Abstract
Substantial progress in understanding mechanisms of immune regulation in allergy, asthma, autoimmune diseases, tumors, organ transplantation and chronic infections has led to a variety of targeted therapeutic approaches. Allergen-specific immunotherapy (AIT) has been used for 100 years as a desensitizing therapy for allergic diseases and represents the potentially curative and specific way of treatment. The mechanisms by which allergen-AIT has its mechanisms of action include the very early desensitization effects, modulation of T- and B-cell responses and related antibody isotypes as well as inhibition of migration of eosinophils, basophils and mast cells to tissues and release of their mediators. Regulatory T cells (Treg) have been identified as key regulators of immunological processes in peripheral tolerance to allergens. Skewing of allergen-specific effector T cells to a regulatory phenotype appears as a key event in the development of healthy immune response to allergens and successful outcome in AIT. Naturally occurring FoxP3+ CD4+CD25+ Treg cells and inducible type 1 Treg (Tr1) cells contribute to the control of allergen-specific immune responses in several major ways, which can be summarized as suppression of dendritic cells that support the generation of effector T cells; suppression of effector Th1, Th2 and Th17 cells; suppression of allergen-specific IgE, and induction of IgG4; suppression of mast cells, basophils and eosinophils and suppression of effector T cell migration to tissues. New strategies for immune intervention will likely include targeting of the molecular mechanisms of allergen tolerance and reciprocal regulation of effector and regulatory T cell subsets.
Introduction
The immune system forms an interactive network with tissues and makes it’s decisions on the basis of signals coming from resident tissue cells, infectious agents, commensal bacteria and almost any environmental agents. Our research during the last years has focused on different aspects for the development of novel concepts on how the immune system tolerates allergens, and how allergic diseases should be redefined accordingly [1-29]. In recent years, induction of immune tolerance has become a prime target for prevention and treatment strategies for many diseases in which dysregulation of the immune system plays an important role [30]. Currently, allergen-specific immunotherapy (AIT) is mainly applied subcutaneously or sublingually and is suitable for both children and adults for pollen, pet dander, house dust mite, and venom allergies [31-34]. It not only affects rhinoconjunctival symptoms but also has documented short- and long-term benefits in asthma treatment. The disease modification effects of AIT leads to decreased disease severity, less drug usage, prevention of future allergen sensitizations, and a long-term curative effect. Increasing safety while maintaining or even augmenting efficiency is the main goal of research for novel vaccine development and improvement of treatment schemes in AIT [32-34].
Immune tolerance to allergens can be defined as establishment of a long-term clinical tolerance against allergens, which immunologically implies changes in memory type allergen-specific T and B cell responses as well as mast cells and basophil activation thresholds that do not cause allergic symptoms anymore [35-39]. In addition, prevention of new allergen sensitizations [40] and progression to more severe disease, such as development of asthma [41] after allergic rhinitis or development of systemic anaphylaxis are main clinical implications of immune tolerance [42-46]. Many different ways of treatments are being pursued to improve efficacy, decrease side effects, decrease long course of treatment and increase patient compliance [47-51]. Currently pursued novel approaches are epicutaneous AIT and combination of peptides of grass pollen allergens with hepatitis B virus Pre S protein and peptide immunotherapy with short and long T cell epitope petides and intralymphatic immunotherapy [52-55]. Studies to provide prophylactic usage are also being performed [56]. Many efforts are being performed for the improvement and standardization of conventional subcutaneous and sublingual AITs as well as oral immunotherapy of food allergy from patient selection, to vaccine applications and treatment schedules [57-63].
The immunologic basis of allergic diseases is observed in two phases: sensitization and development of memory T and B cell responses and IgE (early phase), and effector functions related to tissue inflammation and injury (late phase) [37]. The differentiation and clonal expansion of allergen-specific CD4+ Th2 cells producing IL-4 and IL-13 are essential to induce class switching to the ε immunoglobulin heavy chain in B cells and the production of allergen-specific IgE antibodies during the sensitization phase. Allergen-specific IgE binds to the high-affinity FcεRI on the surface of mast cells and basophils, thus leading to the patient’s sensitization [64]. When a new encounter with the allergen causes cross-linking of the IgE-FcεRI complexes on sensitized basophils and mast cells, they are activated and subsequently release of anaphylactogenic mediators responsible for the classical symptoms of the immediate phase (type 1 hypersensitivity).
Depending on the innate immune response activating capacity of the substances co-exposed with the antigen, co-signals for cell differentiation and status of the cells and cytokines in the microenvironment, CD4+ naive T cells can differentiate into Th1, Th2, Th9, Th17 or Th22 type memory and effector cells. Based on their respective cytokine profiles, responses to chemokines and interactions with other cells, these T-cell subsets can promote different types of inflammatory responses. During the development of allergic disease, effector Th2 cells produce IL-4, IL-5, IL-9, IL-13 [35-37,65,66] and probably other recently identified cytokines such as IL-25, IL-31, IL-33 mainly secreted from epithelial cells and dendritic cells contribute to Th2 responses [67-73]. These cytokines play a role in the production of allergen-specific IgE, eosinophilia, permissiveness of endothelium for the recruitment of inflammatory cells to inflamed tissues, production of mucus and decreased threshold of contraction of smooth muscles [74]. The commonly observed Th2 profile in atopic diseases might be a result of a) increased differentiation and clonal expansion of Th2 cells [75] or b) increased tendency to activation-induced cell death of high IFN-γ-producing Th1 cells [76]. Th1 cells also efficiently contribute to the effector phase in allergic diseases with their role in apoptosis of the epithelium in asthma and atopic dermatitis [77-79], and apoptosis of smooth muscle cells in fatal asthma [80].
The discovery of the Th17 cells is filling an essential gap in our understanding of inflammatory processes. Th17 cells are characterized by IL-17A, IL-17 F, IL-6, IL-8, TNF-α, IL-22 and IL-26 expression [81-87]. Neutralization of IL-17 and Th17-related functions resolves tissue pathology in autoimmunity models, reduces joint destruction in experimental arthritis and reduces neutrophil infiltration in an experimental asthma model, while increasing eosinophil infiltration [88-91]. It was shown in two recent studies that TGF-β in the presence of IL-4 reprograms Th2 cell differentiation and leads to the development of a new population of Th9 cells that produce IL-9 and IL-10 [92,93].
T cell subset known as Th22 cells has been demonstrated in T cells that independently express IL-22 with low expression levels of IL-17 and play a role in atopic dermatitis [94]. All these T subsets and related events represent targets in the treatment of allergic diseases and the induction of Treg cells and allergen tolerance can balance their over activation.
The pivotal role of Treg cells in inducing and maintaining immune tolerance has been demonstrated during the last 15 years, where their adoptive transfer was shown to prevent or cure several T-cell mediated disease models, including asthmatic lung inflammation, autoimmune diseases and allograft rejection [95]. In the clinical setting, both injection and sublingual versions of AIT have been shown to induce allergen-specific Treg cells in humans. In addition to Treg cells, several other factors appear to play a mechanistic role in AIT. It is now essential to identify biomarkers and predict the response to AIT. Novel understanding of disease endotypes will help to further develop this concept [96]. Novel developments in molecular biology such as microRNAs may provide novel targets [97]. miRNAs function together with partner proteins and mainly cause gene silencing through degradation of target mRNAs or inhibition of translation. A particular miRNA can have hundreds of target genes including interleukins and their co expressed pro or anti-inflammatory genes, and thereby influence the expression of a large proportion of proteins [97-100].
