Abstract
Background
Human immunodeficiency virus (HIV)-infected persons have increased risk of chronic kidney disease (CKD). Serum creatinine may underestimate prevalence of CKD in subjects with decreased lean body mass or liver disease. Serum cystatin C, an alternative kidney function marker, is independent of lean body mass.
Study Design
Cross-sectional
Setting and Participants
250 HIV-infected subjects taking highly active antiretroviral therapy in the Nutrition for Healthy Living (NFHL) cohort; 2628 NHANES 2001–2002 subjects.
Predictors and Outcomes
Comparison of serum creatinine in NFHL to NHANES subjects; comparison of CKD in NFHL subjects ascertained using serum creatinine versus cystatin C.
Measurements
Standardized serum creatinine, serum cystatin C, glomerular filtration rate (GFR) estimated from serum creatinine and cystatin C.
Results
Creatinine was lower in NFHL than in NHANES despite higher rates of hepatitis, diabetes, and drug use (mean difference −0.18 mg/dl, p<0.001 adjusted for age, sex, and race). Among NFHL subjects, only 2.4% had creatinine-based estimated GFR <60ml/min/1.73m2, but 15.2% had a cystatin-based estimated GFR <60 ml/min/1.73m2.
Limitations
GFR was estimated rather than measured. Other factors beside GFR may affect creatinine and cystatin C levels. Measures of proteinuria were not available.
Conclusions
Serum creatinine may overestimate GFR in HIV-infected subjects. Kidney disease prevalence may be higher than previously appreciated.
Key phrases: HIV, Cystatin C, Creatinine, GFR, Kidney Disease, Hepatitis, Nutrition for Healthy Living
Introduction
People with human immunodeficiency virus (HIV) infection have an increased risk for kidney disease for a variety of reasons, including the high prevalence of hepatitis B and C co-infection 1–3, drug abuse 4, direct effects of HIV on kidney cells 5–9 and nephrotoxicity of several highly active antiretroviral (HAART) medications 10–14. Kidney disease in HIV infected patients is associated with increased complications and utilization of health care resources 15–18. Early detection of kidney disease in persons with HIV would allow for appropriate evaluation and treatment, as well as modification of medication regimens to avoid systemic toxicity and worsening of kidney function 19.
Chronic kidney disease (CKD) is defined as either kidney damage or reduced glomerular filtration rate (GFR) for three months or more 20. Serum creatinine is the most commonly used index of GFR. However, the serum creatinine level is also determined by factors other than GFR, in particular creatinine generation due to muscle mass, the presence of liver disease, and diet 21;22. Creatinine-based GFR estimating equations account for some of the variability in muscle mass based on age, sex, race and weight, however these equations overestimate GFR in patients who have lower creatinine generation for other reasons such as decreased hepatic synthesis of creatine, or abnormally decreased lean body mass. Because persons with HIV may have decreased lean body mass 23, and have a relatively high prevalence of liver disease, GFR estimates based on serum creatinine may be too high, and CKD may be overlooked. Serum cystatin C, a protein produced by all nucleated cells and cleared by glomerular filtration, is a promising alternative endogenous filtration marker which is not influenced by muscle mass 24 or liver function, and may therefore be a more accurate index of kidney disease in persons with HIV 25;25.
In this study we measured serum creatinine and serum cystatin C in 250 HIV-infected subjects taking HAART in the Nutrition for Healthy Living (NFHL) Study, and generated GFR estimates based on serum creatinine (eGFRcreat) and serum cystatin C (eGFRcys). Our objectives were to determine whether there were differences in creatinine levels in HIV-infected subjects on HAART compared to persons of similar age, race and sex in the National Health and Nutrition Examination Survey 2001–2002 (NHANES) and to compare differences in prevalence of CKD when using creatinine-based versus cystatin C-based glomerular filtration rate (GFR) estimates in HIV infected subjects.
Methods
Participants
NFHL was a prospective cohort study of HIV and nutritional status in HIV infected adults (18 years of age or older) from Boston, Massachusetts and Providence, Rhode Island. Methods have been previously published 26. In brief, questionnaires, laboratory studies, bioelectrical impedance analysis (BIA), and anthropometry measurements were obtained at baseline and at six-month intervals. Dual x-ray absorptiometry (DEXA) was obtained at two-year intervals. The protocol was approved by the Human Investigations Research Committees (HIRC) at the New England Medical Center in Boston, Massachusetts and at Miriam Hospital in Providence, Rhode Island and written informed consent was obtained from each participant. The NFHL study was funded in two cycles (September 1995 to August 2000, and September 2000 to August 2005). The second cycle added analysis of several metabolic parameters including insulin. The kidney function sub-study, which is the basis for this manuscript, was funded in November 2005 to analyze serum creatinine and serum cystatin on stored frozen serum samples for 250 HIV-infected subjects taking HAART. The goal of the sub-study was to study the association of kidney function parameters with metabolic complications of HIV infection, including insulin resistance. Therefore, of the 881 subjects ever enrolled in the NFHL study, only the 567 subjects who were in the study from September 2000 onward (for whom serum insulin was routinely obtained) were potentially eligible. The sub-study included subjects who were taking HAART for at least six months, had fasting insulin, glucose, alanine aminotransferase (ALT), high-density lipoprotein (HDL) and triglyceride results available, and had a dual x-ray absorptiometry scan either at the prior, same, or succeeding visit. All 76 women with eligible visits were included, and 174 men were randomly selected from the 216 men with eligible visits to achieve a total of 250 subjects. If a subject had more than one eligible visit, the earliest eligible visit was selected for this study. The demographics of the 250 subjects included in the sub-study are similar to both the total cohort (N=881), and to those active in the study after September 2000 (N=597) in age, sex, race, Body mass index (BMI), and history of injection drug use.
Data from 2628 NHANES 2001–2002 participants aged 20–50 years with available creatinine values were analyzed 27.
Variables
Measurement of Kidney Function
Serum creatinine for NFHL was determined on stored frozen samples in the Tufts-New England Medical Center (T-NEMC) clinical laboratory in January 2006 using the picric acid Jaffe rate method on the Beckman LX20 analyzer. Creatinine samples for NHANES 2001–2002 were analyzed at the White Sands Laboratory using the Jaffe kinetic alkaline picrate rate method.
Serum cystatin C for NFHL was measured on stored frozen samples at the Cleveland Clinic Laboratory using an automated particle-enhanced immunonephelometric assay (PENIA) on the N Latex Cystatin C, Dade Behring, IL.
Calibration of an analyte is important for optimal use of GFR estimating equations in populations other than those in which the equations were derived 28. In 2006, the T-NEMC and NHANES 2001–2002 creatinine instruments were both calibrated to the Roche P-Module creatinine enzymatic assay at the Cleveland Clinic Research Laboratory, which has been shown to be equivalent to creatinine reference values 29–31(Selvin, E et al, Amer Jour Kid Disease, in press 2007). For T-NEMC, 200 samples were used. Linear regression was used to assess significance of the slope and intercept in comparison of the assigned creatinine values from the T-NEMC and Cleveland Clinic laboratories. Based on these calibration exercises, a value of 0.07 mg/dl (6 µmol/L) was subtracted from creatinine values reported from the Tufts-NEMC laboratory. No correction was required for the NHANES samples 27 (Selvin, E et al, Amer Jour Kid Disease, in press 2007). Thus all creatinine values reported in this study are standardized.
GFR Estimating Equations
GFR was estimated from creatinine using the isotope dilution mass spectrometry-traceable four-variable Modification of Diet in Renal Disease (MDRD) Study equation for standardized creatinine 29;32.
eGFRcreat=(175) (serum creatinine)−1.154 (age)−0.203 (0.742 if female) (1.21 if black)
GFR was estimated from cystatin C using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) cystatin C GFR estimating equation 33. Cystatin C for the CKD-EPI study was determined at the Cleveland Clinic Laboratory using an automated particle-enhanced immunonephelometric assay (PENIA) on the N Latex Cystatin C, Dade Behring, IL. This is the same laboratory and methods used for cystatin C in the NFHL sub-study. Thus there are no inter-laboratory calibration issues for cystatin C.
eGFRcys =76.7*(serum cystatin C−1.18)
For both equations, eGFR is reported as ml/min/1.73m2.
