Abstract
There have been significant breakthroughs over the past decade in the development and use of pluripotent stem cells as a potential source of cells for applications in regenerative medicine. It is likely that this methodology will begin to play an important role in human clinical medicine in the years to come. This review describes the plasticity of one type of pluripotent cell, spermatogonial stem cells (SSCs), and their potential therapeutic applications in regenerative medicine and male infertility. Normally, SSCs give rise to sperm when in the testis. However, both human and murine SSCs can give rise to cells with embryonic stem (ES) cell-like characteristics that can be directed to differentiate into tissues of all three embryonic germ layers when placed in an appropriate inductive microenvironment, which is in contrast to other postnatal stem cells. Previous studies have reported that SSCs expressed an intermediate pluripotent phenotype before differentiating into a specific cell type and that extended culture was necessary for this to occur. However, recent studies from our group using a tissue recombination model demonstrated that SSCs differentiated rapidly into another tissue, in this case, prostatic epithelium, without expression of pluripotent ES cell markers before differentiation. These results suggest that SSCs are capable of directly differentiating into other cell types without going through an intermediate ES cell-like stage. Because SSCs do not require reprogramming to achieve a pluripotent state, they are an attractive source of pluripotent cells for use in regenerative medicine.
Keywords: pluripotency, regenerative medicine, spermatogenesis, testis
INTRODUCTION
Spermatogonial stem cells (SSCs) are relatively rare cells that are found along the basement membrane of the seminiferous tubule in the testis, and their progeny give rise to all stages of the spermatogenic lineage. Our understanding of how spermatogonia undergo the complex structural, molecular and functional changes that eventually result in the generation of fully functional spermatozoa has evolved during the past 3 centuries.
The initial histological techniques used to study spermatogenesis in the 19th century have been supplemented over the years by an increasingly sophisticated array of techniques to identify, isolate and study SSCs. Early studies were directed toward developing a descriptive and mechanistic understanding of how spermatogenesis occurred. However, our recent ability to obtain and manipulate SSCs1,2,3,4 has led to a better understanding of the developmental capabilities of these cells and has opened up the possibility of therapeutic applications in human reproductive medicine and other fields.
Therapeutic treatment with SSCs to restore fertility is now feasible for men in whom spermatogenesis has been impaired or lost due to chemotherapy or other causes. The recent demonstration by Hermann et al.1 that autologous or allogeneic SSCs could be used to restore spermatogenesis in nonhuman primates where endogenous spermatogenesis had been ablated by use of chemotherapeutic drugs indicates that this type of approach might soon be possible in humans. The possibilities of successfully producing sperm in vitro as a therapeutic approach for certain types of infertility was emphasized by recent work from Sato et al.5 showing that fully mature sperm capable of fertilization can be produced in vitro from early-stage mouse germ cells. These advances have implications not only for human medicine, but also for applications that facilitate reproduction in threatened or endangered species and could greatly advance conservation efforts.
The use of transgenic mice has transformed many fields of biology and allowed fundamental questions to be addressed that could not be tested otherwise. Methodologies for producing transgenic animals through the use of SSCs have been pursued for many years, and recent results indicate that this technique is not only feasible but may have unique advantages over other commonly used approaches.6
Over the past decade, a rapidly increasing body of literature has indicated that SSCs isolated from the testis and placed into a different environment in vitro or in vivo can acquire or manifest pluripotency and differentiate into tissues belonging to all three embryonic germ layers. These studies were originally performed in animal models.7,8,9 However, subsequent descriptions of methodologies for obtaining and culturing human SSCs revealed that the human cells appeared to share this ability under certain conditions.2,3,4,10,11 The use of human SSCs in regenerative medicine would face many of the same hurdles associated with other adult cell types, such as identification, isolation and propagation of these cells, as well as questions related to how to deliver these cells and how to induce them to differentiate into the tissue of interest, but the potential pluripotency of these cells also underlines their clinical promise.
This review will focus on the ability of SSCs to differentiate into other cell lineages than sperm, with an emphasis on the promise and limitations of SSCs for use in regenerative medicine. We will first discuss the history and current state of other cell types that have played a critical role in the emerging field of regenerative medicine, and then discuss how SSCs may provide a unique tool for regeneration of human organs and tissues.
