Abstract
AMG 386 is an investigational first-in-class peptide-Fc fusion protein (peptibody) that inhibits angiogenesis by preventing the interaction of angiopoietin-1 (Ang1) and Ang2 with their receptor, Tie2. Although the therapeutic value of blocking Ang2 has been shown in several models of tumorigenesis and angiogenesis, the potential benefit of Ang1 antagonism is less clear. To investigate the consequences of Ang1 neutralization, we have developed potent and selective peptibodies that inhibit the interaction between Ang1 and its receptor, Tie2. Although selective Ang1 antagonism has no independent effect in models of angiogenesis-associated diseases (cancer and diabetic retinopathy), it induces ovarian atrophy in normal juvenile rats and inhibits ovarian follicular angiogenesis in a hormone-induced ovulation model. Surprisingly, the activity of Ang1 inhibitors seems to be unmasked in some disease models when combined with Ang2 inhibitors, even in the context of concurrent vascular endothelial growth factor inhibition. Dual inhibition of Ang1 and Ang2 using AMG 386 or a combination of Ang1- and Ang2-selective peptibodies cooperatively suppresses tumor xenograft growth and ovarian follicular angiogenesis; however, Ang1 inhibition fails to augment the suppressive effect of Ang2 inhibition on tumor endothelial cell proliferation, corneal angiogenesis, and oxygen-induced retinal angiogenesis. In no case was Ang1 inhibition shown to (a) confer superior activity to Ang2 inhibition or dual Ang1/2 inhibition or (b) antagonize the efficacy of Ang2 inhibition. These results imply that Ang1 plays a context-dependent role in promoting postnatal angiogenesis and that dual Ang1/2 inhibition is superior to selective Ang2 inhibition for suppression of angiogenesis in some postnatal settings. Mol Cancer Ther; 9(10); 2641–51.
Introduction
Angiogenesis inhibition holds great promise as a strategy to treat diseases in which progression is dependent on neovascularization. These illnesses include cancer, certain ocular diseases (e.g., diabetic retinopathy and age-related macular degeneration), and inflammatory conditions (e.g., rheumatoid arthritis, osteoarthritis, and psoriasis; refs. 1, 2). The four antiangiogenic therapies currently approved by the U.S. Food and Drug Administration [bevacizumab (Avastin), sorafenib (Nexavar), sunitinib (Sutent), and pazopanib (Votrient)] for the treatment of cancer all target vascular endothelial growth factor (VEGF) or its receptors. Despite clinical benefit, resistance is common, and class-specific side effects have been seen (3). These side effects include hypertension, thromboemboli, hemorrhage, gastrointestinal perforations, and wound dehiscence, with some patients experiencing serious or life-threatening events. Although the safety profile of systemically administered VEGF-targeted drugs is acceptable in selected populations of cancer patients, these characteristics are unfavorable for agents to be used in chronic nononcology indications. To avoid the systemic safety issues and maximize drug exposure at the site of action, two VEGF antagonists, ranibizumab (4), and pegaptanib (5), have been developed for local (intravitreal) administration in patients with age-related macular degeneration. However, repeated intraocular injections are required, conferring a risk for endophthalmitis and retinal detachment.
The expanded use of antiangiogenic therapies would be facilitated by improvements in the efficacy and safety of this class of agents. To this end, intervention in other biochemical pathways may be necessary to augment or replace VEGF pathway inhibition in the suppression of neovascularization. One pathway that has received significant attention in this regard is the angiopoietin (Ang) axis. Like the VEGF pathway, the Ang pathway involves a receptor tyrosine kinase, Tie2, the expression of which is restricted to a limited number of cell types, including the vascular endothelium (6). Tie2 binds three known secreted ligands in humans: Ang1, Ang2, and Ang4. Ang2 is upregulated at sites of postnatal angiogenesis, and Ang2 inhibitors suppress angiogenesis and tumor growth in preclinical models, suggesting that Ang2 is a key regulator or mediator of neovessel formation (7). Ang2 is upregulated in the vasculature of many human tumors, and higher levels of Ang2 upregulation have been associated with more advanced disease and poorer prognosis (8–10).
Ang1 plays an important role in developmental angiogenesis (11), but its function in postnatal neovascularization is less clear. Ang1 has been shown to mediate proangiogenic and antiangiogenic effects in various postnatal settings (12). In the first few weeks of rodent postnatal life, systemically administered Ang1 induced widespread circumferential venous enlargement by promoting endothelial cell proliferation (13). In adult rodents, Ang1 made blood vessels resistant to plasma leakage and induced lymphatic sprouting (14, 15). In tumor xenografts, ectopic overexpression of Ang1 enhanced (16, 17) and retarded (18–21) tumor growth. In accompanying tumor angiogenesis studies, Ang1 stimulated (16, 17) and inhibited (18) neovascularization as measured by assessments of vessel numbers (16–18), vessel diameter (19), vessel branching (17), endothelial cell proliferation (18), pericyte coverage (16, 17, 21), and perfusion (17). In a loss-of-function study, stably transfected Ang1 antisense RNA reduced tumor xenograft growth and decreased tumor microvessel density (22). In conditional transgenic mice, Ang1 induction suppressed choroidal and retinal neovascularization (23). Lastly, Ang1 overexpression increased angiogenesis in models of ischemia (24, 25).
The conflicting results of these studies highlight the challenges encountered in trying to draw general conclusions about the role of Ang1 in postnatal angiogenesis. To circumvent the possibility that some of the observed inconsistencies may be a consequence of administering supraphysiologic levels of exogenous Ang1, we chose to investigate the function of Ang1 by inhibiting endogenous Ang1. To that end, we have developed Ang1-neutralizing peptibodies and tested them alone or in combination with Ang2 inhibitors in preclinical models of postnatal angiogenesis.
Determining the consequences of Ang1 neutralization has important therapeutic implications. Four biologics targeting the Tie2 axis are currently under clinical evaluation in oncology (AMG 386, AMG 780, CVX-060, and CVX-241), but only AMG 386 and AMG 780 are dual inhibitors of Ang1 and Ang2. CVX-060 (26) and CVX-241 (http://clinicaltrials.gov/ct2/show/NCT01004822), as well as a preclinical-stage biologic called 3.19.3 (27), have only one Ang target, Ang2 (although CVX-241 also targets VEGF). Of these five agents, the most advanced is AMG 386, an investigational recombinant peptide-Fc fusion protein (peptibody) that reduces tumor angiogenesis by neutralizing Ang1 and Ang2 [Half maximal inhibitory concentration (IC50) of 0.9 and 0.023 nmol/L, respectively; ref. 7]. In preclinical studies, AMG 386 [previously named 2×Con4(C)] blocked tumor endothelial cell proliferation and tumor xenograft growth in mice but had no effect on cultured tumor cells. In addition, AMG 386 suppressed corneal angiogenesis in a VEGF-driven rat model (7). Together, these data are consistent with an antiangiogenic mechanism of action (7). In a first-in-human study in patients with advanced solid tumors, weekly administration of AMG 386 seemed to be well tolerated (a maximum-tolerated dose was not reached; ref. 28). Toxicities typically associated with inhibitors of the VEGF pathway (29) either did not occur (bleeding, thromboembolic events, gastrointestinal perforations) or were not considered to be treatment related. AMG 386 mediated antitumor efficacy and reduced tumor blood flow/permeability (28).Inarandomized phase 2 study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer, dose-dependent increases in progression-free survival and CA-125 response were seen (30). These data suggest that dual inhibition of Ang1 and Ang2 by AMG 386 confers promising clinical activity, but the component of this activity attributable to Ang1 inhibition remains to be evaluated.
