Abstract
Spermatogonial stem cells (SSCs), the stem cells responsible for male fertility, are one of a small number of cells with the abilities of both self-renewal and generation of large numbers of haploid cells. Technology improvements, most importantly, transplantation assays and in vitro culture systems have greatly expanded our understanding of SSC self-renewal and differentiation. Many important molecules crucial for the balance between self-renewal and differentiation have been recently identified although the exact mechanism(s) remain largely undefined. In this review, we give a brief introduction to SSCs, and then focus on extrinsic and intrinsic factors controlling SSCs self-renewal and differentiation.
Keywords: differentiation, self-renewal, signal, spermatogonial stem cells
INTRODUCTION
Germline stem cells play crucial roles in transmitting genetic information to subsequent generations.1,2,3,4 In rodents, spermatogonial stem cells (SSCs) comprise a subpopulation of undifferentiated spermatogonia derived from gonocytes approximately 6 days after birth, which in turn are derived from primordial germ cells during embryonic development.5,6 The existence of SSCs has long been proposed; however, lacking of appropriate technology to define SSCs greatly retards its progress. Traditional study of spermatogonia is morphology dependent, making it tough to study in vitro.5 In 1994, Brinster and Avarbock7 developed transplantation technique that firstly allows us to identify SSCs functionally, and this technique has still been the golden standard to confirm SSCs. Briefly, dissociated donor-derived testicular cells are transplanted into the efferent duct of the sterile recipient mice, and offspring is produced with the donor haplotype. This technique is of fundamental importance, for it firstly allows us to identify SSCs and even to count the number of SSCs in the seminiferous tubule.8 Another progress is the development of long-term culture system for mouse SSCs (DBA/2 background) in 2003 by Kanatsu-Shinohara et al.9 In the presence of glial cell line-derived neurotrophic factor (GDNF), fibroblast growth factor 2 (FGF2), leukemia inhibitory factor (LIF) and epidermal growth factor (EGF), germ cells from neonatal mouse termed germline stem (GS) cells are able to proliferate in clusters in vitro for a long time. And transplantation assay confirms greatly increased GS cells number in this system. In vitro culture system allows us to obtain large numbers of cells for basic study, such as signal transducing and regulation mechanism.10 These two technologies combined with other methods, such as gene-modified model, have greatly facilitated SSCs study.
SPERMATOGONIAL STEM CELL IDENTIFICATION AND CHARACTERIZATION
As noted above, SSCs comprise a sub-fraction of undifferentiated spermatogonial cells.5 In rodents, there are three types of undifferentiated type A spermatogonial cells, classified according to cell morphological arrangement: As (single), Apr (two paired cells), and Aal (aligned cells of 4, 8, 16 and sometimes even 32 cells). The traditional model considers As cells as SSCs while Apr and Aal cells are progenitors committed to proliferate and eventually produce haploid cells. As cells will either undergo division, producing two daughter As cells, or division, producing Apr cells connected by a cytoplasmic bridge that with further division produce connected Aal cells of 4, 8, 16 and then eventually haploid cells.6 However, this classical model has been challenged in recent years.11 Firstly, not all As cells are actual SSCs and transplantation assays show that only 10% of total As cells are able to populate the recipient testis, indicating that only a small number of As are actual stem cells.10 Additionally, Apr and Aal cells are not always transit-amplifying cells. In fact, they may act as colony-forming cells during tissue regeneration or even in normal situations, and have been proposed as potential stem cells when compared with actual stem cells. Through a tamoxifen-inducible pulse-label system, Nakagawa et al. proved that the number of colonogenic cells are more abundant than the actual stem cells after transplantation or regeneration and they determined that the transit-amplifying is the main contributor to the extra colony-forming cells.12 Furthermore, cells expressing c-Kit, the major signal marking spermatogonial differentiation, which can be detected in Apr, are also able to form colonies after transplantation, albeit with much lower abilities, demonstrating the strong regenerative ability among the undifferentiated spermatogonial cells.13 In 2010, Nakagawa et al.14 proposed an extended model, whereby “stemness” is present at all stages of undifferentiated spermatogonia, but the potential decreases with progression to later stages. Overall, these experiments largely demonstrate stemness among the undifferentiated type A spermatogonia, not the previous thought As. Moreover, lineage tracing and topology feature experiments have revealed heterogeneity among SSCs.15,16 Fragmentation of Aal and Apr cells occurs infrequently, but can reverse molecular expression changes during normal rounds of spermatogenesis and further increase study complexity.17,18