Other Variables
Age, race, and ethnicity were self-reported. BMI was calculated as weight (in kg) divided by height2 (in meters). CD4 lymphocyte counts for NFHL participants were determined using a specific monoclonal antibody and fluorescence-activated cell-sorter analysis. HIV RNA viral load (VL) for NFHL participants was measured with the Roche Amplicor Monitor reverse transcriptase PCR assay (Roche Molecular Systems, Somerville, NJ, USA) (lower detection limit 400 copies/ml). If VL was undetectable, a value of 200 copies/ml was assigned for statistical analyses. Log VL was used in tables and regression models.
HAART use was defined as treatment with either a) two protease inhibitors; b) two nucleoside reverse transcriptase inhibitors (NRTI) with one protease inhibitors; c) two NRTI’s with one non-NRTI; d) a combination of protease inhibitors and non-NRTI with NRTI; or e) three NRTI’s.
Bioelectrical impedance analysis (BIA) at 50 KHz was available for 246 NFHL subjects (RJL Systems, Clinton Twp, MI, USA) and 1815 NHANES subjects (Hydra ECF/ICF BioImpedance Spectrum Analyzer Model 4200, Xitron Technologies, Inc, San Diego, CA, USA). Lean body mass was determined using a two-compartment model 34.
ALT was available for 243 of the 250 NFHL participants, and hepatitis B surface antigen (HBsAg) and hepatitis C antibody (HCV Ab) were available for 233 of the 250 NFHL participants. If HCV Ab was positive, HCV RNA was obtained. Hepatitis serologic tests were not measured at the index visit for most subjects, but were assumed to be constant. ALT, and HBsAg were available for all NHANES subjects, and HCV Ab was available for 2618 NHANES subjects. Hepatitis was defined as detectable Hepatitis B surface Ag, and/or Hepatitis C RNA.
High sensitivity C-reactive protein (CRP) for NFHL participants was assayed using a turbidimetric immunoassay obtained from Wako Chemicals USA (Richmond, VA). CRP for NHANES participants was assayed using latex-enhanced nephelometry on a Behring nephelometer for quantitative CRP determination 35. Two published studies comparing turbidimetric and nephelometric techniques in the same patients found excellent correlation between these methodologies 36;37.
Statistical Analysis
All analyses used SAS Version 9.1 (SAS Institute, Inc, Cary, NC).
For analyses within NFHL, significant differences in variables were assessed using Wilcoxon nonparametric test (if continuous), and χ2 or Fisher exact test (if discrete). We assessed agreement using the Kappa statistic.
An upper limit of normal for serum creatinine was determined using the 95% upper confidence limit for NHANES III 38, which were then expressed as standardized creatinine values 27, resulting in cut-offs of >0.97 mg/dl (>86 µmol/L) for females and >1.16 mg/dl (>103 µmol/L) for males. The creatinine values for NFHL were not separable into tertiles because of the narrow distribution of values, therefore creatinine groups were created using sex specific cut-offs: Group 1 included women with serum creatinine values ≤ 0.53 mg/dl (≤ 47 µmol/L) and men with serum creatinine values ≤ 0.73 (≤ 65 µmol/L); Group 2 included women with serum creatinine values between 0.53 and 0.70 mg/dl (47 to 62 µmol/L), and men with values between 0.73 and 0.93 mg/dl (65–82 µmol/L); Group 3 included women with serum creatinine values ≥ 0.70 mg/dl (≥ 62 µmol/L) and men with values ≥ 0.93 mg/dl (≥ 82 µmol/L).
Several epidemiologic studies have assessed a normal range for serum cystatin C using a nephelometric assay method, with the upper limit of normal ranging from 0.65 to 0.92 mg/L 39–41. In this study, we therefore considered a conservative cut-point of cystatin C >1.00 mg/L to be elevated. NFHL participants were stratified into tertiles of cystatin C40.
Estimated GFR (eGFR) was categorized into less than 60, between 60 to 89, and greater than or equal to 90ml/min/1.73m2 (<1, between 1 and 1.48, and >1.50 ml/s/1.73m2) which are categories consistent with staging of CKD 20. Participants were classified into tertiles based on eGFRcys and eGFRcreat.
Analysis of NHANES 2001–2002 used appropriate weight, stratum and PSU variables in SAS Proc SurveyMeans, Proc SurveyFreq, and Proc SurveyReg, accounting for the complex sampling design of NHANES. These methods result in nationally representative means for similar persons in the non-institutionalized US adult population.
Combined analysis of the NFHL and NHANES 2001–2002 studies was accomplished by merging the two datasets (after assigning a unique stratum number for NFHL, setting NFHL participant ID to the PSU with a weight of 1, and creating a binary indicator for ‘study’ (1=NFHL, or 0=NHANES) as the covariate of interest), using SAS Proc Surveyreg. All multivariate analyses of the combined dataset were adjusted for age, race, sex, and BMI because of substantial differences in these covariates between the studies.
We performed an analysis of the association of hepatitis and abnormal kidney function in NFHL participants using Fisher’s exact test or the χ2 where appropriate. We obtained the common Mantel-Haenzsel relative risk after adjusting for sex, age group (20–30, 31–40, 41–50 years), BMI group (<20, 20–25, 26–30, >30), and race (White, Black, Hispanic, Other).
Results
Table 1 shows socio-demographic and clinical characteristics of NFHL and NHANES 2001–2002 subjects. Compared to NHANES subjects, NFHL subjects were slightly older, and a higher percentage were African American, mean diastolic pressures were higher, mean levels of hemoglobin and serum albumin were lower, and a higher percentage had diagnosed diabetes. There was no significant difference in Lean body mass between NFHL and NHANES participants, after adjusting for age, race, sex, and BMI (mean difference 0.75 kg, p=0.2). However, NFHL subjects had a significantly higher prevalence of liver disease than NHANES subjects, as documented by a higher mean level of ALT (43.6 mg/dl versus 26.6 mg/dl), percent with HCV antibody (36.6% versus 3.0%), or HBsAg (10.3% versus 0.3%). CRP was substantially higher in the 93 NFHL participants with available results, (2.4 mg/dl) compared to NHANES (0.29 mg/dl for males and 0.47 mg/dl for females). Mean CD4 cell count in NFHL participants was >400 cells/mm3 and the majority had an undetectable VL.
Table 1.
Demographic, Laboratory and Examination Characteristics in NFHL and NHANES given as mean (SEM)*
| NFHL (HIV+) |
NHANES 2001–2002† |
|||||
|---|---|---|---|---|---|---|
| Mean (SEM) | Total | Male | Female | Total | Male | Female |
| (N=250) | (N= 174) | (N=76) | (N=2628) | (N=1202) | (N=1426) | |
| Age (years) | 41.8 (0.3) | 42.0 (0.4) | 41.3 (0.6) | 35.3 (0.3) | 35.9 (0.3) | 34.8 (0.4) |
| Race (%):African American | 31.6 | 25.9 | 44.7 | 11.5 | 10.2 | 12.7 |
| White | 52.8 | 60.3 | 35.5 | 67.7 | 69.4 | 66.1 |
| Hispanic | 10.4 | 9.2 | 13.2 | 15.8 | 15.8 | 15.9 |
| Other | 5.2 | 4.6 | 6.6 | 5.0 | 4.7 | 5.3 |
| CD4 cell count (cells/mm3) | 442 (17) | 434 (20) | 461 (36) | ---- | ---- | ---- |
| % Undetectable viral load | 67.2 | 67.1 | 67.6 | ---- | ---- | ---- |
| BMI (kg/m2) | 26.5 (0.3) | 25.8 (0.3) | 28.1 (0.9) | 27.7 (0.16) | 27.7 (0.20) | 27.7 (0.24) |
| Creatinine (mg/dl) | 0.77 (0.01) | 0.81 (0.02) | 0.66 (0.02) | 0.86 (0.01) | 0.98 (0.01) | 0.75 (0.01) |
| Cystatin C (mg/L) | 1.03 (0.02) | 1.04 (0.02) | 1.00 (0.03) | ---- | ---- | ---- |
| eGFRcreat (ml/min/1.73m2) | 120.0 (2.8) | 120.9 (3.7) | 117.5 (4.1) | 96.0 (1.1) | 94.3 (1.6) | 97.7 (1.07) |
| eGFRcys (ml/min/1.73m2) | 79.3 (1.2) | 77.6 (1.3) | 83.3 (2.5) | ---- | ---- | ---- |
| Lean Body Mass (kg) | 57.6 (0.7) | 62.1 (0.6) | 47.3 (1.0) | 53.8 (0.44) | 62.5 (0.4) | 45.2 (0.4) |
| ALT (IU/L) | 43.6 (2.4) | 48.6 (3.1) | 31.6 (3.3) | 26.6 (1.0) | 31.4 (0.6) | 21.9 (1.9) |
| HCV Antibody Positive ‡ (%) | 38.6 | 36.3 | 43.8 | 3.0 | 3.8 | 2.3 |
| HBsAg (%) | 10.3 | 10.0 | 11.0 | 0.25 | 0.46 | 0.04 |
| IDU Ever§ (%) | 31.6 | 29.3 | 36.8 | ---- | ---- | ---- |
| Systolic Blood Pressure (mm Hg)║ | 117 (1.0) | 120 (1.1) | 111 (2.1) | 116 (0.4) | 120 (0.6) | 113 (0.4) |
| Diastolic Blood Pressure (mm Hg) ¶ | 76 (0.7) | 76 (0.8) | 74 (1.3) | 72 (0.4) | 74 (0.4) | 70 (0.5) |
| Diabetes Mellitus# (%) | 6.4 | 4.6 | 10.5 | 3.3 | 3.7 | 2.9 |
| Albumin** (mg/dl) | 3.94 (0.03) | 3.99 (0.03) | 3.84 (0.05) | 4.30 (0.01) | 4.42 (0.01) | 4.18 (0.01) |
| Hemoglobin (g/dl) †† | 14.0 (0.1) | 14.5 (0.1) | 12.7 (0.2) | 14.5 (0.1) | 15.5 (0.1) | 13.5 (0.1) |
| Hematocrit‡‡ | 41.0 (0.3) | 42.3 (0.3) | 38.1 (0.5) | 42.5 (0.2) | 45.7 (0.2) | 39.5 (0.2) |
| CRP (mg/dl) | 2.39 (0.28) | 2.40 (0.34) | 2.37 (0.52) | 0.38 (0.02) | 0.29 (0.02) | 0.47 (0.03) |
Abbreviations used in this table: Nutrition for Healthy Living (NFHL), National Health and Nutrition Survey 2001–2002 (NHANES), standard error of the mean (SEM), body mass index (BMI), estimated glomerular filtration rate based on the Modification of Diet in Renal Disease estimating equation (eGFRcreat), estimated GFR based on the U01 study cystatin estimating equation (eGFRcys), hepatitis C virus (HCV), hepatitis B surface antigen (HBSAg), alanine aminotransferase (ALT), injection drug use (IDU), and C-reactive protein (CRP).
