Abstract
Aim
Phosphorus (P) tends to become limiting in aging terrestrial ecosystems, and its resorption efficiency is higher than for other elements such as nitrogen (N). We thus hypothesized that trees should store more P than those other elements such as N when tree size increases and that this process should be enhanced in slow-growing late successional trees.
Location
Catalan forests.
Methods
We have used data from the Catalan Forest Inventory that contains field data of the P and N contents of total aboveground, foliar and woody biomasses of the diverse Mediterranean, temperate and alpine forests of Catalonia (1018 sites). We used correlation and general lineal models (GLM) to analyze the allometric relationships between nutrient contents of different aboveground biomass fractions (foliar, branches and stems) and total aboveground biomass.
Results
Aboveground forest P content increases proportionally more than aboveground forest N content with increasing aboveground biomass. Two mechanisms underlie this. First, woody biomass increases proportionally more than foliar biomass having woody biomass higher P:N ratio than foliar biomass. Second, wood P:N ratio increases with tree size. These results are consistent with the generally higher foliar resorption of P than of N. Slow-growing species accumulate more P in total aboveground with size than fast-growing species mainly as a result of their large capacity to store P in wood.
Main conclusions
Trees may have thus developed long-term adaptive mechanisms to store P in biomass, mainly in wood, thereby slowing the loss of P from the ecosystems, reducing its availability for competitors, and implying an increase in the P:N ratio in forest biomass with aging. This trend to accumulate more P than N with size is more accentuated in slow-growing, large, long-living species of late successional stages. This way they partly counterbalance the gradual decrease of P in the soil.
Keywords: Early-succesional species, forest, late-succesional species, leaf:wood ratio, N:P, nitrogen, phosphorus, soil, stoichiometry
INTRODUCTION
An increasing number of studies in recent decades have shown that P is a key nutrient in determining the structure and function of both aquatic and terrestrial ecosystems (Walker & Syers, 1976; Margaleff, 1998; Aerts & Chapin, 2000; Richardson et al., 2005; Sardans et al., 2005; Gradowsky & Thomas, 2006; Turner et al., 2012; Peñuelas et al., 2013). The limiting role of P has been observed in all types of terrestrial ecosystems (Vitousek et al., 2010), from cold-temperate regions (Richardson et al., 2005; Sardans et al., 2005; Gradowsky & Thomas, 2006; Turner et al., 2012) to tropical areas, where P is most frequently the limiting nutrient (Walker & Syers, 1976; Quesada et al., 2010; Nottingham et al., 2012).
Phosphorus is progressively transferred from rock parental material to living and dead biomass and/or becomes occluded in secondary minerals such as organo-clay aggregates and Al, Ca and Fe phosphates, depending on soil traits such as pH, bedrock type, and other general ecosystem characteristics such as climatic conditions or vegetation type (Walker & Syers, 1976). A wide variety of conditions influence P and/or N limitation in both temperate (Finzi, 2009; Sackett et al., 2013) and tropical forests (Tanner et al., 1998; Wright et al., 2011), but despite this soils generally evolve toward increasing P limitation over time after their formation (Walker & Syers, 1976; Vitousek et al., 2010), and several studies have observed a P-limiting role for tree growth in all types of forests and world areas (Sardans et al., 2004; Boyce et al., 2006; Gradowski & Thomas, 2006; Ehlers et al., 2010; Alvarez-Clare et al., 2013; Huang et al., 2013; Zhou et al., 2013). Moreover, the P-limitation of N2-fixation (Binkley et al., 2003; Augusto et al., 2013) and of C and N cycling and N availability (Cleveland et al., 2002; Kranabetter et al., 2005) in forests can control the ecosystem level mass balance of N. Phosphorus is thus often the ultimate limiting nutrient in forests (Vitousek et al., 2010). Related to this limiting role of P in many soils, trees could have developed an efficient resorption of P from leaves. Several studies have observed that more P and N are resorbed in nutrient-poor soil-plant systems (Richardson et al., 2004; Lü et al., 2012). Moreover, P:N resorption ratios generally decrease when the soil is N limited and generally increase when the soil is P limited (van Heerwaarden et al., 2003; Zotz, 2004). The available information indicates broader ranges for P than for N resorption efficiencies (Hättenschwiler et al., 2008; Vergutz et al., 2012), despite the large variabilities (Vergutz et al., 2012). Third, the proportional concentrations of C and N relative to P are higher in litter than in leaves, 3007:45:1 and 1212:28:1, respectively (Vergutz et al., 2012), indicating a generally higher resorption efficiency of P than of N, especially under nutrient poor conditions (Mulder et al., 2013). It is logical to hypothesize that if the trees reabsorb P more efficiently than N from leaves, P should accumulate more than N in other plant organs.
Natural selection should favor the capacity to retain P, more than other elements such as N. Species of advanced successional stages that are adapted to remain long periods should be particularly benefited from storing P in biomass. In fact, the trend to make more internal the control of nutrient cycles has been associated to more advanced successional stages (McDonald & Healey, 2000; Parsons & Congdon 2008; Celi et al., 2013). In this way, the old tropical forests growing on old nutrient-poor soils store a large amount of nutrients in wood biomass (Tanner et al., 1998; Wright et al., 2011). In tropical forest, total soil P was a better predictor of wood production rates than any of the fractionated organic- or inorganic-P pools (Quesada et al., 2012). This suggests that it is not only the immediately available P forms, but probably the entire soil phosphorus pool that is interacting with forest growth on longer timescales. Because late successional species tend to occupy the soil for long periods of time, it may be adaptive to internalize nutrient cycles, making the P supply more dependent on the relatively fast organic matter cycle than from soil minerals leaching (Vinegla et al., 2006).
Moreover, in certain situations the time-scale of the ecosystem P-cycle and of the successional process can be similar. An increase of nutrient cycling rates and losses occurs after disturbance and secondary succession with early successional species substituting late-successional species (Valdespino et al., 2009). Forest disturbances such as fires frequently imply increases in P availability that are accompanied by the recruitment of early successional species as observed in Mediterranean (Escudey et al., 2010; Yildiz et al., 2010; Lane et al., 2011; Turkmean & Duzenli, 2011) tropical (Hughes et al., 2000; Kennard & Gholz, 2001; Ilstedt et al., 2003; Blair, 2005) wet temperate (Saa et al., 1993; 1998; Michalzik & Martin, 2013) and cold forests (Lagenstrom et al., 2009; Mitchell & Ruess, 2009). During post-fire events, soil P-availability tends to decrease returning to the values before fire with aging at the time scale of successional processes, because soil-P immobilization increases (Mitchell & Ruess, 2009; Turkmean & Duzenli, 2011; Celi et al., 2013) and soil-P availability decreases (Turkmean & Duzenli, 2011; Huang et al., 2013; Zhou et al., 2013) during plant community succession (decades). Consequently, are logical hypotheses that species tend to retain the nutrient most likely to decrease in soil with time, and that this retention should be greater in species of more advanced successional stages because the evolution pressure at this regard should be greater.