Another surprising characteristic of SSCs is the ability to convert into pluripotent cells without gene manipulation under certain culture conditions. This was first reported by Kanatsu-Shinohara et al.19 who found that cultured SSCs from neonatal mice occasionally convert into embryonic stem cell-like cells (ES-LCs) with similar phenotypes and the ability to form teratomas in all three embryonic germ layers after subcutaneous injection, thereby confirming functional pluripotency. However, the ES-LCs were only able to be produced from young mice, raising the possibility that SSCs lose their ability to convert into pluripotent cells as they mature. Nevertheless, soon after, several groups successfully generated ES-LCs from cultured SSCs of both young and adult mice using different models and conditions, further confirming that SSC can convert into pluripotent cells in certain culture conditions.20,21,22 Although the pluripotent cells of different sources show different behavior after transplantation, some generate teratomas while others contribute to germline chimeras following blastocyst injection.21 This is possibly due to culture of different SSC subpopulations using different isolation methods. The exact mechanism that SSC can convert into pluripotent cells remains unknown, but it is likely to involve the expression of Oct4, Sox 2, c-Myc, and Klf4, core transcription factors for induction of iPSs.23 Nanog, another core pluripotent transcription factor, is not detected in SSCs and may be the last barrier to pluripotency. It is feasible that Nanog is relatively easily activated under certain conditions (e.g., the expression of Sox2 and Oct4, since it's well known that Nanog expression can be activated by Oct4 and Sox2), and combined with other pluripotent factors, pluripotent cell behavior is induced.24 Intriguingly, the ability to convert into pluripotent cells was also seemed possible in human SSCs.25,26,27
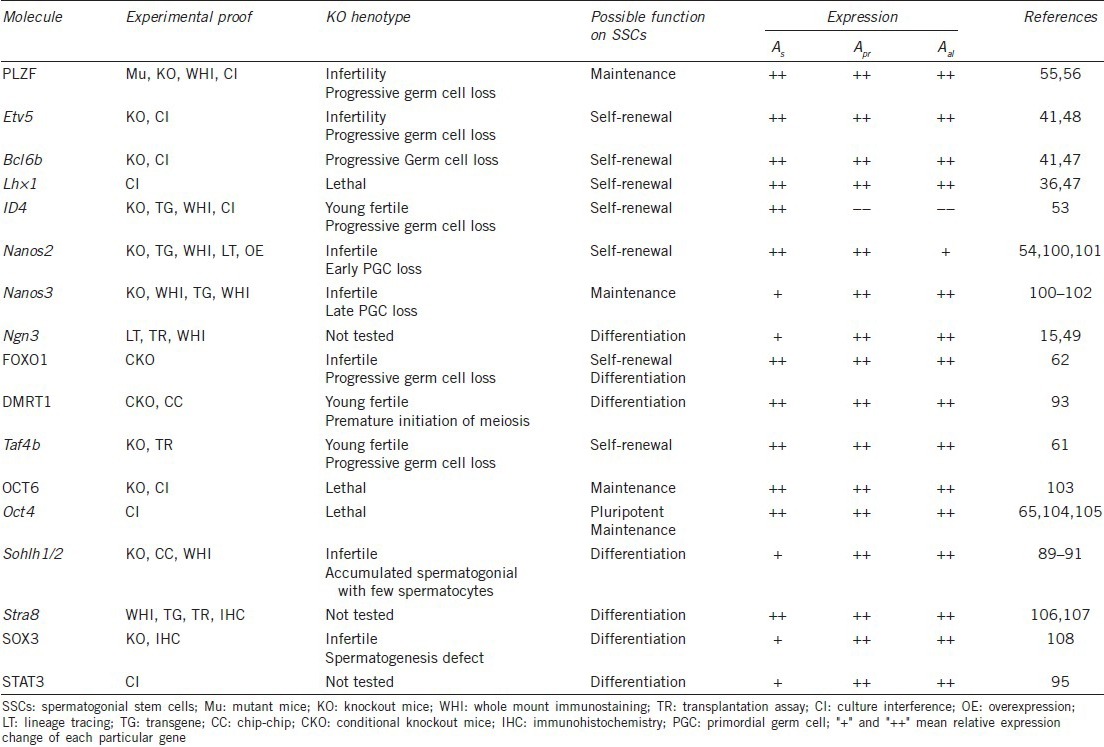
Spermatogonial stem cells are difficult to distinguish morphologically among undifferentiated spermatogonial cells, and until now, no specific marker molecules have been identified.28 SSC rarity (0.002% of total testis cells),29 determined by transplantation assay, hinders progress of SSC mechanistic studies.10 Encouragingly, a large number of genes related to SSC regulation have recently been identified. Surface markers, such as β1-integrin, a6-integrin, EPCAM, GFRA1, RET, and GPR125, are of relevance to SSCs and many are chosen as antigens to enrich SSCs by fluorescence-activated cell sorting or magnetic-activated cell sorting.22,30,31,32,33,34 Many transcription factors crucial for SSC self-renewal, maintenance, and differentiation have also been found (Table 1).
Table 1.
Important transcription factors for SSCs
REGULATION OF SPERMATOGONIAL STEM CELL SELF-RENEWAL AND DIFFERENTIATION
In male mammals, self-renewal and differentiation properties of SSCs lay the foundations for continual genesis of mature spermatozoa, and this process requires coordinated and balanced gene expression of both extrinsic and intrinsic factors. In this section, we discuss recent findings regarding the intrinsic and extrinsic factors regulating the SSC fates of self-renewal and differentiation (major in the mouse model).
Self-renewal and maintenance
Spermatogonial stem cell self-renewal provides the basis for spermatogenesis. SSCs with self-renewal problems lead to defects in their maintenance, with few advanced germ cells and occasional fertility in early days, but definite infertility later coupled with severe testicular atrophy. Genetically modified or mutant mice provide a powerful tool for identifying the molecules underlying regulation of SSC status. Here, we introduce in detail recent findings regarding SSC self-renewal.
Extrinsic factors
Of the extrinsic factors, GDNF, a molecule secreted by Sertoli cells in seminiferous tubules, was first identified with a crucial role in both in vivo development and in vitro culture. Meng et al. were the first to find that GDNF regulates undifferentiated spermatogonia in a dosage-dependent manner, specifically, gene-targeted mice with decreased expression of GDNF show age-dependent germ cell loss while mice overexpressing GDNF exhibit accumulation of undifferentiated spermatogonia.35 Based on these extraordinary findings, a long-term SSC culture system with the addition of GDNF, FGF2, EGF and LIF was successfully developed from neonatal mice.9 In this system, cultured cells termed GS cells proliferate for more than 2 years as confirmed by transplantation assay, implicating a critical role for GDNF signaling in SSC self-renewal. GDNF acts through a multicomponent receptor complex consisting of GFRA1 and RET, and depletion of both receptors results in the same phenotype as GDNF ablation, with SSCs self-renewal defect and germ cell depletion.36 Using combined in vitro culture and transplantation assays, several groups have presented evidence for activation of phosphoinositide 3-kinase (PI3K)-AKT by GDNF.37,38,39 Significant phosphorylation in ser-476 is detected 20 min after adding GDNF to culture medium following overnight starvation, while preincubation with a PI3K chemical inhibitor prevents this activation. Moreover, when AKT inhibitor IV is added to the medium, single cells, and rarely clusters are observed, indicating that AKT plays a central role in both self-renewal and survival.38 GDNF also activates Src family kinase (SFK) signaling, promoting self-renewal partly through AKT signaling. Incorporation of the SFK chemical inhibitor, SU6656, into culture medium results in slower cluster formation compared with controls. AKT ser476 phosphorylation is also downregulated. Compared with the SFK pathway, AKT appears to be dominant molecular for proliferation as AKT inhibitor completely prevents SSCs from proliferating while SFK inhibitor partly impedes proliferation. Intriguingly, He et al. found that the RAS/ERK1/2 pathway is also involved in GDNF-induced self-renewal and proliferation, via upregulation of CREB/activating transcription factor 1 family member phosphorylation and c-FOS transcription factor.40 Overall, GDNF promotes SSC self-renewal and proliferation through multiple signaling pathways, making the process complex.