To convert to SI units: for creatinine mg/dl to µmol/L, multiply value by 88.4; for albumin g/dL to g/L multiply value by 10; for hemoglobin g/dL to g/L, multiply value by 10.
Values represent mean and standard error of the mean (SEM) unless otherwise noted.
N= Actual number of Nutrition for Healthy Living (NFHL) and National Health and Nutrition Survey 2001–2002 (NHANES) participants included in this analysis. All other information for NHANES has been derived using the proper weight, and accounting for the complex sampling design of NHANES 2001–2002, and thus should constitute nationally representative data for non-institutionalized persons in the US who would meet the entry criteria of this study.
Percent of participants with detectable hepatitis C virus (HCV) antibody.
A history of ever using injection drugs either at baseline or at any study visit.
Systolic blood pressure (mm Hg) was available for 173 men and 71 women in NFHL, and for 1159 men and 1359 women in NHANES
Diastolic blood pressure (mm Hg) was available for 173 men and 71 women in NFHL, and for 1159 men and 1359 women in NHANES
History of diabetes was determined by self-report of health professional diagnosed diabetes on the NFHL or the NHANES questionnaire.
Albumin was available for 170 men and 69 women in NFHL, and for 1202 men and 1426 women in NHANES
Hemoglobin was available for 174 men and 75 women in NFHL, and for 1201 men and 1425 women in NHANES
Hematocrit was available for 174 men and 75 women in NFHL, and for 1201 men and 1425 women in NHANES
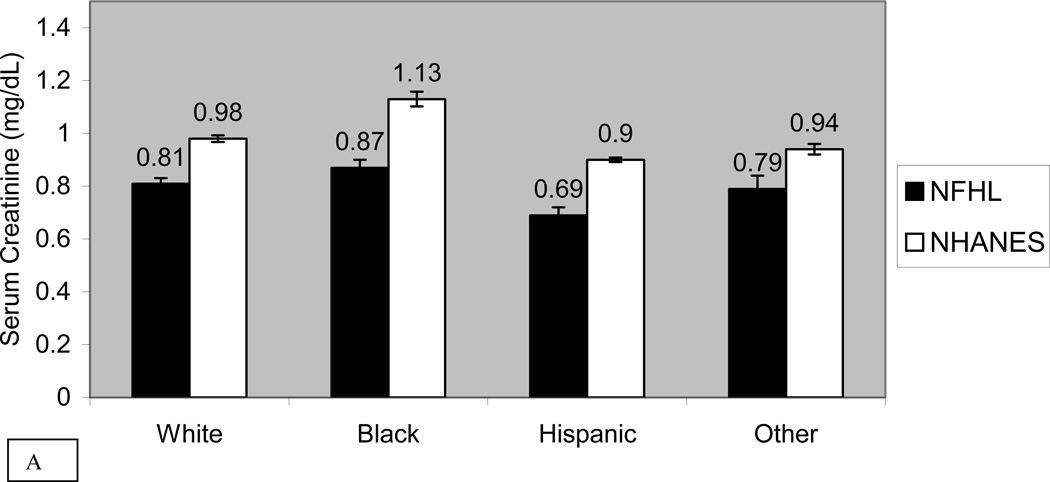
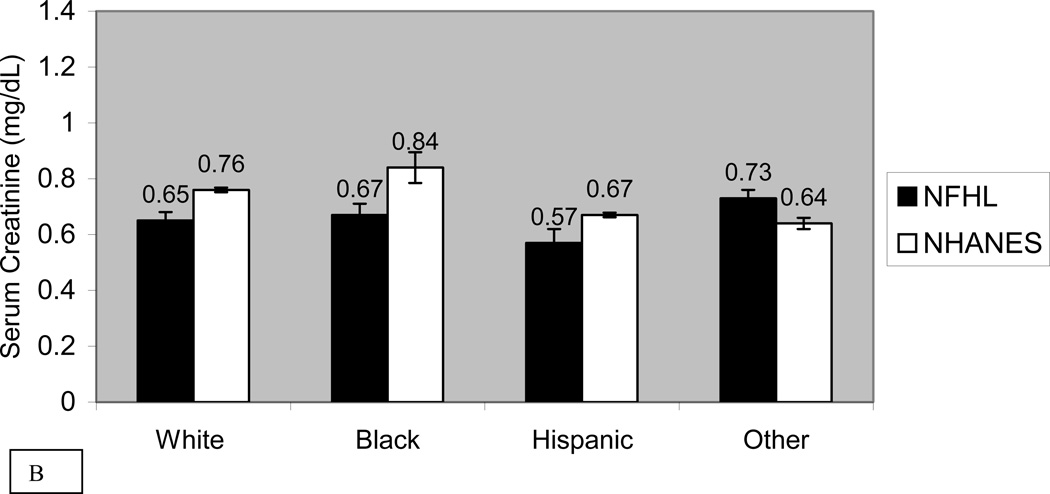
Figure 1 compares the mean standardized serum creatinine by race and sex in NFHL and NHANES 2001–2002 subjects. Mean serum creatinine was significantly lower in NFHL than NHANES in every race-sex group except “other” women. In regression analysis, creatinine in NFHL was significantly lower than in NHANES (−0.10 mg/dL or −9µmol/L, p<0.001). After adjusting for age, race and sex, creatinine in NFHL was even lower compared with NHANES (−0.18 mg/dl or −16 µmol/L, p<0.001). In sensitivity analysis we restricted NHANES data to creatinine values ≤ 2.0 mg/dl (177 µmol/L), and found that NFHL continued to have significantly lower creatinine (−0.15 mg/dl or −13 µmol/L, p<0.001). Because of the marked difference in prevalence of hepatitis C exposure between NFHL and NHANES, and increased rates of progression to cirrhosis in HIV/HCV co-infected patients, we examined correlates of serum creatinine adjusting for age, race, sex, ALT and HCV antibody. NFHL and HCV antibody status were both independently associated with lower levels of creatinine (−0.15 mg/dl (−13 µmol/L), p<0.001 and −0.14 mg/dl (−12 µmol/L), p<0.001, respectively).
Figure 1. Mean Serum Creatinine in Men and Women in NFHL and NHANES 2001–2002.