Materials and Methods
Phage display selection and characterization of Ang1-binding peptides
A filamentous phage peptide library containing 2.3 × 109 independent transformants (Dyax Corp.) was used to select for Ang1-binding phage. Phage display selection was carried out as described using Ang1 protein-coated 96-well plates (7). Peptide sequences were selected based on enzyme-linked immunosorbent assay (ELISA) results and DNA sequencing of phage clones and expressed in a peptibody format. Peptibodies were evaluated in a homogeneous time-resolved fluorescence assay, and several were chosen for affinity maturation, which was done by generating and panning nucleotide-doped phage display libraries (31). These focused libraries, with >1 × 109 independent transformants, were panned by a procedure similar to that used for panning the primary library.
Peptibody mL4-3 was expressed and purified as described (7). The amino acid sequence of mL4-3 is as follows, in which Fc denotes the human IgG1 Fc sequence as described previously (7): MREWTEEMQVIF-DAMMFGPRNDRGGSGSATGSGSTASSGSGSATHREW-TEEMQVIFDAMMFGPRNDRGGGGG-Fc.
Methodology and results for pharmacokinetic assessments of mL4-3 in CD-1 mice and Sprague-Dawley rats are described in the supplementary data. Because the pharmacokinetic properties of mL4-3, L1-7, and AMG 386 (7) were dissimilar, the dose levels and schedules of each agent were chosen, where possible, to achieve equimolar serum steady-state Cmin concentrations within pharmacology studies.
Ang:Tie2 neutralization homogeneous time-resolved fluorescence assay
The homogeneous time-resolved fluorescence assay used to evaluate Ang:Tie2 neutralization is detailed in the supplementary data. Neutralization potency was determined by calculating the percentage inhibition of each peptibody dilution in reference to the maximum (no Ang in the assay mixture) and minimum inhibition (no peptibody in the assay mixture) controls. IC50 values were calculated by plotting percentage inhibition using XLfit4, in which fit = A + {(B - A) /[1 + (C / X)^D]} (IDBS).
Ang:Tie2 neutralization ELISA
The ELISA was done as described (7) using Ang-coated plates and mL4-3 or Fc serially diluted in a solution of 1 nmol/L Tie2-Fc/1% bovine serum albumin/PBS. Optical density was measured at 650 nmol/L using Spectra-Max plate reader (Molecular Devices), and the degree of Ang:Tie2 neutralization (IC50) was determined by comparison against a Tie2 standard curve (the binding activity of serially diluted Tie2 in the absence of competitor) using XLfit.
Animal studies
All procedures were approved by the Amgen Animal Care and Use Committee and met Association for Assessment and Accreditation of Laboratory Animal Care standards. All animals were purchased from Charles River Laboratories.
Tumor xenograft models
Female nude mice were injected s.c. with 5 × 106 HT-29 or 2 × 106 Colo205 human colon cancer cells mixed with one-third volume Matrigel (BD Biosciences). Once tumors were established, animals were randomly assigned to treatment groups (n = 10 per group except where noted). Tumor measurements and body weights were recorded twice per week. All tumor studies were done in a blinded fashion. Tumor volume was calculated as length × width × height in cubic millimeters. Data were analyzed using repeated measures ANOVA followed by a Scheffé or Dunnett’s post hoc test. Terminal tumor volume assessments were analyzed using ANOVA followed by a Bonferroni-Dunn post hoc test. To assess differences between selective Ang2 inhibition and combined Ang1/2 inhibition across multiple Colo205 studies, two meta-analyses were done using either terminal tumor volumes (terminal-time-point meta-analysis) or all tumor volumes from the initiation to the termination of dosing (all-time-point meta-analysis). The data were log transformed before the statistical analysis for each individual study. For the terminal-time-point meta-analysis, final tumor volumes for each study were analyzed using a one-way ANOVA with group (dual Ang1/2 inhibition, selective Ang2 inhibition, and negative control) as a fixed effect. For the all-time-point meta-analysis, the data were analyzed using a mixed-effect model that included group, day, and the two-way interaction between day and time as fixed effects and subject as a random effect. The random-effects model was used to pool the results from the nine studies in a meta-analysis.
Histologic tumor analyses
Tumor blood vessel area
Blood vessel area assessments were done on Colo205 tumors that were immersion-fixed in cold zinc Tris solution (32) and then paraffin embedded by standard methods (n = 9 or 10 per group). Sections were immunostained for vascular endothelium (anti-CD31 antibody MEC 13.3; BD Biosciences Pharmingen) using 3,3’-diaminobenzidine as the chromogen and lightly counterstained with hematoxylin. The total blood vessel area (square millimeter) for every section was calculated (viable tumor area × respective vessel area fraction). Details are outlined in the supplementary data.
Viable tumor burden
Colo205 tumor viability was determined histologically as described (n = 10 per group; ref. 33). Viable tumor area was analyzed by RGB thresholding (using a Nikon DXM1200 camera mounted on a Nikon FXA compound microscope with a 1× objective or using Aperio virtual slide scanning) and automated pixel counting (Visiopharm Integrator System) and was expressed as a fraction of total tumor area. For each tumor, the tumor burden (gram) was calculated as viable fraction × corresponding terminal tumor weight. All histologic analyses were done in a blinded fashion. Statistical analyses of histologic data were done by ANOVA, followed by a Bonferroni/Dunn post hoc test.
Basement membrane histology
Empty basement membrane sleeves were evaluated as described (34). Briefly, tissues were collected from mice perfused with 1% paraformaldehyde and frozen in Tissue-Tek Optimal Cutting Temperature compound (Sakura Finetek). Cryostat sections (80 µm) were stained for endothelial cells (anti-CD31; Clone 2H8, 1:500; Thermo Scientific) and basement membrane (anti-type IV collagen; 1:8,000; Cosmo Bio Co. Ltd.) and examined using a Zeiss LSM 510 confocal microscope.
Tumor endothelial cell proliferation assay
Tumor endothelial cell proliferation was assayed as described (7). Briefly, when tumors were ~500 mm3 in size, Colo205 tumor-bearing mice (n = 3 per group) were treated s.c. for 3 days with Fc (5.7 mg/kg daily), AMG 386 (6 mg/kg single dose), L1-7(N) (2.2 mg/kg daily), mL4-3 (3.5 mg/kg daily), or L1-7(N) combined with mL4-3 (at the same doses and schedules used in the single-agent groups). Statistical analysis was done using an unpaired t test.
Corneal angiogenesis model
VEGF- and basic fibroblast growth factor (bFGF)-induced angiogenesis studies were done in 8- to 12-week-old female CD rats (n = 8 per group) as described (35). Treatment (i.v.) with Fc (60 mg/kg), L1-7(N) (5 mg/kg), mL4-3 (60 mg/kg), or the combination of L1-7(N) and mL4-3 (at the same doses used in the single-agent groups) was initiated on the day before corneal implantation and continued on days 3 and 6. On day 8, the study was terminated, and the corneas were photographed as described (7). For each corneal image, the number of blood vessels intersecting the midpoint between the implanted disc and the limbus was counted. All evaluations were done in a blinded fashion. Statistical significance was assessed by ANOVA followed by Fisher’s post hoc test.