Fibroblast growth factor 2 is another indispensable extrinsic factor in GS cell culture. Both GDNF and FGF2 activate MAP2K1, but FGF2 is a stronger stimulator, and an activated form of MAP2K1 can substitute for FGF2, confirming that FGF2 promotes proliferation through MAP2K1 pathways. Three transcription factors (Bcl6b, Etv5, and Lhx 1) act downstream of FGF2 pathways as they are downregulated following MAP2K1 inactivation.41 However, in a previous study, these three factors were shown to act downstream of GDNF through SFK signaling, suggesting that GDNF and FGF2 signaling may be partially redundant.38 In another study, they found that MEK (mitogen-activated protein kinase/ERK1 kinase), whose activation is a key signal for cell cycle progression, seems to be the downstream of FGF2. Inhibitor of MEK markedly suppresses GS cells proliferation, while GS cells with activated MEK successfully proliferate only with GDNF, indicating that MEK can be a substitution for FGF2.41 Intriguingly, Shinohara lab found that GS cells with activated H-RAS proliferate without extra cytokine additions, while GS cells with transduction of K-RAS, another activated form of RAS, which activates AKT pathway more strongly compared to H-RAS, fail to proliferate, indicating the importance of balanced pathway between AKT and MEK.42
In addition to these essential factors, others such as CSF1 and WNT5A, produced by Leydig and Sertoli cells, respectively, are essential constituents of the SSC niche and may act as enhancers for SSC self-renewal. Microarray transcript profiling, performed by Oatley et al. found approximately 200 genes expressing 10-fold or higher in THY1+ cells compared with THY1− depleted testis cells, including colony stimulating factor 1 receptor (Csf1r). Csf1r expression is highly enriched in a subpopulation of cultured THY1+ germ cells, implying a potential role for CSF1 in undifferentiated spermatogonial cells. Adding recombinant soluble CSF1 to chemically defined and serum-free culture medium caused a significant increase in SSC number in vitro without total THY1+ germ cell expansion, confirmed by functional transplantation assay.43 Thus, it is reasonable to speculate that CSF1 promotes SSC propagation only, and not the entire population of undifferentiated spermatogonial cells, although the mechanism remains unknown. Wnt5a expression is confined to Sertoli cells in mouse testis with all WNT5A receptors present on the surface of undifferentiated spermatogonia, and since β-catenin-dependent Wnt signaling promotes self-renewal of various stem cell types, it's reasonable that this signal is involved in SSCs self-renewal. Although transgenic reporter mice showed that β-catenin-dependent signaling was not active in SSCs in vitro and most spermatogonia in vivo, WNT5A antagonist significantly reduced SSC colonies in vitro, while exogenous WNT5A enhanced SSC colony formation in a β-catenin-independent manner, indicating a potential role for WNT5A in enhancing SSCs self-renewal.44 Our study showed that short-type PB-cadherin promoted self-renewal of SSCs via activating Janus kinase/signal transducer and activator of transcription (STAT) and PI3K/AKT, and blocking transforming growth factor-beta1 signaling.45 Morimoto et al.46 reported that reactive oxygen species are required for mouse SSC self-renewal.
Intrinsic factors
According to the response to GDNF, there are two categories of transcription factors: GDNF-inducible and-independent. Microarray analysis identified six transcripts (Bcl6b, Etv5, Lhx 1, Egr2/3, and Tspan8) that were first downregulated with GDNF removal from culture medium, and then significantly up-regulated with addition to the medium. Culture intervention by siRNA and knockout experiments implicated Bcl6b as an important maintenance factor.47 Etv5 ablation in mice causes infertility, although the first wave of spermatogenesis is not impaired, the following waves are severely impaired.48 The first wave of spermatogenesis is a distinct process independent of SSC self-renewal while subsequent spermatogenesis relies on SSC amplification.49 SiRNA-mediated ablation of Etv5 in THY1+ cultured spermatogonial cells results in reduced SSC numbers, as determined by functional assay.38 Microarray analysis shows that RNAi depletion of Etv5 leads to downregulation of Bcl6b, Lhx 1, and Brachyury, all important molecules for SSC proliferation.50 Interestingly, Chen et al. found that Etv5 in Sertoli cells promotes many adhesion molecules (e.g. CXCL-12, CXCR4, and CCL9), some are known for regulating the SSCs niche and indicative of a role for Etv5 in niche formation.48,51,52 ID4, an inhibitor of DNA binding protein 4, is another important transcription factor induced by GDNF. ID4 is unique in that it is exclusively detected in As spermatogonia. ID4 knockout mice exhibit age-dependent germ cell depletion, suggesting a role in SSC maintenance.53 Nanos2, a zinc-finger RNA-binding protein, plays an important role in SSC maintenance. Nanos2 knockout mice display progressive germ cell loss, while Nanos2 overexpression in mice causes accumulation of promyelocytic leukemia zinc finger (PLZF+) spermatogonia.54 Although many downstream factors of GDNF signaling have emerged, the underlying gene networks remain largely undefined and require further investigation.