Very few studies have provided field data for the changes that occur in the contents of P and N in the different ecosystem compartments as forests age and grow, even though P and N play key roles in the function and structure of organisms and ecosystems. In a study of 10 Amazonian tropical forests ranging in age from 0 to 14 years since agricultural abandonment, Feldpausch et al. (2004) observed that the total stocks of P in soil decreased, with asymmetric behaviors in different compartments: the accumulation of P in stand biomass increased whereas the Olsen P (the P available for plants) of the soil decreased. Similar studies in other forests, however, particularly in temperate regions, have not been conducted. Moreover, regional data sets are lacking that would allow an extensive study of the trends in P and N accumulation in forests over time in the different components of tree biomass, including wood.
Given the frequently limiting role of P, the impoverishment of soil P with succession, and the higher resorption efficiency of P than of N, we hypothesized that the evolutionary processes under increasing P-limitation should have selected a greater capacity of retaining and storing P in biomass and do it even more than for other nutrients such as N. We also hypothesized that this storage of P is larger in species of advanced successional stages adapted to remain for longer periods than early-successional fast-growing species. Here, we tested these hypotheses, which have some experimental support in tropical forests (Tanner et al., 1998; Wright et al., 2011), using a large data set from the Catalan Forest Inventory.
MATERIALS AND METHODS
Study area and climatic data
The study was based on data in the Catalan Forest Inventory (Gracia et al., 2004a). These databases contain information on the concentrations of C, N and P in branches, stems and leaves in 1018 plots, and the corresponding biomasses. The plots were uniformly distributed throughout 19 568 Km2 of the forested areas of Catalonia. Catalonia, which has an area of 32 114 Km2, is located on the shores of the Mediterranean Sea, and the presence of the Pyrenees and continental gradients generate contrasting climatic regions, including semiarid-Mediterranean, wet-Mediterranean, Atlantic wet temperate and alpine. Coastal areas have Mediterranean climates, and inland areas have mostly continental Mediterranean climates. To the north, the Pyrenees have montane or, at the highest elevations, alpine climates. Data for mean annual precipitation and temperature were obtained from the “Atlas climàtic digital de Catalunya” (Ninyerola et al., 2000).
Estimation of biomass and growth
In each plot (minimum 28.3 m2), all living trees with a diameter at breast height (DBH) of at least 5 cm were identified to species, and their height and DBH were measured. Bark was measured at a 1.3 m height with a bark calibrator (with an accuracy of 1 mm) in two orientations (north and south) in three trees of each plot. We used the scaling relationships between the DBH without bark and bark thickness to calculate the bark thickness for all the other trees of the same species of this plot. To calculate wood biomass we used the DBH without bark. To calculate the biomass of the different aboveground organs and also total aboveground biomass we used the allometric equations shown in Tables S1-S4 (Appendices 1-4). In these equations we take into account wood density (g cm−3) determined in each plot by weighting wood samples dried in a dry-oven at 75 °C during 48 hours. The volume was obtained by wood cores obtained from a stem extraction with a Pressler drill and measured with a Vernier caliper.
Then current biomass (t ha−1) per plot of the other aboveground organs of the different species was estimated using allometric equations (See Tables S1-S4 in the Appendices 1-4) obtained for each species and region (Vilà et al., 2003). Briefly, each plot had a minimum diameter of 6 m. This plot area was function of tree density in the plot and was variable to include a minimum of 15-25 trees with a DBH higher than 5 cm. Within the plot all trees were measured. The biomass of the plot was the sum of the biomasses of all the trees of each species within. The 1018 plots used to conduct the chemical analyses plus the biomass estimation were mostly monospecific forest (with more than 90% of trees belong to the same target species). Total wood per tree was the sum of branch and stem wood.
Sampling and chemical analyses
In each plot, samples of leaves, stems and branches were collected and analyzed. These samples were pools of the leaves, stems and branches, respectively, of at least three different trees collected and sampled in all directions of the canopy. The leaves were sampled from the upper central part of the crown by using extensible loppers. The final foliar sample included all foliar cohorts present in the different branches sampled from the selected trees. For more information on the method of sampling, see Vilà et al. (2003).
Samples were ground with a Braun Mikrodismembrator-U (B. Braun Biotech International, Melsungen, Germany). Concentrations of N were determined by combustion coupled to gas chromatography using a Thermo Electron Gas Chromatograph (model NA 2100, CE instruments-Thermo Electron, Milan, Italy). To determine the concentrations of P, samples were solubilized in 50 mL Teflon centrifuge tubes (Nalge Nunc International, Rochester, NY, USA) containing a 2:1 acidic mixture of HNO3 (60%) (143255, purissimum, PANREAC, Barcelona) and HClO4 (60%) (141054, purissimum, PANREAC, Barcelona) in a microwave oven (SAMSUNG, TDS, Seoul, South Korea). A standard certified biomass (DC73351, poplar leaf, China National Analysis Centre for Iron & Steel) assessed the accuracy of the digestions and analytical procedures. After digestion, the concentrations of P were determined using ICP-OES (Optic Emission Spectroscopy with Inductively Coupled Plasma) (JOBIN YBON JI 38 Jobin, France).
Statistical analyses
We analyzed all allometric relationships among log-transformed biomasses and P and N concentrations (in mass basis), contents and ratios and conducted a GLM to test for differences between fast-growing species (Pinus halepensis, Pinus sylvestris, Pinus nigra, Pinus pinaster, Castanea sativa, Pseudotsuga menziesii, Pinus radiata, Populus tremula, Populus hybrides, Populus nigra, Fraxinus angustifolia, Fraxinus excelsior, Prunus avium, Cedrus deodara) versus slow-growing species (Pinus uncinata, Quercus ilex, Quercus suber, Abies alba, Quercus cerrioides, Quercus humilis, Quercus petraea, Fagus sylvatica). We used major axis regression (MA) and standardized major axis (SMA) using SMATR package (http://www.bio.mq.edu.au/ecology/SMATR) (Warton & Weber, 2002; Warton et al., 2006) to compare differences in regression slopes between allometric relationships (Figures 1a, 2a, 4, 5a, 5b, Supplementary Figures S1 and S3). We conducted general linear models using successional status, mean annual temperature (MAT) and mean annual precipitation (MAP) as independent factors and P:N ratios in tree organs as dependent variables. We used with Statistica 6.0 (StatSoft, Inc. Tule, Oklahoma, USA). The simple allometric relationships (Figures S2a, S2b in Appendix 6, Figure S4 in Appendix 8 and Figures 2b, 3a, 3b) were analyzed by using the unbiased Theil-Sen’s slope estimator (Sen, 1968; Theil, 1950) from the R (Core Team, 2013) package “mblm” (median-based linear models) to avoid outlier influence in scaling relationships (Komsta, 2012).