Unadjusted mean serum creatinine and standard error of the mean (SEM) bars in NFHL and NHANES 2001–2002 subjects, stratified by race for men (panel A) and women (panel B). Units for creatinine are mg/dl. Abbreviations used in this figure include: NFHL (Nutrition for Healthy Living) and NHANES (National Health and Nutrition Examination Survey 2001–2002)
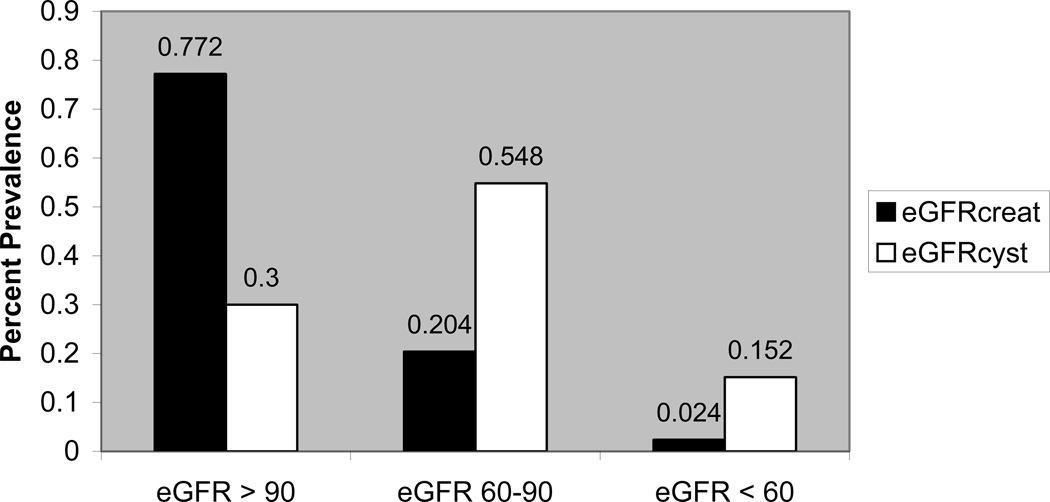
In comparing creatinine and cystatin within the NFHL cohort, 5.2% of participants were identified with abnormal kidney function using a creatinine-based cut-off, compared with 42% using the cystatin C cut-off. The kappa statistic for agreement in identifying abnormal kidney function was poor (0.12). With eGFRcys, prevalence of eGFR less than 60 and 60–89 ml/min/1.73m2 was 15.2% and 55%, respectively, compared to 2.4% and 20% respectively with the eGFRcreat (Figure 2). The within-individual kappa statistic showed poor agreement for eGFR <60 ml/min/1.73m2 (kappa 0.15 for eGFRcreat versus eGFRcys).
Figure 2. Percent Prevalence of Kidney Disease in NFHL by eGFRcreat and eGFRcystatin.
Percent prevalence of estimated GFR >90, 60–89, and <60 ml/min/1.73m2 in NFHL subjects as assessed by the Modification of Diet in Renal Disease (MDRD) GFR estimating equation (eGFRcreat), and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) cystatin GFR estimating equation (eGFRcys). Abbreviations used in this figure include: NFHL (Nutrition for Healthy Living), eGFR (estimated glomerular filtration rate).
Table 2 shows the association of clinical characteristics with serum creatinine sex-specific groups and cystatin C tertiles in NFHL. Higher serum creatinine group was significantly associated with the presence of HCV antibody, history of injection drug use, and with lower CD4 cell count. Higher cystatin C tertile was more strongly associated with HCV antibody, and history of injection drug use, and was also significantly associated with lower CD4 cell count, higher log viral load, and higher ALT.
Table 2.
Association of Demographic, and Laboratory Characteristics with Cystatin C Tertiles and Creatinine Groups
| Cystatin C Tertiles* |
Creatinine Groups† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD)‡ | Tertile 1 <0.88mg/L (N=80) |
Tertile 2 88-1.05mg/L (N=85) |
Tertile 3 ≥1.06 mg/L (N=85) |
P-value | Group1 (N=102) |
Group2 (N=89) |
Group3 (N=59) |
P-value |
| Age (years) | 41.6 (4.8) | 40.8 (5.5) | 43.0 (5.0) | 0.02 | 41.7 (5.6) | 41.0 (5.0) | 43.1 (4.4) | 0.07 |
| % Female | 44 | 22 | 26 | 0.006 | 24 | 28 | 46 | 0.01 |
| Race(%): | ||||||||
| African American | 43 | 31 | 22 | 0.1 | 27 | 30 | 41 | 0.03 |
| White | 41 | 55 | 61 | 52 | 55 | 51 | ||
| Hispanic | 10 | 8 | 13 | 18 | 8 | 2 | ||
| Other | 6 | 6 | 4 | 3 | 7 | 7 | ||
| BMI (kg/m2) | 26.8 (6.8) | 27.1 (4.8) | 25.5 (4.1) | 0.2 | 26.3 (5.1) | 26.7 (5.8) | 26.5 (5.3) | 0.7 |
| Creatinine (mg/dl) | 0.68 (0.17) | 0.76 (0.18) | 0.85 (0.27) | 0.006 | 0.60 (0.12) | 0.80 (0.12) | 1.00 (0.23) | <0.0001 |
| Cystatin C (mg/L) | 0.80 (0.07) | 0.97 (0.05) | 1.30 (0.26) | <0.0001 | 0.97 (0.19) | 0.99 (0.20) | 1.18 (0.36) | <0.0001 |
| eGFRcreat(ml/min/1.73m2) | 131 (37) | 122 (46) | 108 (49) | <0.0001 | 155 (49) | 105 (12) | 81 (15) | <0.0001 |
| eGFRcys (ml/min/1.73m2) | 101 (11) | 80 (5) | 58 (10) | <0.0001 | 83 (18) | 81 (19) | 69 (20) | <0.0001 |
| HCVAntibody (%) | 20.6 | 36.4 | 56.6 | <0.0001 | 39.0 | 29.6 | 50.9 | 0.04 |
| ALT (IU/L) | 37 (30) | 40 (33) | 53 (47) | 0.001 | 45 (42) | 42 (36) | 44 (34) | 0.4 |
| CD4 cell count (cells/mm3) | 522 (262) | 434 (228) | 373 (303) | 0.0002 | 446 (272) | 489 (299) | 367 (208) | 0.05 |
| Log10 viral load (copies/ml) | 2.48 (0.45) | 2.74 (0.75) | 3.25 (1.21) | <0.0001 | 2.85 (0.95) | 2.80 (0.89) | 2.81 (0.94) | 1.0 |
| % undetectable viral load | 84 | 66 | 52 | 0.0001 | 68 | 69 | 66 | 1.0 |
| IDU ever (%) | 17.5 | 24.7 | 51.8 | <0.0001 | 30 | 25 | 44 | 0.04 |
| Fat Free Mass (kg) | 56.5 (11.3) | 59.5 (10.5) | 56.7 (10.3) | 0.1 | 58.1 (10.5) | 58.9 (11.3) | 54.6 (9.9) | 0.9 |
| Fat Free Mass Percent | 74.9 (11.0) | 75.4 (10.7) | 75.5 (10.3) | 1.0 | 75.3 (10.5) | 76.6 (11.0) | 73.1 (10.2) | 0.1 |
| Years HIV Positive | 9.0 (4.8) | 8.7 (4.1) | 10.0 (4.3) | 0.1 | 9.0 (4.4) | 8.9 (4.6) | 10.3 (4.3) | 0.2 |
| Systolic Blood Pressure | 116 (14) | 119 (15) | 118 (18) | 0.7 | 118 (14) | 117 (15) | 118 (19) | 0.8 |
| Diastolic Blood Pressure | 75 (10) | 76 (8) | 76 (12) | 0.7 | 74 (8) | 76 (11) | 77 (13) | 0.5 |
| Albumin | 4.08 (0.31) | 3.96 (0.36) | 3.80 (0.49) | 0.0001 | 3.93 (0.44) | 3.94 (0.36) | 3.96 (0.45) | 0.9 |
| Hemoglobin | 13.9 (1.6) | 14.1 (1.8) | 13.9 (1.8) | 0.6 | 14.0 (1.7) | 14.1 (1.7) | 13.7 (1.9) | 0. 5 |
| % on Tenofovir | 10.0 | 14.1 | 15.3 | 0.6 | 9 | 13 | 20 | 0.1 |
| % on Indinavir | 12.5 | 7.1 | 10.6 | 0.5 | 9 | 13 | 7 | 0.4 |
| CRP§ | 2.23 (2.56) | 1.98 (1.80) | 3.23 (3.83) | 0.4 | 2.46 (2.27) | 2.06 (2.60) | 2.70 (3.44) | 0.3 |
Abbreviations used in this table: standard deviation (SD), body mass index (BMI), estimated glomerular filtration rate based on the Modification of Diet in Renal Disease estimating equation (eGFRcreat), estimated GFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) cystatin estimating equation (eGFRcys), hepatitis C virus (HCV), hepatitis B surface antigen (HBSAg), alanine aminotransferase (ALT), injection drug use (IDU), human immunodeficiency virus (HIV), C-reactive protein (CRP).
