Abstract
To investigate the subcortical efferent connections of visual area V2, we injected tritiated amino acids under electrophysiological control into 15 V2 sites in 14 macaques. The injection sites included the fovea representation as well as representations ranging from central to far peripheral eccentricities in both the upper and lower visual fields. The results indicated that V2 projects topographically to different portions of the inferior and lateral pulvinar and to the superficial and intermediate layers of the superior colliculus. Within the pulvinar, the V2 projections terminated in fields P1, P2, and P4, with the strongest projection being in P2. Central visual field injections in V2 labeled projection zones in P1 and P2, whereas peripheral field injections labeled P1, P2, and P4. No projections were found in P3. Both central and peripheral field injections in V2 projected topographically to the superficial and intermediate layers of the superior colliculus. Projections from V2 to the pulvinar and the superior colliculus constituted cortical–subcortical loops through which circuits serving spatial attention are activated.
INTRODUCTION
There is extensive literature on the cortical connections of V2 that contrast with the paucity of studies on its subcortical connections. In macaques, V2 is the major cortical projection target of area V1 (Lund, Hendrickson, Ogren, & Tobin, 1991; Weller & Kaas, 1983; Rockland & Pandya, 1979, 1981; Zeki, 1969, 1971, 1976; Cragg & Ainsworth, 1969; Kuypers, Szwarcbart, Mishkin, & Rosvold, 1965). V2 projects back to V1 and forward to several visuotopically organized extrastriate areas, including V3, V4, MT, and PO (Nakamura, Gattass, Desimone, & Ungerleider, 1993; Boussaoud, Desimone, & Ungerleider, 1991; Shipp & Zeki, 1985, 1989; Zeki & Shipp, 1989; Colby, Gattass, Olson, & Gross, 1988; Burkhalter, Felleman, Newsome, & Van Essen, 1986; Ungerleider & Desimone, 1986b; DeYoe & Van Essen, 1985; Kennedy & Bullier, 1985; Felleman & Van Essen, 1983, 1984; Fenstemaker, Olson, & Gross, 1984; Maunsell & Van Essen, 1983; Rockland & Pandya, 1981; Zeki, 1971). In our previous study of the cortical projections of V2 (Gattass, Sousa, Mishkin, & Ungerleider, 1997), we found that the peripheral but not central visual field representation of V2 projects to a number of other visual areas located in occipitoparietal cortex, including PO, MST, and VIP, as well as to a portion of area TF (area VTF; Boussaoud et al., 1991) located within the posterior parahippocampal gyrus. Previously, injections of tritiated amino acids were made in area V4 to study the subcortical projections of that area (see Gattass, Galkin, Desimone, & Ungerleider, 2014). However, whereas the representation of the visual field in V4 is restricted to the central 30°–40°, V2 encompasses the entire visual field (Gattass, Sousa, & Gross, 1988; Gattass, Sousa, & Covey, 1986). Thus, the study of V2 projections may better reveal central versus peripheral asymmetries than would the study of V4 projections.
The asymmetry in central versus peripheral field projections suggests that information from the peripheral visual field is preferentially relayed to areas concerned with spatial vision.
Prior studies have shown that the subcortical projections from V2 target the pulvinar (Soares, Botelho, & Gattass, 2001), the superior colliculus, and the claustrum (Pearson, Brodal, Gatter, & Powell, 1982), but the organization of these projections remains unclear, as does their relation to the now well-defined subdivisions within the pulvinar.
We report here on the subcortical projections of area V2 in 15 cases, using tritiated amino acid injections placed under physiological control into different retinotopic locations. Our results indicate that V2 sends topographically organized projections to three fields of the pulvinar and to the superficial and intermediate layers of the superior colliculus.
METHODS
Autoradiographic material from 14 adult Macaca mulatta weighing between 3.2 and 4.5 kg was used. In all animals except one, injections of tritiated amino acids were placed into retinotopically specified sites that were determined by electrophysiological recordings. The injection sites spanned eccentricities covering central and peripheral vision in both the upper and lower visual fields (Rosa, Sousa, & Gattass, 1988; Gattass, Gross, & Sandell, 1981; see Figure 1). In the one case without physiological recordings (Case 1), the injection was placed into the foveal representation of V2 under direct visualization.
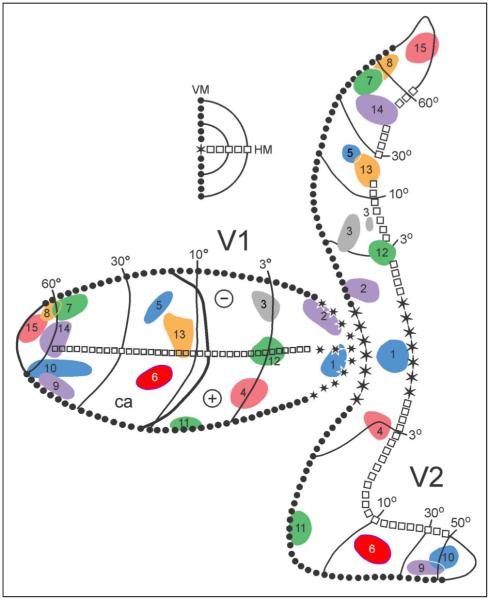
Figure 1.
Location of the injection sites in V2 and of the feedback projections to V1, shown on a 2-D reconstruction of the cortex. In the inset at left, the representation of the vertical meridian (VM) is illustrated with circles, the horizontal meridian (HM) with squares, the foveal representation with asterisks, and the isoeccentricity lines with thin lines. Injection sites, shown without the surrounding halo, were plotted on the flattened map to best retain their locations relative to sulcal (thick continuous lines) and myeloarchitectonic borders. Each injection site and its corresponding connections with V1 are numbered and colored accordingly.
Receptive Field Recording
The experimental procedures for multiunit recordings have been described in detail elsewhere (Gattass & Gross, 1981). Briefly, under ketamine and sodium pentobarbital anesthesia and before the first recording session, the animal was implanted with a bolt to hold the head in the stereotaxic apparatus and with a stainless steel recording chamber. In each recording session, the animal was anesthetized with 2% halothane, followed by a 70%:30% mixture of N2/O2. Muscular paralysis was induced by pancuronium bromide, and artificial ventilation was maintained by a respiratory pump connected to an endotracheal cannula. Heart rate, rectal temperature, and the level of expired CO2 were continuously monitored and kept within the normal physiological range. The right eye was fitted with a contact lens, which focused the eye to the surface of a 30-cm radius translucent hemisphere placed in front of the animal. The locations of the fovea and the center of the optic disc were projected onto the hemisphere using the target of an ophthalmoscope reflected by a corner cube prism (Edmund Scientifics, Barrington, NJ). The horizontal meridian was taken to be a line through both these points, and the vertical meridian was an orthogonal line passing through the fovea.
Varnish-coated tungsten microelectrodes were used to record from small clusters of neurons. Visual receptive fields were plotted by moving white or colored bars onto the surface of the translucent hemisphere under light-adapted conditions. Recordings continued until the desired visual field representation within V2 was located.