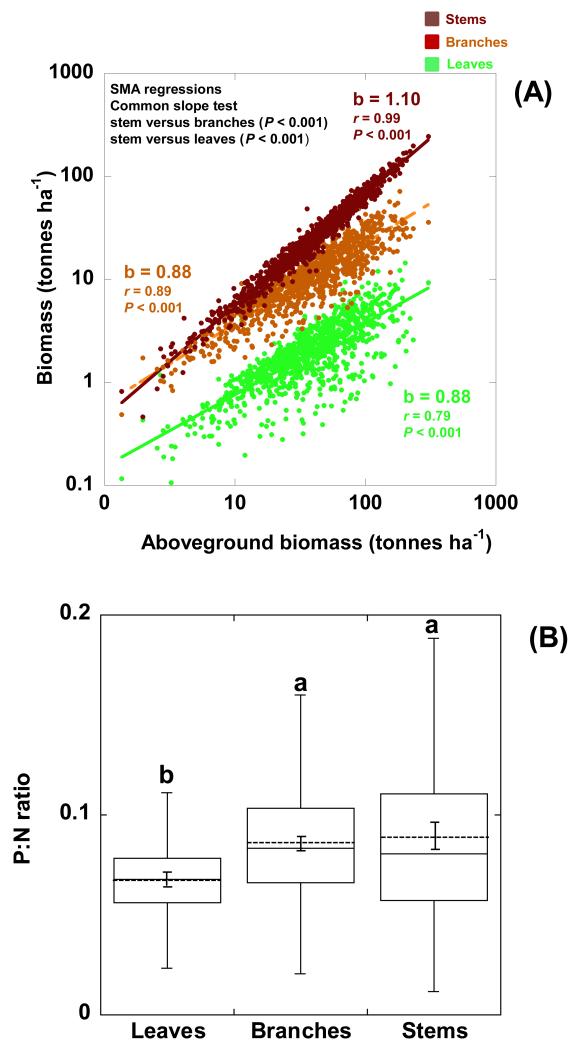
Figure 1.
Allometric relationships of foliar, branch and stem biomasses with total aboveground biomass (A). Boxplots of the P:N ratios of leaves, branches and stems median (the continuous line into the boxes) with the corresponding quartiles. The dashed lines into the boxes represent the mean values ± (S.E.). Different letters indicate statistically significant differences (P<0.05) (B). (SMA = Slope Major Axis).
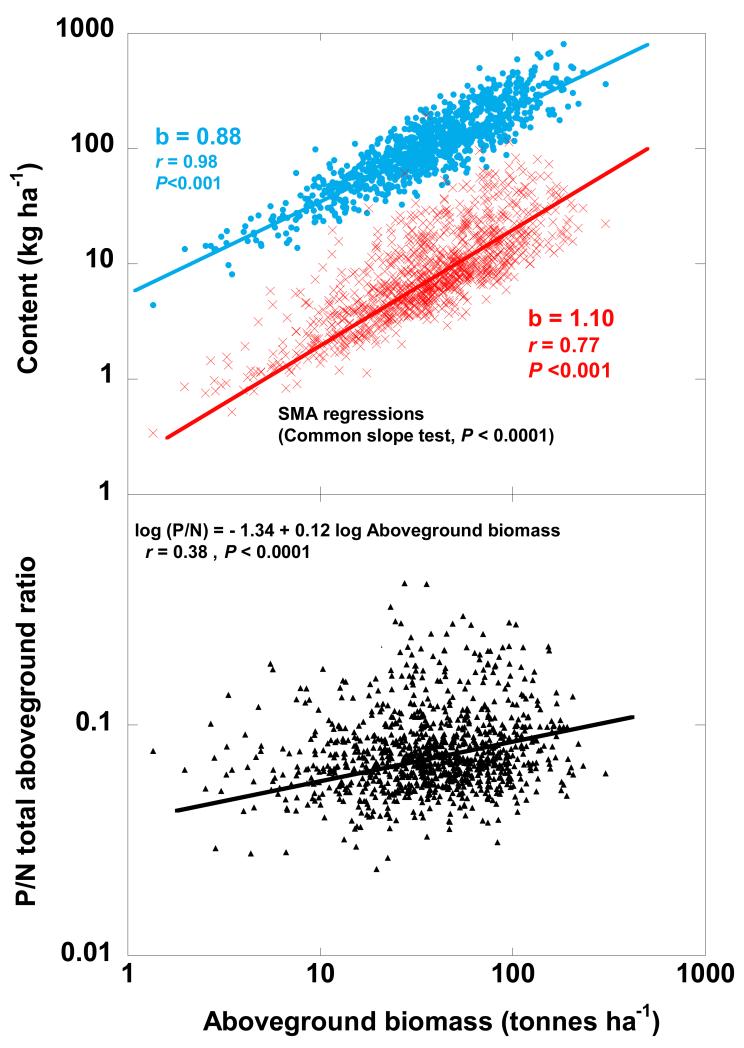
Figure 2.
Allometric relationships of P and N contents in total aboveground biomass with total aboveground biomass (A). Allometric relationships of P:N ratios in total aboveground biomass with total aboveground biomass (B). (SMA = Slope Major Axis).
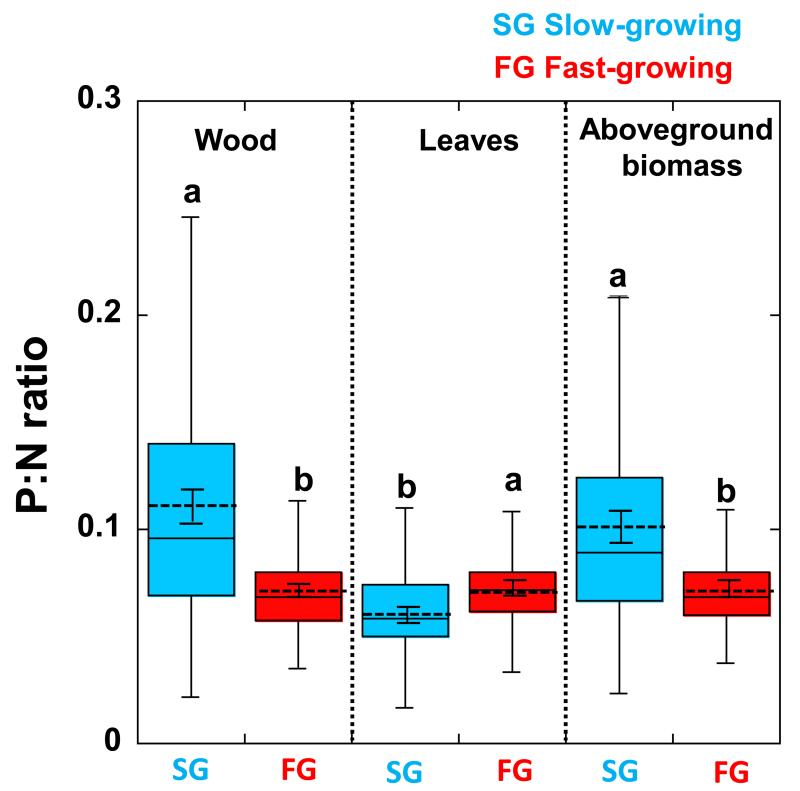
Figure 4.