Molecular and cellular events in AIT and their underlying mechanisms
Very early mast cell and basophil suppression-related desensitization effect
Although decreases in IgE antibody levels and IgE-mediated skin sensitivity normally requires years of AIT, most patients are protected against bee stings or tolerate skin late phase response challenges at early stages of respective venom or grass pollen SITs [101,102]. An important observation starting from the first injection is an early decrease in mast cell and basophil activity for degranulation and systemic anaphylaxis (Figure 1). There is surprisingly little information about the mechanisms by which AIT modifies and/or suppresses immune responses of basophils and mast cells, in particular during repetitive administration of increasing doses of allergens within the first hours. Although it seems similar to rapid desensitization for hypersensitivity reactions to drugs, the mechanism of this desensitization effect for AIT is yet unknown. Acute oral desensitization in mice demonstrated that antigen-specific mast cell desensitization is one of the main underlying mechanisms for oral desensitization [103]. It has been shown that mediators of anaphylaxis (histamine and leukotrienes) are released during AIT and sting challenges without inducing a systemic anaphylactic response [104]. Their piecemeal release below the threshold of systemic anaphylaxis may decrease the granule content of mediators and also may affect the threshold of activation of mast cells and basophils, because decreased mediator release in these cells is a well demonstrated feature a short time after the start of AIT [104-106]. One of the main soluble factors liberated by effector cells following allergen challenge is histamine, which mediates its effects via histamine receptors (HRs). So far, four different human HR-types have been identified as H1-4 [107]. Both the expression pattern of HRs and modifications in the intensity of the expression of a single HR type are decisive for the nature of the developing immune response [108,109]. H1R has significant proinflammatory and cell activating properties, while H2R has been shown to be coupled to adenylate cyclase and phosphoinositide second messenger systems and is supposed to be involved primarily in tolerogenic immune responses [110]. Although there are individual differences and risks for developing systemic anaphylaxis during the course of AIT, the suppression of mast cells and basophils continues to be affected by changes in other immune parameters such as the generation of allergen-specific Treg cells and decreased specific IgE. In a recent study, significantly enhanced tryptophan degradation and elevated human Ig receptors inhibitory transcript (ILT4) expression in monocytes were found within a few hours after the first injection on day 1 representing markers of very early changes [111]. In addition, early improvement in basophil sensitivity predicts symptom relief with grass pollen immunotherapy [112]. Furthermore, basophil expression of diamine oxidase shows a significant increase after AIT and suggested as a novel biomarker of allergen immunotherapy response [113].
Figure 1.
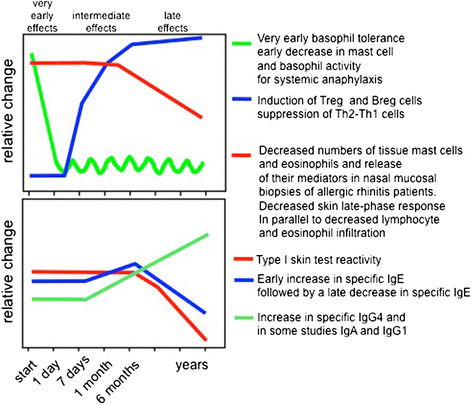
Immunologic changes during the course of AIT. Starting with the first injection, decreases in mast cell and basophil activity, degranulation and tendency for systemic anaphylaxis degranulation takes place within the first hours. This is followed by generation of allergen-specific Treg and Breg cells and suppression of allergen-specific Th1 and Th2 cells. Specific IgE shows an early increase and decreases relatively late. These events are in parallel to increases of IgG4 that continuously increases as long as the treatment continues. After several months, the allergen-specific IgE/IgG4 ratio decreases. After a few months, decreases in tissue mast cells and eosinophils and release of their mediators and skin late phase response occurs. A significant decrease in type I skin test reactivity is also observed relatively late in the course. It has to be noted that there is significant variation between donors and protocols.
Very early effects related to antigen-presenting cells and adjuvants
Aluminium hydroxide is a commonly used adjuvant in AIT vaccines. While generally proven to be efficacious and having a good safety profile, novel adjuvants are needed to overcome current problems in conventional immunotherapy. For example depending on the type of toll-like receptor (TLR), different types of antigen-presenting cells can be targeted. TLR-triggering compounds that can control the overexpression of Th2 cytokines or skew the Th1-Th2 balance towards a Th1 and Treg profile have been effective in murine models of allergy [114].
The epidermis contains high numbers of potent antigen-presenting Langerhans cells. Accordingly, transcutaneous or epicutaneous AIT was recently introduced as a treatment option for allergies [115]. A few applications of allergens using skin patches with treatment duration of a few weeks were sufficient to achieve lasting relief. Similarly oral mucosal Langerhans cells bind allergens after resorbtion, which significantly increased their migratory capacity but attenuated their maturation [116]. Allergen challenge promoted the release of TGF-beta1 and IL-10 by oral mucosal Langerhans cells themselves as well as by cocultured T cells.
The tolerogenic function of different types of DC depends on certain maturation stages and subsets of different ontogenies and can be influenced by immunomodulatory agents. A role for DC in the induction of different subsets of Treg cells in defined microenvironments has been supported by several studies. In intestinal lamina propria, several subsets of DC reside and are in close contact with commensal bacteria and food antigens/allergens [117,118]. DC from the lamina propria of the small intestine and from the mesenteric lymph node are noticeably better than splenic DC at inducing the expression of Foxp3 in naive T cells in the presence of exogenous TGF-β [117,118]. Treg cells can be induced in the microenvironment of tumors and chronic infections due to DC that promote them. In some cases, DC conditioned by Foxp3+ Treg cells; pathogen-derived molecules such as, filamentous hemagglutinin [119]; exogenous signals such as histamine via its receptor 2 [110], adenosine [120], Vitamin D3 metabolites [121] or retinoic acid [122] can induce new populations of Treg cells. Antigen presentation by partially mature airway DC that express IL-10 induce the formation of Tr1-like cells, which inhibit subsequent inflammatory responses [123]. In addition, depletion and adoptively transfer of pulmonary plasmocytoid DC has demonstrated an important role for these cells in protection from allergen sensitization and asthma development in mice [124].
Virus-like particles as a novel, modular, acellular antigen-presenting system and as strong adjuvants are able to modulate the responses of allergen-specific T cells. Displaying Fel d1 on virus-like particles prevents type I hypersensitivity despite greatly enhanced immunogenicity and represents a novel therapy for cat allergy. A single vaccination was sufficient to induce protection in mice [125,126].