Injections of V2
In Case 1, the injection was placed into the foveal representation of V2 under direct visualization of the cortex. In Case 5, the injection was made with a 1-μl Hamilton syringe attached to a tungsten microelectrode. In the remaining cases, after the desired injection site was located electrophysiologically, a guide tube was advanced through the dura and placed approximately 300 μm above the intended injection site. The microelectrode was then advanced through the guide tube, and the visuotopic location of the injection site was confirmed. The electrode was then withdrawn from the guide tube and replaced by a 1-μl Hamilton syringe. We injected 0.15–0.3 μl of an equal parts mixture of tritiated proline (New England Nuclear L-[2,3,4,5-3H], specific activity 100–140 Ci/mmol) and tritiated leucine (New England Nuclear L-[3,4,5-3H (N)], specific activity 100 140 Ci/mmol). The labeled amino acids, which had been evaporated and then reconstituted in 0.9% saline to give a final concentration of 50 μCi/μl, were injected at a rate of 0.02 μl/2 min. To minimize leakage of the tracer up the electrode track, the syringe was left in place for 30 min after the injection and then withdrawn into the guide tube, which was then removed from the brain. In the first 13 animals, we made unilateral V2 injections. Because no contralateral projections were observed in these cases, we injected V2 bilaterally in the remaining animal (Cases 4 and 14), confident that no ambiguity would result provided we avoided the representation of the vertical meridian.
Figure 1 summarizes the injection sites in area V2 on a composite 2-D flattened map of the cerebral cortex, which was generated as follows. For each case, we created a 2-D “wire map” of the cortex (Gattass, Sousa, & Rosa, 1987; Ungerleider & Desimone, 1986a), and the locations of the tracers, myeloarchitectonic borders, and recording sites were transferred onto the flattened maps. We then created a composite map from the individual cases.
The injection sites ranged from the fovea of V2 to eccentricities of 65° in the lower and upper visual fields (Figure 1, right). We only used injections that did not invade the white matter and that showed consistent, topographically organized connections with V1. In all cases, there were one or more labeled zones in V1, the visuotopic locus of which (Figure 1, left) was highly consistent with the visuotopic locus of the injection site in V2 (Gattass et al., 1981).
Histological Processing
After survival times of 6–8 days, the animals received a lethal dose of sodium pentobarbital and were then perfused transcardially with 0.9% saline followed by 10% formol-saline. Their brains were blocked stereotaxically, removed from the skull, photographed, and stored in 30% sucrose in 10% formol-saline until they sank. Frozen sections, 33 μm in thickness, were cut in the frontal plane. Every fifth section was mounted onto gelatinized slides, dehydrated, defatted, and processed for autoradiography according to the procedures of Cowan, Gottleib, Hendrickson, Price, and Woolsey (1972). Subsequently, the autoradiographs were developed in Kodak D19, fixed, and counterstained with thionin. Alternate sections were stained for myelin using the Gallyas (1979) procedure or, in one case, using the Spielmeyer method (Lillie, 1965). For the purpose of analysis, the locations of concentrations of silver grains were charted onto enlarged photographs of the myelin-stained sections. We prepared the illustrations based on tracing of the enlarged photographs and on observations of selected autoradiographs.
Correspondence of Receptive Fields to Injection Sites
In addition to the receptive field recorded at the injection site, for each case we calculated a back-transformed receptive field, using a method similar to the one described by Maunsell and Van Essen (1983). Briefly, back-transformed receptive fields were initially determined by mapping the projection to V1 on a flattened map of V1 and then by overlaying this map onto previously published visuotopic maps of V1 (Daniel & Whitteridge, 1961). Finally, the coordinates overlaid by the V1 projections were used to draw the back-transformed receptive field (see Figures 3–6).
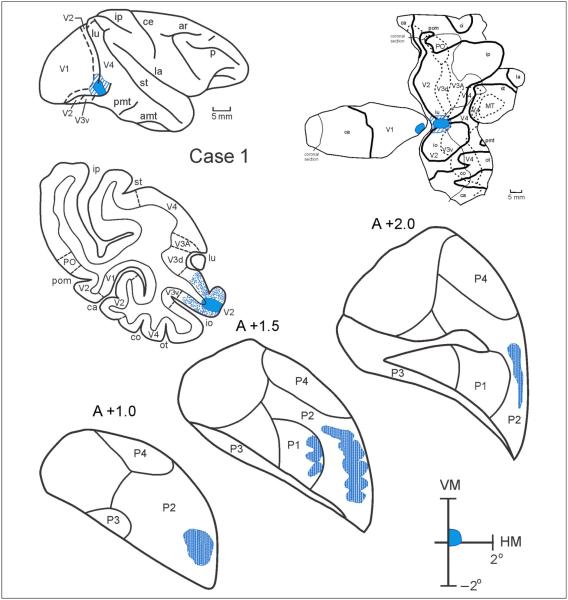
Figure 3.
Case 1: Distribution of labeled terminals following an injection into the foveal representation of V2, shown in coronal sections through the pulvinar. In coronal sections of the cortex, the injection site is shown in blue, the dots indicate the relative density and laminar distribution of labeled terminals, and the dashed lines indicate the myeloarchitectonic borders of visual areas. In the lateral view of the hemisphere, the injection site is shown in blue and the halo surrounding the injection site is illustrated with stripes. The portion of the visual field corresponding to the back-transformation of the projection to V1 is shown in blue at upper right. Top right: Flat map of V1, V2, and surrounding areas showing the injection site in V2 and the back-projection to V1. Abbreviations: Cortical visual areas: LIP = lateral intraparietal area; LIPv = ventral portion of LIP; MIP = medial intraparietal area; MST = medial superior temporal area; MT = middle temporal area; PIP = posterior intraparietal area; PO = parieto-occipital area; PRO = area prostriata; TEO = posterior inferior temporal cortex; V1 = primary visual cortex; V2 = visual area 2; V3A = visual complex V3 part A; V3d = dorsal portion of visual area 3; V3v = ventral portion of visual area 3; V4 = visual area 4; V4t = V4 transition zone; VIP = ventral intraparietal area; VTF = visual part of parahippocampal TF. Cortical sulci: amt = anterior middle temporal sulcus; ar = arcuate sulcus; ca = calcarine fissure; ce = central sulcus; co = collateral sulcus; ec = external calcarine sulcus; io = inferior occipital sulcus; ip = intraparietal sulcus; la = lateral sulcus; lu = lunate sulcus; orb = orbital sulcus; ot = occipitotemporal sulcus; p = principal sulcus; pmt = posterior middle temporal sulcus; rh = rhinal sulcus; sp = subparietal sulcus; st = superior temporal sulcus. For other conventions, see Figure 2.
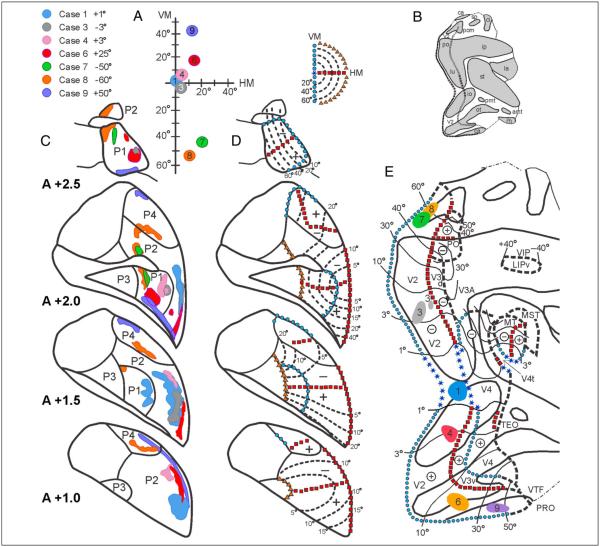
Figure 6.