Retinal neovascularization
Ischemic retinopathy was induced in C57BL/6J mice as described (35) using postnatal day 7 (P7) pups (n = 7 per group). Details are outlined in the supplementary data. Fc control (200 mg/kg), mL4-3 (100 mg/kg), L1-7 (N) (100 mg/kg), or mL4-3/L1-7(N) combination (100 mg/kg each) was administered s.c. daily for 9 days starting on P8. All counts were done in a blinded fashion. Statistical analysis was done by ANOVA followed by Fisher’s post hoc test.
Ovarian follicular angiogenesis
Superovulation was induced in C57BL/6J mice using standard methodology; details are provided in the supplementary data. Fixed sections of ovaries (32) were stained either with hematoxylin and eosin or immunostained for vascular endothelium (anti-CD31; rat anti-mouse monoclonal MEC 13.3). Two replicates of this experiment were done on different days (n = 8 to 10 per group). Statistical analysis was done by ANOVA, followed by Dunnett’s post hoc test.
Evaluation of ovaries and epiphyseal plates in treated rats
Sprague-Dawley rats received 300 mg/kg of AMG 386, L1-7(N), or mL4-3 i.v. twice weekly for 28 days (n = 10 animals per sex per group). At scheduled necropsy, ovaries and epiphyseal plates were sectioned, stained with hematoxylin and eosin, and observed for microscopic changes. All statistical analyses were done using StatView 5.0.1 software (SAS Institute, Inc.).
Results
Identification and characterization of Ang1-selective inhibitors
Ang1-neutralizing peptibodies were generated and one, mL4-3, was chosen for use in these studies. mL4-3 exhibited similar potency against several Ang1 orthologs and displayed >40,000-fold selectivity over Ang2 (Table 1; Supplementary Table S1). The pharmacokinetic parameters of mL4-3 in rodents are shown in Supplementary Table S2. Peptibodies L1-7(N), a potent and selective Ang2 inhibitor, and AMG 386 [also known as 2×Con4 (C)], a dual Ang1/2 inhibitor, have been described (7) and are shown in Table 1. None of these Ang inhibitors had any effect on the proliferation or permeability of cultured human umbilical vein endothelial cells (Supplementary Figs. S1 and S2).
Table 1.
Peptibodies competitively inhibit Ang: Tie2 interactions
| Agent | hAng1 IC50 (nmol/L) | hAng2 IC50 (nmol/L) |
|---|---|---|
| L1-7(N) | >10,000 | 0.064 |
| mL4-3 | 0.022 | 3,085 |
| AMG 386 | 6.2 | 0.029 |
| Fc | >10,000 | >10,000 |
Abbreviation: h, human.
mL4-3 can be used as a reagent for interrogating Ang1 function in vivo
To assess whether mL4-3 was capable of selectively sequestering Ang1 in vivo, mL4-3, L1-7(N), and Fc (negative control) were administered s.c. to mice, followed by an i.v. challenge with recombinant Ang1. Ang1 induced Tie2 phosphorylation in mouse lung endothelium (~5-fold), an effect that could be prevented by mL4-3, but not by L1-7(N) or Fc (Supplementary Fig. S3A).
Next, we wanted to determine whether mL4-3 could neutralize endogenous Ang1 in a setting in which Ang1 was known to play a physiologically relevant role. Developmental genetic knockout studies have shown that Ang1 deletion reduces cardiac size and endocardial folding in embryos (11). E12.5 mouse embryos exposed systemically to mL4-3 at early and middle gestation had reduced cardiac size and trabeculation similar to but less dramatic than that observed in Ang1-null embryos (Supplementary Fig. S3B; Supplementary Table S3). Unlike Ang1 knockout embryos, which display a less complex vascular network than their wild-type counterparts (11), no extracardiac vascular changes were observed by light microscopy in mL4-3 treated embryos (data not shown). The less pronounced phenotype of the mL4-3 treated embryos may have been a consequence of suboptimal embryonic mL4-3 exposures and incomplete Ang1 sequestration. Nonetheless, mL4-3 clearly induced embryonic cardiac defects that phenocopy those of Ang1 genetic knockout mice, confirming the utility of mL4-3 as a reagent for investigating Ang1 function in vivo.
Ang1 antagonism augments Ang2 antagonism in suppressing tumor growth
In a previous report, we showed that systemically administered L1-7(N) and AMG 386 could inhibit the growth of Colo205 tumor xenografts implanted into nude mice (7). In that study, the antitumor effects of AMG 386 and L1-7(N) seemed to be similar, although AMG 386 mediated modestly superior efficacy to that of L1-7(N) (P = 0.006). To confirm that dual Ang1/2 inhibition confers better tumor growth suppression than Ang2 inhibition alone, a similar experiment was done, but this time, groups treated with mL4-3 or a combination of mL4-3 and L1-7(N) were also tested. The AMG 386 treatment group and the mL4-3/L1-7(N) combination treatment group showed comparable antitumor efficacy; moreover, both groups exhibited efficacy superior to that mediated by either L1-7(N) or mL4-3 alone (Fig. 1A). In fact, mL4-3 had no discernable single-agent effect on tumor growth, implying that combining Ang2 antagonism with Ang1 antagonism may have unmasked the antitumor effect of Ang1 inhibition (Fig. 1A). Selective Ang2 inhibitors and dual Ang1/2 inhibitors were tested head-to-head in seven additional Colo205 tumor xenograft studies, and in six of these, the groups treated with the dual inhibitors exhibited smaller terminal tumor volumes than the groups treated with the selective Ang2 inhibitors (ref. 36 and data not shown). In some cases, these differences did not reach statistical significance. However, a meta-analysis comparing terminal tumor volumes over all nine Colo205 tumor xenograft studies showed a highly significant difference between these two treatments (P = 0.0018; 26% greater average tumor growth suppression with dual Ang1/2 inhibition than with Ang2 inhibition alone). A separate meta-analysis of these studies, comparing all tumor volumes from the initiation of dosing to the termination of dosing, yielded similarly significant differences between these two treatments (P < 0.0002). To determine whether these findings were specific to the Colo205 model, we did experiments comparing selective Ang1 inhibition, selective Ang2 inhibition, and dual Ang1/2 inhibition in the HT-29 colon tumor xenograft model. Two representative experiments are shown (Supplementary Fig. S4). Dual Ang1/2 inhibition mediated statistically superior efficacy to selective Ang2 inhibition in one of the two experiments and similar efficacy to Ang2 inhibition in the other experiment. Selective Ang1 inhibition had no activity in any of the HT-29 experiments in which it was tested.