Innate lymphoid cells are a recently introduced cell subset that may play a role in enhancing inflammation in many diseases. Particularly Type 2 innate lymphoid cells play a role in asthma and upper respiratory inflammation [127]. Type 2 immunity consists of GATA-3+ ILC2s, TC2 cells, and Th2 cells producing IL-4, IL-5, and IL-13, which induce mast cell, basophil, and eosinophil activation, as well as IgE antibody production, thus protecting against helminthes and venoms [128]. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy [129].
Treg cells and peripheral T cell tolerance to allergens
The induction of a tolerant state in peripheral T cells represents an essential step in AIT (Figure 2). Peripheral T cell tolerance is characterized mainly by generation of allergen-specific Treg cells [130-132] and decrease in Th2 and Th1 cells [133]. It is initiated by IL-10 and TGF-β, which are increasingly produced by the antigen-specific Treg cells [130-132,134]. Subsets of Treg cells with distinct phenotypes and mechanisms of action include the naturally occurring, thymic selected CD4+CD25+ Treg cells, and the inducible type 1 Treg cells (Tr1) (Figure 1) [135]. Different studies show roles for both subsets suggesting an overlap in particularly the inducible subsets of Treg cells in humans. Their first effect is realized by suppression of allergen-specific Th2 and Th1 cells. The suppression by these cells could partially be blocked by the use of neutralizing antibodies against secreted or membrane-bound IL-10 and TGF-β. In coherence with this, it has been shown that CD4+CD25+ Treg cells from atopic donors have a reduced capability to suppress the proliferation of CD4+CD25− T cells [136]. The presence of local Foxp3+CD25+CD3+ cells in the nasal mucosa, their increased numbers after immunotherapy, and their association with clinical efficacy and suppression of seasonal allergic inflammation strengthen the concept of allergen tolerance based on Treg cells in humans [137]. These findings were coined by tracking specific T cells with allergen class-II tetramers: clinical tolerance induction in humans is associated with a marked loss of IL-4-producing T-cells and the acquisition of IL-10-producing and FOXP3-positive antigen-specific CD4+ T-cells [138]. In addition to conventional immunotherapy, peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. Treatment with selected epitopes from a single allergen resulted in suppression of responses to other (“linked”) epitopes within the same molecule [139]. Treg cells and suppression of allergen-specific immune response in the course of AIT has been shown in many different AITs [140]. Similar findings of induction of IL-10 and Treg cells have been observed in mouse models of AIT only when prolonged schedules are used [141].
Figure 2.
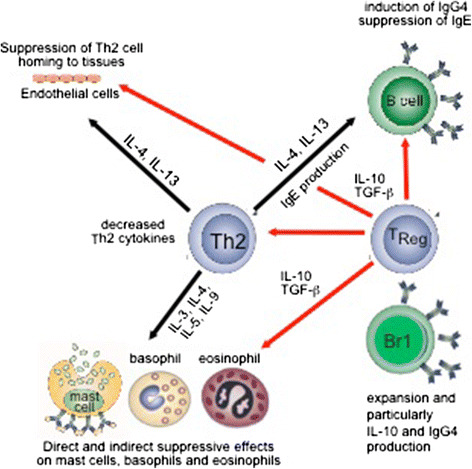
Role of Treg and Breg cells in the suppression of allergic inflammation. The balance between Th2 cells and Treg cells is decisive for the development or suppression of allergic inflammation. Treg cells and their cytokines suppress Th2 type immune responses and contribute to the control of allergic diseases in several major ways. Red arrows indicate the regulatory and suppressive effects of Treg cells, which exert their regulatory functions directly or indirectly on B cells by inducing IgG4 and IgA and suppressing IgE; on vascular endothelium by suppressing Th2 cell homing to tissues; on mast cells, basophils and eosinophils via direct and indirect suppressive effects; and on directly and indirectly suppression of epithelial cell activation and proinflammatory properties. In addition, B reg cells also suppress effector T cells and contribute to IgG4 synthesis.
IL-10-producing antigen-presenting cells, such as B cells [142] and dendritic cells [123] as well as clonally expanded IL-10-producing allergen-specific Tr1 cells [131,143] all contribute to the suppressive effects of IL-10 in different models. IL-10 suppresses T cells by blocking CD2, CD28 and inducible co-stimulator (ICOS) co-stimulatory signals in a rapid signal transduction cascade [144]. In the presence of IL-10, a direct inhibition on CD2, CD28 and ICOS signaling in T cells occurs via utilization of Src-homology-2 domain containing tyrosine phosphatase (SHP-1) by IL-10 [144,145]. SHP-1 rapidly binds to CD28 and ICOS and dephosphorylates them [144]. Supporting these findings, spleen cells from SHP-1-deficient mice show increased proliferation with CD2, CD28 and ICOS stimulation in comparison to wild-type mice, which was not suppressed by IL-10. Generation of dominant negative SHP-1-overexpressing T cells or silencing of the SHP-1 gene by small inhibitory RNA (siRNA) both altered SHP-1 functions and abolished the suppressive effect of IL-10 [144-146]. Interestingly, the suppressive effect of IL-10 was not observed in other IL-10 family cytokines IL-19, IL-20, IL-22, IL-24 [147]. In addition to T cells, IL-10 also exerts inhibitory effect on activated monocytes and macrophages [148]. It has been shown in monocytes and DC that IL-10 suppresses co-stimulatory molecules and down regulates MHC class-II molecules and APC capacity [149]. Furthermore, IL-10 induces the expression of the suppressor of the cytokine-signalling-3 (SOCS3) gene that might play a role in the inhibition of the IFN-γ-induced tyrosine phosphorylation of Stat1 [150].
TGF-β is essential for the maintenance of immunological self-tolerance [151]. TGF-β induces the conversion of naive CD4+CD25− T cells into CD4+CD25+ T cells by the induction of FoxP3 [152], and TGF-β signaling is required for in vivo expansion and immunosuppressive capacity of CD4+CD25+ T cells [153]. In addition, RUNX1 and RUNX3 transcription factors play an essential role in FOXP3 development both in humans and mice [154]. However, the exact suppressive mechanisms behind TGF-β activation of Smad pathways remain to be elucidated.
Treg and Breg cells in healthy immune response to allergens in high dose exposed individuals
Two high dose allergen exposure models have been studied in humans. These are immune response to bee venom allergens in bee keepers and immune response to cat allergens in cat owners [135,155]. If a detectable immune response is mounted, Tr1 cells specific for common environmental allergens consistently represent the dominant subset in healthy individuals. They use multiple suppressive mechanisms, IL-10 and TGF-β as secreted cytokines, and cytotoxic T lymphocyte antigen 4 and programmed death 1 as surface molecules. Healthy and allergic individuals exhibit all three, i.e. Th1, Th2, Tr1 type allergen-specific subsets in different proportions [143]. Accordingly, a change in the dominant subset and the balance between Th2 and Treg cells may lead to either allergy development or recovery.