Three topographically organized projection zones (P1, P2, and P4) of the pulvinar revealed after injections of anterograde tracers into V2 at the eccentricities shown in seven selected cases (top left). The list of the cases and the locations of the receptive field centers in V2 are shown in A. (B) Flat map of extrastriate cortex showing the areas of the sulci in gray. (C) Reconstructions of the projection in coronal sections (A +2.5–A +1.0) of the pulvinar from anterior (+2.5) to posterior (+1.0) regions. (D) Representations of the topographical maps in the projection zones P1, P2, and P4 of the pulvinar are drawn at four coronal sections through the pulvinar (right). (E) Two-dimensional reconstruction of the macaque cortex, showing the location of V2 and surrounding extrastriate visual areas. Heavy lines indicate the boundaries of the sulci and the dotted-dashed lines indicate the boundaries between the neocortex and allocortex. The gray area on the small 2-D reconstruction indicates cortex within sulci. For other conventions, see Figures 2 and 3.
RESULTS
The results are based on data from 15 injections of tritiated amino acids in V2 (Figure 1).
Projections from V2 to the Pulvinar
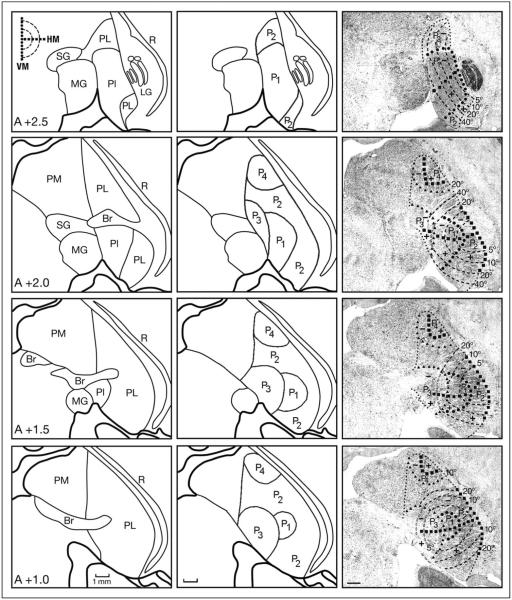
The relationship between the P1–P4 fields and the architectonic subdivisions of the pulvinar are shown in Figure 2. The visuotopic maps of P1 and P2 were charted onto Nissl-stained sections based on previously published work by Ungerleider et al. (Ungerleider, Desimone, Galkin, & Mishkin, 1984; Ungerleider, Galkin, & Mishkin, 1983) and Bender (1981). The first estimate of the P3 borders was guided by Ungerleider et al. (1984) and, subsequently, according to the distribution of labeled V4 projections (Gattass et al., 2014). The borders of P3 and P4 were guided by the distribution of calbindin immunoreactivity presented by Adams, Hof, Gattass, Webster, and Ungerleider (2000) and the distribution of cells and terminals in our V4 cases (Gattass et al., 2014). Thus, our assignment of terminals to P1–P4 is based on estimated borders of these regions.
Figure 2.
Representative coronal sections stained for Nissl through the rostral-to-caudal (top-to-bottom) extent of the pulvinar. Left: Cytoarchitectonic subdivisions, according to Olszewski (1952). Middle: Limits of the pulvinar fields. Right: The pulvinar fields P1, P2, P3, and P4 are shown superimposed on each section. Solid circles indicate the representation of the vertical meridian, solid squares indicate the representation of the horizontal meridian, heavy dashes indicate isoeccentricity lines, open (white) dashed lines indicate isoeccentricity lines in the areas of coarse topography (P3 and P4), small solid triangles indicate the borders of P3 and P4, and small dotted lines indicate the borders of the pulvinar fields. The plus sign indicates the upper visual field representation, and the minus sign indicates the lower visual field representation. The sections are spaced 0.5 mm apart, and they do not reach the caudal extent of the pulvinar. Abbreviations: Br = brachium of superior colliculus; Cd = caudate nucleus; LG = lateral geniculate nucleus; MG = medial geniculate nucleus; P1–P4 = subdivisions of pulvinar; PI = inferior pulvinar; PL = lateral pulvinar; PM = medial pulvinar; R = thalamic reticular formation; SC = superior colliculus; SG = supra geniculate nucleus. Scale bars = 1 mm.
Several clusters of labeled terminals were found in the pulvinar after V2 injections at different topographic locations. These projections are illustrated in coronal sections in five representative cases (Figures 3–5). Examples of such data are illustrated in Figures 3 and 4, where labeled terminals were found in the P1 and P2 projection zones, as defined previously (Ungerleider et al., 1984; Ungerleider et al., 1983). The labeled terminals were not restricted to these projection fields, however, but rather extended dorsally into the field that we term P4 (Figure 5). As shown in Figure 5, the distribution of the label appeared in small clusters in the inferior pulvinar (PI), in part of the lateral pulvinar (PL), and, to a lesser extent, in the medial pulvinar (PM). The borders of these small clusters appeared to coincide with the limits of the chemoarchitectonic subdivisions of the pulvinar (see Adams et al., 2000); however, because the tissue was processed many years ago, no direct comparison between chemoarchitecture and projections was possible in our study.
Figure 5.
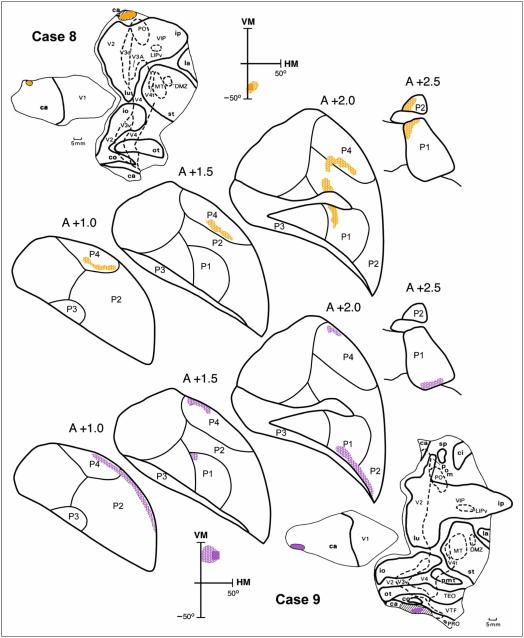
Connections of the pulvinar with V2 in Cases 8 and 9. Distribution of labeled terminals following an injection into the upper and lower peripheral visual field representations in V2, shown in coronal sections through the pulvinar. The receptive field recorded at the locations of the recording site is illustrated by a colored square (golden orange, Case 8 and purple, Case 9). For other conventions, see Figures 2 and 3. For details, see text.
Figure 4.