To convert to SI units: for creatinine mg/dl to µmol/L, multiply value by 88.4; for albumin g/dL to g/L multiply value by 10; for hemoglobin g/dL to g/L, multiply value by 10.
Normal range for cystatin C is considered to be <1.00. Cystatin C was normally distributed with a mean (SD) of 1.03 (0.26) mg/L and range 0.62–2.42 mg/L.
Creatinine groups were created using sex specific cut-offs of standardized creatinine: Group 1 had creatinine ≤ 0.53 if female and ≤ 0.73 if male; Group 2 had creatinine > 0.53 and <0.7 if female, and >0.73 and <0.93 if male; Group 3 had creatinine ≥ 0.7 if female and ≥ 0.93 if male. These groups are not tertiles but come as close to tertiles as possible given the resolution and spread of the data.
Values represent mean (SD) unless otherwise indicated.
Of those in cystatin tertile 1, 2, and 3, the number of participants with available CRP was 28, 40, and 25 respectively. Of those in creatinine groups 1, 2, and 3, the number of participants with available CRP was 36, 32,and 25 respectively.
Table 3 shows demographic and laboratory factors associated with eGFRcys <60ml/min/1.73m2 . Those with eGFRcys <60ml/min/1.73m2 (1 ml/s/1.73m2), were significantly more likely to have active viral hepatitis, history of injection drug use, and lower CD4 cell count, and were less likely to have an undetectable viral load.
Table 3.
Association of Demographic, and Laboratory Characteristics with eGFRcys <60 ml/min/1.73m2
| Mean (SD)* |
eGFRcys ≥60 (N=212) |
eGFRcys <60 (N=38) |
P-value |
|---|---|---|---|
| Creatinine (mg/dl) | 0.74 (0.19) | 0.90 (0.32) | 0.003 |
| Cystatin C (mg/L) | 0.94 (0.14) | 1.50 (0.27) | <0.0001 |
| eGFRcreat (ml/min/1.73m2) | 123 (40) | 105 (64) | <0.0001 |
| eGFRcys (ml/min/1.73m2) | 85 (15) | 49 (8) | <0.0001 |
| Age (years) | 41.4 (5.2) | 44.1 (4.7) | 0.05 |
| HCVAntibody (%) | 33.9 | 63.2 | 0.0007 |
| ALT (IU/L) Mean (SD) | 40 (31) | 62 (61) | 0.003 |
| Active Viral Hepatitis† | 33.3 | 73.7 | <0.0001 |
| Albumin | 3.99 (0.40) | 3.67 (0.41) | <0.0001 |
| CD4 cell count (cells/mm3) | 466 (271) | 311 (235) | 0.006 |
| Log viral load (copies/ml) | 2.74 (0.80) | 3.30 (1.33) | 0.009 |
| % undetectable log viral load | 70.3 | 52.6 | 0.03 |
| IDU ever (%) | 26.4 | 60.5 | <0.0001 |
| Fat Free Mass (kg) | 58.3 (10.8) | 53.3 (9.5) | 0.01 |
| Hemoglobin | 14.1 (1.7) | 13.5 (1.8) | 0.04 |
Abbreviations used in this table: standard deviation (SD), estimated glomerular filtration rate based on the Modification of Diet in Renal Disease estimating equation (eGFRcreat), estimated GFR based on the U01 study cystatin estimating equation (eGFRcys), hepatitis C virus (HCV), hepatitis B surface antigen (HBSAg), alanine aminotransferase (ALT), injection drug use (IDU).
To convert to SI units: for creatinine mg/dl to µmol/L, multiply value by 88.4; for albumin g/dL to g/L multiply value by 10; for hemoglobin g/dL to g/L, multiply value by 10.
None of the following covariates were significantly associated with eGFRcys < 60 ml/min/1.73m2: Race, sex, BMI, fat-free mass percent, years of known HIV infection, systolic or diastolic blood pressure , % taking tenofovir, % taking indinavir, or CRP.
Values represent mean and standard deviation (SD) unless otherwise indicated. P-values are from Wilcoxon models.
Participants with active viral hepatitis had either detectable hepatitis C virus (HCV) RNA or hepatitis B virus surface antigen (HBSAg). Hepatitis testing was available for 233 participants.
Table 4 shows prevalence of abnormal kidney function predictors in NFHL participants with and without hepatitis. A greater proportion of participants with viral hepatitis compared to those without viral hepatitis had eGFR <60ml/min/1.73m2 (1 ml/s/1.73m2) when determined using cystatin-based estimates of GFR but not with creatinine-based estimates.
Table 4.
N (%) of NFHL Participants with Abnormal Kidney Parameters by Hepatitis Status*
| Hepatitis (N=93) |
No Hepatitis (N=140) |
Mantel-Haentzel RR† (95% CI) |
|
|---|---|---|---|
| Elevated Creatinine‡ | 9 (10%) | 4 (3%) | 2.54 (0.91, 7.09) |
| Elevated Cystatin C‡ | 57 (61%) | 43 (31%) | 2.32 (1.59, 3.39) |
| eGFRcreat <60 | 5 (5%) | 1 (1%) | 5.41 (0.94, 31.07) |
| eGFRcys <60 | 28 (30%) | 10 (7%) | 4.04 (1.75, 9.33) |
Abbreviations used in this table: Nutrition for Healthy Living study (NFHL), National Health and Nutrition Examination Survey 2001–2002 (NHANES), estimated glomerular filtration rate based on the Modification of Diet in Renal Disease estimating equation (eGFRcreat), estimated GFR based on the U01 study cystatin estimating equation (eGFRcys), relative risk (RR), body mass index (BMI).
Two hundred thirty three NFHL subjects had hepatitis serology (160 men and 73 women). Hepatitis was defined as either detectable Hepatitis B surface Ag, or Hepatitis C RNA. Of those with hepatitis, 28 (30%) were female. Of those without hepatitis, 48 (34%) were female.
This RR is a Mantel-Haenszel relative risk of kidney disease in those with viral hepatitis versus without viral hepatitis, after stratification by sex, age group (20–30, 31–40, 41–50 years), BMI group (<20, 20–25, 26–30, >30), and race (White, Black, Hispanic, Other).
Elevated creatinine: Cr > 0.97 mg/dl (86µmol/L) if female, or 1.16 mg/dl (103 µmol/L)if male. Elevated cystatin C: Cystatin C > 1.00 mg/L for both sexes
Discussion
This is the largest study to date examining the prevalence of decreased kidney function using serum cystatin C in HIV-infected participants. Our data show two main findings. First, serum creatinine values in the HIV-infected NFHL cohort were significantly lower than those from the NHANES 2001–2002 cohort (adjusted for age, race and sex). Second, using a GFR estimating equation based on cystatin C, we observed a higher prevalence of kidney disease compared to creatinine-based estimates.
We suspect that the lower serum creatinine concentrations in the NFHL cohort than in the general population reflects decreased creatinine generation, probably related to the prevalence of liver disease in the NFHL cohort. Liver disease is a well-recognized cause of decreased creatinine generation due either to reduced muscle mass or decreased creatine synthesis 22. While the prevalence of extensive hepatic fibrosis or cirrhosis in the NFHL cohort is unknown, hepatic fibrosis progresses more rapidly in persons co-infected with hepatitis C and HIV 42–44 and liver disease is currently the leading cause of death in NFHL.
Muscle disease related to HIV itself or HIV antiretroviral medications (especially azidothymidine) could also be responsible for decreased creatinine generation. Nucleoside reverse transcriptase inhibitors are associated with mitochondrial toxicity and myopathy 45–47. Although we did not find decreased muscle mass in NFHL compared with NHANES, it is possible that sub-clinical myopathy occurs in asymptomatic patients on HAART, and this may be associated with decreased muscle creatine content or decreased creatinine production.