Boxplot of P:N rations of wood, leaves and total aboveground biomass median (the continuous line into the boxes) with the corresponding quartiles in slow- and fast-growing tree species. The dashed lines into the boxes represent the mean values ± (S.E.). Different letters indicate statistically significant differences (P<0.05) between slow- and fast-growing species. Slow-growing (Pinus uncinata, Quercus ilex, Quercus suber, Abies alba, Quercus cerrioides, Quercus humilis, Quercus petraea, Fagus sylvatica) and fast-growing tree species (Pinus halepensis, Pinus sylvestris, Pinus nigra, Pinus pinaster, Castanea sativa, Pseudotsuga menziesii, Pinus radiata, Populus tremula, Populus hybrides, Populus nigra, Fraxinus angustifolia, Fraxinus excelsior, Prunus avium, Cedrus deodara).
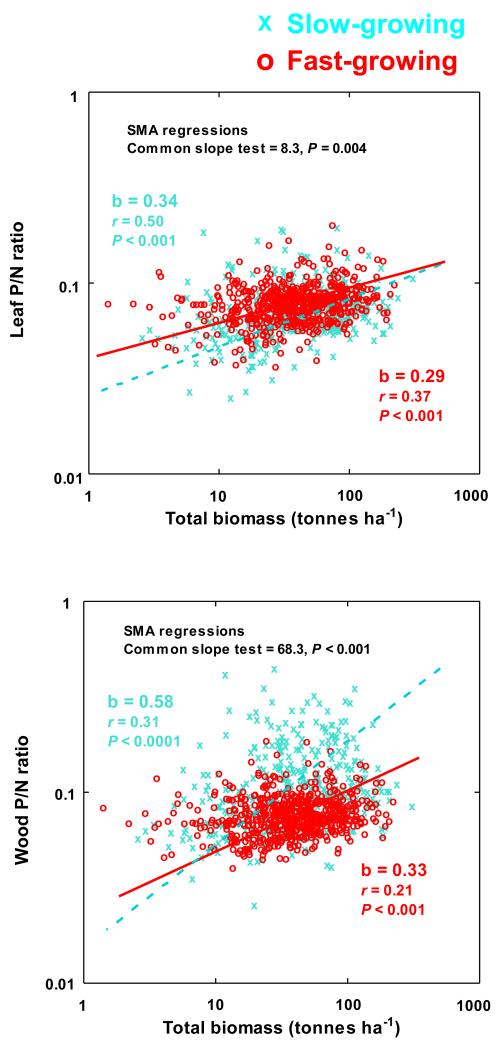
Figure 5.
Allometric relationships of (A) woody P:N ratios and (B) foliar P:N ratios with log-transformed total aboveground biomass in slow- and fast-growing. (SMA = Slope Major Axis).
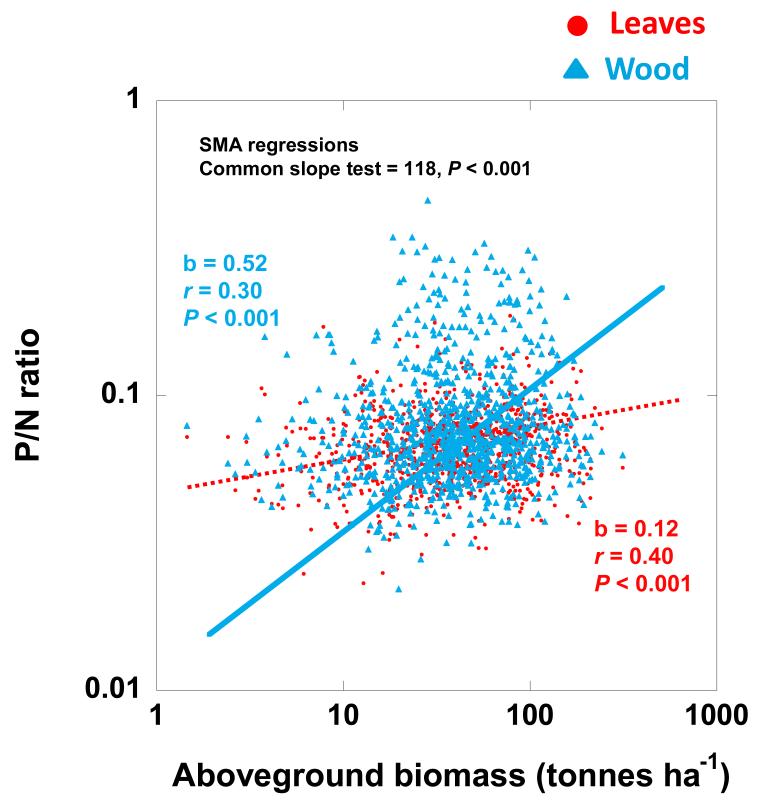
Figure 3.
Allometric relationships of (A) woody P:N ratios and (B) foliar P:N ratios with log-transformed total aboveground biomass. (SMA = Slope Major Axis).
RESULTS
P and N accumulation patterns
Woody biomass, especially stem biomass, increases proportionally more than foliar biomass as total aboveground biomass increases (Fig. 1A). This difference implies that the ratio of woody (branches + stem) to foliar biomass increases with increasing total aboveground biomass in all forest types (See Fig. S1 in Appendix 5). This increase is higher in wet temperate deciduous forests than in the other forest types (coniferous and Mediterranean evergreen) (See Fig. S1 in Appendix 5). On the other hand, P:N ratio is higher in branches and stems than in foliar biomass (Fig 1B). The large increase in the wood/leaf ratio with increasing forest aboveground biomass, however, indicates that the ratio of P stocks in wood over foliage also increases when total aboveground biomass increases, thus functional P (leaf) ratio versus no-functional P (wood) decreases (See Fig. S2 in Appendix 6). In contrast, the increase of functional N (leaf) versus non functional decreases less (P < 0.001, SMA test of common slopes) when total aboveground biomass increases (See Fig. S2 in Appendix 6). As a result, the total P content of the aboveground biomass increases more than the total N content (P < 0.001, SMA test of common slopes) (Fig. 2A), so the P:N ratio in the aboveground biomass consequently increases as the aboveground biomass rises (Fig. 2B). This higher proportional increase of the total P content than N content with increasing aboveground biomass is observed both in angiosperms (P < 0.001 SMA test of common slopes) (See Fig. S3A in Appendix 7) and in gymnosperms (P < 0.001, test of common slopes) (See Fig. S3B in Appendix 7).
The P:N ratios of the foliar biomass only increase slightly in forests, but the P:N ratios of the woody biomass increase strongly when the total aboveground biomass also increases (P < 0.001 SMA test of common slopes) (Fig. 3), and similar results are observed with the total P content of aboveground biomass also increases (P < 0.001 SMA test of common slopes) (See Fig. S4 in Appendix S8).