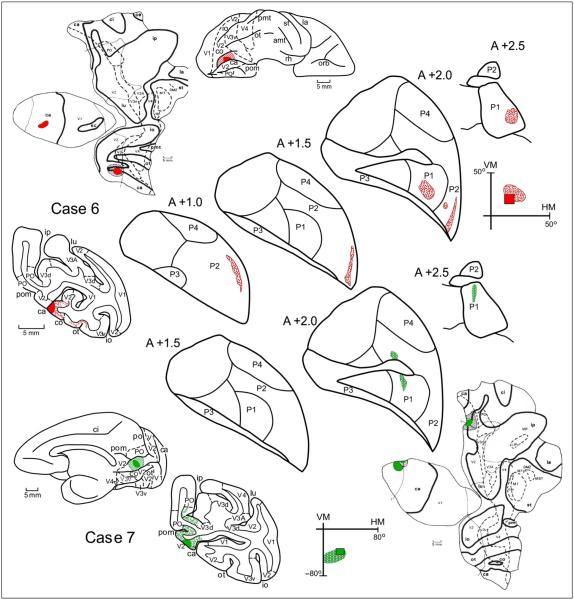
Connections of the pulvinar with V2 in Cases 6 and 7. Distribution of labeled terminals following an injection into the upper and lower intermediate visual field representations in V2, shown in coronal sections through the pulvinar. In the coronal sections of the cortex, the injection site is shown in red (Case 6) or green (Case 7), the dots indicate the relative density and laminar distribution of labeled terminals, and the dashed lines indicate the myeloarchitectonic borders of visual areas. In the lateral view of the hemisphere, the injection site is shown in red or green, and the halo surrounding the injection site is illustrated with stripes. The receptive field recorded at the locations of the recording site is illustrated by a colored square. The portion of the visual field corresponding to the back-transformation of the projection to V1 is shown in colored dots at lower left. For other conventions, see Figures 2 and 3. For details, see text.
Figure 3 shows, in Case 1, the distribution of labeled terminals in the pulvinar after injections of an anterograde tracer (3H) into the central representation area of V2, located on the lateral convexity of the prelunate gyrus. The anterograde projections from V2 extended from the anterior portion of PI (section A +2.0) to the posterior portion of PL (section A +1.0). These projections encompass the P1 (section A +1.5) and P2 (section A +2.0– A +1.0) projection fields. No projections were found in P3. Similarly, no projections were observed in the interlaminar zones of the LGN or in the thalamic reticular nucleus, unlike what we had observed after V4 injections (Gattass et al., 2014).
Figure 4 shows the distribution of labeled terminals in the pulvinar after injections into the intermediate upper and lower field representations in Cases 6 and 7, respectively. In Case 6, the injection was located in the lower bank of the calcarine fissure extending to the ventral convexity. Several clusters of labeled terminals were found in P1 (sections A +2.5–A +2.0) and P2 (sections A +1.5–A +1.0). No label was found in P3. In Case 7, the injection was located in the upper calcarine gyrus extending to its medial convexity. Several clusters of labeled terminals were found in P1 (sections A +2.5–A +2.0) and P2 (section A +1.5). Again, no label was found in P3. The projections observed after injection into the lower field representation of V2 were located more dorsally than those emanating from the upper field representation.
Figure 5 shows the distribution of labeled terminals in the pulvinar after injections of an anterograde tracer (3H) into the peripheral lower and upper field representations in Cases 8 and 10, respectively. In Case 8, the injection was located on the medial convexity of the cortex, above the calcarine fissure (see Figure 1). Several clusters of labeled terminals were found in P1 (sections A +2.5–A +1.5), P2 (sections A +2.0–A +1.0), and P4 (sections A +2.0–A +1.0). No label was found in P3. In Case 10, the injection was located in the lower bank of the calcarine fissure (see Figure 1). Several clusters of labeled terminals were found in P1 (sections A +2.5–A +2.0), P2 (section A +2.0), and P4 (sections A +2.0–A +1.0). Again, no label was found in P3. In both Case 8 and Case 10, the projections visualized after injection of the lower field representation were located more dorsally in P1 and P2 than those from the upper field representation. This upper and lower visual field trend did not apply to P4, which has a complex topography similar to that of Pμ in the Cebus monkey (Gattass, Sousa, & Oswaldo-Cruz, 1978).
Figure 6 shows a summary of the regions containing labeled terminals in seven selected cases after injections into V2. In addition, the inferred visuotopic organization of the pulvinar is illustrated, with well-defined topographic maps in P1 and P2 and a cruder map in P4, which may exhibit some separation of the upper and lower visual fields. The projections from V2 in these seven cases encompass almost the entire extent of the P1, P2, and P4 fields of the pulvinar. The injection sites of Cases 2, 6, and 10, located in the upper field representation of V2, led to ventral patches in P1 (Figure 6, A +2.5–A +2.0) and P2 (Figure 6, A +2.0–A +1.0) and to a central patch in P4 (Figure 6, A +2.0–A +1.0). The injections into the lower field representation of V2 led to dorsal patches in P1 (Figure 6, A +2.5–A +2.0) and P2 (Figure 6, A +2.0–A +1.0) and to both dorsal and ventral patches in P4 (Figure 6, A +2.0–A +1.0). The patches revealed by the 14 injections into V2 show considerable overlap in all pulvinar fields, suggesting coarser topographic organization in these fields as a result of convergent input and/or larger receptive fields in the pulvinar as compared with those in V2.
Visual Topography of the Pulvinar Projection Zones
Injections of tritiated amino acids into V2 revealed topographically organized projections in P1, P2, and P4. The map in P1 was originally described by Bender (1981) in his recording study of the pulvinar. According to Bender, P1 is located in the inferior pulvinar but also includes a small portion of the immediately adjacent lateral pulvinar. The peripheral visual field is represented anteriorly in the medial portion of the inferior pulvinar, and the central visual field is represented more posteriorly in the medial portion of the lateral pulvinar. The vertical meridian is represented on the lateral edge of the nucleus, and the horizontal meridian is represented obliquely from lateral to medial across the nucleus and tilted slightly downward. The upper field is represented ventrally, and the lower field is represented dorsally. In coronal sections, P1 resembles a first-order transformation of the visual field (Almann & Kaas, 1974). P2 is located lateral to P1 and contains the representations of the peripheral region in the anterior portion of the nucleus and the central region located in the posterior portion of the lateral pulvinar. P2 and P1 share the representation of the vertical meridian, whereas the P2 representation of the horizontal meridian is a continuation of the horizontal meridian of P1 that subsequently splits to constitute the lateral side border of the pulvinar. As in P1, in P2 the upper field is represented ventrally and the lower field dorsally. In coronal sections, the P2 map resembles a second-order transformation of the visual field (Almann & Kaas, 1970). The P4 field has a complex topographic arrangement. The representation of the vertical meridian is located on the dorsal edge of P4, and the representation of the horizontal meridian extends from the dorsal edge of P4 in an oblique curve toward the inferior border of P4. The upper field occupies the most dorsal and anterior portions of P4, and the representation of the lower field is located more medially, adjacent to the lower field representation of P2. In coronal sections, the P4 map resembles a distorted first-order transformation of the visual field.