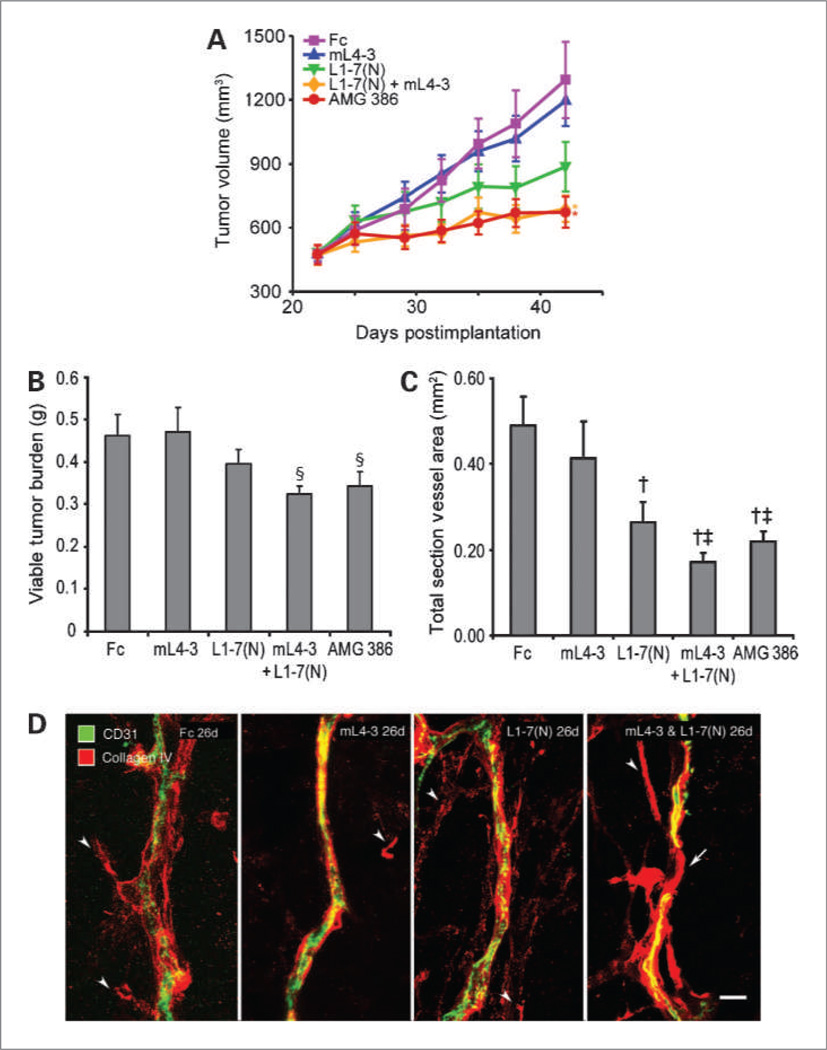
Figure 1.
The effect of combined Ang1 and Ang2 inhibition on the growth of Colo205 tumor xenografts. A, mice implanted with Colo205 cells were treated with either AMG 386 (5.6 mg/kg twice per week), Fc control (5.2 mg/kg), L1-7(N) (2.0 mg/kg), or mL4-3 (3.2 mg/kg) daily; or the combination of L1-7(N) and mL4-3 (at the same dosing regimens used in the single-agent groups). Where necessary, Fc control protein was added to match the total amount of protein delivered in the combination group (5.2 mg/kg). Viable tumor burden (B) and total blood vessel area (C) were determined following 7 days of peptibody administration. Data are mean values ± SE. *, P < 0.05 versus L1-7(N), mL4-3, and Fc; §, P < 0.05 versus Fc; †, P < 0.01 versus Fc; ‡, P = 0.02 versus mL4-3. D, confocal microscopic images of tumor endothelial cells (CD31; green) and basement membrane (type IV collagen; red). After 26 days of treatment with Fc, mL4-3, or L1-7(N), tumor vessels were accompanied by basement membrane with varying abnormalities (arrowheads), but the vessels are continuous. By comparison, a discontinuous (regressing) vessel in a tumor treated with both mL4-3 and L1-7 (N) was observed (right). The region of vessel regression, which lacks endothelial cells, is spanned by an empty basement membrane sleeve (arrow). Scale bar, 15 µm.
Additional Colo205 tumor xenograft studies were done to evaluate the histologic consequences of Ang inhibition. Treatment with AMG 386 or the mL4-3/L1-7(N) combination induced reductions in viable tumor burden (Fig. 1B) and total blood vessel area (Fig. 1C). L1-7(N) tended to show less activity than the dual Ang1/2 inhibitors, and mL4-3 had no statistically significant effects in either assay. To determine whether blood vessel regression occurred, tumors were stained for blood vessels (CD31) and basement membrane (type IV collagen). All treatment groups had some scattered type IV collagen staining not associated with the tumor vessels (Fig. 1D). In the groups treated with Fc, mL4-3, or L1-7(N), the tumor vessels were covered by basement membrane sleeves. After combination treatment with mL4-3 and L1-7(N), however, tumor vascularity was reduced, and empty basement membrane sleeves were present, indicative of vessel regression (34). We attempted to bolster this finding by measuring tumor endothelial cell apoptosis by fluorescence-activated cell sorting (using an antibody against activated caspase-3) and histologically (by TUNEL staining), but there were too few events to reliably detect differences between the treatment groups (data not shown). Tumor vessels are typically leaky, tortuous, and dilated, with loose or absent pericyte associations. Tumor vessel normalization, a reversal of these aberrant features, has been observed with inhibition of the VEGF (37) and Ang (36) pathways. Ang2 inhibition, but not Ang1 inhibition or dual Ang1/2 inhibition, resulted in tumor vessel normalization as evidenced by enhanced pericyte coverage (Supplementary Fig. S5).
We next evaluated the combined effects of inhibiting Ang and VEGF activity. Suboptimal doses of AMG 386 or bevacizumab alone failed to confer significant tumor growth inhibition, but combining the two agents at these dose levels yielded cooperative antitumor activity (Fig. 2A and B). To assess whether dual Ang1/2 inhibition was more efficacious than selective Ang2 inhibition in the context of concurrent VEGF antagonism, optimal doses of L1-7(N) and AMG 386 were combined with the same suboptimal dose of bevacizumab used in Fig. 2A and B. Both combination treatments reduced tumor growth. AMG 386 plus bevacizumab mediated better antitumor activity than L1-7(N) plus bevacizumab over the latter time points of the study (Fig. 2C), and terminal assessment of viable tumor burden substantiated this finding (Fig. 2D). These results confirm that dual Ang1/2 inhibition is superior to selective Ang2 inhibition for suppressing Colo205 tumor growth, regardless of whether VEGF activity is simultaneously antagonized.
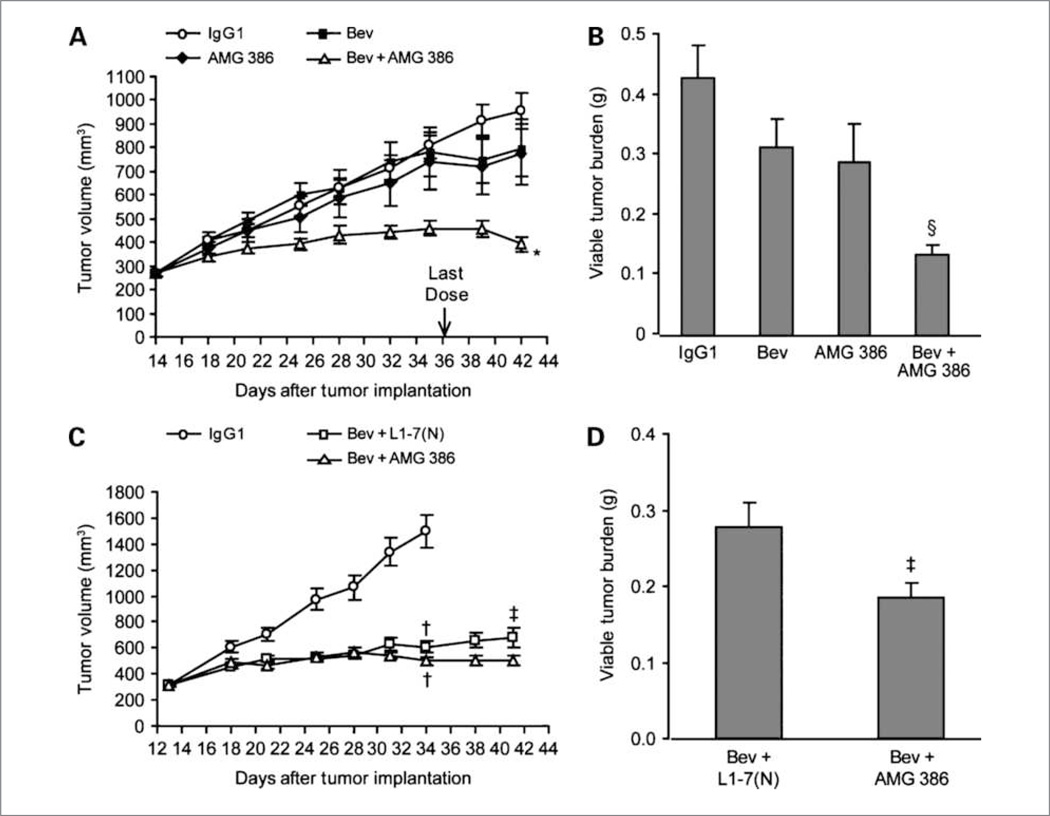
Figure 2.