In principle, lower serum creatinine could be due to increased GFR, increased tubular secretion or increased extra-renal elimination of creatinine. However no studies have identified these factors in HIV infected patients treated with HAART.
The high prevalence of CKD defined by eGFRcys<60ml/min/1.73m2 (1 ml/s/1.73m2) in the NFHL cohort is consistent with the high prevalence of CKD risk factors including liver disease, diabetes, and a history of injection drug use 48.
Cystatin C has been reported to be superior to creatinine for accurate estimation of GFR in a number of populations, particularly those with decreased Lean body mass or chronic liver disease 24;49–67. Studies of cirrhotic patients that evaluated serum cystatin C and serum creatinine as filtration markers compared to measured GFR using inulin clearance 53 or 99mTc-DTPA clearance 63 found that cystatin C performed better than serum creatinine 56, consistent with the findings reported here. Although studies in cirrhotic patients have not specifically compared cystatin C-based GFR estimating equations to creatinine-based GFR estimating equations, given that serum creatinine is substantially reduced in cirrhotic patients, these equations would be expected to perform similarly poorly.
However, cystatin C has not been thoroughly evaluated as a filtration marker and preliminary evidence suggests that it may also be affected by factors other than GFR. A particular concern is inflammation. Knight et al 68 suggested that levels of cystatin C are associated with increased inflammation independent of level of kidney function (estimated by measured creatinine clearance). In our study, CRP levels in NFHL were significantly higher than in NHANES, and cystatin C levels were significantly correlated with CRP (r=0.37, p<0.001). Few studies have measured cystatin C in HIV infected persons, and these did not examine kidney function 69 or excluded persons with diabetes, hypertension or cirrhosis 11. Inflammation may be a partial explanation for the marked difference in prevalence of decreased GFR using the two GFR estimating equations.
This study illustrates that eGFRcys can identify kidney disease in HIV-infected persons with chronic liver disease that would be undetected by serum creatinine-based measurements. Among HIV infected subjects with and without chronic hepatitis, for example, the eGFRcreat identified kidney impairment in only 5% and 1% respectively, not showing the increased prevalence that is expected with chronic hepatitis. In contrast the eGFRcys identified kidney impairment in 7% of HIV-infected subjects without hepatitis, which increased to 30% in HIV-infected subjects with hepatitis.
A strength of this study is use of serum creatinine and cystatin C values calibrated to the same instrument used for development of the respective estimating equations. In addition, we were able to assess lean body mass, and to look at other variables associated with risk of abnormal kidney function. This is the first study comparing lean body mass of HIV infected persons taking HAART versus a similarly aged national cohort.
The primary limitation is that we did not use a gold standard method for determination of GFR. We are relying on serum creatinine and cystatin C-based GFR estimating equations in a population in whom the accuracy of these equations is unknown 70. In addition, NFHL did not collect urine samples for proteinuria or renal biopsy, and this analysis had only one creatinine and cystatin C sample per individual. Thus we could not make a formal diagnosis of CKD, which requires evidence of kidney damage or eGFR <60 ml/min/1.73m2 (1 ml/s/1.73m2) that persists for three or more months 20.
These data suggest that CKD may be more prevalent in HIV-infected patients than is currently believed, and that cystatin C may be a better marker of abnormal kidney function in the HIV population, particularly those with chronic viral hepatitis co-infection 71. Both have important implications for care of HIV-infected patients. Studies using gold-standard exogenous clearance methods for measuring GFR are required for more definitive answers.
Acknowledgements
Support: This study was supported by the NIH National Center for Research Resources GCRC Grant M01 RR00054, the NIH Grant 3P01-DK-045734-10S1, and the NIDDK grant 1P01DK45734-10 U01 DK053869-07
Financial Disclosure: Dr Ira Wilson is the recipient of a Mid-career Investigator Award in Patient-Oriented Research (K24 RR020300) from the National Center for Research Resources. Dr Lesley Stevens is the recipient of the American Society of Nephrology-Association of Specialty Professors Junior Development Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest:
Clara Y. Jones, MD: None
Camille A. Jones, MD: None
Ira B. Wilson, MD: None
Tamsin A. Knox, MD: None
Andrew S. Levey, MD: None
Donna Spiegelman, PhD: None
Sherwood L.Gorbach, MD: None
Frederick Van Lente, PhD: None
Lesley A. Stevens, MD: None
REFERENCES
- 1.Amin J, Kaye M, Skidmore S, Pillay D, Cooper D, Dore G. HIV and Hepatitis C Coinfection Within The CAESAR Study. HIV Medicine. 2004;5:174–179. doi: 10.1111/j.1468-1293.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 2.Strader D. Coinfection with HIV and Hepatitis C virus in Injection Drug Users and Minority Populations. Clinical Infectious Disease. 2005;41:S7–S13. doi: 10.1086/429489. [DOI] [PubMed] [Google Scholar]
- 3.DiGiambenedetto S, Baldini F, Cingolani A, Tamburrini E, Cauda R, DeLuca A. The Influence of Hepatitis C Virus Coinfection on the Risk of Lipid Abnormalities in a Cohort of HIV-1-Infected Patients After Initiation of Highly Active Antiretroviral Therapy. JAIDS. 2004;36:641–642. doi: 10.1097/00126334-200405010-00015. [DOI] [PubMed] [Google Scholar]
- 4.Schneider E, Glynn M, Kajese T, McKenna M. Epidemiology of HIV/AIDS - United States, 1981–2005. MMWR. 2006;55:589–590. [Google Scholar]
- 5.Conaldi P, Biancone L, Bottelli A, Wade-Evans A, Racusen L, Boccellino M, Orlandi V, Serra C, Camussi G, Toniolo A. HIV-1 Kills Renal Tubular Epithelial Cells in vitro by Triggering an Apoptotic Pathway Involving Caspase Activation and Fas Upregulation. J Clin Invest. 1998;102:2041–2049. doi: 10.1172/JCI3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray P, Liu X-H, Henry D, Dye L, III, Xu L, Orenstein J, Schuztbank T. Infection of Human Primary Renal Epithelial Cells with HIV-1 From Children with HIV-Associated Nephropathy. Kidney International. 1998;53:1217–1229. doi: 10.1046/j.1523-1755.1998.00900.x. [DOI] [PubMed] [Google Scholar]
- 7.Kimmel P, Ferreira-Centeno A, Farkas-Szallasi T, Abraham A, Garrett C. Viral DNA in Microdissected Renal Biopsy Tissue From HIV Infected Patients With Nephrotic Syndrome. Kidney International. 1992;43:1347–1352. doi: 10.1038/ki.1993.189. [DOI] [PubMed] [Google Scholar]
- 8.Bruggeman L, Ross M, Tanji N, Cara A, Dikman S, Gordon R, Burns G, D'Agati V, Winston J, Klotman M, Klotman P. Renal Epithelium is a Previously Unrecognized Site of HIV-1 Infection. J Am Soc Nephrol. 2000;11:2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 9.Marras D, Bruggeman L, Gao F, Tanji N, Mansuknani M, Cara A, Ross M, Gusella G, Benson G, D'Agati V, Hahn B, Klotman M, Klotman P. Replication and Compartmentalization of HIV-1 in Kidney Epithelium of Patients with HIV-Associated Nephropathy. Nature Medicine. 2002;8:522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- 10.Barrios A, Garcia-Benayas T, Gonzalez-Lahoz J, Soriano V. Tenofovir-related Nephrotoxicity in HIV-infected Patients. AIDS. 2003;18:960–963. doi: 10.1097/00002030-200404090-00019. [DOI] [PubMed] [Google Scholar]
- 11.Mauss S, Berger F, Schmutz G. Antiretroviral Therapy with Tenofovir is Associated with Mild Renal Dysfunction. AIDS. 2005;19:93–99. doi: 10.1097/00002030-200501030-00012. [DOI] [PubMed] [Google Scholar]