Projections from V2 to the Superior Colliculus
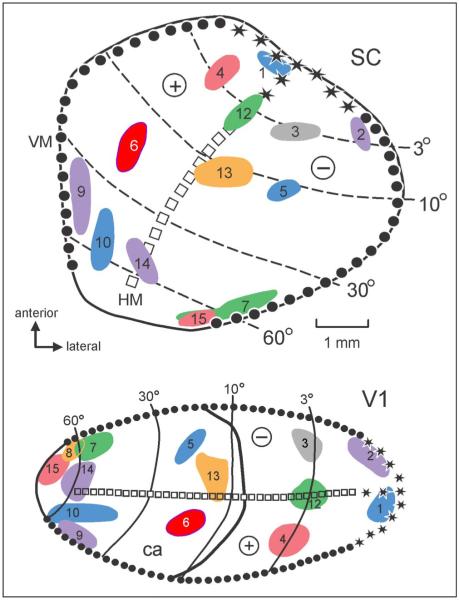
In 14 of the 15 cases with anterograde tracers injected into V2, projections were found in the upper layers of the superior colliculus. These projections followed a topographic pattern compatible with the known visuotopic map of the macaque superior colliculus (Tabareau, Bennequin, Berthoz, Slotine, & Girard, 2007; Cynader & Berman, 1972). According to Cynader and Berman (1972), the fovea is represented anteriorly, the peripheral visual field is represented posteriorly, the lower visual field is represented laterally, and the upper visual field is represented medially. As illustrated in Figure 7, the location of the labeled patches in the superior colliculus after V2 injections was consistent with this visuotopic organization. To facilitate a direct comparison between the back projection from V2 to V1 and the V2 projections to the superior colliculus, we renumbered the projections accordingly. Central field injections showed projections to the anterior portion of the colliculus (Projections 1–3, 8–9), whereas peripheral field injections showed projections to more posterior locations (Projections 6–7, 12–14). Lower field injections showed projections to the lateral portion of the colliculus (Projections 3, 5, 7, 13), whereas upper field injections showed projections to more medial locations (Projections 2, 4, 6, 14). Thus, the projections from V2 are in topographic register with the visuotopic organization of the superior colliculus. We did observe, however, that in some cases the projection zone appeared to be elongated in the anterior-to-posterior plane compared with what one would expect from the receptive field recordings (e.g., see Cases 6, 7, and 14; Figure 7); the reason for this observation is currently unclear. In all cases, projections to the colliculus from V2 extended ventrally from the stratum griseum superficiale to include the stratum opticum, although this projection was less dense.
Figure 7.
Topographically organized projections of V2 to the superficial layers of the superior colliculus in 14 cases. The flattened map of the superior colliculus shows topographically organized projections after the injection of an anterograde tracer into V2. The visuotopic locations of the injection sites are shown in the representation of the flattened map of V1 (bottom) of the right hemisphere, and the reconstruction of the projection zones are shown on the right superior colliculus surface (top).
DISCUSSION
The results of this study demonstrate that V2 projects topographically to P1, P2, and P4 of the pulvinar and to the superficial and intermediate layers of the superior colliculus. In this section, we first discuss the topographic organization of the projection fields of V2 and then compare the projections of V2 in macaques to those that have been described in other primate species. Finally, we discuss the relevance of central versus peripheral field projections.
Injections of tritiated amino acid s in visual area V4 (Gattass et al., 2014) revealed projections to both the pulvinar and superior colliculus, which was found after similar injections in V2. Injections in V4, however, also showed projections to the claustrum, amygdala, caudate nucleus, putamen, reticular nucleus, and intralaminar layers of the LGN. Because the amount and concentration of the tritiated amino acids and the injection techniques were identical in the studies of V4 and V2, we are confident that differences observed in the V4 and V2 projection zones are real. We therefore conclude that subcortical projections from V2 are restricted to the three visual field maps within the pulvinar and to the superficial and intermediate layers of the superior colliculus. Interestingly, the projections from V2 and V4 to these two structures had a similar topographic arrangement, although those from V2 appeared to be more limited in extent, which may reflect the smaller receptive field sizes and larger magnification factor in V2 (Gattass et al., 1981, 1988).
The Pulvinar
On the basis of electrophysiological recordings in the pulvinar, Bender (1981) described two separate fields, both of which are visuotopically organized. The first was termed the “PI” map, which is found mainly in rostrolateral PI and extends into medial portions of adjacent PL. The second was termed the “PL” map, which partially surrounds the PI map and is located entirely in ventrolateral PL. Subsequently, Ungerleider et al. (Ungerleider et al., 1984; Ungerleider et al., 1983) termed the PI and PL maps the “P1” and “P2” fields, respectively, based on connections of the pulvinar with V1 and MT. A third field, “P3,” was described by Ungerleider et al. (1984) based on its preferential connections with MT. It is located posteromedially in PI but also includes small adjacent portions of PL and PM that lie dorsal to the brachium of the superior colliculus (see also Standage & Benevento, 1983). Unlike its neighbor P1, P3 does not seem to have a well-defined retinotopic map, although it has not yet been mapped electrophysiologically. Dorsal to the P1–P3 fields, near the boundary between dorsal PL and PM, lies a region termed “Pdm” (Petersen, Robinson, & Keys, 1985). Like P3, Pdm has little, if any, visuotopic organization. We use the term “P4” to describe the projection field of area V4 that is located dorsal to P1 and P2 (Figure 2). P4 may be at least in part coextensive with Pdm (Petersen et al., 1985) or with Pμ of Cebus (Gattass et al., 1978).
Adams and collaborators (Adams et al., 2000) showed that projections from the pulvinar to V1 and V2 in macaques are overlapping in two separate fields that are in register with the visual field maps of P1 and P2. In some but not all cases, an additional projection was found from P3 to V2; we did not observe a reciprocal projection from V2 to P3 in the current cases. MT projecting cells were also found in P1 and P2 but were mainly concentrated in the most medial portion of P3. Adams et al. (2000) also showed an extensive projection to V4 from P2, with sparser projections from P1 and still sparser projections from P3. Our current results showed that V2 projecting neurons terminate in P1, P2, and P4, similar to the projection pattern from V4.
Immunohistochemical studies in macaque, capuchin, and squirrel monkeys have revealed five similar subdivisions of the pulvinar, which include all of the inferior pulvinar and encompass portions of the lateral and medial pulvinar; these have been named PIP, PIM, PIC, PI, and PILS (Soares, Gattass, et al., 2001; Adams et al., 2000; Gray, Gutierrez, & Cusick, 1999; Gutierrez, Yaun, & Cusick, 1995; Cusick, Scripter, Darensberg, & Weber, 1993). The similarities in the chemoarchitectonic subdivisions contrast with the distinct connectivity and the different visuotopic organization found in the pulvinar among these species. In Cebus, Soares, Gattass, et al. (2001) were unable to clearly segregate P1 from P2 based on the connectivity with V1, V2, MT, and V4, despite the chemoarchitectural similarities of the pulvinar of macaques and capuchin monkeys. Areas V2 and V4 in Cebus have preferential connections with P1, which may correspond to the ventrolateral complex of the Cebus (Gattass et al., 1978) and may correspond to both P1 and P2 of Macaca. A similar segregation was described by Stepniewska and Kaas (1997) and Cusick et al. (1993), who also established that the subdivisions of PI that receive ascending connections from the superior colliculus are distinct from the portion of the nucleus that projects to area MT.