The effect of combined Ang1 and Ang2 inhibition in the presence of VEGF pathway antagonism. A, mice implanted with Colo205 cells were treated with human IgG1 (0.4 mg/kg, i.p.), bevacizumab (0.4 mg/kg, i.p.), AMG 386 (0.11 mg/kg, s.c.), or the combination of bevacizumab and AMG 386 (at the same dose and schedule as the single-agent groups) twice per week. B, viable tumor burden was determined at the end of the experiment (n = 10). Bev, bevacizumab. C, Colo205 tumor-bearing mice were treated with IgG1 (0.4 mg/kg, i.p.; n = 10) or bevacizumab (0.4 mg/kg, i.p.) in combination with either AMG 386 (2.8 mg/kg, s.c.) or L1-7(N) (2.8 mg/kg, s.c.) twice per week (n = 25 per group). Fc or IgG1 control proteins were added to match the total amount of protein delivered in the combination group in both experiments. D, viable tumor burden (n = 23–25). Data are mean values ± SE. *, P < 0.0001 versus bevacizumab or AMG 386; §, P < 0.05 versus bevacizumab or AMG 386; †, P ≤ 0.05 versus IgG1; ‡, P < 0.05 versus bevacizumab + L1-7(N) at terminal measurement.
Ang2 antagonism, but not Ang1 antagonism, inhibits tumor endothelial cell proliferation, corneal angiogenesis, and retinal angiogenesis
We previously showed that dual Ang1/2 inhibition suppressed Colo205 tumor endothelial cell proliferation in vivo (7). To investigate whether this effect was conferred through Ang1 inhibition, Ang2 inhibition, or a combination of the two, Colo205 tumor-bearing mice were treated with mL4-3, L1-7(N), mL4-3/L1-7(N), or AMG 386. As with the tumor volume results described in the previous section, mL4-3 had no single-agent effect on tumor endothelial cell proliferation, whereas L1-7(N) was inhibitory (Fig. 3A). Dual Ang1/2 inhibition conferred no greater effect on endothelial cell proliferation than Ang2 inhibition alone (Fig. 3A) in contrast to the apparently cooperative effects of combined Ang1/2 inhibition on Colo205 tumor growth (Fig. 1). This dissimilarity implies that repression of endothelial cell proliferation is only one component underlying the tumor growth inhibition mediated by Ang antagonism.
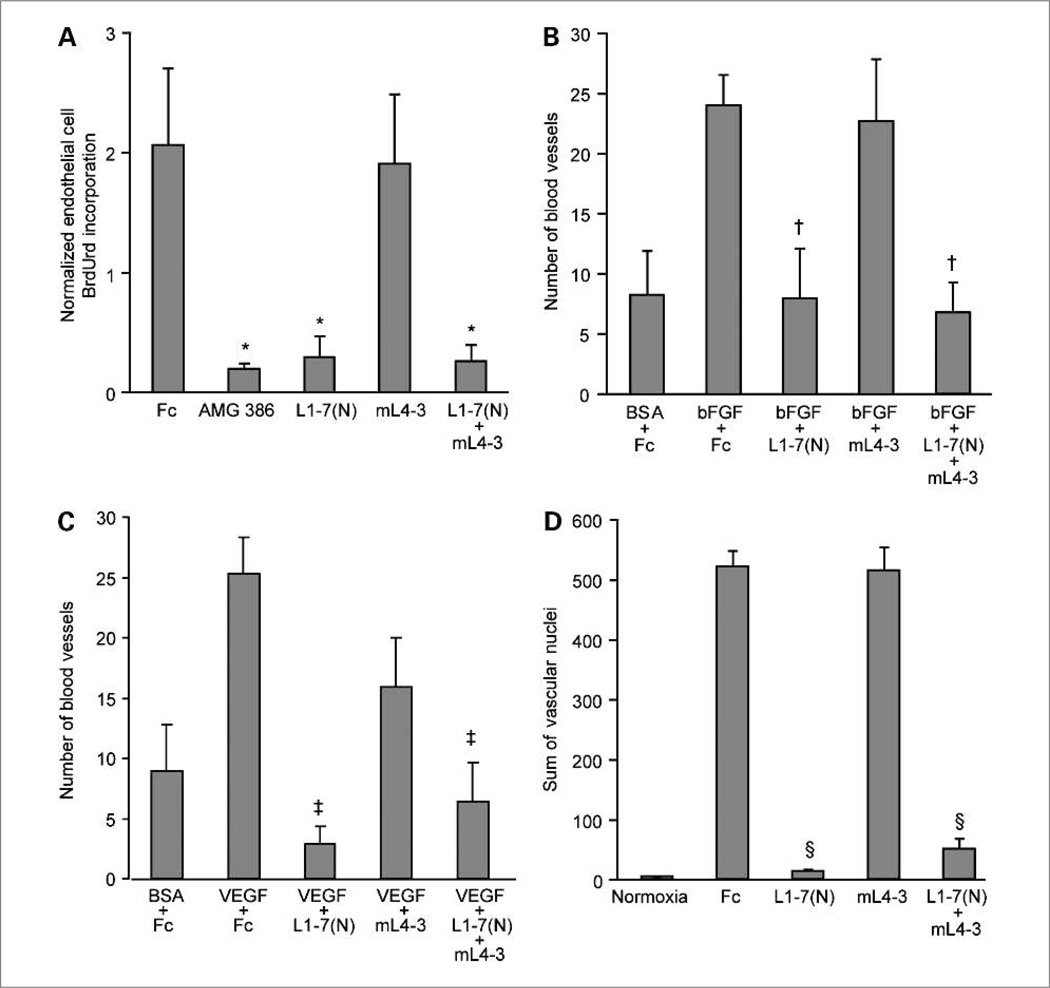
Figure 3.
The effect of Ang1 and Ang2 antagonism on tumor endothelial cell proliferation, corneal angiogenesis, and retinal angiogenesis. A, BrdUrd incorporation in endothelial cells. Colo205 tumor-bearing mice were treated with Fc, AMG 386, L1-7(N), mL4-3, or L1-7(N) plus mL4-3. Bar, mean endothelial:total mouse cell BrdUrd ratios. Data are mean values ± SE. *, P < 0.05 versus Fc. The effect of inhibition of Ang1 and Ang2 on bFGF-induced (B) and VEGF-induced (C) corneal angiogenesis following treatment with Fc, L1-7(N), mL4-3, and L1-7(N) plus mL4-3. Data are mean values ± SE. †, P < 0.002 versus Fc + bFGF; ‡, P < 0.01 versus Fc + VEGF. D, inhibition of Ang2 prevents oxygen-induced neovascularization in the mouse retina. Pups were treated with Fc, L1-7(N), mL4-3, or L1-7(N) plus mL4-3. Data are mean values ± SE. §, P < 0.0001 versus Fc.
