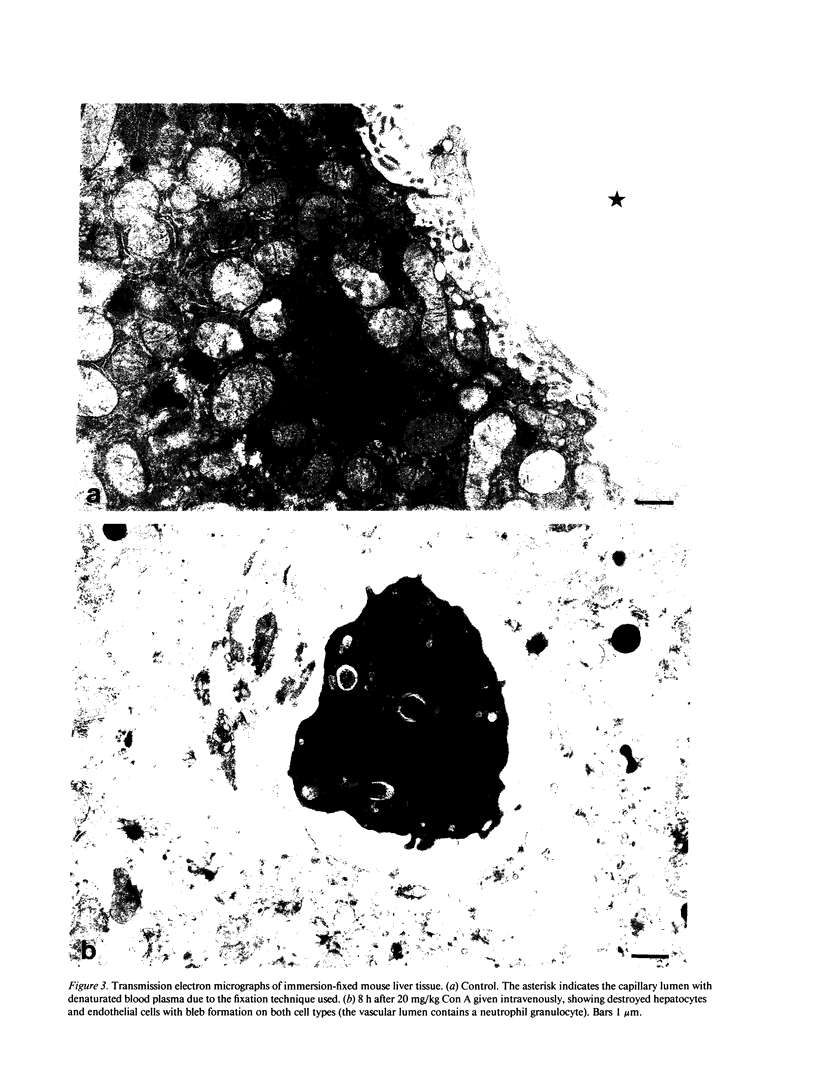
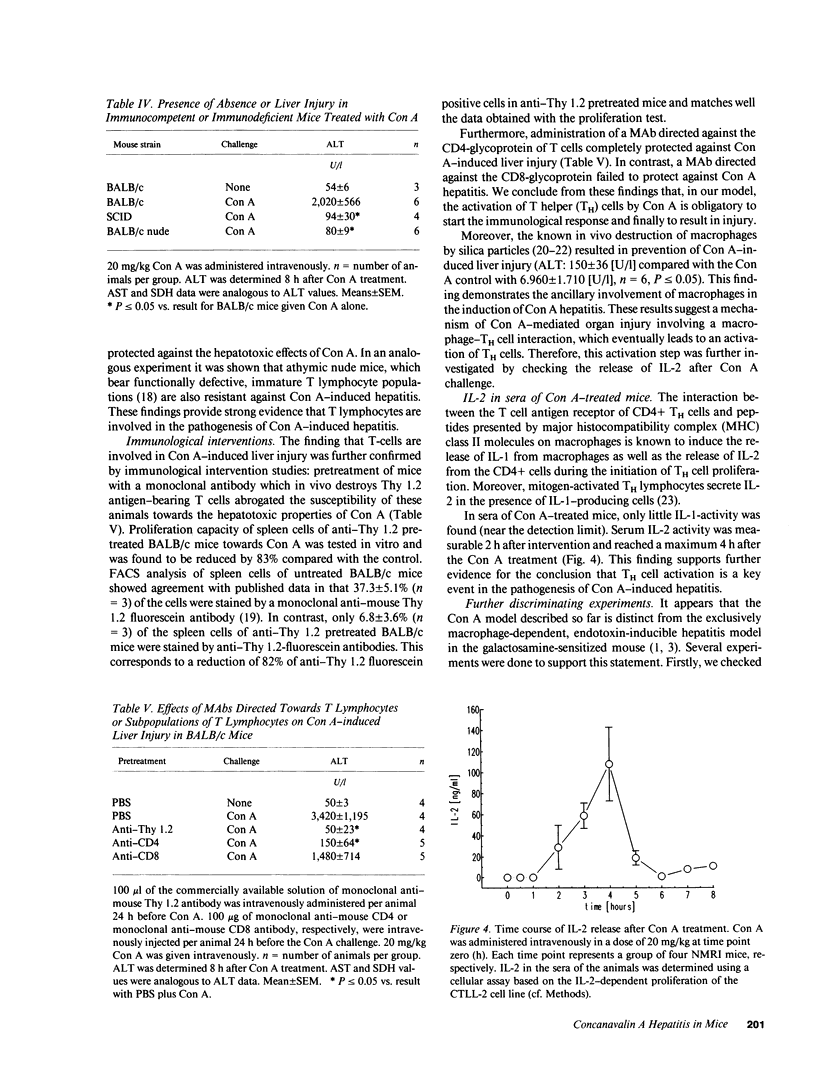
Male NMRI or BALB/c mice developed severe liver injury as assessed by transaminase release within 8 h when an intravenous dose greater than 1.5 mg/kg concanavalin A (Con A) was given. Histopathologically, only the liver was affected. Electron micrographs revealed leukocyte sticking to endothelial cells and bleb formation of hepatocytes. The hepatotoxicity of the lectin correlated neither with its agglutination activity nor with its sugar specificity. Administration of 0.5 mg/kg dexamethasone or 50 mg/kg cyclosporine A or 50 mg/kg FK 506 (Fujimycin) resulted in protection of the animals whereas indomethacin pretreatment failed to protect. Con A hepatitis was accompanied by the release of IL-2 into the serum of the animals. Mice with severe combined immunodeficiency syndrome lacking B as well as T lymphocytes were resistant against Con A. Athymic nude mice with immature T lymphocytes were also resistant. Pretreatment of mice with an antibody against T lymphocytes fully protected against Con A as did monoclonal anti-mouse CD4. Monoclonal anti-mouse CD8 failed to protect. Pretreatment of mice with silica particles, i.e., deletion of macrophages, prevented the induction of hepatitis. These findings provide evidence that Con A-induced liver injury depends on the activation of T lymphocytes by macrophages in the presence of Con A. The model might allow the study of the pathophysiology of immunologically mediated hepatic disorders such as autoimmune chronic active hepatitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Ferluga J., Janossy G. Non-specific cytotoxicity by T cells activated with plant mitogens in vitro and the requirement for plant agents during the killing reaction. Clin Exp Immunol. 1973 Dec;15(4):573–589. [PMC free article] [PubMed] [Google Scholar]
- Beuscher H. U., Günther C., Röllinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990 Mar 15;144(6):2179–2183. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Bornstein M. B., Bloom B. R. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J Immunol. 1981 Feb;126(2):614–620. [PubMed] [Google Scholar]
- DeFranco A. L. Signal transduction. Immunosuppressants at work. Nature. 1991 Aug 29;352(6338):754–755. doi: 10.1038/352754a0. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Andrus L., Steinman R. M. Lymphokine and nonlymphokine mRNA levels in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1986 Apr 1;163(4):922–937. doi: 10.1084/jem.163.