Spermatogonial stem cell maintenance can be regulated independent of GDNF, and in this regards, several nuclear factors have been identified. PLZF was first described as an important maintenance factor specifically expressed in undifferentiated spermatogonia. A nonsense mutation in Zbtb16, the gene encoding PLZF, causes infertility and progressive germ cell loss in mice,55 and appears to promote SSC proliferation, not survival, as no significant apoptosis is detected. Additionally, SSCs from PLZF-null mice cannot repopulate the testis when transplanted into germ cell deficient mice,56 but can be cultured in medium with excessive GDNF, indicating PLZF and GDNF work independently. However, the exact mechanism by which PLZF maintains the SSC pool is not clear. Filipponi et al. found that PLZF binds to the c-Kit promoter region and thus directly represses the expression of c-Kit, a marker for spermatogonia differentiation.57 Moreover, Hobbs et al. showed that SALL4 antagonizes PLZF, and increasing SALL4 expression leads to c-Kit transcription.58 Relative levels and mutual effects of SALL4 and PLZF determine SSC status. Another transcription factor directly affected by PLZF is Redd1, an inhibitor of mammalian target of rapamycin complex 1 (mTORC1). In stem cells, mTORC1 hyperactivity leads to an abnormal translation of downstream targets and eventually results in cell exhaustion.59,60 Additionally, activated mTORC1 in SSCs represses expression of the GDNF receptor, RET. Another important GDNF-independent transcription factor, Taf4b, although not exclusively expressed in testis, is detected in both spermatogonia and Sertoli cells, and shows an essential role in SSC maintenance, with Taf4b loss in mice inducing age-dependent germ cell loss.61 Transplantation of normal SSCs into Taf4b-depleted testis induces normal and sustained spermatogenesis, suggesting that Taf4b behaves in a cell-autonomous manner. FOXO1 has also been shown to play an indispensable role in SSC maintenance, and may be at the upper levels of GDNF-independent transcription regulation. FOXO1 knockout mice exhibit similar defects of SSC maintenance to the previous maintenance factors (e.g., PLZF, Taf4b, Etv5) with progressive age-dependent decline in spermatogenesis, however microarray analysis revealed decreasing expression of SSC maintenance genes (e.g., Lhx 1, c-Ret) and increasing expression of SSC differentiating genes (e.g., Egr2, Tex 19).62 Furthermore, in gonocytes, FOXO1 is located within the cytoplasm but resides in nuclei of spermatogonia, indicating a change in subcellular location during gonocyte transition to SSCs. Previous studies using transgenic models show that this shift is controlled by AKT signaling.63,64 Conditional knockout of Pten or Pdk1 (repressor or stimulator of AKT activity, respectively) both result in germ cell deficiency in mice. Conditional Pten ablation causes AKT hyperactivity, FOXO1 phosphorylation, and subsequent cytoplasmic localization of FOXO1 in spermatogonia, while Pdk1 conditional knockout shows FOXO1 nuclear preference in gonocytes rather than spermatogonia. This indicates that AKT activity is important to normal FOXO1 function.62 Other GDNF-independent transcription factors, Oct4 for instance, though much less well understood, they also play an important role in SSC self-renewal.65
Differentiation
In this section, we discuss the extrinsic and intrinsic factors contributing to SSC differentiation.5 Two types of differentiation are involved here. One is SSC conversion to progenitors or transit amplifying cells, and the other is generation of differentiated spermatogonia from undifferentiated spermatogonia. It is a challenge to identify factors in the former process since progenitors share the same phenotype as SSCs, and can reverse to SSCs in certain cases. The second differentiation process is retinoic acid (RA) dependent and largely marked by expression of c-Kit, the receptor for KITL known as stem cell factor (SCF).
Extrinsic factors
It has been a while since the finding that mice receiving a long-term vitamin A deficient diet develop spermatogenesis defects, with only undifferentiated spermatogonial and Sertoli cells found in the seminiferous epithelium. Replacement of vitamin A in the diet counters this spermatogenic arrest.66 Vitamin A is normally stored and transported as retinol, and RA biosynthesis undergoes two sequential oxidative steps in both Sertoli and spermatogenetic cells via alcohol dehydrogenases and retinaldehyde dehydrogenases (RALDH).67 Bis-[dichloroacetyl]-diamines, WIN 18,446, inhibits spermatogenesis and acts as an inhibitor of RALDH2, an essential enzyme for RA biosynthesis. Adding WIN 18,446 to organ culture medium causes a germ cell developmental defect compared with controls.68 RA receptor (RAR) is the receptor for RA, and when formed as a complex they can bind to RA response elements and induce transcriptional changes, including expression of the meiotic initiator gene, Stra8. In organ culture, the RAR antagonists, BMS-204493 and AGN193109, inhibit expression of the meiotic marker genes, Stra8, Scp3, and Dmc1, while RA agonists do not.69,70 In males, RA is degraded by CYP26B1, and RA degradation may protect from premeiosis during embryonic development. Although Cyp26b1 knockout mice are embryonic lethal, high Stra8 and Scp3 expression is detected, and even more surprisingly, the pachytene stage of meiosis I germ cells are present at 16.5 dpc.70,71 Besides, in vitro study proved that RA induces differentiation of GS cells and down-regulation the expression of Oct4 and PLZF.65 Taking all these studies together, RA is an indispensable extrinsic factor for normal differentiation of spermatogonia, and Stra8 is the main target for the meiosis initiation.
Stem cell factor is also known as KIT ligand or the steel factor and is encoded at the Steel locus (Sl). Mutation at this site results in disruption of hematopoiesis, melanogenesis, and gametogenesis. Similarly, c-Kit mutation results in the same phenotype. It has been shown that SCF is a ligand for c-Kit, a tyrosine kinase receptor. The SCF/c-Kit interaction is critical for spermatogonia survival and proliferation, differentiation, and subsequent meiosis.72,73 Here, we briefly introduce its role in proliferation and differentiation of spermatogonia. In the testis, SCF is secreted by Sertoli cells while c-Kit is expressed in differentiated type A and B spermatogonia.74 In Sl17H/Sl17H mice, with SCF splicing defects, As, Apr and Aal undifferentiated spermatogonia are noted according to their morphology and arrangement. However, no advancing germ cells exist, not even Aal spermatogonia expressing c-Kit, suggesting arrest of spermatogonia differentiation due to SCF/c-Kit signaling defect.75 Intraperitoneal injection of anti-c-Kit antibody in adult mice leads to reduced proliferation of differentiating spermatogonia, whereas undifferentiated spermatogonia are unaffected.76 Sl/Sld and Sl17H/Sl17H mice are both SCF mutant, while W/Wv mice are c-Kit mutant, and all of them are infertile. Transplantation of green fluorescent protein-labeled undifferentiated spermatogonia into the testis of Sl/Sld or Sl17H/Sl17H mice results in proliferation of undifferentiated spermatogonia.75 W mutant mice receiving the same transplantation have spermatogenesis restored, demonstrating the essential role of SCF/c-Kit in spermatogonia differentiation and proliferation.77 The PI3K-AKT pathway may be involved in SCF/c-Kit induced proliferation and differentiation, as shown by both in vitro and in vivo experiments.78 Blume-Jensen et al. took advantage of the Cre-loxP system to mutate the codon for Tyr719 within the SCF/c-Kit binding site for PI3k. This mutant shows significantly decreased (90%) PI3K − dependent activation of AKT. Moreover, homozygous mutant mice are infertile owing to a spermatogenesis defect, with an initial reduced proliferation rate and then widespread apoptosis occurring in undifferentiated spermatogonia.79 Further experiments have found that SCF/c-Kit promotes rapamycin-sensitive proliferation, through activation of p70s6k by PI3K-AKT and then the cell progression transcription factors, cyclin D3 and RB.80 Co-transfection of constitutively active v-AKT or dominant negative AKT-K179M (using the p70s6k plasmid) in spermatogonia cultured in serum-free medium, remarkably promotes or inhibits, respectively, p70s6k phosphorylation and can be blocked by rapamycin, suggesting that AKT activates p70s6k via FRAP/mTOR kinase. Dolci et al. reported that SCF administration transiently activates ERK1/2 kinase and sustained activation of PI3K-dependent AKT kinase, indicating that MEK signaling also plays a role in spermatogonia proliferation. Inhibition of either signaling pathway in spermatogonia completely blocks SCF-induced proliferation, showing both signaling pathways are indispensable. Additionally, both pathways converge when cyclinD3 translocates from the cytoplasm to the nucleus.81 As opposed to the proliferation, it is still not clear how the differentiation marker c-Kit is induced.