- 12.Dieleman J, van Rossum A, Stricker B, Sturkenboom M, de Groot R, Telgt D, Blok W, Burger D, Blijenberg B, Zietse R, Gyssens I. Persistent Leukocyturia and Loss of Renal Function in a Prospectively Monitored Cohort of HIV-Infected Patients Treated With Indinavir. Journal of Acquired Immune Deficiency Syndrome. 2003;32:135–142. doi: 10.1097/00126334-200302010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Tashima K, Horowitz J, Rosen S. Indinavir Nephropathy. New England Journal of Medicine. 1997;336:138–139. doi: 10.1056/NEJM199701093360215. [DOI] [PubMed] [Google Scholar]
- 14.Dieleman J, Sturkenboom M, Jambroes M, et al. Risk Factors for Urological Symptoms in a Cohort of Users of the HIV Protease Inhibitor Indinavir Sulfate: the ATHENA cohort. Archives of Internal Medicine. 2002;162:1493–1501. doi: 10.1001/archinte.162.13.1493. [DOI] [PubMed] [Google Scholar]
- 15.Gardner L, Klein R, Szczech L, Phelps R, Tashima K, Rompalo A, Schuman P, Sadek R, Tong T, Greenberg A, Holmberg S. Rates and Risk Factors for Condition-Specific Hospitalizations in HIV-Infected and Uninfected Women. Journal of Acquired Immune Deficiency Syndromes. 2003;34:320–330. doi: 10.1097/00126334-200311010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gardner L, Holmberg S, Williamson J, Szczech L, Carpenter C, Rompalo A, Schuman P, Klein R. Development of Proteinuria or Elevated Serum Creatinine and Mortality in HIV-Infected Women. Journal of Acquired Immune Deficiency Syndromes. 2003;32:203–209. doi: 10.1097/00126334-200302010-00013. [DOI] [PubMed] [Google Scholar]
- 17.Szczech L, Hoover D, Feldman J, Cohen M, Gange S, Agin A, Gooze L, Rubin N, Young M, Cai X, Shi Q, Gao W, Anastos K. The Association Between Renal Disease and Outcomes Among HIV-Infected Women Taking and Not Taking Antiretroviral Therapy. Clinical Infectious Disease. 2004;30:1150–1200. doi: 10.1086/424013. [DOI] [PubMed] [Google Scholar]
- 18.Becker B, Schulman G. Nephrotoxicity of Antiviral Therapies. Current Opinion in Nephrology and Hypertension. 1996;5:375–379. doi: 10.1097/00041552-199607000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Eustace J, Winston J, Boydstun I, Ahuja T, Rodriguez R, Tashima K, Roland M, Franceschini N, Palella F, Lennox J, Klotman P, Nachman S, Hall S, Szczech L. Guidelines for the Management of Chronic Kidney Disease in HIV-Infected Patients: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases. 2005;40:1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 20.Levey A, Coresh J, Balk E, Kausz A, Levin A, Steffes M, Hogg R, Perrone R, Lau J, Eknoya G. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Annals of Internal Medicine. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 21.Stevens L, Levey A. Measurement of Kidney Function. The Medical Clinics of North America. 2005;89:457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Cocchetto D, Tschanz C, Bjornsson T. Decreased Rate of Creatinine Production in Patients with Hepatic Disease: Implications for Estimation of Creatinine Clearance. Therapeutic Drug Monitoring. 1983;5:161–168. doi: 10.1097/00007691-198306000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kotler D. Nutritional Alterations Associated with HIV Infection. JAIDS. 2000;25:S81–S87. doi: 10.1097/00042560-200010001-00013. [DOI] [PubMed] [Google Scholar]
- 24.Filler G, Bokenkamp A, Hofmann W, LeBricon T, Martinez-Bru C, Grubb A. Cystatin C As A Marker of GFR -History, Indications, and Future Research. Clinical Biochemistry. 2005;8:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Massey D. Commentary: Clinical Diagnostic Use of Cystatin C. Journal of Clinical Laboratory Analysis. 2004;18:55–60. doi: 10.1002/jcla.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knox T, Spiegelman D, Skinner S, et al. Diarrhea and Abnormalities of Gastrointestinal Function in a Cohort of Men and Women with HIV Infection. American Journal of Gastroenterology. 2000;95:3482–3489. doi: 10.1111/j.1572-0241.2000.03365.x. [DOI] [PubMed] [Google Scholar]
- 27.U.S.Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, and NHANES Web site: http/www.cdc.gov/nchs/nhanes.htm. NHANES 2001–2002. 2006 [Google Scholar]
- 28.Coresh J, Astor B, McQuillan G, Kusek J, Greene T, Van Lente F, Levey A. Calibration and Random Variation of the Serum Creatinine Assay as Critical Elements of Using Equations to Estimate Glomerular Filtration Rate. American Journal of Kidney Diseases. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 29.Levey A, Coresh J, Greene T, Stevens L, Zhang Y, Hendriksen S, Kusek J, Van Lente F for the Chronic Kidney Disease Epidemiology Collaboration. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Annals of Internal Medicine. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F and Chronic Kidney Disease Epidemiology Collaboration. “Expressing the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate with Standardized Serum Creatinine Values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 31.Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, Poggio ED, Schmid CH, Steffes MW, Zhang YL, Van Lente F, Coresh J. “Impact of Creatinine Calibration on Performance of GFR Estimating Equations in a Pooled Individual Patient Database. American Journal of Kidney Diseases. 2007;50(1):21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Levey A, Bosch J, Lewis J, Greene T, Rogers N, Roth D. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Annals of Internal Medicine. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Coresh J, Stevens LA, Greene T, Feldman H, Froissart M, Kusek J, Rossert J, Schmid C, Van Lente F, Zhang L, Levey A and for CKD-EPI. Serum Cystatin C GFR Estimation Equation: Pooled Analysis of 3134 Individuals. Journal of the American Society of Nephrology. 2006;17:189A. [Google Scholar]
- 34.Lukaski H. Use of Bioelectrical Impedance Analysis to Assess Human Body Composition: A Review, in Livingston G (ed): Nutritional Status Assessment of the Individual. 1989:189–204. [Google Scholar]
- 35.NHANES III Laboratory Data File:U.S. Department of Health and Human Services (DHHS). National Center for Health Statistics. Third National Health and NutritionExamination Survey, 1988–1994, NHANES III Laboratory Data File (CD-ROM)Public Use Data File Documentation Number 76200. Hyattsville, MD: Centers for Disease Control and Prevention; 1996. Available from National Technical Information Service (NTIS), Springfield, VA. Acrobat. PDF format; includes access software: Adobe Systems, In. Acrobat Reader 2.1. 2004. [Google Scholar]
- 36.Correia L, Lima J, Gerstenblith G, Magalhaes L, Moreira A, Barbosa O, Dumet J, Passos L, D'Oliveira A, Esteves J. Correlation Between Turbidimetric and Nephelometric Methods of Measuring C-reactive Protein in Patients with Unstable Angina or non-ST Elevation Acute Myocardial Infarction. Arquivos Brasileiros de Cardiologia. 2003;81:133–136. doi: 10.1590/s0066-782x2003001000002. [DOI] [PubMed] [Google Scholar]
- 37.Roberts W, Moulton L, Law T, et al. Evaluation of Nine Automated High-sensitivity C-reactive Protein Methods: Implications for Clinical and Epidemiological Applications. Clinical Chemistry. 2001;47:418–425. [PubMed] [Google Scholar]
- 38.Jones C, McQuillan G, Kusek J, Eberhardt M, Herman W, Coresh J, Salive M, Jones C, Agodoa L. Serum Creatinine Levels in the US Population: Third National Health and Nutrition Examination Survey. American Journal of Kidney Diseases. 1998;32:992–999. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 39.Finney H, Newman D, Thakkar H, Fell J, Price C. Reference Ranges for Plasma Cystatin C and Creatinine Measurements in Premature Infants, Neonates, and Older Children. Arch Dis Child. 2000;82:71–75. doi: 10.1136/adc.82.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galteau M-M, Guyon M, Gueguen R, Siest G. Determination of Serum Cystatin C: Biological Variation and Reference Values. Clin Chem Lab Med. 2001;3981:850–857. doi: 10.1515/CCLM.2001.141. [DOI] [PubMed] [Google Scholar]