Differences in P accumulation intensity depending on forest species
The slow-growing tree species have higher aboveground P contents than fast-growing species and even increase their total P contents with biomass more than do the fast-growing species (See Fig. S5 in Appendix 9) (P < 0.001, SMA test of common slopes). Slow growing species had higher P:N ratios wood and total aboveground biomass and lower leaf P:N ratios than fast-growing species (Figure 4). Within angiosperms and within gymnosperms by separate this general tendencies were also observed (see Table S5 in Appendix 10) and also when comparing slow- and fast-growing species within species growing under Mediterranean climate and also within the species growing under wet-temperate climatic conditions (see Table S6 in Appendix 11). Moreover, the increases of P:N ratio with total aboveground biomass also increase more in slow-growing than in fast-growing species, mainly in wood (Figure 5).
Different increases in total P contents with biomass were also observed in comparing the angiosperms with the gymnosperms of those Catalan forests (P < 0.001, SMA test of common slopes) and also between evergreen angiosperms and deciduous angiosperms (P < 0.001, SMA test of common slopes) (see Fig. S6 in Appendix 12).
DISCUSSION
Trees respond to the frequent P limitation by the P losses during soil aging and ecological succession by increasingly accumulating P (Walker & Syers, 1976; Izquierdo et al., 2013; Celi et al., 2013; Huang et al., 2013; Zhou et al., 2013). This allows slowing down the plant-soil-plant P-cycle for longer time and avoiding P losses by soil leaching. Two mechanisms appear to underlie the increasing capacity to store P and the increasing P:N ratio as aboveground biomass increases. First, woody biomass increases proportionally more than foliar biomass. Second, wood has higher P:N ratio than foliar biomass and moreover wood P:N ratio increases when plants increase in size. Increasing size thus not only increases the proportion of wood but also the P:N ratio in the wood. This trend is observed both in angiosperm and in gymnosperm forests.
Some previous studies have focused on the scaling relationships among total plant biomass and nutrient content and stoichiometric relationships (Kerkhoff et al., 2005; Kerkhoff & Enquist, 2006). These studies focused on all types of plants and considered that the total plant nutrient content reflects the metabolically active fraction of plant mass, which in turn should scale isometrically in direct proportion with total leaf mass, and that whole plant P:N ratio should be independent of plant biomass and equivalent to leaf P:N ratio. This can be adequate in herbs, but our results demonstrate that in morphologically more complex plants such as trees, we cannot expect that foliar nutrient content and stoichiometryc relationships reflect directly all tree nutrient content and stoichiometric relationships. Several studies have observed that the scaling relationships between N and P observed in leaves are also confirmed in other major plant organs (Kerkhoff et al., 2006; Elser et al., 2010). Although these relationships can differ between woody and herbaceous species and among different tissues of the same species, P increases disproportionately with N in all them (Niklas et al., 2005; Reich et al., 2010).
Despite some studies and models observed and projected decreases of P:N with plant size, this relationship can be very complex, among several reasons, because it can vary in function of how the stoichiometry of plant organs affect growth and production and how allocation to different organs vary with plant size (Niklas et al., 2005; Elser et al., 2010). Moreover, Elser et al. (2010) i observed that “functional” and “non functional” pools of nutrients can change with plant size which has not been adequately assessed in most current models. Our data also shows that the proportion of functional (in leaves) versus non functional (in wood) P tends to decrease with size in higher proportion than for N, because P accumulates proportionally more than N in wood and less in leaves. This suggests that in the stoichiometrical allometrical relationships apart from plant production considerations other factors such as storing capacity can strongly determine the N and P scaling relationships, especially in species with most biomass (wood) not directly linked to production. Our study also agrees with the previous studies reporting that leaf P(F/noF):N(F/noF) ratio tends to decrease with increasing size of plants (Elser et al., 2010). Our study showed that large size and long life trees are related with their great capacity to continuously accumulate wood tissues not directly related with active mechanisms underlying production capacity. This wood can thus become an effective reservoir of sources. The slopes of the relationships of P content and N content with total aboveground biomass were 1.1 and 0.88 respectively, showing that the overall aboveground P concentration increases whereas N aboveground concentration decreases. This phenomenon occurs as a result of the increase in wood/leaf ratio (with wood with higher P:N ratio) and also as direct result of the higher P:N ratio in leaves and mainly in wood when aboveground biomass increases. In other words, P concentration in aboveground biomass increases because P has a higher proportional content enhancement in leaves and mainly in wood than N when aboveground biomass increases and also by the increasingly wood/leaf ratio with size. This higher retention of P in wood resulting from internal mobility differences between N and P could be interpreted as an evolutionarily acquired mechanism resulting from the selective pressure to retain as much P as possible to counteract the natural trend of soils to lose P thus also taking profit of the wood accumulation with age. The higher resorption efficiency of P than of N at global scale (Mulder et al., 2013) is a key process that can generate these differences between foliar P and N concentrations and P:N ratios in leaves and wood. The trend to accumulate P is even more accentuated and important in species of advanced successional stages. In this case, the slow-growing species (see Figure 4) such as Q. ilex, Q. suber, A. alba, Q. petraea, F. sylvatica, Q. humilis, Q. cerrioides or Pinus uncinata) are species of advanced forest succesional stages (Gracia et al., 2004b). The slow-growing plant species have been associated with low foliar P:N ratio (Elser et al., 2010; Reich et al., 2010), large growth and long life, coupled to a large capacity to store P in wood (Reich et al., 2010; Sardans & Peñuelas, 2013). In contrast, the capacity to store P in wood is less important in species of early successional stages with higher foliar P:N ratios, that are usually fast-growing species in this study (Gracia et al., 2004b). In fact, fast-growing plants have normally higher P:N ratios in photosynthetic tissues (Yu et al., 2010; 2011). The fact that most early successional species in Catalan forests are gymnosperms that present low wood/foliar ratio can influence the previous results. This is translated into lower allometrical slopes in the increase of P accumulation with biomass in gymnosperms than in angiosperms. In any case, gymnosperms, as angiosperms, also increase the accumulation of P proportionally more than the accumulation of N with biomass increase. Thus, the results show that all types of forests accumulate more P than N with increasing biomass, and suggest that early-succesional fast-growing tree species allocate proportionally more P to leaves than to wood with respect to N, favoring higher foliar P:N ratios and lower wood P:N ratios than late-successional slow-growing species. However, they have lower global capacity of storing P than slow-growing long-living late-successional species since wood/leaf ratio increases with tree aging. Slow-growing species make a conservative use of limiting resources such as P because wood/leaf ratios increase with age and their higher allocation of P to wood favors P storing in stand biomass. Thus, the natural successional process seems to favor the ecosystem capacity to retain P (mainly in wood), more than N. This differential retention is of particular importance in preventing ecosystem P losses with soil aging. In addition, this different capacity of tree species to accumulate more or less P, may have significant consequences in many forest ecosystem due to the outstanding role of P:N at the community level including in soil trophic webs (Sterner & Elser, 2002; Sardans et al., 2012).