Other factors secreted by Sertoli cells, such as BMP4 and Activin A, may also be involved in extrinsic regulation of differentiation.82 In a 7-day spermatogonia primary culture system, BMP4 or activin A decrease colony forming ability after transplantation, while total cell number remains unchanged, suggesting these two factors promote differentiation.83 Accordingly, Carlomagno et al. found that in rat SSC lines, BMP4 upregulated expression of c-Kit. Furthermore, A time-course DNA micro-array analysis highlights the pivotal role for adhesion molecules in BMP4-induced differentiation, with many adhesion molecules upregulated, thereby facilitating migration out of the niche.84 The BMP4 receptor is a serine/threonine kinase, exclusively expressed in spermatogonia. Adding BMP4 to cultured spermatogonia induces phosphorylation, nuclear translocation of the SMAD4/5 heteromeric complex, and the formation of a DNA-binding complex with the various transcription co-activator p300/CBP.85
Intrinsic factors
Neurogenin 3 (Ngn3), a bHLH family transcription factor, is well known for its role in promoting differentiation of the endocrine pancreas, enteroendocrine cells, and other systems. In the testis, Ngn3 expression is restricted to undifferentiated spermatogonia, indicating a potential role in SSC regulation.86 In vitro culture of THY1+ cells exposed to GDNF suppresses Ngn3 expression, and transient Ngn3 knockdown by siRNA enhances colony formation after transplantation whereas the total germ cell number is not changed, indicating a potential role in SSC differentiation to progenitors. Using a shRNA approach that stably knocks down Ngn3 caused impaired spermatogenesis after transplantation, although patches of As, Apr, and Aal cells are present, showing that Ngn3 is indispensable for SSC differentiation.87 Lineage tracing experiments provided further evidence. A tamoxifen-inducible Ngn3-Cre system allows the fate of labeled cells to be traced by pulse-chase analysis. Mating these mice with mice carrying the LacZ gene activated by Cre excision, Nakagawa et al. reported that predominantly, labeled cells progressed to advanced stages of spermatogenesis and only a few patches remained in the testis 2 months after tamoxifen administration, the period for a spermatogenesis cycle, arguing that most Ngn3+ spermatogonia are progenitors and committed to differentiation.12 It is still not clear what the downstream effector of Ngn3 signaling is, and how it promotes differentiation, since its expression is parallel to that of c-Ret.86 It is known, however, to be regulated by several established differentiation factors, including STAT and Sohlh1/2, which are discussed below.
Sohlh1 is also a bHLH family member, and its expression is limited to premeiotic germ cells in both males and females, indicating a potential role in germ cell development.88 Sohlh1 knockout mice are infertile with severely decreased differentiating spermatogonia, whereas PLZF+ spermatogonia are slightly increased, evidence for its important role in differentiation.89 Interestingly, although there are severely decreased spermatocytes at 10 days postnatal, increasing spermatocytes are detected at 3 weeks, with a few still detected at 7 weeks of age. This transient leaky phenotype is possible owing to compensation by a homologous transcription factor, Sohlh2, since a two-fold higher expression of Sohlh2 is observed in Sohlh1 knockout mice. Like Sohlh1, Sohlh2 also exhibits germ cell specific expression, limited to a subpopulation of undifferentiated and the majority of differentiating spermatogonia. Sohlh2-deficient mice exhibit almost the same phenotype as Sohlh1 knockout mice, raising the possibility of a related function.90 Suzuki et al. found that Sohlh1 and Sohlh2 not only heterodimerize with each other, but also individually homodimerize. Further, they found via chip analysis that these two genes bind to each other's promoter, elaborating their cross-regulation.91 It is not surprising therefore that the Sohlh1 and Sohlh2 double knockout mouse is infertile, and exhibits a similar phenotype as single knockout mice. Gene arrays using double or single knockout mice reveal molecular features of differentiation at both earlier and later steps, suggesting that Sohlh induces spermatogonial differentiation. Increasing the expression of Gfra1, c-Ret, Nanos2, and Oct4, traditional maintenance factors, were found in Sohlh knockout mice, while expression of the earlier differentiation factors, SOX3 and Ngn3, was decreased. Chip analysis confirms these findings, as either Sohlh1 or Sohlh2 is able to bind to promoters of these genes. Simultaneously, c-Kit (the marker gene for Aal to A1 spermatogonia transition) is significantly decreased in Sohlh knockout mice. Chip and electrophoretic mobility shift assay analysis show that both Sohlh1 and Sohlh2 are able to bind to the E-box-containing Kit promoter region. Transfection of Sohlh1 and/or Sohlh2 enhanced endogenous c-Kit expression, with these two genes functioning independently and cooperatively.92 The upstream regulator of Sohlh remains largely unknown, and DMRT1 is a possibility, which we will discuss later. Sohlh is an important intrinsic factor that regulates both SSC and spermatogonia differentiation. Other intrinsic factors, including DMRT1, SOX3, and STAT3 are also involved in SSC differentiation. Through restricting spermatogonial RA responsiveness, directly inhibiting Stra8 expression, and activating Sohlh1 transcription, DMRT1 guarantees continuous and sufficient sperm production.93 DMRT1 knockout mice exhibit the premature meiosis initiation and Stra8 expression in early stages of spermatogonia. ChIP-chip analysis found that Stra8 and Sohlh1 were DMRT1 targets, and DMRT1 may perform its role in SSC differentiation through Stra8 inhibition and Sohlh1 activation.94 Transient STAT3 knockdown in cultured THY1+ cells causes increased SSCs, but not total cell number, indicating a possible role for STAT3 in SSC differentiation.95 Stable shRNA-mediated STAT3 knockout, results in failed spermatogenesis following transplantation into recipient mice. The STAT3 target remains largely unknown, but Ngn3 is a possible one. STAT3 inactivation in cultured THY1+ cells exposed to GDNF, a key factor for self-renewal, suppressed Ngn3 gene expression. More direct evidence comes from the finding that STAT3 is able to bind to the distal Ngn3 promoter/enhancer region in vitro.96 Compared with self-renewal, more unknowns remain regarding the signaling pathway(s) underlying SSC differentiation. Developing an in-vitro culture system may greatly facilitate this (Figures 1 and 2).