It was found in allergic children that Treg cells increase during pollen season [156]. Whether these CD4+CD25high T cells directly contribute to inflammation or their increased levels keep the inflammation at low levels remains as an important research question. Circulating allergen-specific CD4+CD25highFoxp3+ T-regulatory cells do not show a major difference between nonatopic and atopic individuals [157]. However, it was demonstrated that FOXP3 expression shows a negative correlation with IgE, eosinophilia and IFN-γ levels and FOXP3+/CD4+ ratio is significantly low in asthma and atopic dermatitis [158]. CD4+CD25+ Treg cells have been associated with the spontaneous remission of Cow’s milk allergy. Children who outgrew their allergy (tolerant children) had higher frequencies of circulating CD4+CD25+ T cells and decreased in vitro proliferative responses to bovine beta-lactoglobulin in peripheral blood mononuclear cells compared with children who maintained clinically active allergy [159]. Peripheral tolerance utilizes multiple mechanisms to suppress allergic inflammation. Treg cells contribute to the control of allergen-specific immune responses by a)Suppression of antigen-presenting cells that support the generation of effector T cells; b) suppression of Th2 and Th1 cells; c) suppression of allergen-specific IgE and induction of IgG4; d) suppression of mast cells, basophils and eosinophils; e) interaction with resident tissue cells and remodeling [135]. In addition to immune suppression, decrease in antigen-specific Th2 repertoire because of central lymphatic organ homing or deletion my play a role in the mechanisms of allergen tolerance [160,161]. In some cases and types of immunotherapies, the suppression of Th2 cells was found to be transient [162].
Allergen tolerance in healthy individuals can be broken under certain conditions. In a recent study, human tonsils were studied that contain allergen-specific T cells but show very low levels of allergen-induced T-cell proliferation, thus representing a very suitable in vivo model to assess mechanisms of breaking allergen-specific T-cell tolerance [163,164]. It was demonstrated that triggering of Toll-like receptor (TLR) 4 or TLR8 and the proinflammatory cytokines IL-1beta or IL-6 break allergen-specific T-cell tolerance in human tonsils and peripheral blood through a mechanism dependent on the adaptor molecule myeloid differentiation primary response gene 88. In particular, myeloid DCs and stimulations that activate them broke the tolerance of allergen-specific CD4 T cells, whereas plasmacytoid DCs and stimulations that activate them, such as TLR7 and TLR9, did not have any effect. Tolerance-breaking conditions induced by different molecular mechanisms were associated with a mixed cytokine profile with a tendency toward increased levels of IL-13 and IL-17, which are T2 and T17 cytokines, respectively. These findings suggest that certain innate immune response signals and proinflammatory cytokines break allergen-specific CD4 T-cell tolerance in healthy subjects, which might lead to the development or exacerbation of allergic diseases after encountering microbes or inflammatory conditions [163]. Viral infections represent important candidates for breaking of allergen tolerance, because as a virus infected lymphoid tissue, human tonsillar cytokine expression is closely related to existing viral infections and shows distinct clusters between antiviral and immune regulatory genes [165].
In addition to Treg cells, IL-10-producing regulatory B cells suppress immune responses, and lack of these cells leads to exacerbated symptoms in mouse models of chronic inflammation, transplantation, and chronic infection [166]. In a recent study human inducible IL-10-secreting B regulatory 1 (BR1) cells were characterized. Human IL-10+ BR1 cells expressed high surface CD25 and CD71 and low CD73 levels. Sorting of CD73-CD25 + CD71+ B cells allowed enrichment of human BR1 cells, which produced high levels of IL-10 and potently suppressed antigen-specific CD4+ T-cell proliferation [166]. IgG4 was selectively confined to human BR1 cells. B cells specific for the major bee venom allergen PLA isolated from nonallergic beekeepers show increased expression of IL-10 and IgG4. Furthermore, the frequency of IL-10+ PLA-specific B cells increased in allergic patients receiving allergen-specific immunotherapy. This study demonstrates two essential in vivo evidence for allergen tolerance: the suppressive B cells and IgG4-expressing B cells that are confined to IL-10+ BR1 cells in human subjects [166]. It was recently demonstrated that solely IL-10-overexpressing B cells acquired a prominent immunoregulatory profile comprising upregulation of suppressor of cytokine signaling 3 (SOCS3), glycoprotein A repetitions predominant (GARP), the IL-2 receptor alpha chain (CD25), and programmed cell death 1 ligand 1 (PD-L1) [167]. These cells showed a significant reduction in levels of proinflammatory cytokines (TNF-alpha, IL-8, and macrophage inflammatory protein 1alpha) and augmented the production of anti-inflammatory IL-1 receptor antagonist and vascular endothelial growth factor. Furthermore, IL-10-overexpressing B cells secreted less IgE and potently suppressed proinflammatory cytokines in PBMCs, maturation of monocyte-derived dendritic cells (rendering their profile to regulatory phenotype), and antigen-specific proliferation [167].
Modulation of allergen-specific IgE and IgG responses during AIT
Peripheral T cell tolerance is rapidly induced during AIT, however there is no evidence for B cell tolerance in the early course [130]. AIT induces a transient increase in serum specific IgE followed by gradual decrease over months or years of treatment (Figure 1) [168,169]. In pollen-sensitive patients, desensitization prevents elevation of the serum specific IgE during the pollen season [170]. The changes in IgE levels cannot explain the diminished responsiveness to specific allergen due to AIT, since the decrease in serum IgE is relatively late and does not correlate with clinical improvement after AIT.
Subclasses of IgG antibodies, especially IgG4 is thought to capture the allergen before reaching the effector cell-bound IgE, and thus to prevent the activation of mast cells and basophils. IgG4 antibodies can be viewed as a marker of introduced allergen dose and they have the ability to modulate the immune response to allergen. However, the relationship between the efficacy of AIT and the induction of allergen-specific IgG subgroups remains a controversial issue with serum concentrations of allergen-specific IgG correlating with clinical improvement in some studies, but not in others [171,172]. Allergen-specific IgG may be directed against the same epitopes as IgE, resulting in direct competition for allergen binding and a “blocking” effect. The concept of blocking antibodies has been revaluated. Analysis of the IgG subtypes induced by AIT has shown specific increases in IgG1 and particularly IgG4, with levels increasing 10-100-fold [173,174]. There is accumulating evidence that specific immunotherapy also influences the blocking activity on IgE-mediated responses by IgG4. Results suggest that successful specific immunotherapy is associated with an increase in IgG blocking activity that is not solely dependent on the quantity of IgG antibodies [175,176]. In a recent study, inhibition by IgG required Fcγ receptor-IIB. One IgG against a single epitope on the major allergen was able to block the degranulation of basophils from individuals with cat allergy. The inhibitory potential of IgG antibodies increased when larger allergen-IgG complexes were formed. It seems to be relevant rather to measure the blocking activity and or affinity of allergen-specific IgG or IgG subsets, particularly IgG4 and also IgG1 instead of their levels in sera [177].