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren Z., Berke G. Selective binding of concanavalin A to target cell major histocompatibility antigens is required to induce nonspecific conjugation and lysis by cytolytic T lymphocytes in lectin-dependent cytotoxicity. Cell Immunol. 1984 Dec;89(2):458–477. doi: 10.1016/0008-8749(84)90347-2. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung J. T. Impaired clonal expansion in athymic nude CD8+CD4- T cells. J Immunol. 1988 Jun 1;140(11):3727–3735. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. Effects of intravenous silica on immune and non-immune functions of the murine host. J Immunol. 1975 Jul;115(1):41–48. [PubMed] [Google Scholar]
- McKenna R. M., Szturm K., Jeffery J. R., Rush D. N. Inhibition of cytokine production by cyclosporine A and G. Transplantation. 1989 Feb;47(2):343–348. doi: 10.1097/00007890-198902000-00032. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Parker W. L., Martz E. Lectin-induced nonlethal adhesions between cytolytic T lymphocytes and antigenically unrecognizable tumor cells and nonspecific "triggering" of cytolysis. J Immunol. 1980 Jan;124(1):25–35. [PubMed] [Google Scholar]
- Robb R. J. Human T-cell growth factor: purification biochemical characterization, and interaction with a cellular receptor. Immunobiology. 1982 Mar;161(1-2):21–50. doi: 10.1016/S0171-2985(82)80018-1. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J., Maddux B. A., Goldfine I. D. Regulation of insulin receptor kinase activity by insulin mimickers and an insulin antagonist. Biochem Biophys Res Commun. 1983 Aug 30;115(1):245–252. doi: 10.1016/0006-291x(83)90996-8. [DOI] [PubMed] [Google Scholar]
- Shakir K. M., O'Brian J. T., Gartner S. L. Enhanced phospholipase A2 activity in rat plasma, liver, and intestinal mucosa following endotoxin treatment: a possible explanation for the protective effect of indomethacin in endotoxic shock. Metabolism. 1985 Feb;34(2):176–182. doi: 10.1016/0026-0495(85)90129-5. [DOI] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Thomson A. W. FK-506 enters the clinic. Immunol Today. 1990 Feb;11(2):35–36. doi: 10.1016/0167-5699(90)90011-w. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Niehörster M., Wendel A. Leukocyte alterations do not account for hepatitis induced by endotoxin or TNF alpha in galactosamine-sensitized mice. Biochem Pharmacol. 1990 Sep 15;40(6):1317–1322. doi: 10.1016/0006-2952(90)90398-5. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Wendel A. Leukotriene-mediated liver injury. Biochem Pharmacol. 1988 Jul 1;37(13):2569–2573. doi: 10.1016/0006-2952(88)90248-1. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989 Feb 15;38(4):627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]