Figure 1.
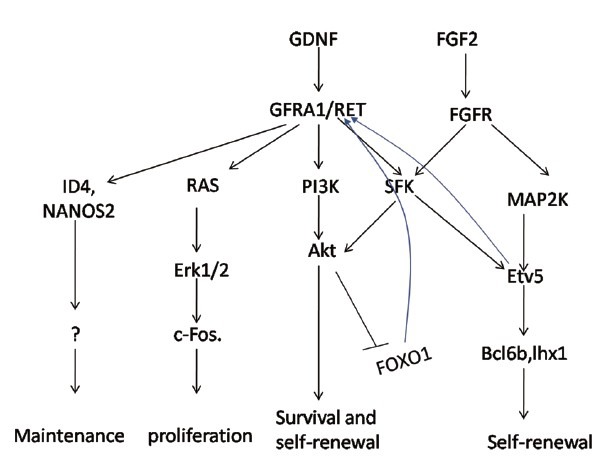
Signaling pathway of the extrinsic factors, glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factor 2 (FGF2). GDNF is the main extrinsic factor for maintaining undifferentiated spermatogonia. FGF2 signaling interacts with GDNF and is more likely to enhance proliferation rate.
Figure 2.
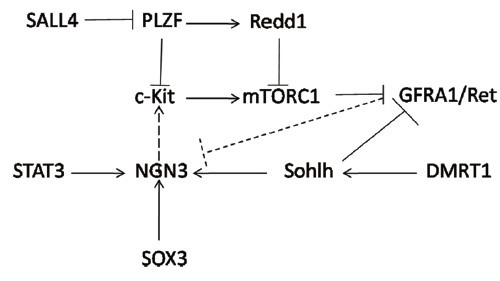
Transcription factor interaction. Solid arrows/lines represent confirmed interactions, and dotted lines/arrows indicate predicted interactions.
PERSPECTIVES
The last decade has witnessed great progress in reproductive research, with technology improvements such as in vitro fertilization and intracytoplasmic sperm injection. However, for those with early stage gametogenesis failure, these technologies cannot help. SSCs are the origin of spermatogenesis, therefore, normal SSC function is of crucial importance and any disruption can result in severe infertility. To help solve this clinical problem, it is important to determine the mechanisms underlying normal functioning SSCs. Many important molecules regulating SSC self-renewal and differentiation have been identified; however, the exact mechanisms remain largely unknown. Difficulties in distinguishing SSCs from progenitors greatly hinder our understanding. It is unclear if there is a specific stage during spermatogonial development that once passed, marks unidirectional differentiation, or if no specific stage exists and a small part of undifferentiated spermatogonia maintain stem potential. If this stage does exist, identification of stage specific markers becomes critical and will aid our understanding.
Proliferation and differentiation of stem cells are regulated intrinsically by the stem cell itself and extrinsically by factors in the stem cell's niche. It is a sophisticated balance ensuring life-long spermatogenesis. Despite the number of extrinsic and intrinsic factors discussed above, SSC fate determination remains uncertain. Currently, the importance of GDNF-dependent signaling pathways is gradually becoming apparent, although GDNF-independent pathways remain to be clarified. The SALL4-PLZF-REDD1-mTORC1 interaction is thought to be an important circuit for maintenance of the undifferentiated state, yet PLZF and SALL4 regulation remains elusive. The maintenance factor, Taf4b, is not affected by the known maintenance signaling pathway, raising the possibility that there are more unknown signals and undiscovered core transcription factors. Culture intervention combined with microarray analysis may help investigate this problem.
At present, an increasing number of people are suffering from infertility because of the cryptorchid testis and acquired the infertility after chemotherapy or radiotherapy in cancer treatment. For those with normal SSC function, a prior testis biopsy combined with in vitro proliferation and transplantation may solve these problems.97 Human SSC culture enabling SSC amplification is now possible; however lack of a differentiation culture system hinders its clinical application. Many are trying to develop a system that supports the entire process of spermatogenesis, but attempts have so far failed. Exposure of cultured SSCs to RA increases the proportion of germ cells expressing c-Kit, nevertheless, premature meiosis affirming the incomplete spermatogenesis restricts its potential application.65 In 2012, Sato et al. developed an organ culture system that supported the whole spermatogenesis process, and produced functional sperm in about 2 months.98 However, the demand for organ fragments for those patients with defected spermatogenesis inhibits its potential application. It is presumed that spermatogenesis is highly conserved among rodent animals as demonstrated by spermatogenesis following transplantation of hamster SSCs into mouse testis.99 Xenotransplantation of human SSCs to primates may solve the problem, but needs further validation. To better improve culture systems supporting differentiation, greater understanding of molecular pathways is of paramount importance while, in reverse, such culture systems will greatly facilitate mechanistic studies.