There are several features of IgG4, which may play a role in its non inflammatory role. IgG4 hinge region has unique structural features that result in a lower affinity for certain Fcγ receptors and the ability to separate and repair by dynamic Fab arm exchange leads to bi-specific antibodies that are functionally monomeric [178,179]. Furthermore, IgG4 does not fix complement and is capable of inhibiting immune-complex formation by other isotypes, giving this isotype anti-inflammatory characteristics. In a clinical trial with five recombinant Phleum allergen mixures, all treated subjects developed very strong allergen-specific IgG4 and also increased IgG1 antibody responses. Some patients who were not initially sensitized to Phl p 5, for example, developed strong specific IgG4, but not IgE antibody responses specifically against that allergen [173]. This demonstrates that extract based antibody measurements may provide a wrong information and studies on mechanisms of AIT should be performed with single allergens.
It is highly possible that the decrease in IgE/IgG4 ratio during AIT is a feature of skew from allergen-specific Th2 to Treg cell predominance. IL-10 is a potent suppressor of both total and allergen-specific IgE, while it simultaneously increases IgG4 production [131,180]. Thus, IL-10 not only generates tolerance in T cells; it also regulates specific isotype formation towards a non-inflammatory phenotype. The healthy immune response to Der p 1 is associated with increased specific IgA and IgG4, small amounts of IgG1 and almost undetectable IgE antibodies in serum [132]. In the same study house dust mite-AIT did not significantly change specific IgE levels after 70 days of treatment; however, a significant increase in specific IgA, IgG1 and IgG4 was observed [132]. The reason for the long-time gap between the change in T cell subsets, but not IgE levels is not easily explainable by the half-life of this antibody. In this context, the role of bone marrow-residing IgE-producing plasma cells with very long life-span remain to be investigated [181].
Conclusion
During the past 20 years, major advances have been made in understanding the molecular and cellular mechanisms of allergen tolerance in humans. The demonstration of allergen-specific T and B cell tolerance, particularly that mediated by the immune-suppressive functions of IL-10, led to a major conceptual change in this area [182]. AIT has multiple mechanisms of action with the involvement of many cell subsets. These effects comprise very early effects related to antigen-presenting cells and adjuvants, desensitization of effector cells, antigen-specific immune tolerance in T and B cells and regulation of IgE and IgG4 (Figure 1). Similar mechanisms are observed in high dose allergen tolerance in healthy bee keepers and non allergic cat owners. The kinetics and intensity of these events change according to the type of AIT vaccine that is used and the place of administration.
Acknowledgements
Support for the dissemination of the WAO Immunotherapy and Biologics Online Monograph is provided by the following sponsors: Circassia, Boehringer-Ingleheim, and ORA Inc.
Footnotes
Competing interests
CAA and MA declare they have no competing interests.
Authors’ contributions
CAA and MA contributed equally to the development of the document.
Contributor Information
Cezmi A Akdis, Email: akdisac@siaf.uzh.ch.
Mübeccel Akdis, Email: akdism@siaf.uzh.ch.
References
- 1.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–31. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 2.Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–49. doi: 10.1038/nm.2754. [DOI] [PubMed] [Google Scholar]
- 3.Akdis M, Akdis AC. Immune Tolerance. In: Franklin Adkinson Jr BSB N, Wesley B, Busse WW, Holgate ST, Lemanske Jr RF, O’Hehir RE, editors. Middleton’s Allergy. 8 2013. [Google Scholar]
- 4.Akdis M, Akdis CA. Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov. 2009;8:645–60. doi: 10.1038/nrd2653. [DOI] [PubMed] [Google Scholar]
- 5.Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol. 2006;18:718–26. doi: 10.1016/j.coi.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Akdis C, Papadopoulos N, Cardona V. Fighting allergies beyond symptoms: the European declaration on immunotherapy. Eur J Immunol. 2011;41:2802–4. doi: 10.1002/eji.201190061. [DOI] [PubMed] [Google Scholar]
- 8.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–21. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 10.Akkoc T, Akdis M, Akdis CA. Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res. 2011;3:11–20. doi: 10.4168/aair.2011.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousquet J, Anto J, Auffray C, Akdis M, Cambon-Thomsen A, Keil T, et al. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy. 2011;66:596–604. doi: 10.1111/j.1398-9995.2010.02534.x. [DOI] [PubMed] [Google Scholar]
- 12.Deniz G, Akdis M. NK cell subsets and their role in allergy. Expert Opin Biol Ther. 2011;11:833–41. doi: 10.1517/14712598.2011.572549. [DOI] [PubMed] [Google Scholar]
- 13.Direskeneli H, Fujita H, Akdis CA. Regulation of TH17 and regulatory T cells in patients with Behcet disease. J Allergy Clin Immunol. 2011;128:665–6. doi: 10.1016/j.jaci.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Eiwegger T, Akdis CA. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol. 2011;41:1535–8. doi: 10.1002/eji.201141668. [DOI] [PubMed] [Google Scholar]
- 15.Eiwegger T, Gruber S, Geiger C, Meyer E, Dehlink E, Bannert C, et al. Impact of systemic immuno-suppression after solid organ transplantation on allergen-specific responses. Allergy. 2011;66:271–8. doi: 10.1111/j.1398-9995.2010.02475.x. [DOI] [PubMed] [Google Scholar]
- 16.Fujita H, Chalubinski M, Rhyner C, Indermitte P, Meyer N, Ferstl R, et al. Claudin-1 expression in airway smooth muscle exacerbates airway remodeling in asthmatic subjects. J Allergy Clin Immunol. 2011;127:1612–21. doi: 10.1016/j.jaci.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011;66:725–32. doi: 10.1111/j.1398-9995.2011.02589.x. [DOI] [PubMed] [Google Scholar]
- 18.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 2011;11:139–45. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–60. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 20.O’Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128:1153–62. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Specific immunotherapy and turning off the T cell: how does it work? Ann Allergy Asthma Immunol. 2011;107:381–92. doi: 10.1016/j.anai.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Mechanisms of immunotherapy to wasp and bee venom. Clin Exp Allergy. 2011;41:1226–34. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 23.Palomares O, O’Mahony L, Akdis CA. The many routes of dendritic cells to ensure immune regulation. J Allergy Clin Immunol. 2011;127:1541–2. doi: 10.1016/j.jaci.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Sackesen C, Birben E, Soyer OU, Sahiner UM, Yavuz TS, Civelek E, et al. The effect of CD14 C159T polymorphism on in vitro IgE synthesis and cytokine production by PBMC from children with asthma. Allergy. 2011;66:48–57. doi: 10.1111/j.1398-9995.2010.02428.x. [DOI] [PubMed] [Google Scholar]
- 25.Sin BA, Akdis M, Zumkehr J, Bezzine S, Bekpen C, Lambeau G, et al. T-cell and antibody responses to phospholipase A2 from different species show distinct cross-reactivity patterns. Allergy. 2011;66:1513–21. doi: 10.1111/j.1398-9995.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 26.Soyer OU, Akdis M, Akdis CA. Mechanisms of subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am. 2011;31:175–90. doi: 10.1016/j.iac.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Vale-Pereira S, Todo-Bom A, Geraldes L, Schmidt-Weber C, Akdis CA, Mota-Pinto A. FoxP3, GATA-3 and T-bet expression in elderly asthma. Clin Exp Allergy. 2011;41:490–6. doi: 10.1111/j.1365-2222.2010.03640.x. [DOI] [PubMed] [Google Scholar]