Kaas and Lyon (2007) have further proposed that the pulvinar nuclei could be segregated into two groups related to the two streams of visual information processing, namely, the ventral and dorsal streams for object vision and spatial vision, respectively (Ungerleider & Mishkin, 1982). According to this proposal, the pulvinar nuclei provide cortico-pulvinar-cortical interactions that spread information both across areas within each visual stream and across streams as well as relay information from the superior colliculus via P3 to areas belonging primarily to the dorsal stream.
There are two feedforward projections to V2, one from the lateral/inferior pulvinar and the other from V1. Inasmuch as neither the pulvinar nor V2 can be activated visually following V1 removal, either or both of these inputs to V2 could be a driving source (Marion, Li, Purushothaman, Jiang, & Casagrande, 2013). Reversibly inactivating the lateral pulvinar in the Galago, a prosimian primate was found to prevent supragranular V1 neurons from responding to visual stimulation (Purushothaman, Marion, Li, & Casagrande, 2012), while reversible, focal excitation of lateral pulvinar receptive fields increased by fourfold the visual responses in coincident V1 receptive fields and shifted partially overlapping V1 receptive fields toward the center of excitation (Purushothaman et al., 2012). V1 responses to regions surrounding the excited lateral pulvinar receptive fields were suppressed. In addition, the excitation of the lateral pulvinar after later geniculate lesions activated supragranular layer V1 neurons. If these results also hold in other primates, then the lateral pulvinar would be in a powerful position to control and gate information outflow from V1 during changes of state of attention (Purushothaman et al., 2012; Sherman & Guillery, 2002). Consistent with this role of the pulvinar in regulating the effects of spatial attention, deactivation of this portion of the pulvinar causes spatial attention deficits in monkeys (Desimone, Wessinger, Thomas, & Schneider, 1990). Finally, joint recordings in V4 and the lateral pulvinar reveal synchronized activity between the two structures that is modulated by attention (Saalmann & Kastner, 2011).
The Superior Colliculus
The superficial layers of the superior colliculus receive direct retinotopically organized projections from the K and M ganglion cells in the retina, which are restricted to the upper half of the stratum griseum superficiale (Graham, 1982; Ogren & Hendrickson, 1976; Hendrickson, Wilson, & Toyne, 1970). Whereas the projections from V1 to the superior colliculus are similarly restricted to the upper half of the stratum griseum superficiale (Ungerleider et al., 1984), those from extrastriate areas V2, MT, and TEO extend through this stratum to also include the stratum opticum (Webster, Bachevalier, & Ungerleider, 1993; Ungerleider et al., 1984). For both striate and extrastriate areas, projections to the colliculus are in register with the visuotopic organization of the structure (Cynader & Berman, 1972). This was also found to be true for the projections from area V4 (Gattass et al., 2014), which terminated in the same strata as projections from other extrastriate visual areas, namely, the stratum griseum superficiale and the stratum opticum. Inasmuch as visuotopic inputs to the colliculus are superimposed on an oculomotor map (Tabareau et al., 2007; Skaliora, Doubell, Holmes, Nodal, & King, 2004; Wallace, McHaffie, & Stein, 1997; Baleydier & Mauguiere, 1978), it may be that projections from V2 and V4 provide visual feature information, which could trigger orienting oculomotor reactions to spatially localized regions based on unexpected forms, colors, or textures (Zénon & Krauzlis, 2012).
The Claustrum
The claustrum is a thin, irregular, sheet-like neuronal structure hidden beneath the inner surface of the neocortex. Gattass et al. (2014) found extensive reciprocal connections between V4 and the ventral portion of the claustrum (vCl) as well as to a more restricted region located farther dorsal, near the middle of the structure (mCl). These portions of the claustrum appear to overlap considerably with those portions connected with other cortical visual areas, including V1 (Doty, 1983; Mizuno et al., 1981), V2 (Pearson et al., 1982), MT (Maunsell & Van Essen, 1983; Ungerleider et al., 1984), MST and FST (Boussaoud, Desimone, & Ungerleider, 1992), TEO (Webster et al., 1993), and TE (Baizer, Desimone, & Ungerleider, 1993; Webster et al., 1993; Turner, Mishkin, & Knapp, 1980; Kemp & Powell, 1970; Nauta & Whitlock, 1956).
Pearson et al. (1982) injected HRP at the central representation of V2 and found projections to the claustrum. Using tritiated amino acid injections, we did not find these projections. This discrepancy may be because of the inclusion of portions of the central representation of other occipital extrastriate areas, such as V4, in their injections. A systematic study with more sensitive tracers is needed to confirm the existence of this projection.
Central versus Peripheral Visual Field Projections
There is accumulating evidence for differences in the cortical projections of central and peripheral visual field representations (Nakamura et al., 1993; Ungerleider & Desimone, 1986b; Ungerleider & Mishkin, 1982; Zeki, 1969, 1980). Colby et al. (1988) demonstrated direct input to PO from peripheral but not central field representations of V1 and V2. Gattass et al. (1997) also found that the peripheral but not central field of V2 projects directly to PO; injections placed at eccentricities of 30° or greater produced label in PO, but those placed at lesser eccentricities did not. In addition, we found that the peripheral but not central field of V2 projects to areas MST, VIP, and VTF. These projections also arose from the portions of V2 representing eccentricities of 30° or greater. The central–peripheral asymmetry found in these cortical projections finds a parallel in the subcortical projections of V2 to the pulvinar; central injections label P1 and P2, and peripheral injections additionally label P4.
Rosa and Tweedale (2001) argued that the fact that only peripheral V2 sends projections to PO may not be as surprising, as we noted, because in their scheme of extrastriate organization, area PO is the peripheral representation of the area named V6 or DM by different investigators. These authors (Rosa & Tweedale, 2001) presented an alternative scheme for the organization of the areas anterior to V2 dorsally. Despite differences in nomenclature, a comparison of the data on visuotopic organization, myeloarchitecture, and connections of the relevant visual areas with that of area PO from previous studies in Old (Colby et al., 1988) and New World monkeys (Neuenschwander, Gattass, Sousa, & Piñon, 1994) reveals a remarkable degree of similarity. In all animals, a caudal visual area named DM or PO (V6) appears to be important for the detection of coherent patterns of movement across wide regions of the visual field, such as those induced during self-motion, as proposed for PO (Neuenschwander et al., 1994).
Differences between peripheral and central field inputs can be related to differences in magnification factor, in receptive field size, in the extent of the visual field represented, or in the visual processing requirements of an area (see Gattass et al., 1997). As originally noted by Ungerleider (1985), ventral stream areas receive preferential inputs from central field representations, which is consistent with the role of these areas in object vision (Ungerleider & Mishkin, 1982). In contrast, dorsal stream areas receive preferential inputs from peripheral field representations, which is consistent with the role of these areas in spatial vision. This notion is supported by the presence of projections from the central field representations of V2 to P1 and P2 and by projections from peripheral field representations of V2 to P1, P2, and P4. In this context, it is tempting to speculate that P4 of the pulvinar might also play a role in spatial vision.
Acknowledgments
We thank Charles G. Gross for his support during several phases of this work, Juliana Soares for her valuable comments on the manuscript, and John N. Sewell III and Joanna Lawrence for their skillful technical assistance. The injections into V2 were made at the Laboratory of Professor Charles Gross in Princeton, in the late 1970s. L. G. U., R. D., and R. G. planned the experiment and placed the injections, and T. W. G. plotted the data. R. G., R. D., and L. G. U. reviewed the data, prepared the illustrations, and wrote the manuscript. R. D. was supported by EY017292, and R. G. was supported by CNPq and FAPERJ.