HISTORICAL BACKGROUND – ISOLATION AND DERIVATION OF CELLS CAPABLE OF GENERATING TISSUES FROM ALL GERM LAYERS
There has been substantial interest in using stem cells for various applications in regenerative medicine since the pioneering work of Martin, Evans and Kaufman in the late 1970's and early 1980's12,13 describing embryonic stem (ES) cells derived from mouse embryos that appeared to have at least the potential to form any tissue or cell type. Interest in this field became more intense with the report of successful production of pluripotent human ES cells in 1998 by Thomson et al.14 These cells attracted intense interest from the scientific community because of their potential ability to differentiate into any cell type, and thus use of these cells could allow tissue or organ function to be restored in humans. Despite rapid technical advances and numerous demonstrations of the ability of these cells to give rise to a wide variety of clinically relevant cell types, the clinical future of human ES cells was always unclear because of the ethical, legal and moral issues involved in the destruction of human embryos to harvest ES cells and the need for therapeutic cloning for their optimal use.
These hurdles were at least partially overcome by Takahashi and Yamanaka et al.15 who derived induced pluripotent stem cells (iPSCs) using viral vectors to deliver the genes for a small combination of transcription factors into terminally differentiated cells. These iPSCs have many qualities of ES cells, and their production does not involve the destruction of embryos or therapeutic cloning. As with ES cells, a technique for producing iPSCs was initially developed with murine cells but was soon applied to human cells, and pluripotency was induced in human skin fibroblasts using the same approach.16,17,18 The iPSC technology has immense potential for clinical therapy, as shown by the recent demonstration that human iPSCs could be differentiated into functional pancreatic beta cells in vitro that showed glucose-responsive insulin production.19 Furthermore, these cells avoid some ethical and moral issues associated with ES cells. Despite the promise of this technique, there was concern initially that genomic insertion of genes introduced into the cellular genome by viral vectors20,21 would result in an increased risk of tumorigenicity22,23,24 either through disrupting endogenous genes at their insertion sites, or through production of exogenous factors introduced by viral vectors. A report that iPSCs could generate an immune response even in an autologous host also increased concern about using these cells clinically.25
To obviate concerns regarding risks of tumorigenicity from cells in which genes for transcription factors have been incorporated into the genome, a variety of new techniques have been developed. These involve new viral vectors that do not result in the introduced genes being inserted into the genome, the use of reprogramming proteins, rather than genes for these factors, to induce pluripotency, and the use of chemical cocktails that mimic the effects of the reprogramming factors.26,27
POTENTIAL USE OF SPERMATOGONIAL STEM CELLS IN REGENERATIVE MEDICINE
Currently, the only stem cell therapy widely accepted and used clinically is adult bone-marrow-derived stem cells for treatment of blood disorders and other conditions, a technique originally developed in the 1950's.28 However, the use of limbal stem cells, another type of adult stem cell, for repair of the cornea has shown success in human patients and may soon be used clinically.29,30,31 Given the limited capacity for expansion and potentially restricted differentiation potential of some adult stem cells, a great deal of attention is now focused on developing therapies from pluripotent stem cells, primarily iPSCs. Despite intense research interest and the unquestioned promise of iPSCs, it is unclear whether they are the best cells to use in regenerative applications, given the need for reprogramming these cells prior to clinical applications. Other multipotent or pluripotent cell types such as SSCs, which are capable of forming other tissue types without requiring reprogramming, may have a role in the future of regenerative medicine. The remainder of this review focuses on the potential use of SSCs in regenerative medicine.