- 28.Vanbervliet B, Akdis M, Vocanson M, Rozières A, Benetière J, Rouzaire P, et al. Histamine receptor H1 signaling on dendritic cells plays a key role in the IFN-gamma/IL-17 balance in T cell-mediated skin inflammation. J Allergy Clin Immunol. 2011;127:943–53. doi: 10.1016/j.jaci.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann M, Koreck A, Meyer N, Basinski T, Meiler F, Simone B, et al. TNF-like weak inducer of apoptosis (TWEAK) and TNF-alpha cooperate in the induction of keratinocyte apoptosis. J Allergy Clin Immunol. 2011;127:200–7. doi: 10.1016/j.jaci.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Smits HH, Akdis CA. In utero priming by worms protects against respiratory allergies. J Allergy Clin Immunol. 2014;134:1280–1. doi: 10.1016/j.jaci.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 31.Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol. 2014;133:612–9. doi: 10.1016/j.jaci.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Soyka MB, van de Veen W, Holzmann D, Akdis M, Akdis CA. Scientific foundations of allergen-specific immunotherapy for allergic disease. Chest. 2014;146:1347–57. doi: 10.1378/chest.14-0049. [DOI] [PubMed] [Google Scholar]
- 33.Jutel M, Akdis CA. Novel immunotherapy vaccine development. Curr Opin Allergy Clin Immunol. 2014;14:557–63. doi: 10.1097/ACI.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 34.Cavkaytar O, Akdis CA, Akdis M. Modulation of immune responses by immunotherapy in allergic diseases. Curr Opin Pharmacol. 2014;17:30–7. doi: 10.1016/j.coph.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–91. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Akdis M. Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol. 2006;18:738–44. doi: 10.1016/j.coi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 38.Durham SR, Walker SM, Varga E-V, Jacobson MR, O'Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 39.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–75. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–7. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 41.Moller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 42.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–96. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 43.Creticos PS, Esch RE, Couroux P, Gentile D, D'Angelo P, Whitlow B, et al. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2014;133:751–8. doi: 10.1016/j.jaci.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 44.Wood RA, Togias A, Wildfire J, Visness CM, Matsui EC, Gruchalla R, et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol. 2014;133:846–52. doi: 10.1016/j.jaci.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez RM, Jacobs RL. Eosinophilic esophagitis treated with immunotherapy to dust mites. J Allergy Clin Immunol. 2013;132:503–4. doi: 10.1016/j.jaci.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 46.Creticos PS, Maloney J, Bernstein DI, Casale T, Kaur A, Fisher R, et al. Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J Allergy Clin Immunol. 2013;131:1342–9. doi: 10.1016/j.jaci.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Witten M, Malling HJ, Blom L, Poulsen BC, Poulsen LK. Is intralymphatic immunotherapy ready for clinical use in patients with grass pollen allergy? J Allergy Clin Immunol. 2013;132:1248–52. doi: 10.1016/j.jaci.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Tam H, Calderon MA, Boyle RJ. Efficacy of allergen-specific immunotherapy for patients with atopic dermatitis. J Allergy Clin Immunol. 2013;132:1012–3. doi: 10.1016/j.jaci.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 49.Patel P, Holdich T, von Weikersthal-Drachenberg KJ F, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014;133:121–9. doi: 10.1016/j.jaci.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 50.Swoboda I, Balic N, Klug C, Focke M, Weber M, Spitzauer S, et al. A general strategy for the generation of hypoallergenic molecules for the immunotherapy of fish allergy. J Allergy Clin Immunol. 2013;132:979–81. doi: 10.1016/j.jaci.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 51.Kiel MA, Roder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Molken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353–60. doi: 10.1016/j.jaci.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol 2014; article in press, published online ahead of print. (doi:10.1016/j.jaci.2014.09.012) [DOI] [PMC free article] [PubMed]
- 53.von Moos S, Johansen P, Tay F, Graf N, Kundig TM, Senti G. Comparing safety of abrasion and tape-stripping as skin preparation in allergen-specific epicutaneous immunotherapy. J Allergy Clin Immunol. 2014;134:965–7. doi: 10.1016/j.jaci.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 54.Spertini F, Perrin Y, Audran R, Pellaton C, Boudousquié C, Barbier N, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–40. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Kundig TM, Johansen P, Bachmann MF, Cardell LO, Senti G. Intralymphatic immunotherapy: time interval between injections is essential. J Allergy Clin Immunol. 2014;133:930–1. doi: 10.1016/j.jaci.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 56.Holt PG, Sly PD, Sampson HA, Robinson P, Loh R, Lowenstein H, et al. Prophylactic use of sublingual allergen immunotherapy in high-risk children: a pilot study. J Allergy Clin Immunol. 2013;132:991–3. doi: 10.1016/j.jaci.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 57.Stringari G, Tripodi S, Caffarelli C, Dondi A, Asero R, Di Rienzo Businco A, et al. The effect of component-resolved diagnosis on specific immunotherapy prescription in children with hay fever. J Allergy Clin Immunol. 2014;134:75–81. doi: 10.1016/j.jaci.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 58.Allam JP, Wuestenberg E, Wolf H, Klimek L, Decot E, Horn A, et al. Immunologic response and safety in birch pollen sublingual versus oral vestibule immunotherapy: a pilot study. J Allergy Clin Immunol. 2014;133:1757–9. doi: 10.1016/j.jaci.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Passalacqua G, Baena-Cagnani CE, Bousquet J, Canonica GW, Casale TB, Cox L, et al. Grading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same language. J Allergy Clin Immunol. 2013;132:93–8. doi: 10.1016/j.jaci.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 60.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–23. doi: 10.1016/j.jaci.2013.12.1040. [DOI] [PubMed] [Google Scholar]
- 61.Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larenas-Linnemann D, Wahn U, Kopp M. Use of omalizumab to improve desensitization safety in allergen immunotherapy. J Allergy Clin Immunol. 2014;133:937. doi: 10.1016/j.jaci.2013.12.1089. [DOI] [PubMed] [Google Scholar]
- 63.Kohler J, Blank S, Muller S, Bantleon F, Frick M, Huss-Marp J, et al. Component resolution reveals additional major allergens in patients with honeybee venom allergy. J Allergy Clin Immunol. 2014;133:1383–9. doi: 10.1016/j.jaci.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 64.Wang M, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, Joetham A, et al. Peanut-induced intestinal allergy is mediated through a mast cell-IgE-FcepsilonRI-IL-13 pathway. J Allergy Clin Immunol. 2010;126:306–16. doi: 10.1016/j.jaci.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. 2008;121:1311–20. doi: 10.1016/j.jaci.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 66.Chatila TA, Li N, Garcia-Lloret M, Kim HJ, Nel AE. T-cell effector pathways in allergic diseases: transcriptional mechanisms and therapeutic targets. J Allergy Clin Immunol. 2008;121:812–23. doi: 10.1016/j.jaci.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 67.Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33:290–6. doi: 10.1165/rcmb.2005-0003OC. [DOI] [PubMed] [Google Scholar]
- 68.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 70.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–25. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 71.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 73.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–54. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 75.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–8. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 76.Akkoc T, de Koning PJ, Ruckert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol. 2008;121:652–8. doi: 10.1016/j.jaci.2007.12.1171. [DOI] [PubMed] [Google Scholar]
- 77.Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trautmann A, Schmid-Grendelmeier P, Krüger K, Crameri R, Akdis M, Akkaya A, et al. T cells and eosinophils cooperate in the induction of bronchial epithelial apoptosis in asthma. J Allergy Clin Immunol. 2002;109:329–37. doi: 10.1067/mai.2002.121460. [DOI] [PubMed] [Google Scholar]