Summarizing, in Catalan forests, P accumulates in forest aboveground biomass more than N; the P concentration of aboveground biomass increases whereas the N concentration decreases with increasing aboveground biomass. This is mainly due to the higher capacity of wood to store P than N, a difference that it is enhanced with increasing aboveground biomass. These data coincided with previous observations in tropical forest and could be a general trend to compensate the long-term soil-P depletion with time. In this way this storage of P in wood would be a strategy to remove P from the soil to avoid soil immobilization and losses and also could be and strategy to reduce its availability to competitors. Whether or not this P accumulated in wood is used as an active reservoir for the growing trees remains to be investigated. However this trend could not exist in forests evolved on soils with specific N-limitation and/or especially rich in P. Moreover, late-successional slow-growing long-living species have higher capacity to store more P in aboveground biomass than early-successional fast-growing species. In any case, this increasing storage of P and the consequent shift in P:N ratios, which are very important in physiological and ecological processes, are likely to have large implications for ecosystem functioning and services.
Supplementary Material
Appendix 1. Table S1. Allometries to the calculation of leaf biomass per tree.
Appendix 2. Table S2. Allometries to the calculation of leaf biomass + branch biomass per tree.
Appendix 3. Table S3. Allometries to the calculation of stem biomass per tree.
Appendix 4. Table S4. Allometries to the calculation of total aboveground biomass per tree.
Appendix 5. Supplementary Figure S1. Relationships of wood/leaf ratios with total aboveground biomass in different forest types
Appendix 6. Supplementary Figure S2. Allometric relationships of log-transformed ratios of leaf P content/total wood P content and of leaf N content/total wood N content with log-transformed total aboveground biomass.
Appendix 7. Supplementary Figure S3. Allometric relationships of log-transformed total aboveground N and P contents with total aboveground biomass in angiosperms and in gymnosperms forest.
Appendix 8. Supplementary Figure S4. Allometric relationships of log-transformed woody an foliar P:N ratios with log-transformed total P in aboveground biomass
Appendix 9. Supplementary Figure S5. Allometric relationships of log-transformed total aboveground P contents with log-transformed aboveground biomass in slow- and fast-growing species.
Appendix 10. Table S5. Allometric relationships of total aboveground P contents and total aboveground biomass in fast-growing species and slow-growing species.
Appendix 11. Table S6. Leaf, wood and total aboveground biomass in slow- and fast growing species.
ACKNOWLEDGEMENTS
This research was supported by the Spanish Government grants CGL2013-48074-P and Consolider-Ingenio Montes CSD2008-00040, the Catalan Government project SGR 2014-274 and the European Research Council Synergy grant ERC-SyG-610028 IMBALANCE-P.
BIOSKETCH
Dr. Jordi Sardans and Prof Josep Peñuelas research is focused on the field of plant ecophysiology, biogeochemical cycles and atmosphere-biosphere interactions. JS mainly focuses his research on the elemental stoichiometry relationships with terrestrial ecosystems structure and function and in the metabolomic plant responses to abiotic and biotic changes. JP research covers a wide range of ecological disciplines: chemical ecology, biogeochemistry, global change, climate change, atmospheric pollution, biogenic VOCs emissions, remote sensing, and functioning and structure of terrestrial plants and ecosystems.
Footnotes
SUPPORTING INFORMATION Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- Aerts R, Chapin FS. The mineral nutrition of wild plants revised: A re evaluation of processes and patterns. Advances in Ecological Research. 2000;30:1–67. [Google Scholar]
- Alvarez-Clare S, Mack MC, Brooks M. A direct test of nitrogen and phosphorus limitation to net productivity in a lowland tropical wet forest. Ecolgy. 2013;94:1540–1551. doi: 10.1890/12-2128.1. [DOI] [PubMed] [Google Scholar]
- Augusto L, Delerue F, Gallet-Budynek A, Achat DL. Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Global Biogeochemical Cycles. 2013;27:804–815. [Google Scholar]
- Binkley D, Senock R, Cromack K. Phosphorus limitation on nitrogen fixation by Falcataria seedlings. Forest Ecology and Management. 2003;186:171–176. [Google Scholar]
- Blair BC. Fire effects on the spatial patterns of soil resources in a Nicaraguan wet tropical forest. Journal of Tropical Ecology. 2005;21:435–444. [Google Scholar]
- Boyce RL, Larson JR, Sanford RL. Phosphorus and nitrogen limitations to photosynthesis in Rocky mountains bristlecone pine (Pinus aristata) in Colorado. Tree Physiology. 2006;26:1477–1486. doi: 10.1093/treephys/26.11.1477. [DOI] [PubMed] [Google Scholar]
- Celi L, Cerli C, Turner BL, Santoni S, Bonifacio E. Biogeochemical cycling of soil phosphorus during natural revegetation of Pinus sylvestris on disused sand quarries in Northwestern Russia. Plant and Soil. 2013;367:121–134. [Google Scholar]
- Cleveland C, Townsend AR, Schmidt SK. Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems. 2002;5:680–691. [Google Scholar]
- Core R Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- Ehlers K, Bakken LR, Frostegard A, Frossard E, Bunemann EK. Phosphorus limitation in a Ferrasol: impact on microbial activity and cell internal P pools. Soil Biology and Biochemistry. 2010;42:558–566. [Google Scholar]
- Elser JJ, Fagan WF, Kerkhoff AJ, Swenson NG, Enquist BJ. Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytologist. 2010;186:593–608. doi: 10.1111/j.1469-8137.2010.03214.x. [DOI] [PubMed] [Google Scholar]
- Escudey M, de la Fuente P, Antilen M, Molina M. Effect of ash from forest fires on phosphorus availability, transport, chemical forms, and content in volcanic soils. Environmental Chemistry. 2010;7:103–110. [Google Scholar]
- Feldpausch TR, Rondon MA, Fernandes ECM, Riha SJ, Wandelli E. Carbon and nutrient accumulation in secondary forest regenerating on pastures in central Amazonia. Ecological Applications. 2004;14:S164–S176. [Google Scholar]
- Finzi AC. Decades of atmospheric deposition have not resulted in widespread phosphorus limitation or saturation of tree demand for nitrogen in southern New England. Biogeochemistry. 2009;92:217–229. [Google Scholar]
- Gradowski T, Thomas SC. Phosphorus limitation of sugar maple growth in central Ontario. Forest Ecology and Management. 2006;226:104–109. [Google Scholar]
- Gracia C, Burriel JA, Ibàñez JJ, Mata T, Vayreda J. Inventari Ecològic i Forestal de Catalunya. Métodes. Vol. 9. CREAF; Bellaterra: 2004a. p. 112. [Google Scholar]
- Gracia C, Burriel JA, Ibàñez JJ, Mata T, Vayreda J. Inventari Ecològic i Forestal de Catalunya. Catalunya. Vol. 10. CREAF; Bellaterra: 2004a. p. 112. [Google Scholar]
- Hättenschwiler S, Aeschlimann B, Coûteaux MM, Roy J, Bonal D. High variation in foliage and leaf litter chemistry among 45 tree species of a neotropical rainforest community. New Phytologist. 2008;179:165–175. doi: 10.1111/j.1469-8137.2008.02438.x. [DOI] [PubMed] [Google Scholar]
- Huang WJ, Liu JX, Wang YP, Zhou GY, Han TF, Li Y. Increasing phosphorus limitation along three successional forest in southern China. Plant and Soil. 2013;364:181–191. [Google Scholar]
- Hughes RF, Kauffman JB, Cummings DF. Fire in the Brazilian Amazon 3. Dynamics of biomass, C, and nutrient pools in regeneration forests. Oecologia. 2000;124:574–588. doi: 10.1007/s004420000416. [DOI] [PubMed] [Google Scholar]
- Ilsdedt U, Giesler R, Nordgren A, Malmer A. Changes in soil chemical and microbial properties after a wildfire in a tropical rainforest in Sabah, Malaysia. Soil Biology and Biochemistry. 2003;35:1071–1078. [Google Scholar]
- Izquierdo JE, Houlton BZ, van Huysen TL. Evidence for progressive phosphorus limitation over long-term ecosystem development: examination of a biogeochemical paradigm. Plant and Soil. 2013;367:135–147. [Google Scholar]
- Kennard DK, Cholz HL. Effects of high- and low-intensity fires on soil properties and plant growth in a Bolivian dry forest. Plant and Soil. 2001;234:119–129. [Google Scholar]
- Kerkhoff AJ, Enquist BJ, Elser JJ, Fagan WF. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Global Ecology and Biogeography. 2005;14:585–598. [Google Scholar]
- Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and functional variation in the scaling of nitrogen and phosphorus in the seed plants. American Naturalist. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- Kerkhoff AJ, Enquist J. Ecosystem allometry: the scaling of nutrient stocks and primary productivity across plant communities. Ecology Letters. 2006;9:419–427. doi: 10.1111/j.1461-0248.2006.00888.x. [DOI] [PubMed] [Google Scholar]
- Komsta L. mblm: Median-Based Linear Models. 2012 R package version 0.11. http://CRAN.R-project.org/package=mblm.