These agents were next tested in models of angiogenesis in the cornea and the retina. The cornea is normally avascular, but pathologic angiogenesis can occur in the cornea secondary to conditions such as keratitis and corneal transplant rejection (38). VEGF- and bFGF-induced models of corneal angiogenesis (35) were used to test the roles of Ang1 and Ang2 antagonism in blood vessel formation. As observed with endothelial cell proliferation, corneal angiogenesis seemed to be dependent on Ang2, but not on Ang1 (Fig. 3B and C). The same conclusion could be drawn from evaluation of these Ang-antagonizing peptibodies in a Tie2-dependent retinal model of angiogenesis (39) in which neovascularization was induced by changes in ambient oxygen tension (Fig. 3D). Thus, in three pre-clinical settings (endothelial cell proliferation, corneal angiogenesis, and retinal angiogenesis), Ang2 inhibition dramatically suppressed neovessel formation, whereas Ang1 inhibition had no effect alone or in combination with Ang2 inhibition.
Selective inhibition of Ang1 or Ang2 induces ovarian atrophy, but not epiphyseal plate thickening
To assess the effects of Ang inhibition in normal animals, rats were treated systemically with mL4-3, L1-7 (N), or AMG 386 for 1 month. AMG 386, like VEGF antagonists, has been observed to induce epiphyseal plate thickening and ovarian atrophy, effects considered to be mechanism-based consequences of antiangiogenic therapy (7, 40). In the present study, AMG 386 provoked epiphyseal plate thickening in all treated animals, whereas remarkably, L1-7(N) and mL4-3 failed to alter epiphyseal morphology in any of the animals (Supplementary Table S4). Thus, induction of epiphyseal plate thickening seems to require inhibition of Ang1 and Ang2. In striking contrast, all three peptibodies produced ovarian atrophy at similar incidence rates, indicating that selective inhibition of Ang1 or Ang2 is sufficient to induce ovarian atrophy.
Ang1 and Ang2 inhibitors cooperatively suppress ovarian follicular angiogenesis
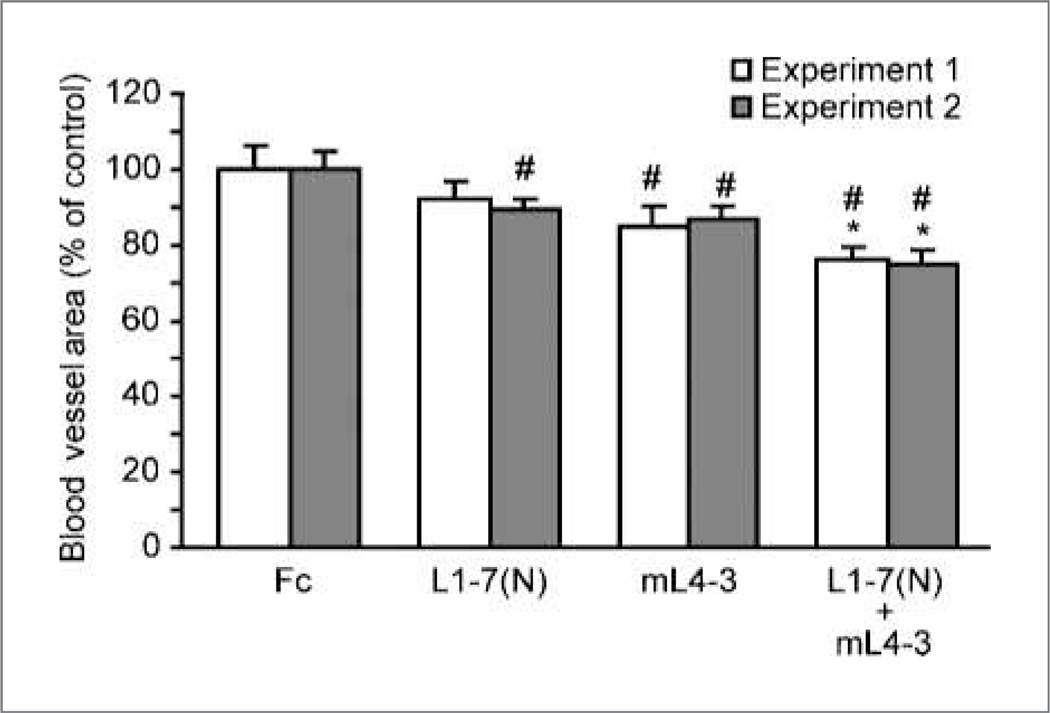
To better understand the effects of Ang inhibition on the ovary, we used a hormone-induced model of ovarian follicular angiogenesis that allowed controlled assessment of neovascularization in mice that had never previously ovulated. Two replicates of this experiment yielded almost identical activity profiles with respect to percentage reduction in blood vessel area fraction (replicate 1, replicate 2): L1-7(N) (8%,11%), mL4-3 (15%, 14%), and mL4-3/L1-7(N) (24%, 26%). All single-agent and combination peptibody groups, with the exception of the L1-7 (N) group in experiment 1, mediated statistically significant inhibition of angiogenesis relative to the Fc control (P < 0.05; Fig. 4). Thus, the inhibition of ovarian angiogenesis and induction of ovarian atrophy could be elicited by inhibiting Ang1, Ang2, or both, consistent with the notion that the ovarian atrophy was a consequence of failed neovessel development.
Figure 4.
Ang1 and Ang2 inhibitors cooperatively suppress ovarian follicular angiogenesis. Fc, mL4-3, L1-7(N), or an mL4-3/L1-7(N) combination were administered to superovulated mice (two independent experiments). Data are mean values ± SE. *, P = 0.005 comparing mL4-3/L1-7(N) combination versus either single-agent alone; #, P < 0.05 versus Fc.
Discussion
We describe here the generation and evaluation of the first systemically administrable pharmacologic agents capable of selectively inhibiting Ang1 in vivo. We show that Ang1 inhibition plays a context-dependent role in the suppression of angiogenesis in preclinical disease models and in normal animals. In utero, pharmacologic Ang1 inhibition substantially phenocopied the genetic ablation of Ang1, consistent with the important role of Ang1 in developmental angiogenesis (11). Postnatally, selective Ang1 antagonism inhibited ovarian angiogenesis and induced ovarian atrophy, effects that could also be achieved by inhibiting Ang2 alone or Ang1 plus Ang2 together. However, in postnatal disease models, Ang1 inhibition had little effect on its own, although its biological activity seemed to be unmasked in some settings when combined with Ang2 suppression. The mechanism underlying the differential dependency on Ang1 in these settings remains to be determined.