- 79.Meyer N, Zimmermann M, Bürgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol. 2010;125:858–65. doi: 10.1016/j.jaci.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 80.Solarewicz-Madejek K, Basinski TM, Crameri R, Akdis M, Akkaya A, Blaser K, et al. T cells and eosinophils in bronchial smooth muscle cell death in asthma. Clin Exp Allergy. 2009;39:845–55. doi: 10.1111/j.1365-2222.2009.03244.x. [DOI] [PubMed] [Google Scholar]
- 81.Burgler S, Ouaked N, Bassin C, Basinski TM, Mantel PY, Siegmund K, et al. Differentiation and functional analysis of human T(H)17 cells. J Allergy Clin Immunol. 2009;123(3):588–95. doi: 10.1016/j.jaci.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 82.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 83.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 85.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 86.Makihara S, Okano M, Fujiwara T, Kariya S, Noda Y, Higaki T, et al. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J Allergy Clin Immunol. 2010;126:397–400. doi: 10.1016/j.jaci.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 87.Martin P, Gomez M, Lamana A, Matesanz Marín A, Cortés JR, Ramírez-Huesca M, et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J Allergy Clin Immunol. 2010;126:355–65. doi: 10.1016/j.jaci.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Sergejeva S, Ivanov S, Lotvall J, Linden A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005;33:248–53. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 89.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 90.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–5. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 91.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–19. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 94.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–52. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 96.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rebane A, Akdis CA. MicroRNAs: essential players in the regulation of inflammation. J Allergy Clin Immunol. 2013;132:15–26. doi: 10.1016/j.jaci.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 98.Nicodemus-Johnson J, Laxman B, Stern RK, Sudi J, Tierney CN, Norwick L, et al. Maternal asthma and microRNA regulation of soluble HLA-G in the airway. J Allergy Clin Immunol. 2013;131:1496–503. doi: 10.1016/j.jaci.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu TX, Rothenberg ME. Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J Allergy Clin Immunol. 2013;132:3–13. doi: 10.1016/j.jaci.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rebane A, Runnel T, Aab A, Maslovskaja J, Rückert B, Zimmermann M, et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol. 2014;134:836–47. doi: 10.1016/j.jaci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 101.Tarzi M, Klunker S, Texier C, Verhoef A, Stapel SO, Akdis CA, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36:465–74. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 102.Alexander C, Ying S, B Kay A, Larche M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma + T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35:52–8. doi: 10.1111/j.1365-2222.2005.02143.x. [DOI] [PubMed] [Google Scholar]
- 103.Woo HY, Kim YS, Kang NI, Chung WC, Song CH, Choi IW, et al. Mechanism for acute oral desensitization to antibiotics. Allergy. 2006;61:954–8. doi: 10.1111/j.1398-9995.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- 104.Eberlein-Konig B, Ullmann S, Thomas P, Przybilla B. Tryptase and histamine release due to a sting challenge in bee venom allergic patients treated successfully or unsuccessfully with hyposensitization. Clin Exp Allergy. 1995;25:704–12. doi: 10.1111/j.1365-2222.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 105.Jutel M, Muller UR, Fricker M, Rihs S, Pichler WJ, Dahinden C. Influence of bee venom immunotherapy on degranulation and leukotriene generation in human blood basophils. Clin Exp Allergy. 1996;26:1112–8. doi: 10.1111/j.1365-2222.1996.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 106.Plewako H, Wosinska K, Arvidsson M, Bjorkander J, Skov PS, Håkansson L, et al. Basophil interleukin 4 and interleukin 13 production is suppressed during the early phase of rush immunotherapy. Int Arch Allergy Immunol. 2006;141:346–53. doi: 10.1159/000095461. [DOI] [PubMed] [Google Scholar]
- 107.Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39:1786–800. doi: 10.1111/j.1365-2222.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 108.Muller UR, Jutel M, Reimers A, Zumkehr J, Huber C, Kriegel C, et al. Clinical and immunologic effects of H1 antihistamine preventive medication during honeybee venom immunotherapy. J Allergy Clin Immunol. 2008;122:1001–7. doi: 10.1016/j.jaci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 110.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010;65(12):1558–65. doi: 10.1111/j.1398-9995.2010.02430.x. [DOI] [PubMed] [Google Scholar]
- 112.Schmid JM, Wurtzen PA, Dahl R, Hoffmann HJ. Early improvement in basophil sensitivity predicts symptom relief with grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:741–4. doi: 10.1016/j.jaci.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 113.Shamji MH, Layhadi JA, Scadding GW, Cheung DK, Calderon MA, Turka LA et al. Basophil expression of diamine oxidase: A novel biomarker of allergen immunotherapy response. J Allergy Clin Immunol 2014;Nov 22. Published online ahead of print. (doi:10.1016/j.jaci.2014.09.049) [DOI] [PubMed]
- 114.Crameri R, Rhyner C. Novel vaccines and adjuvants for allergen-specific immunotherapy. Curr Opin Immunol. 2006;18:761–8. doi: 10.1016/j.coi.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Senti G, Graf N, Haug S, Rüedi N, von Moos S, Sonderegger T, et al. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;124:997–1002. doi: 10.1016/j.jaci.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 116.Allam JP, Wurtzen PA, Reinartz M, Winter J, Vrtala S, Chen KW, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–45. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 117.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22:3491–9. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Urry Z, Xystrakis E, Richards DF, McDonald J, Sattar Z, Cousins DJ, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119(2):387–98. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mucida D, Park Y, Kim G, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 123.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 124.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leb VM, Jahn-Schmid B, Kueng HJ, Schmetterer KG, Haiderer D, Neunkirchner A, et al. Modulation of allergen-specific T-lymphocyte function by virus-like particles decorated with HLA class II molecules. J Allergy Clin Immunol. 2009;124:121–8. doi: 10.1016/j.jaci.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 126.Schmitz N, Dietmeier K, Bauer M, Maudrich M, Utzinger S, Muntwiler S, et al. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206:1941–55. doi: 10.1084/jem.20090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–8. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2014. [DOI] [PubMed]
- 129.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193–5. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 130.Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Müller U, et al. Epitope specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996;98:1676–83. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 133.Suarez-Fueyo A, Ramos T, Galan A, Jimeno L, Wurtzen PA, Marin A. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol. 2014;133:130–8. doi: 10.1016/j.jaci.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 134.Francis JN, Till SJ, Durham SR. Induction of IL-10 + CD4 + CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–61. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 135.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116:961–8. doi: 10.1016/j.jaci.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 136.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4 + CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 137.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–72. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 138.Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS One. 2010;5:e11028. doi: 10.1371/journal.pone.0011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009; : –. [DOI] [PMC free article] [PubMed]