- Kranabetter JM, Banner A, Groot A. An assessment of phosphorus limitation to soil nitrogen availability across forest ecosystems of north coastal British Columbia. Canadian Journal of Forest Research. 2005;35:530–540. [Google Scholar]
- Lagerstrom A, Esberg C, Wardle DA, Giesler R. Soil phosphorus and microbial response to a long-term wildfire chronosequence in northern Sweden. Biogeochemistry. 2009;95:199–213. [Google Scholar]
- Lane PNJ, Noske PJ, Sheridan GJ. Phosphorus enrichment from point to catchment scale following fire in eucalyptus forests. Catena. 2011;87:157–162. [Google Scholar]
- Lü XT, Freschet GT, Flynn DFB, Han XG. Plasticity in leaf and stem nutrient resorption proficiency potentially reinforces plant-soil feedbacks and microscale heterogeneity in a semi-arid grassland. Journal of Ecology. 2012;100:144–150. [Google Scholar]
- Margalef R. Our Biosphere. Ecology Institute; Oldendorf/Luhe: 1998. [Google Scholar]
- McDonald MA, Healey JR. Nutrient cycling in secondary forests in the Blue Mountains of Jamaica. Forest Ecology and Management. 2000;139:257–278. [Google Scholar]
- Michalzik B, Martin S. Effects of experimental duff fires on C, N and P fluxes into the mineral soil at a coniferous and broadleaf forest site. Geoderma. 2013;197:169–176. [Google Scholar]
- Mitchell JS, Ruess RW. N2-fixing alder (Alnus viridis spp. Fructicosa) effects on soil properties across a secondary successional chronosequence in interior Alaska. Biogeochemistry. 2009;95:215–229. [Google Scholar]
- Mulder C, Ahrestani FS, Bahn M, Bohan DA, Bonkowski M, Griffiths BS, Guicharnaud RA, Kattge J, Krogh PH, Lovorel S, Lewis OT, Mancinelli G, Naeem S, Peñuelas J, Poorter H, Reich PB, Rossi L, Rusch GM, Sardans J, Wright IJ. Connecting the green and brown worlds: elemental factors and trait-driven predictability of ecological networks. Advances in Ecological Research. 2013;49:69–175. [Google Scholar]
- Niklas KJ, Owens T, Reich PB. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecology Letters. 2005;8:636–642. [Google Scholar]
- Ninyerola M, Pons X, Roure JM. A methodological approach of climatological modelling of air temperature and precipitation through GIS techniques. International Journal of Climatology. 2000;20:1823–1841. [Google Scholar]
- Nottingham AT, Turner BL, Chamberlain PM, Stott AW, Tanner EVJ. Priming and microbial nutrient limitation on lowland tropical forest soils of contrasting fertility. Biogeochemistry. 2012;111:219–237. [Google Scholar]
- Parsons SA, Congdon RA. Plant litter decomposition and nutrient cycling in north Queensland tropical rain-forest communities of differing successional status. Journal of Tropical Ecology. 2008;24:317–327. [Google Scholar]
- Peñuelas J, Poulter B, Sardans J, Ciais P, Van Der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, Nardin E, Vicca S, Obersteiner M, Janssens IA. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nature Communications. 2013;4:2934. doi: 10.1038/ncomms3934. [DOI] [PubMed] [Google Scholar]
- Quesada CA, Lloyd J, Schwarz M, Patiño S, Baker TR, Czimczik C, Fyllas NM, Martinelli L, Nardoto GB, Schmerler J, Santos AJB, Hodnett MG, Herrera R, Luizao FJ, Arneth A, Lloyd G, Dezzeo N, Hilke I, Kuhlmann I, Raesseler M, Brand WA, Geilmann H, Moraes Filho JO, Carvalho FP, Araujo Filho RN, Chaves JE, Cruz Junior OF, Pimentel TP, Paiva R. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences. 2010;7:1515–1541. [Google Scholar]
- Quesada CA, Phillips OL, Schwarz M, Czimczik CI, Baker TR, Patino S, Fyllas NM, Hodnett MG, Herrera R, Almeida S, Alvarez Dávila E, Arneth A, Arroyo L, Chao KJ, Dezzeo N, Erwin T, di Fiore A, Higuchi N, Coronado EH, Jimenez EM, Killen T, Lezama AT, Lloyd G, López-González G, Luizao FJ, Malhi Y, Monteagudo A, Neill DA, Núñez-Vargas P, Paiva R, Peacock J, Peñuelas MC, Peña Cruz A, Pitman N, Priante Filho N, Prieto A, Ramírez H, Rudas A, Salomao R, Santos AJB, Schmerler J, Silva N, Silveira M, Vásquez R, Viera I, Terborgh J, Lloyd J. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences. 2012;9:2203–2246. [Google Scholar]
- Richardson SJ, Peltzer DA, Allen RB, MaGlone MS, Parfitt RL. Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia. 2004;139:267–276. doi: 10.1007/s00442-004-1501-y. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Peltzer DA, Allen RB, McGlone MS. Resorption proficiency along a chronosequence: responses among communities and within species. Ecology. 2005;86:20–25. [Google Scholar]
- Reich PB, Oleksyn J, Wright IJ, Niklas KJ, Hedin L, Elser JJ. Evidence of s general 2/3-power law of scaling leaf nitrogen to phosphorus among major plant groups and biomes. Proceedings of the Royal Society of London B Biological Sciences. 2010;277:877–883. doi: 10.1098/rspb.2009.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saa A, Trasar-Cepeda MC, Cilsotres F, Carballas T. Chages in soil-phosphorus and acid-phosphatase-activity immediately following forest-fires. Soil Biology and Biochemistry. 1993;25:1223–1230. [Google Scholar]
- Saa A, Trasar-Cepeda MC, Carballas T. Soil P status and phosphomonoesterase activity of recently burnt and unburnt soil following laboratory incubation. Soil Biology and Biochemistry. 1998;30:419–428. [Google Scholar]
- Sackett TE, Smith SM, Basiliko N. Indirect and direct effects of exotic earthworms on soil nutrient and carbon pools in North American temperate forests. Soil Biology and Biochemistry. 2013;57:459–467. [Google Scholar]
- Sardans J, Rodà F, Peñuelas J. Phosphorus limitation and competitive capacities of Pinus halepensis and Quercus ilex subsp rotundifolia on different soils. Plant Ecology. 2004;174:305–317. [Google Scholar]