Footnotes
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 U.S.C. 105, no copyright protection is available for such works under U.S. law.
REFERENCES
- Adams MM, Hof PR, Gattass R, Webster MJ, Ungerleider LG. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. Journal of Comparative Neurology. 2000;419:377–393. doi: 10.1002/(sici)1096-9861(20000410)419:3<377::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH. The organization of the second visual area (V II) in the owl monkey: A second order transformation of the visual hemifield. Brain Research. 1974;76:247–265. doi: 10.1016/0006-8993(74)90458-2. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Desimone R, Ungerleider LG. Comparison of subcortical connections of inferior temporal and posterior cortex in monkeys. Visual Neuroscience. 1993;10:59–72. doi: 10.1017/s0952523800003229. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. Projections of the ascending somesthetic pathways to the cat superior colliculus visualized by the horseradish peroxidase technique. Experimental Brain Research. 1978;31:43–50. doi: 10.1007/BF00235803. [DOI] [PubMed] [Google Scholar]
- Bender DB. Retinotopic organization of macaque pulvinar. Journal of Neurophysiology. 1981;46:672–693. doi: 10.1152/jn.1981.46.3.672. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Desimone R, Ungerleider LG. Visual topography of area TEO in the macaque. Journal of Comparative Neurology. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Desimone R, Ungerleider LG. Subcortical connections of MST and FST in the macaque. Visual Neuroscience. 1992;9:291–302. doi: 10.1017/s0952523800010701. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Felleman DJ, Newsome WT, Van Essen DC. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Research. 1986;26:63–80. doi: 10.1016/0042-6989(86)90071-4. [DOI] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: A dual tracer study. Journal of Comparative Neurology. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Gottleib DI, Hendrickson AE, Price JL, Woolsey TA. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Research. 1972;37:21–51. doi: 10.1016/0006-8993(72)90344-7. [DOI] [PubMed] [Google Scholar]
- Cragg BG, Ainsworth A. The topography of the afferent projections in the circunstriate cortex of the monkey studied by the Nauta method. Vision Research. 1969;9:733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Scripter JL, Darensberg JG, Weber JT. Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr. Journal of Comparative Neurology. 1993;336:1–30. doi: 10.1002/cne.903360102. [DOI] [PubMed] [Google Scholar]
- Cynader M, Berman N. Receptive-field organization of monkey superior colliculus. Journal of Neurophysiology. 1972;35:187–201. doi: 10.1152/jn.1972.35.2.187. [DOI] [PubMed] [Google Scholar]
- Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. Journal of Physiology (London) 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Wessinger M, Thomas L, Schneider W. Attention control of visual perception: Cortical and mechanisms. Cold Spring Harbor Symposium. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Van Essen DC. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature (London) 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Doty RW. Nongeniculate afferents to striate cortex in macaques. Journal of Comparative Neurology. 1983;218:159–173. doi: 10.1002/cne.902180204. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. The connections of area V4 of macaque monkey extrastriate cortex. Society for Neuroscience Abstract. 1983;9:153. [Google Scholar]
- Felleman DJ, Van Essen DC. Cortical connections of area V3 in macaque extrastriate cortex. Society for Neuroscience Abstract. 1984;10:933. [Google Scholar]
- Fenstemaker SB, Olson CR, Gross CG. Afferent connections of macaque visual areas V4 and TEO. Investigative Ophthalmology Visual Science. 1984;25:213. [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurology Research. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Gattass R, Galkin TW, Desimone R, Ungerleider LG. Subcortical connections of V4 in the macaque. Journal of Comparative Neurology. 2014;522:1–25. doi: 10.1002/cne.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Gross CG. Visual topography of the striate projection zone in the posterior temporal sulcus (MT) of the macaque. Journal of Neurophysiology. 1981;46:621–638. doi: 10.1152/jn.1981.46.3.621. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross CG, Sandell JH. Visual topography of V2 in the macaque. Journal of Comparative Neurology. 1981;201:519–540. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa APB, Covey E. Cortical visual areas of the macaque: Possible substrates for pattern recognition mechanisms. Experimental Brain Research (Supplement) 1986;11:1–20. [Google Scholar]
- Gattass R, Sousa APB, Gross CG. Visuotopic organization and extent of V3 and V4 of the macaque. Journal of Neuroscience. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Mishkin M, Ungerleider LG. Cortical projections of area V2 in the macaque. Cerebral Cortex. 1997;7:110–129. doi: 10.1093/cercor/7.2.110. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa APB, Oswaldo-Cruz E. Single unit response types in the pulvinar of Cebus monkey to multisensory stimulation. Brain Research. 1978;158:75–88. doi: 10.1016/0006-8993(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa APB, Rosa MGP. Visual topography of V1 in the Cebus monkey. Journal of Comparative Neurology. 1987;259:529–548. doi: 10.1002/cne.902590404. [DOI] [PubMed] [Google Scholar]
- Graham J. Some topographical connections of the striate cortex with subcortical structures in Macaca fascicularis. Experimental Brain Research. 1982;47:1–14. doi: 10.1007/BF00235880. [DOI] [PubMed] [Google Scholar]
- Gray D, Gutierrez C, Cusick CG. Neurochemical organization of inferior pulvinar complex in squirrel monkeys and macaques revealed by acetylcholinesterase histochemistry, calbindin and CAT-301 immunostaining, and Wisteria floribunda agglutinin binding. Journal of Comparative Neurology. 1999;409:452–468. doi: 10.1002/(sici)1096-9861(19990705)409:3<452::aid-cne9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Yaun A, Cusick CG. Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. Journal of Comparative Neurology. 1995;363:545–562. doi: 10.1002/cne.903630404. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Wilson ME, Toyne MJ. The distribution of optic nerve fibers in Macaca mulatta. Brain Research. 1970;23:425–427. doi: 10.1016/0006-8993(70)90068-5. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Research Rev. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JM, Powell TPS. The cortico-striate projection in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Bullier J. A double labeling investigation of the afferent connectivity of cortical areas V1 and V2 of the macaque monkey. Journal of Neuroscience. 1985;5:2815–2830. doi: 10.1523/JNEUROSCI.05-10-02815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HGJM, Szwarcbart MK, Mishkin M, Rosvold HE. Occipitotemporal corticocortical connections in the rhesus monkey. Experimental Neurology. 1965;11:245–262. doi: 10.1016/0014-4886(65)90016-6. [DOI] [PubMed] [Google Scholar]
- Lillie RD. Histopathologic technique and practical histochemistry. McGraw Hill; New York: 1965. [Google Scholar]
- Lund JS, Hendrickson AE, Ogren MP, Tobin EA. Anatomical organization of primate visual cortex area VII. Journal of Comparative Neurology. 1991;202:19–45. doi: 10.1002/cne.902020104. [DOI] [PubMed] [Google Scholar]