- 140.Tsai YG, Lai JC, Yang KD, Hung CH, Yeh YJ, Lin CY. Enhanced CD46-induced regulatory T cells suppress allergic inflammation after Dermatophagoides pteronyssinus-specific immunotherapy. J Allergy Clin Immunol. 2014;134:1206–9. doi: 10.1016/j.jaci.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 141.Fox EM, Torrero MN, Evans H, Mitre E. Immunologic characterization of 3 murine regimens of allergen-specific immunotherapy. J Allergy Clin Immunol 2014; Oct 2. Published online ahead of print. (doi:10.1016/j.jaci.2014.07.052) [DOI] [PubMed]
- 142.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of athritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor A, Akdis M, Joss A, Akkoç T, Wenig R, Colonna M, et al. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol. 2007;120:76–83. doi: 10.1016/j.jaci.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 145.Taylor A, Verhagen J, Akkoc T, Wenig R, Flory E, Blaser K, et al. IL-10 suppresses CD2-mediated T cell activation via SHP-1. Mol Immunol. 2009;46(4):622–9. doi: 10.1016/j.molimm.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 146.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000;14:1666–8. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 147.Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, et al. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–8. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 148.de Waal MR, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 150.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, et al. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–63. [PubMed] [Google Scholar]
- 151.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 152.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4 + CD25- naive T cells to CD4 + CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4 + CD25+ T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 154.Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, Ziegler M, et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206:2701–15. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Custovic A, Simpson BM, Simpson A, Hallam CL, Marolia H, Walsh D, et al. Current mite, cat, and dog allergen exposure, pet ownership, and sensitization to inhalant allergens in adults. J Allergy Clin Immunol. 2003;111:402–7. doi: 10.1067/mai.2003.55. [DOI] [PubMed] [Google Scholar]
- 156.Jartti T, Burmeister KA, Seroogy CM, Jennens-Clough ML, Tisler CJ, Salazar LP, et al. Association between CD4(+)CD25(high) T cells and atopy in children. J Allergy Clin Immunol. 2007;120:177–83. doi: 10.1016/j.jaci.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 157.Maggi L, Santarlasci V, Liotta F, Frosali F, Angeli R, Cosmi L, et al. Demonstration of circulating allergen-specific CD4 + CD25highFoxp3+ T-regulatory cells in both nonatopic and atopic individuals. J Allergy Clin Immunol. 2007;120:429–36. doi: 10.1016/j.jaci.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 158.Orihara K, Narita M, Tobe T, Akasawa A, Ohya Y, Matsumoto K, et al. Circulating Foxp3 + CD4+ cell numbers in atopic patients and healthy control subjects. J Allergy Clin Immunol. 2007;120:960–2. doi: 10.1016/j.jaci.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 159.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4 + CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199:1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wambre E, DeLong JH, James EA, Torres-Chinn N, Pfützner W, Möbs C, et al. Specific immunotherapy modifies allergen-specific CD4(+) T-cell responses in an epitope-dependent manner. J Allergy Clin Immunol. 2014;133:872–9. doi: 10.1016/j.jaci.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zaleska A, Eiwegger T, Soyer O, van de Veen W, Rhyner C, Soyka MB, et al. Immune regulation by intralymphatic immunotherapy with modular allergen translocation MAT vaccine. Allergy. 2014;69:1162–70. doi: 10.1111/all.12461. [DOI] [PubMed] [Google Scholar]
- 162.Gorelik M, Narisety SD, Guerrerio AL, Chichester KL, Keet CA, Bieneman AP, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol 2014; Dec 24. Published online ahead of print. (doi:10.1016/j.jaci.2014.11.010) [DOI] [PMC free article] [PubMed]
- 163.Kucuksezer UC, Palomares O, Ruckert B, Jartti T, Puhakka T, Nandy A, et al. Triggering of specific Toll-like receptors and proinflammatory cytokines breaks allergen-specific T-cell tolerance in human tonsils and peripheral blood. J Allergy Clin Immunol. 2013;131:875–85. doi: 10.1016/j.jaci.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 164.Palomares O, Rückert B, Jartti T, Kücüksezer UC, Puhakka T, Gomez E, et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol. 2012;129:510–20. doi: 10.1016/j.jaci.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 165.Jartti T, Palomares O, Waris M, Tastan O, Nieminen R, Puhakka T, et al. Distinct regulation of tonsillar immune response in virus infection. Allergy. 2014;69:658–67. doi: 10.1111/all.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–12. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 167.Stanic B, van de Veen W, Wirz OF, Rückert B, Morita H, Söllner S, et al. IL-10-overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol. 2014;135(3):771–780.e8. doi: 10.1016/j.jaci.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 168.Van Ree R, Van Leeuwen WA, Dieges PH, Van Wijk RG, De Jong N, Brewczyski PZ, et al. Measurement of IgE antibodies against purified grass pollen allergens (Lol p 1, 2, 3 and 5) during immunotherapy. Clin Exp Allergy. 1997;27:68–74. doi: 10.1111/j.1365-2222.1997.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 169.Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70:261–71. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 170.Bousquet J, Maasch H, Martinot B, Hejjaoui A, Wahl R, Michel FB. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. II. Comparison between parameters assessing the efficacy of immunotherapy. J Allergy Clin Immunol. 1988;82:439–46. doi: 10.1016/0091-6749(88)90017-6. [DOI] [PubMed] [Google Scholar]
- 171.Golden DB, Meyers DA, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Clinical relevance of the venom-specific immunoglobulin G antibody level during immunotherapy. J Allergy Clin Immunol. 1982;69:489–93. doi: 10.1016/0091-6749(82)90172-5. [DOI] [PubMed] [Google Scholar]
- 172.Müller UR, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989;44:412–8. doi: 10.1111/j.1398-9995.1989.tb04172.x. [DOI] [PubMed] [Google Scholar]
- 173.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 174.Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, König F, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116:347–54. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 175.Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–8. doi: 10.1097/01.all.0000136753.35948.c0. [DOI] [PubMed] [Google Scholar]
- 176.Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113:1025–34. doi: 10.1016/j.jaci.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 177.Shamji MH, Francis JN, Wurtzen PA, Lund K, Durham SR, Till SJ. Cell-free detection of allergen-IgE cross-linking with immobilized phase CD23: inhibition by blocking antibody responses after immunotherapy. J Allergy Clin Immunol. 2013;132:1003–5. doi: 10.1016/j.jaci.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 178.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van der Neut KM, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–7. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 180.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63:1455–63. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 181.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 182.Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest. 2014;124:4678–80. doi: 10.1172/JCI78891. [DOI] [PMC free article] [PubMed] [Google Scholar]