SPERMATOGONIAL STEM CELLS DERIVED FROM MICE ARE PLURIPOTENT
In the embryo, primordial germ cells (PGCs) arise as a small cluster of cells in the proximal epiblast near the extra-embryonic ectoderm. Following their initial development, PGCs migrate into the developing gonads at about 4–5 weeks of gestation in humans and 11–13 days postcoitus in rodents.32 The PGCs then become mitotically quiescent until birth and are called gonocytes. Gonocytes resume mitosis and migrate to the basement membrane of seminiferous tubules shortly after birth, where they form SSCs and subsequently remain throughout life. Interestingly, gene expression in early germ cells is very similar to ES cells, and their overall gene expression pattern suggests that SSCs have a gene profile that is more undifferentiated than other adult stem cells.33,34
In the testicular microenvironment, SSCs typically produce only cells of the spermatogenic lineage and thus appear to be unipotent. Freshly isolated SSCs may likely also be unipotent, but it appears that altering the SSC environment in certain ways may lead to the generation of pluripotent cells from these SSCs. The groundbreaking demonstration that isolated neonatal murine SSCs can produce ES-like cells when grown for extended periods in ES cell culture conditions7,9 suggested that at least neonatal SSCs might be capable of giving rise to a wide variety of tissue and cell types. This initial work was followed by studies showing that adult mouse SSCs produced ES-like colonies, which in turn gave rise to cell types derived from all three embryonic germ layers and produced teratomas when injected subcutaneously into nude mice.35,36,37,38 In addition, these ES-like cells contributed to embryonic development when injected into blastocysts.36,37,38 Subsequent work has shown that the SSC-derived ES-like cells differentiate into mature cardiac and endothelial cells, and functional neurons and glia.35,39,40,41 Additional results have shown that SSCs can be induced to dedifferentiate in vitro and then develop into hematopoietic tissue, kidney and hepatocytes, as well as oocyte-like cells.38,39,40,41,42
ADULT HUMAN SPERMATOGONIAL STEM CELLS ARE PLURIPOTENT
Studies showing that neonatal and adult murine SSCs were multipotent or pluripotent suggested possible applications in human regenerative medicine. A key question then was whether human SSCs could be effectively isolated and whether they had similar potential to give rise to other cell types. This was quickly shown to be the case, and protocols were published for isolation and extended culture of human SSCs. Importantly, cell populations derived from human SSCs using methodologies similar to the original mouse work had ES-like characteristics and were pluripotent.2,4,10,11 Some studies used human testicular biopsies4,10 illustrating the feasibility of obtaining and propagating patient-specific SSCs that could be converted into tissue types needed for therapy.
SPERMATOGONIAL STEM CELLS DIFFERENTIATE INTO VARIOUS EPITHELIA IN A TISSUE RECOMBINATION SYSTEM
Despite the potential of human and mouse SSCs to give rise to ES-like cells that differentiate into a variety of tissues, there were obvious impediments to using these cells in clinical applications. For example, cultured SSCs give rise to ES-like cells at low frequency and only after extended culture time, and the extended time period necessary for subculturing and multiplication of these cells to therapeutically useful numbers is a practical hindrance to use of SSCs in a clinical setting.
Based on their unique developmental history, their ability to give rise to ES-like cells in culture and the similarity of gene expression between early germ cells and ES cells,33,34 we hypothesized that neonatal SSCs could be directly converted into other tissue types without the need to derive intermediate ES-like cells from the SSC populations in vitro. We demonstrated that neonatal SSCs gave rise to prostatic, uterine and skin epithelium8 using a tissue recombination technology in which neonatal SSCs were combined with an inductive fetal or neonatal mesenchyme and then grafted under the renal capsule of nude mouse hosts. Subsequent work by another group showed that SSCs injected into the bone-marrow can give rise to hematopoietic cells in vivo.9 Thus, in addition to cultured SSCs giving rise to an undifferentiated cell type that is then capable of differentiating into other tissue, SSCs may be able to directly give rise to other cell types, and this further emphasizes their clinical potential.
Despite our results indicating that SSCs formed tissues of all germ layers when recombined with inductive mesenchymes, the steps by which SSCs are converted into these tissue types were not clear from these initial experiments. One possibility was that SSCs recombined with inductive mesenchyme de-differentiated into ES-like cells, and then differentiated into a new tissue under the inductive influence of the mesenchyme. Alternatively, SSCs may have directly differentiated into prostatic or other epithelial cells without transition through an intermediate pluripotent ES-like cell stage under the influence of differentiation-inducing factors produced by the inductive tissue.