- Sardans J, Peñuelas J. Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Global Ecology and Biogeography. 2013;22:494–507. [Google Scholar]
- Sardans J, Rodà F, Peñuelas J. Phosphorus limitation and competitive capacities of Pinus halepensis and Quercus ilex subsp. rotundifolia on different soils. Plant Ecology. 2005;174:305–317. [Google Scholar]
- Sardans J, Rivas-Ubach A, Peñuelas J. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry. 2012;111:1–39. [Google Scholar]
- Sen PK. Estimates of the regression coefficient based on Kendall’s tau. J. Am. Stat. Assoc. 1968;63:1379–1389. [Google Scholar]
- Sterner RW, Elser JJ. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princenton University Press; 2002. [Google Scholar]
- Tanner EVJ, Vitousek PM, Cuevas E. Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology. 1998;79:10–22. [Google Scholar]
- Theil H. A rank-invariant method of linear and polynomial regression analysis. Nederlandse Akademie Wetenschappen Series A. 1950;53:386–392. [Google Scholar]
- Turkmen N, Duzenli A. Early post-fire changes in Pinus brutia forests (Amanos Mountains, Turkey) Acta Botanica Croata. 2011;70:9–21. [Google Scholar]
- Turner BL, Condron LM, Wells A, Andersen KM. Soil nutrient dynamics during podzol development under lowland temperate rain forest in New Zealand. Catena. 2012;97:50–62. [Google Scholar]
- Valdespino P, Romualdo R, Cadenazzi L, Campo J. Phosphorus cycling in primary and secondary seasonally dry tropical forests in Mexico. Annals of Forest Sciences. 2009;66:107. [Google Scholar]
- Van Heerwaarden LM, Toet S, Aerts R. Nitrogen and phosphorus resorption efficiency and proficiency in six sub-artic bog species after 4 years of nitrogen fertilization. Journal of Ecology. 2003;91:1060–1070. [Google Scholar]
- Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecological Monographs. 2012;82:205–220. [Google Scholar]
- Vilà M, Vayreda J, Gracia C, Ibàñez JJ. Does tree diversity increase wood production in pine forests? Oecologia. 2003;135:299–303. doi: 10.1007/s00442-003-1182-y. [DOI] [PubMed] [Google Scholar]
- Vinegla B, Garcia-Ruiz R, Lietor J, Ochoa V, Carreira JA. Soil phosphorus availability and transformation rates in relictic pinsapo fir forests from southern Spain. Biogeochemistry. 2006;78:151–172. [Google Scholar]
- Vitousek PM, Porder S, Houlton BZ, Chadwick OA. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecological Applications. 2010;20:5–15. doi: 10.1890/08-0127.1. [DOI] [PubMed] [Google Scholar]
- Walker TW, Syers JK. The fate of phosphorus during pedogenesis. Geoderma. 1976;15:1–19. [Google Scholar]
- Warton DI, Weber NC. Common slope tests for errors-in-variables models. Biometrical Journal. 2002;44:161–174. [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biological Reviews. 2006;81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Yavitt JB, Wurzburger N, Turner BL, Tanner EVJ, Sayer EJ, Santiago LS, Kaspari M, Hedin LO, Harms KK, Garcia MN, Corre MD. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology. 2011;92:1616–1625. doi: 10.1890/10-1558.1. [DOI] [PubMed] [Google Scholar]
- Yildiz O, Esen D, Sarginci M, Toprak B. Effects of forest fire on soil nutrients in Turkish pine (Pinus brutia, Ten) ecosystems. Journal of Environmental Biology. 2010;1-2:11–13. [PubMed] [Google Scholar]
- Yu Q, Chen Q, Elser JJ, He N, Wu H, Zhang G, Wu J, Bai Y, Han X. Linking stoichiometry homeostasis with ecosystem structure, functioning and stability. Ecology Letters. 2010;13:1390–1399. doi: 10.1111/j.1461-0248.2010.01532.x. [DOI] [PubMed] [Google Scholar]
- Yu Q, Elser JJ, He N, Wu H, Chen Q, Zhang G, Han X. Stoichiometry homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia. 2011;166:1–10. doi: 10.1007/s00442-010-1902-z. (2011) [DOI] [PubMed] [Google Scholar]
- Zhou J, Wu YH, Joerg P, Bing HJ, Yu D, Sun SQ, Luo J, Sun HY. Changes of soil phosphorus speciation along a 120-year soil chronosequence in the Hailuogou Glacier retreat area (Gongga Mountain, SW China) Geoderma. 2013;195:251–259. [Google Scholar]
- Zotz G. The resorption of phosphorus is greater than that on nitrogen in senescing leaves of vascular epiphytes from lowland Panama. Journal of Tropical Ecology. 2004;20:693–696. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Table S1. Allometries to the calculation of leaf biomass per tree.
Appendix 2. Table S2. Allometries to the calculation of leaf biomass + branch biomass per tree.
Appendix 3. Table S3. Allometries to the calculation of stem biomass per tree.
Appendix 4. Table S4. Allometries to the calculation of total aboveground biomass per tree.
Appendix 5. Supplementary Figure S1. Relationships of wood/leaf ratios with total aboveground biomass in different forest types
Appendix 6. Supplementary Figure S2. Allometric relationships of log-transformed ratios of leaf P content/total wood P content and of leaf N content/total wood N content with log-transformed total aboveground biomass.
Appendix 7. Supplementary Figure S3. Allometric relationships of log-transformed total aboveground N and P contents with total aboveground biomass in angiosperms and in gymnosperms forest.
Appendix 8. Supplementary Figure S4. Allometric relationships of log-transformed woody an foliar P:N ratios with log-transformed total P in aboveground biomass
Appendix 9. Supplementary Figure S5. Allometric relationships of log-transformed total aboveground P contents with log-transformed aboveground biomass in slow- and fast-growing species.
Appendix 10. Table S5. Allometric relationships of total aboveground P contents and total aboveground biomass in fast-growing species and slow-growing species.
Appendix 11. Table S6. Leaf, wood and total aboveground biomass in slow- and fast growing species.