AUTHOR CONTRIBUTIONS
XXM, JWa and JW wrote and revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by National Basic Research Program of China (grant numbers 2013CB967401; http://www.most.gov.cn) and the National Nature Science Foundation of China (grant numbers 81370675, 81200472 and 81421061; http://www.nsfc.gov.cn), and Shanghai Jiao Tong University Medicine-Engineering Fund (grant numberYG2013ZD04; http://www.sjtu.edu.cn).
REFERENCES
- 1.Zou K, Yuan Z, Yang Z, Luo H, Sun K, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Jiang M, Bi H, Chen X, He L, et al. Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol. 2014;6:164–71. doi: 10.1093/jmcb/mju004. [DOI] [PubMed] [Google Scholar]
- 3.Xie W, Wang H, Wu J. Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep. 2014;4:5580. doi: 10.1038/srep05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Yang Z, Yang Y, Wang S, Shi L, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–41. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- 5.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–98. [PubMed] [Google Scholar]
- 6.de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- 7.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–7. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanatsu-Shinohara M, Inoue K, Miki H, Ogonuki N, Takehashi M, et al. Clonal origin of germ cell colonies after spermatogonial transplantation in mice. Biol Reprod. 2006;75:68–74. doi: 10.1095/biolreprod.106.051193. [DOI] [PubMed] [Google Scholar]
- 9.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 10.Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–87. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- 11.Hara K, Nakagawa T, Enomoto H, Suzuki M, Yamamoto M, et al. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658–72. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, et al. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS One. 2009;4:e7909. doi: 10.1371/journal.pone.0007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–7. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida S, Nabeshima Y, Nakagawa T. Stem cell heterogeneity: actual and potential stem cell compartments in mouse spermatogenesis. Ann N Y Acad Sci. 2007;1120:47–58. doi: 10.1196/annals.1411.003. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–31. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.de Rooij DG, Griswold MD. Questions about spermatogonia posed and answered since 2000. J Androl. 2012;33:1085–95. doi: 10.2164/jandrol.112.016832. [DOI] [PubMed] [Google Scholar]
- 18.Lok D, de Rooij DG. Spermatogonial multiplication in the Chinese hamster. III. Labelling indices of undifferentiated spermatogonia throughout the cycle of the seminiferous epithelium. Cell Tissue Kinet. 1983;16:31–40. [PubMed] [Google Scholar]
- 19.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–12. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Izadyar F, Pau F, Marh J, Slepko N, Wang T, et al. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771–84. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]
- 21.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 22.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, et al. Generation of functional multipotent adult stem cells from GPR125+germline progenitors. Nature. 2007;449:346–50. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–49. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, et al. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–26. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, et al. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158–67. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- 28.Chuykin I, Stauske M, Guan K. In: Regenerative Medicine. Rostock: Springer; 2013. Spermatogonial stem cells; pp. 219–49. [Google Scholar]
- 29.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60:1429–36. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100:6487–92. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson R, Schaible K, Heasman J, Wylie C. Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil. 1999;116:379–84. doi: 10.1530/jrf.0.1160379. [DOI] [PubMed] [Google Scholar]
- 32.Shinohara T, Avarbock MR, Brinster RL. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96:5504–9. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanatsu-Shinohara M, Toyokuni S, Shinohara T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol Reprod. 2004;70:70–5. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- 34.Ebata KT, Zhang X, Nagano MC. Expression patterns of cell-surface molecules on male germ line stem cells during postnatal mouse development. Mol Reprod Dev. 2005;72:171–81. doi: 10.1002/mrd.20324. [DOI] [PubMed] [Google Scholar]
- 35.Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 36.Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–21. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, et al. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–9. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 38.Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–51. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, et al. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–78. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishii K, Kanatsu-Shinohara M, Toyokuni S, Shinohara T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. 2012;139:1734–43. doi: 10.1242/dev.076539. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, et al. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell. 2009;5:76–86. doi: 10.1016/j.stem.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–9. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh JR, Zhang X, Nagano MC. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci. 2011;124:2357–66. doi: 10.1242/jcs.080903. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Zhang Y, Tian GG, Zou K, Lee CM, et al. Short-type PB-cadherin promotes self-renewal of spermatogonial stem cells via multiple signaling pathways. Cell Signal. 2008;20:1052–60. doi: 10.1016/j.cellsig.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, et al. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12:774–86. doi: 10.1016/j.stem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103:9524–9. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–4. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 50.Wu X, Goodyear SM, Tobias JW, Avarbock MR, Brinster RL. Spermatogonial stem cell self-renewal requires ETV5-mediated downstream activation of Brachyury in mice. Biol Reprod. 2011;85:1114–23. doi: 10.1095/biolreprod.111.091793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon L, Ekman GC, Garcia T, Carnes K, Zhang Z, et al. ETV5 regulates sertoli cell chemokines involved in mouse stem/progenitor spermatogonia maintenance. Stem Cells. 2010;28:1882–92. doi: 10.1002/stem.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang QE, Kim D, Kaucher A, Oatley MJ, Oatley JM. CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J Cell Sci. 2013;126:1009–20. doi: 10.1242/jcs.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod. 2011;85:347–56. doi: 10.1095/biolreprod.111.091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–8. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 55.Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–52. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 56.Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–9. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 57.Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, et al. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–81. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobbs RM, Fagoonee S, Papa A, Webster K, Altruda F, et al. Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell. 2012;10:284–98. doi: 10.1016/j.stem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 60.Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–9. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falender AE, Freiman RN, Geles KG, Lo KC, Hwang K, et al. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. 2005;19:794–803. doi: 10.1101/gad.1290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011;121:3456–66. doi: 10.1172/JCI57984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 64.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, et al. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–37. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 66.van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod. 1990;43:363–67. doi: 10.1095/biolreprod43.3.363. [DOI] [PubMed] [Google Scholar]