- Marion R, Li K, Purushothaman G, Jiang Y, Casagrande VA. Morphological and neurochemical comparisons between pulvinar and V1 projections to V2. Journal of Comparative Neurology. 2013;521:813–832. doi: 10.1002/cne.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. Journal of Neuroscience. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N, Uchida K, Nomura S, Nakamura Y, Sugimoto T, Uemura-Sumi M. Extrageniculate projections to the visual cortex in the macaque monkey: An HRP study. Brain Research. 1981;212:454–459. doi: 10.1016/0006-8993(81)90477-7. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Gattass R, Desimone R, Ungerleider LG. The modular organization of projections from areas V1 and V2 to areas V4 and TEO in macaques. Journal of Neuroscience. 1993;13:3681–3691. doi: 10.1523/JNEUROSCI.13-09-03681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ, Whitlock DG. Subcortical projections from the temporal neocortex in Macaca mulatta. Journal of Comparative Neurology. 1956;106:183–212. doi: 10.1002/cne.901060107. [DOI] [PubMed] [Google Scholar]
- Neuenschwander S, Gattass R, Sousa APB, Piñon MCGP. Indentification and visuotopic organization of areas PO and POd in Cebus monkey. Journal of Comparative Neurology. 1994;340:65–86. doi: 10.1002/cne.903400106. [DOI] [PubMed] [Google Scholar]
- Ogren M, Hendrickson A. Pathways between striate cortex and subcortical regions in Macaca mullata and Saimiri sciureus: Evidence for a reciprocal pulvinar connection. Experimental Neurology. 1976;53:780–800. doi: 10.1016/0014-4886(76)90155-2. [DOI] [PubMed] [Google Scholar]
- Olszewski J. The thalamus of the Macaca mulatta (an atlas for use with the stereotaxic instrument) S. Karger; Basel, Switzerland: 1952. [Google Scholar]
- Pearson RC, Brodal P, Gatter KC, Powell TP. The organization of the connections between the cortex and the claustrum in the monkey. Brain Research. 1982;234:435–441. doi: 10.1016/0006-8993(82)90883-6. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: Visual responses and their modulation. Journal of Neurophysiology. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- Purushothaman G, Marion R, Li K, Casagrande VA. Gating and control of primary visual cortex by pulvinar. Nature Neuroscience. 2012;15:905–912. doi: 10.1038/nn.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Research. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Cortical connections of the occipital lobe in the rhesus monkey: Interconnections between areas 17, 18, 19 and the superior temporal sulcus. Brain Research. 1981;212:249–270. doi: 10.1016/0006-8993(81)90461-3. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Sousa APB, Gattass R. Representation of the visual field in the second visual area in the Cebus monkey. Journal of Comparative Neurology. 1988;275:326–345. doi: 10.1002/cne.902750303. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Tweedale R. The dorsomedial visual areas in New World and Old World monkeys: Homology and function. European Journal of Neuroscience. 2001;13:421–427. doi: 10.1046/j.0953-816x.2000.01414.x. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki SM. The organization of connections between areas V5 and V2 in macaque monkey visual cortex. European Journal of Neuroscience. 1989;1:333–354. doi: 10.1111/j.1460-9568.1989.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Skaliora I, Doubell TP, Holmes NP, Nodal FR, King AJ. Functional topography of converging visual and auditory inputs to neurons in the rat superior colliculus. Journal of Neurophysiology. 2004;92:2933–2946. doi: 10.1152/jn.00450.2004. [DOI] [PubMed] [Google Scholar]
- Soares JGM, Botelho EP, Gattass R. Distribution of calbindin, parvalbumin and calretinin in the superior colliculus and lateral geniculate nucleus of Cebus apella monkeys. Journal of Chemical Neuroanatomy. 2001;22:139–146. doi: 10.1016/s0891-0618(01)00123-5. [DOI] [PubMed] [Google Scholar]
- Soares JGM, Gattass R, Sousa APB, Rosa MGP, Fiorani M, Brandão BL. Connectional and neurochemical subdivisions of the pulvinar in Cebus monkeys. Visual Neuroscience. 2001;18:25–41. doi: 10.1017/s0952523801181034. [DOI] [PubMed] [Google Scholar]
- Standage GP, Benevento LA. The organization of connections between the pulvinar and visual area MT in the macaque monkey. Brain Research. 1983;262:288–294. doi: 10.1016/0006-8993(83)91020-x. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Kaas JH. Architectonic subdivisions of the inferior pulvinar in New World and Old World monkeys. Visual Neuroscience. 1997;14:1043–1060. doi: 10.1017/s0952523800011767. [DOI] [PubMed] [Google Scholar]
- Tabareau N, Bennequin D, Berthoz A, Slotine JJ, Girard B. Geometry of the superior colliculus mapping and efficient oculomotor computation. Biological Cybernetics. 2007;97:279–292. doi: 10.1007/s00422-007-0172-2. [DOI] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, Knapp M. Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. Journal of Comparative Neurology. 1980;191:515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG. The corticocortical pathways for object recognition and spatial perception. In: Chagas C, Gattass R, Gross CG, editors. Pattern recognition mechanisms. Political Academy of Sciences; Vatican City: 1985. pp. 21–37. [Google Scholar]
- Ungerleider LG, Desimone R. Projections to the superior temporal sulcus from the central and peripheral field representations of V1 and V2. Journal of Comparative Neurology. 1986a;248:147–163. doi: 10.1002/cne.902480202. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. Journal of Comparative Neurology. 1986b;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R, Galkin TW, Mishkin M. Subcortical projections of area MT in macaque. Journal of Comparative Neurology. 1984;223:368–386. doi: 10.1002/cne.902230304. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Mishkin M. Visuotopic organization of projections from striate cortex to the inferior and lateral pulvinar in rhesus monkey. Journal of Comparative Neurology. 1983;217:135–157. doi: 10.1002/cne.902170203. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- Wallace MT, McHaffie JG, Stein BE. Visual response properties and visuotopic representation in the newborn monkey superior colliculus. Journal of Neurophysiology. 1997;78:2732–2741. doi: 10.1152/jn.1997.78.5.2732. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Subcortical connections of inferior temporal areas TE and TEO in macaque monkeys. Journal of Comparative Neurology. 1993;335:73–91. doi: 10.1002/cne.903350106. [DOI] [PubMed] [Google Scholar]
- Weller RE, Kaas JH. Retinotopic pattern of connections of area 17 with areas V-II and MT in macaque monkeys. Journal of Comparative Neurology. 1983;222:253–279. doi: 10.1002/cne.902200302. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Representation of the central visual field in prestriate cortex of the monkey. Brain Research. 1969;14:271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Cortical projections from two prestriate areas in the monkey. Brain Research. 1971;34:19–35. doi: 10.1016/0006-8993(71)90348-9. [DOI] [PubMed] [Google Scholar]
- Zeki SM. The projections to the superior temporal sulcus from areas 17 and 18 in the rhesus monkey. Proceedings of the Royal Society B. 1976;193:199–207. doi: 10.1098/rspb.1976.0040. [DOI] [PubMed] [Google Scholar]
- Zeki SM. A direct projection from area V1 to area V3A of rhesus monkey visual cortex. Proceedings of the Royal Society B. 1980;207:499–506. doi: 10.1098/rspb.1980.0038. [DOI] [PubMed] [Google Scholar]
- Zeki SM, Shipp S. Modular connections between areas V2 and V4 of macaque monkey visual cortex. European Journal of Neuroscience. 1989;1:494–506. doi: 10.1111/j.1460-9568.1989.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]