It is intriguing that the ovary was the only organ in which a biological consequence of selective Ang1 inhibition was observed postnatally. The ovary, by virtue of its role in reproductive cycling, is one of the few organs that undergo normal angiogenesis in adults. Based upon an extensive analysis of ovarian expression patterns of Ang1 and Ang2 in hormone-induced ovulating rats, Ang2 is thought to play two distinct roles at separate phases of follicular development: (a) preovulatory follicles and (b) aging corpora lutea (41). In preovulatory follicles, Ang2 expression is increased around blood vessels of the theca interna. Because Ang1 expression is subsequently increased around blood vessels in the early corpus luteum, Ang2 and Ang1 are hypothesized to have opposing functions, in which Ang2 initially displaces Ang1 from Tie2, resulting in vessel destabilization and angiogenesis. The temporal expression patterns imply that this state of plasticity is later reversed when Ang1 ousts Ang2 from the receptor to re-establish vascular quiescence and stability. In conflict with this model, the data from the current study imply that Ang1 and Ang2 play proangiogenic roles in the ovary.
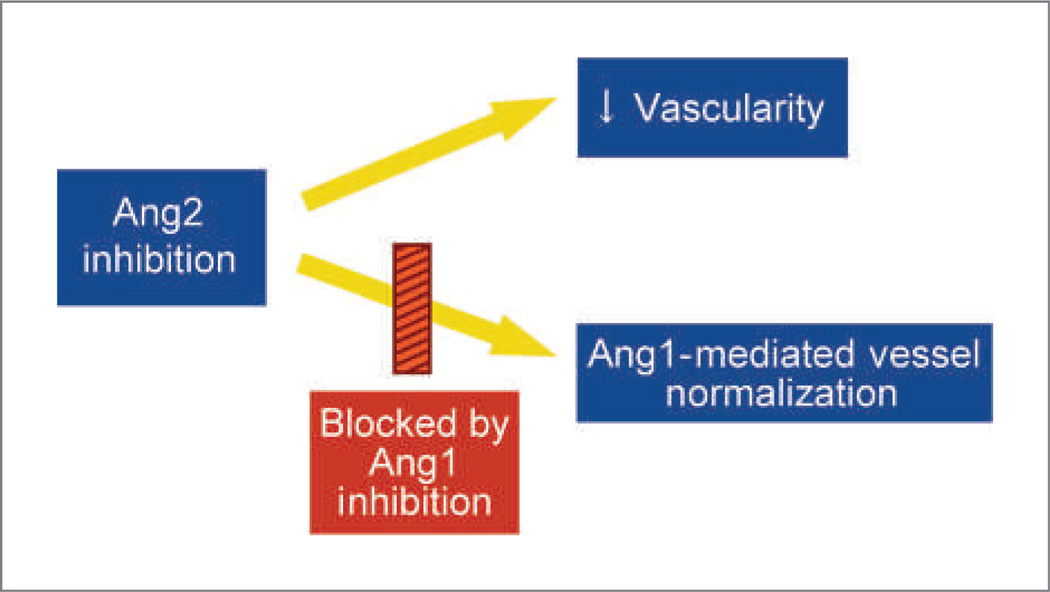
In the Colo205 tumor xenograft model, the antagonism of Ang1 and Ang2 either by combining individual inhibitors or by using AMG 386 mediated greater tumor suppression than was achieved by inhibiting Ang1 or Ang2 individually, indicating that this tumor model is dependent on both angiopoietins. A potential mechanism through which inhibition of Ang1 and Ang2 could cooperatively suppress tumor growth has recently been identified (36). In the Colo205 tumor xenograft model, Ang2 inhibition mediated two vascular outcomes: (a) reduction in the total number of tumor vessels and (b) normalization of the remaining tumor vessels (Fig. 5). Selective Ang1 inhibition had no vascular effect. However, in combination with Ang2 inhibition, Ang1 neutralization prevented Ang2 inhibitor-mediated vessel normalization without antagonizing the antivascular effects of Ang2 inhibition. Normalization is thought to hinder vessel regression (42), which may prevent selective Ang2 inhibitors from reaching their full antitumor potential. Consistent with this concept, dual Ang1/2 inhibition induced vessel regression, whereas selective Ang2 inhibition did not. Thus, combining Ang1 inhibition with Ang2 inhibition may counteract a disadvantageous consequence of selective Ang2 suppression (vessel normalization) while maintaining or enhancing the beneficial antivascular effects of Ang2 antagonism.
Figure 5.
Mechanistic consequences of Ang2 and Ang1 inhibition in the Colo205 tumor model.
In contrast to the subtle and context-dependent effects of Ang1 inhibition, Ang2 inhibition frequently mediated effects that were equivalent or nearly equivalent to those conferred by combined antagonism of Ang1 and Ang2, implying that Ang2 may be the dominant Ang involved in postnatal angiogenesis. Ang1 seems to be the dominant Ang involved in prenatal angiogenesis (11, 43), suggesting a shift in the dependency on these two factors around the time of birth.
Ang1 and Ang2 have been shown to play similar and opposing functional roles in various in vitro and in vivo systems (18, 41, 44–47). The inability to draw consistent conclusions from these studies may be in part a consequence of differing experimental conditions. These differences include (a) in vitro versus in vivo systems, (b) prenatal versus postnatal angiogenesis, (c) varying vascular beds, (d) pathologic versus normal angiogenesis, and (e) gain-of-function versus loss-of-function experimental designs. This final difference may be particularly important because the addition of exogenous factors to a model system may be a less physiologically relevant means to elucidate function than removal of endogenous factors. Perhaps the most informative published experiments in this regard are those in which Ang1 and Ang2 have been genetically deleted in the germline of rodents (11, 41, 48). These studies provide significant insight into the developmental roles of Ang1 and Ang2. However, it is more difficult to genetically examine the postnatal in vivo function of Ang1 and Ang2 without the availability of conditional knockout systems; the constitutive Ang1 knockout mouse dies in utero (as does the constitutive Ang2 knockout on some strain backgrounds; refs. 11, 41), and the postnatal pheno-type of surviving Ang2 knockout mice may be influenced by residual effects of developmental gene deletion. By using pharmacologic Ang1 and Ang2 inhibitors to examine the postnatal roles of Ang1 and Ang2 in vivo, we have circumvented these issues. The results of the current study show that Ang1 neutralization does not counteract and, in some cases augments, the efficacy mediated by Ang2 neutralization.
Pathologic angiogenesis is associated with altered Ang levels in a number of diseases, including cancer, diabetic retinopathy, macular degeneration, rheumatoid arthritis, osteoarthritis, and psoriasis (1). Ang-targeted interventions in these therapeutic indications may provide clinical benefit alone or in combination with VEGF pathway antagonists. The data presented here suggest that, in some settings, combined inhibition of Ang1 and Ang2, as achieved with AMG 386, may provide superior therapeutic efficacy to that mediated by targeting Ang2 alone. Furthermore, this enhanced activity is also observed in the presence of concurrent VEGF pathway antagonism. In early clinical studies, AMG 386 has shown promising antitumor activity, as well as pharmacodynamic activity. In the first-in-human study in patients with advanced solid tumors, AMG 386 was well tolerated and showed a toxicity profile that did not include treatment-related adverse events typically associated with angiogenesis inhibitors targeting the VEGF pathway (28). Two additional phase 1 studies of AMG 386 in combination with VEGF pathway inhibitors (49) or various chemotherapy regimens (50) showed AMG 386 to be well tolerated with evidence of antitumor efficacy. A randomized phase 2 study of AMG 386 combined with paclitaxel in patients with recurrent ovarian cancer yielded dose-dependent increases in progression-free survival and CA-125 response (30). Additional controlled studies of AMG 386 in cancer and other angiogenesis-associated diseases will further inform the potential clinical benefit to patients.