- 67.Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod. 2012;86:35. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, et al. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod. 2011;84:957–65. doi: 10.1095/biolreprod.110.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–9. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 71.MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology. 2007;148:4560–7. doi: 10.1210/en.2007-0492. [DOI] [PubMed] [Google Scholar]
- 72.Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, et al. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl. 1993;119:125–37. [PubMed] [Google Scholar]
- 73.Mithraprabhu S, Loveland KL. Control of KIT signalling in male germ cells: what can we learn from other systems? Reproduction. 2009;138:743–57. doi: 10.1530/REP-08-0537. [DOI] [PubMed] [Google Scholar]
- 74.Vincent S, Segretain D, Nishikawa S, Nishikawa SI, Sage J, et al. Stage-specific expression of the Kit receptor and its ligand (KL) during male gametogenesis in the mouse: a Kit-KL interaction critical for meiosis. Development. 1998;125:4585–93. doi: 10.1242/dev.125.22.4585. [DOI] [PubMed] [Google Scholar]
- 75.de Rooij DG, Okabe M, Nishimune Y. Arrest of spermatogonial differentiation in jsd/jsd, Sl17H/Sl17H, and cryptorchid mice. Biol Reprod. 1999;61:842–7. doi: 10.1095/biolreprod61.3.842. [DOI] [PubMed] [Google Scholar]
- 76.Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–99. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 77.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blume-Jensen P, Janknecht R, Hunter T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol. 1998;8:779–82. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- 79.Blume-Jensen P, Jiang G, Hyman R, Lee KF, O’Gorman S, et al. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3’- kinase is essential for male fertility. Nat Genet. 2000;24:157–62. doi: 10.1038/72814. [DOI] [PubMed] [Google Scholar]
- 80.Feng LX, Ravindranath N, Dym M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J Biol Chem. 2000;275:25572–6. doi: 10.1074/jbc.M002218200. [DOI] [PubMed] [Google Scholar]
- 81.Dolci S, Pellegrini M, Di Agostino S, Geremia R, Rossi P. Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J Biol Chem. 2001;276:40225–33. doi: 10.1074/jbc.M105143200. [DOI] [PubMed] [Google Scholar]
- 82.de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72:580–5. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- 83.Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68:2207–14. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- 84.Carlomagno G, van Bragt MP, Korver CM, Repping S, de Rooij DG, et al. BMP4-induced differentiation of a rat spermatogonial stem cell line causes changes in its cell adhesion properties. Biol Reprod. 2010;83:742–9. doi: 10.1095/biolreprod.110.085456. [DOI] [PubMed] [Google Scholar]
- 85.Pellegrini M, Grimaldi P, Rossi P, Geremia R, Dolci S. Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116:3363–72. doi: 10.1242/jcs.00650. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, et al. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–58. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 87.Kaucher AV, Oatley JM. Neurogenin3 is a regulator of mouse spermatogonial stem cell differentiation. Biol Reprod. 2009;80:146. doi: 10.1095/biolreprod.109.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rajkovic A, Yan MS, Klysik M, Matzuk M. Discovery of germ cell-specific transcripts by expressed sequence tag database analysis. Fertil Steril. 2001;76:550–4. doi: 10.1016/s0015-0282(01)01966-5. [DOI] [PubMed] [Google Scholar]
- 89.Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006;294:161–7. doi: 10.1016/j.ydbio.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 90.Hao J, Yamamoto M, Richardson TE, Chapman KM, Denard BS, et al. Sohlh2 knockout mice are male-sterile because of degeneration of differentiating type A spermatogonia. Stem Cells. 2008;26:1587–97. doi: 10.1634/stemcells.2007-0502. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, et al. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol. 2012;361:301–12. doi: 10.1016/j.ydbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barrios F, Filipponi D, Campolo F, Gori M, Bramucci F, et al. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci. 2012;125:1455–64. doi: 10.1242/jcs.092593. [DOI] [PubMed] [Google Scholar]
- 93.Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, et al. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19:612–24. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy MW, Sarver AL, Rice D, Hatzi K, Ye K, et al. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc Natl Acad Sci U S A. 2010;107:13360–5. doi: 10.1073/pnas.1006243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oatley JM, Kaucher AV, Avarbock MR, Brinster RL. Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling. Biol Reprod. 2010;83:427–33. doi: 10.1095/biolreprod.109.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaucher AV, Oatley MJ, Oatley JM. NEUROG3 is a critical downstream effector for STAT3-regulated differentiation of mammalian stem and progenitor spermatogonia. Biol Reprod. 2012;86(1):164–11. doi: 10.1095/biolreprod.111.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vlajkovic S, Cukuranovic R, Bjelakovic MD, Stefanovic V. Possible therapeutic use of spermatogonial stem cells in the treatment of male infertility: a brief overview. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/374151. 374151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protoc. 2013;8:2098–104. doi: 10.1038/nprot.2013.138. [DOI] [PubMed] [Google Scholar]
- 99.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod. 1999;60:515–21. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- 100.Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, et al. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–41. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki A, Tsuda M, Saga Y. Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development. Development. 2007;134:77–83. doi: 10.1242/dev.02697. [DOI] [PubMed] [Google Scholar]
- 102.Lolicato F, Marino R, Paronetto MP, Pellegrini M, Dolci S, et al. Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol. 2008;313:725–38. doi: 10.1016/j.ydbio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Wu X, Oatley JM, Oatley MJ, Kaucher AV, Avarbock MR, et al. The POU domain transcription factor POU3F1 is an important intrinsic regulator of GDNF-induced survival and self-renewal of mouse spermatogonial stem cells. Biol Reprod. 2010;82:1103–11. doi: 10.1095/biolreprod.109.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 105.Pesce M, Wang X, Wolgemuth DJ, Schöler H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 106.Antonangeli F, Giampietri C, Petrungaro S, Filippini A, Ziparo E. Expression profile of a 400-bp Stra8 promoter region during spermatogenesis. Microsc Res Tech. 2009;72:816–22. doi: 10.1002/jemt.20724. [DOI] [PubMed] [Google Scholar]
- 107.Oulad-Abdelghani M, Bouillet P, Décimo D, Gansmuller A, Heyberger S, et al. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–77. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raverot G, Weiss J, Park SY, Hurley L, Jameson JL. So×3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Dev Biol. 2005;283:215–25. doi: 10.1016/j.ydbio.2005.04.013. [DOI] [PubMed] [Google Scholar]