To understand the steps involved and potential mechanisms of induction of SSCs into prostatic epithelium, wild-type urogenital sinus mesenchyme (UGM; a potent instructive inducer of prostatic epithelium) derived from day 16 mouse fetuses was recombined with SSCs derived from 6-day-old mice expressing green fluorescent protein (GFP) as a lineage marker and grafted under the renal capsule of host mice. Tissue recombinants were then examined 1, 2, 4 and 8 days after grafting. The UGM + SSCs tissue recombinants harvested 4 days after grafting expressed a key prostatic marker, Nkx3.1 (Figure 1a). Nkx3.1, a murine homolog of drosophila bagpipe, is not only a marker of prostatic epithelium but is also critically involved in epithelial ductal branching and proliferation in prostate.43 Eight days following grafting, SSCs had formed histologically normal prostatic epithelium that was of SSC origin based on GFP expression (Figure 1a–1c).
Figure 1.
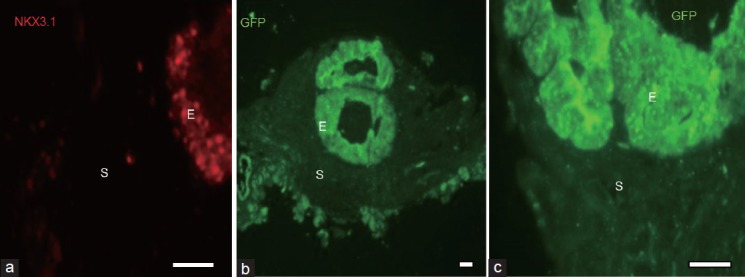
Expression of prostatic epithelial marker and phenotype at 4 and 8 days following tissue recombination. Neonatal spermatogonial stem cells (SSCs) isolated from 6-day-old green fluorescent protein (GFP+) mice were recombined with 16 days fetal wild-type urogenital sinus mesenchyme and grafted under the renal capsule of syngeneic nude male hosts for 4 (a) or 8 (b and c) days. Tissue recombinants expressed Nkx 3.1 (red staining), a prostatic epithelial marker, after 4 days of renal grafting (a). Tissue recombinants formed histologically identifiable prostatic ducts by 8 days (b), and the epithelium in the tissue recombinants was of SSC origin, as shown by GFP immunostaining (b) and the higher power view of these ducts in (c). The scale bars in the lower right corner of all photos = 10 μm. S: stroma; E: epithelium.
In addition to Nkx3.1 expression, the tissue recombinants also expressed mRNA for p63 (Cooke and Simon, unpublished results), a selective marker of prostatic basal cells.44 Basal cells are the precursors to secretory cells in the prostate. Thus, UGM + SSCs tissue recombinants show histologically identifiable prostatic epithelium 8 days after grafting and evidence of early expression of key genes involved in prostatic development and morphogenesis (Nkx3.1 and p63 expression) after only 4 days of grafting. This rapid differentiation of SSCs into prostatic epithelium suggests that SSCs could be directly differentiating into prostatic epithelium without intermediate differentiation into ES-like cells (Simon and Cooke, unpublished).
TISSUE RECOMBINATIONS OF UROGENITAL SINUS MESENCHYME + SPERMATOGONIAL STEM CELLS DO NOT EXPRESS PLURIPOTENCY MARKERS
One hallmark of ES cells is that they express pluripotency markers.33,34 To determine if SSCs in tissue recombinants expressed pluripotency markers during differentiation into prostatic epithelium, tissue recombinants of UGM + SSCs were examined 1, 2 and 4 days after grafting for pluripotency markers such as stage-specific embryonic antigen 1, sex determining region Y-box 2 and Nanog.33,34 None of the pluripotency markers were detected through quantitative reverse transcriptase polymerase chain reaction at any of the time points tested (Simon and Cooke, unpublished results). Furthermore, SSCs markers such as GDNF family receptor alpha-1 and promyelocytic leukemia zinc finger were also down-regulated in the tissue recombinants at day 1, 2, and 4 (Simon and Cooke, unpublished results). These results suggest that differentiation of SSCs into prostatic epithelium appears to be a direct transition that involves the rapid loss of SSC characteristics and may not involve an intermediate ES cell-like stage prior to manifestation of prostatic epithelial differentiation.