Supplementary Material
Acknowledgments
We thank Drs. Glenn Begley, David Weinreich, and Beate Quednau for their critical reading of the manuscript; Dr. Julia Gage, Jennifer Keysor, and Heather Hartley for manuscript preparation; Michael Mann for technical assistance; and Lei Zhou for statistical analysis.
Grant Support
Commercial research grant from Amgen, Inc. (B.L. Falcón and D.M. McDonald), NIH grants HL24136 and HL59157 from the National Heart, Lung and Blood Institute and CA82923 from the National Cancer Institute (D.M. McDonald).
Footnotes
Note: Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
All authors (with the exception of B.L. Falcón and D.M. McDonald) are current or former employees of Amgen, Inc.
References
- 1.Perry BN, Arbiser JL. The duality of angiogenesis: implications for therapy of human disease. J Invest Dermatol. 2006;126:2160–2166. doi: 10.1038/sj.jid.5700462. [DOI] [PubMed] [Google Scholar]
- 2.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Cruijsen H, van der Veldt A, Hoekman K. Tyrosine kinase inhibitors of VEGF receptors: clinical issues and remaining questions. Front Biosci. 2009;14:2248–2268. doi: 10.2741/3377. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 6.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Oliner J, Min HS, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–516. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. 1999;155:1967–1976. doi: 10.1016/S0002-9440(10)65515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. [print]. [PubMed] [Google Scholar]
- 10.Sfiligoi C, de Luca A, Cascone I, et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003;103:466–474. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 11.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 12.Yu Q. The dynamic roles of angiopoietins in tumor angiogenesis. Future Oncol. 2005;1:475–484. doi: 10.2217/14796694.1.4.475. [DOI] [PubMed] [Google Scholar]
- 13.Thurston G, Wang Q, Baffert F, et al. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–3326. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- 14.Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 15.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 16.Shim WSN, Teh M, Bapna A, et al. Angiopoietin 1 promotes tumor angiogenesis and tumor vessel plasticity of human cervical cancer in mice. Exp Cell Res. 2002;279:299–309. doi: 10.1006/excr.2002.5597. [DOI] [PubMed] [Google Scholar]
- 17.Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165:1557–1570. doi: 10.1016/S0002-9440(10)63413-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad SA, Liu WB, Jung YD, et al. The effects of angiopoietin-1 and −2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001;61:1255–1259. [PubMed] [Google Scholar]
- 19.Tian S, Hayes AJ, Metheny-Barlow LJ, Li LY. Stabilization of breast cancer xenograft tumour neovasculature by angiopoietin-1. Br J Cancer. 2002;86:645–651. doi: 10.1038/sj.bjc.6600082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes AJ, Huang WQ, Yu J, et al. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83:1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawighorst T, Skobe M, Streit M, et al. Activation of the Tie2 receptor by angiopoietin-1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am J Pathol. 2002;160:1381–1392. doi: 10.1016/S0002-9440(10)62565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim WS, Teh M, Mack PO, Ge R. Inhibition of angiopoietin-1 expression in tumor cells by an antisense RNA approach inhibited xenograft tumor growth in immunodeficient mice. Int J Cancer. 2001;94:6–15. doi: 10.1002/ijc.1428. [DOI] [PubMed] [Google Scholar]
- 23.Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11:865–873. doi: 10.1038/sj.gt.3302230. [DOI] [PubMed] [Google Scholar]
- 24.Shyu KG, Manor O, Magner M, Yancopoulos GD, Isner JM. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation. 1998;98:2081–2087. doi: 10.1161/01.cir.98.19.2081. [DOI] [PubMed] [Google Scholar]
- 25.Zhou YF, Stabile E, Walker J, et al. Effects of gene delivery on collateral development in chronic hypoperfusion: diverse effects of angiopoietin-1 versus vascular endothelial growth factor. J Am Coll Cardiol. 2004;44:897–903. doi: 10.1016/j.jacc.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Do J, Li L, et al. Angiopoietin-2 specific CVX-060 inhibits tumor growth cooperatively with chemotherapy. 99th Annual Meeting of the American Association for Cancer Research; 2008 Apr 12–16; San Diego, CA. 2008. p. Abstract 2493. [Google Scholar]
- 27.Brown JL, Cao ZA, Pinzon-Ortiz M, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther. 2010;9:145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS, Hong D, Chap L, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–3565. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 29.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42:3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Karlan BY, Oza AM, Hansen VL, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients (pts) with recurrent ovarian carcinoma [abstract 5000] J Clin Oncol. 2010;28(Suppl):15s. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 31.Lowman HB. Bacteriophage display and discovery of peptide leads for drug development. Annu Rev Biophys Biomol Struct. 1997:26. doi: 10.1146/annurev.biophys.26.1.401. [DOI] [PubMed] [Google Scholar]
- 32.Beckstead JH. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cyto-chem. 1994;42:1127–1134. doi: 10.1177/42.8.8027531. [DOI] [PubMed] [Google Scholar]
- 33.Coxon A, Bush T, Saffran D, et al. Broad antitumor activity in breast cancer xenografts by motesanib, a highly selective, oral inhibitor of vascular endothelial growth factor, platelet-derived growth factor, and Kit receptors. Clin Cancer Res. 2009;15:110–118. doi: 10.1158/1078-0432.CCR-08-1155. [DOI] [PubMed] [Google Scholar]
- 34.Inai T, Mancuso M, Hashizume H, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coxon A, Bolon B, Estrada J, et al. Inhibition of interleukin-1 but not tumor necrosis factor suppresses neovascularization in rat models of corneal angiogenesis and adjuvant arthritis. Arthritis Rheum. 2002;46:2604–2612. doi: 10.1002/art.10546. [DOI] [PubMed] [Google Scholar]
- 36.Falcón BL, Hashizume H, Koumoutsakos P, et al. Contrasting actions of selective inhibitors of angiopoietins-1 and −2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 38.Cursiefen C, Hofmann-Rummelt C, Kuchle M, Schlotzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultra-structural study with clinicopathological correlation. Br J Ophthalmol. 2003;87:101–106. doi: 10.1136/bjo.87.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P. Angiopoietin/Tek interactions regulate MMP-9 expression and retinal neovascularization. Lab Invest. 2003;83:1637–1645. doi: 10.1097/01.lab.0000097189.79233.d8. [DOI] [PubMed] [Google Scholar]
- 40.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation [comment] Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 41.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 42.Bicknell R, Harris AL. Anticancer strategies involving the vasculature: vascular targeting and the inhibition of angiogenesis. Semin Cancer Biol. 1992;3:399–407. [PubMed] [Google Scholar]
- 43.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 44.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 45.Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 46.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 47.Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endo-thelial growth factor (VEGF) Proc Natl Acad Sci U S A. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasarre P, Thomas M, Kruse K, et al. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 2009;69:1324–1333. doi: 10.1158/0008-5472.CAN-08-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong D, Gordon M, Appleman L, et al. From a phase 1b study of safety, pharmacokinetics and tumor response of the angiopoietin1/2-neutralizing peptibody AMG 386 in combination with AMG 706 (motesanib), bevacizumab or sorafenib in advanced solid tumors. Ann of Oncol. 2008;19 Abstract 462D. [Google Scholar]
- 50.Mita AC, Wang D, Takimoto CH, et al. AMG 386, a selective angiopoietin 1/2-neutralizing peptibody, in combination with chemotherapy in adult patients with advanced solid tumors. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25 Abstract 14033. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.