- 41.Uhlmann E, Hock K, Issitt C, Sneeringer M, Cervelli D, Gorman R, Scott M. Reference Intervals for Plasma Cystatin C in Healthy Volunteers and Renal Patients, as Measured by the Dade Behring BN II System, and Correlation with Creatinine. Clinical Chemistry. 2001;47:2031–2033. [PubMed] [Google Scholar]
- 42.Giordano T, Kramer J, Souchek J, Richardson P, El-Serag H. Cirrhosis and Hepatocellular Carcinoma in HIV-infected Veterans With And Without The Hepatitis C Virus: A Cohort Study, 1992–2001. Archives of Internal Medicine. 2004;164:2349–2354. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 43.Poynard T, Mathurin P, Lai C, Guyader D, Poupon R, Tainturier M, Myers R, et al. A Comparison of Fibrosis Progression in Chronic Liver Diseases. Journal of Hepatology. 2003;38:265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 44.Martin-Carbonero L, Benhamou Y, Puoti M, Berenguer J, Mallolas J, Quereda C, Arizcorreta A, et al. Incidence and Predictors of Severe Liver Fibrosis in Human Immunodeficiency Virus-Infected Patients with Chronic Hepatitis C: A European Collaborative Study. Clinical Infectious Disease. 2004;38:128–133. doi: 10.1086/380130. [DOI] [PubMed] [Google Scholar]
- 45.Moyle G. Mitochondrial Toxicity: Myths and Facts. Journal of HIV Therapy. 2004;9:45–47. [PubMed] [Google Scholar]
- 46.Authier F-J, Chariot P, Gherardi R. Skeletal Muscle Involvement in Human Immunodeficiency Virus (HIV)-Infected Patients in the Era of Highly Active Antiretroviral Therapy (HAART) Muscle & Nerve. 2005;32:247–260. doi: 10.1002/mus.20338. [DOI] [PubMed] [Google Scholar]
- 47.Moyle G. Mechanisms of HIV and Nucleoside Reverse Transcriptase Inhibitor Injury to Mitochondria. Antiviral Therapy. 2005;10:M47–M52. [PubMed] [Google Scholar]
- 48.El-Serag H, Hampel H, Yeh C, Rabeneck L. Extrahepatic Manifestations of Hepatitis C Among United States Male Veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 49.Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A. Relationships Among Serum Cystatic C, Serum Creatinine, Lean Tissue Mass and Glomerular Filtration Rate in Healthy Adults. Scand J Clin Lab Invest. 1999;59:587–592. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 50.Villa P, Jimenez M, Soriano M-C, Manzanares J, Cassanovas P. Serum Cystatin C Concentration as a Marker of Acute Renal Dysfunction in Critically Ill Patients. Critical Care. 2005;9:R139–R143. doi: 10.1186/cc3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan G, Lewis A, James T, Altmann P, Taylor R, Levy J. Clinical Usefulness of Cystatin C for the Estimation of Glomerular Filtration Rate in Type 1 Diabetes. Diabetes Care. 2002;25:2004–2009. doi: 10.2337/diacare.25.11.2004. [DOI] [PubMed] [Google Scholar]
- 52.Samyn M, Cheeseman P, Bevis L, Taylor R, Samaroo B, Buxton-Thomas M, Heaton N, Rela M, Mieli-Vergani G, Dhawan A. Cystatin C, an Easy and Reliable Marker for Assessment of Renal Dysfunction in Children with Liver Disease and After Liver Transplantation. Liver Transplantation. 2005;11:344–349. doi: 10.1002/lt.20330. [DOI] [PubMed] [Google Scholar]
- 53.Randers E, Ivarsen P, Erlandsen E, Hansen E, Aagaard N, Bendtsen F, Vilstrup H. Plasma Cystatin C As A Marker of Renal Function in Patients With Liver Cirrhosis. Scand J Clin Lab Invest. 2002;62:129–134. doi: 10.1080/003655102753611753. [DOI] [PubMed] [Google Scholar]
- 54.Randers E, Erlandsen E, Pedersen O, Hasling C, Danielsen H. Serum Cystatin C as an Endogenous Parameter of the Renal Function in Patients with Normal to Moderately Impaired Kidney Function. Clinical Nephrology. 2000;54:203–209. [PubMed] [Google Scholar]
- 55.Perlemoine C, Beauvieux M-C, Rigalleau V, Baillet L, Barthes N, Derache P, Gin H. Interest of Cystatin C in Screening Diabetic Patients for Early Impairment of Renal Function. Metabolism. 2003;52:1258–1264. doi: 10.1016/s0026-0495(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 56.Orlando R, Mussap M, Plebani M, Piccoli P, DeMartin S, Floreani M, Padrini R, Palatini P. Diagnostic Value of Plasma Cystatin C as a Glomerular Filtration Marker in Decompensated Liver Cirrhosis. Clinical Chemistry. 2002;48:850–858. [PubMed] [Google Scholar]
- 57.O'Riordan S, Webb M, Stowe H, Simpson D, Kandarpa M, Coakley A, Newman D, Saunders J, Lamb E. Cystatin C Improves the Detection of Mild Renal Dysfunction in Older Patients. Ann Clin Biochem. 2003;40:648–655. doi: 10.1258/000456303770367243. [DOI] [PubMed] [Google Scholar]
- 58.Larsson A, Malm J, Grubb A, Hansson L-O. Calculation of Glomerular Filtration Rate Expressed in mL/min from Plasma Cystatin C Values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 59.Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindstrom V, Grubb A. Serum Cystatin C, Determined by a Rapid, Automated Particle-Enhanced Turbidimetric Method, Is A Better Marker than Serum Creatinine for Glomerular Filtration Rate. Clinical Chemistry. 1994;40:1921–1926. [PubMed] [Google Scholar]
- 60.Kazama J, Kutsuwada K, Maruyama H, Gejyo F. Serum Cystatin C Reliably Detects Renal Dysfunction in Patients with Various Renal Diseases. Nephron. 2002;91:13–20. doi: 10.1159/000057599. [DOI] [PubMed] [Google Scholar]
- 61.Hoek F, Kemperman F, Krediet R. A Comparison Between Cystatin C, Plasma Creatinine and the Cockcroft and Gault Formula for the Estimation of Glomerular Filtration Rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 62.Dharnidharka V, Kwon C, Stevens G. Serum Cystatin C is Superior to Serum Creatinine as a Marker of Kidney Function: A Meta-Analysis. American Journal of Kidney Diseases. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 63.Demirtas S, Bozbas A, Akbay A, Yavuz Y, Karaca L. Diagnostic Value of Serum Cystatin C for Evaluation of Hepatorenal Syndrome. Clinica Chimica Acta. 2001;311:81–89. doi: 10.1016/s0009-8981(01)00546-0. [DOI] [PubMed] [Google Scholar]
- 64.Christensson A, Grubb A, Nilsson J, Norrgren K, Sterner G, Sundkvist G. Serum Cystatin C Advantageous Compared with Serum Creatinine in the Detection of Mild but Not Severe Diabetic Nephropathy. Journal of Internal Medicine. 2004;256:510–518. doi: 10.1111/j.1365-2796.2004.01414.x. [DOI] [PubMed] [Google Scholar]
- 65.Woitas P, Stoffel-Wagner B, Flommersfeld S, Poege U, Schiedermaier P, Klehr H-U, Spengler U, Bidlingmaier F, Sauerbruch T. Correlation of Serum Concentrations of Cystatin C and Creatinine to Inulin Clearance in Liver Cirrhosis. Clinical Chemistry. 2000;46:712–715. [PubMed] [Google Scholar]
- 66.Wasen E, Isoaho R, Mattila K, Vahlberg T, Kivela S, Irjala K. Estimation of Glomerular Filtration Rate in the Elderly: A Comparison of Creatinine-based Formulae with Serum Cystatin C. Journal of Internal Medicine. 2004;256:70–78. doi: 10.1111/j.1365-2796.2004.01340.x. [DOI] [PubMed] [Google Scholar]
- 67.Hojs R, Bevc S, Antolinc B, Gorenjak M, Puklavec L. Serum Cystatin C as an Endogenous Marker of Renal Function in the Elderly. Int J Clin Pharm Res. 2004;XXIV:49–54. [PubMed] [Google Scholar]
- 68.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. “Factors Influencing Serum Cystatin C Levels Other Than Renal Function and the Impact on Renal Function Measurement. Kidney International. 2004;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 69.Colle A, Tavera C, Prevot D, Leung-Tack J, Thomas Y, Manuel Y, Benveniste J, Leibowitch J. Cystatin C Levels in Sera of Patients with Human Immunodeficiency Virus Infection. Journal of Immunoassay. 1992;13:47–60. doi: 10.1080/15321819208019824. [DOI] [PubMed] [Google Scholar]
- 70.Stevens LA, Coresh J, Greene T, Levey AS. Assessing Kidney Function - Measured and Estimated Glomerular Filtration Rate. The New England Journal of Medicine. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 71.Reisler RB, Jacobson L, Gupta S, Qiao W, Margolick J, Riddler S, Visscher B, Williams C, Palella F. Chronic Kidney Disease and the Use of HAART; 12th Conference on Retroviruses and Opportunistic Infections (CROI) Abstract 818.2005. [Google Scholar]