If SSCs directly differentiate into other tissue types without an intermediate ES-cell like stage, this may circumvent some limitations associated with the current methodology of differentiating SSCs into other cells. For example, it might be unnecessary to culture SSCs for an extended time for them to give rise to ES-like cells if under the appropriate conditions SSCs can directly convert to other tissue types. Additional studies are required to understand the mechanisms involved in direct differentiation of SSCs, but these results highlight the clinical potential of these cells.
ARE OTHER STEM CELLS POTENTIALLY PLURIPOTENT?
The ability of SSCs to give rise to other tissues derived from all germ layers suggests that these cells could potentially have important applications in regenerative medicine. However, a critical question is whether this capability is unique to SSCs or is common to many or all other stem cells. This question has major implications not only for understanding stem cell plasticity, but also for various regenerative applications. The current intense research focus on iPSCs results in part from the assumption that these cells may be more plastic than stem cells derived from other tissues or organs. Our work8 and that of others2,3,9,11,37,38,39,40,41,42 suggest that SSCs could potentially have plasticity rivaling that of iPSCs or ES cells. Is there something unique about SSCs, due to their developmental history or other factors, which makes them potentially more plastic than other stem cells? Our research with other stem cell populations suggests that this might be the case.
MESOANGIOBLASTS FORM MESODERMAL DERIVATIVES BUT MAY BE DEVELOPMENTALLY LIMITED COMPARED TO SPERMATOGONIAL STEM CELLS
Mesoangioblasts are blood vessel-derived stem cells that express markers of endothelial cells, hematopoietic stem cells, and pericytes.45,46,47,48 Upon transplantation into quail and chick embryos, mesoangioblasts isolated from embryonic vessels differentiated into cell types of mesodermal origin, including smooth, skeletal and cardiac muscle cells, pericytes in capillaries, bone, hyaline cartilage, and blood.46 However, mesoangioblasts express genes specific to neural stem cells49 and following transplantation into severely damaged skeletal muscle in a murine model of severe dystrophy, mesoangioblasts from juvenile vessels differentiated into Schwann cells as well as smooth, skeletal, and cardiac muscle cells and skeletal muscle stem cells.47,50,51,52 These data suggest that mesoangioblasts have ectodermal potential as well as mesodermal potential. In vitro studies of purified mesoangioblast cultures and transplantation of mesoangioblasts into murine brain supported their potential to generate myelinating glial cells.48
Tissue recombination with instructive mesenchymes was conducted to explore the potential of mesoangioblasts to differentiate into derivatives of all germ layers53 and compare the differentiation ability of mesangioblasts to that of SSCs. Recombining uterine mesenchyme with mesoangioblasts resulted in the formation of morphologically and functionally normal simple columnar uterine epithelium by mesoangioblasts,53 a result similar to what we obtained previously using SSCs combined with uterine mesenchyme.8 This uterine epithelium expressed all uterine epithelial markers tested, including cytokeratin 8 and estrogen receptor 1. Furthermore, estrogen administration to ovariectomized graft hosts stimulated proliferation and down-regulated the progesterone receptor in epithelial cells of mesoangioblast origin in the grafts, which are typical responses of the uterine epithelium to estrogen. These data indicate that similar to SSCs, mesoangioblasts can differentiate as morphologically and functionally normal uterine epithelium in response to cues from neonatal uterine mesenchyme and represent the first report of the potential of mesoangioblasts to generate epithelial cells.53
Recombining UGM, which successfully induced prostatic epithelial differentiation in SSCs,8 with mesoangioblasts did not yield prostatic epithelium,53 indicating that mesoangioblasts were not capable of differentiating into this epithelium of endodermal origin in response to UGM. This result is consistent with previous reports that mesoangioblasts did not give rise to cells of endodermal origin following transplantation into embryos of different species.46 Similarly, recombination with fetal dermis also failed to result in differentiation of mesoangioblasts into epidermis,53 in contrast to SSCs,8 indicating that the mesoangioblasts did not generate epithelial cells of ectodermal origin. These data indicate that while mesoangioblasts generate myelinating glial cells,48 their potential to give rise to ectodermal derivatives is limited. In agreement with this assertion, independent attempts by other researchers to induce neuronal differentiation from mesoangioblasts were also unsuccessful.49 Collectively, our tissue recombination studies are consistent with previous studies showing the potential of mesoangioblasts to give rise to many mesodermal derivatives. However, mesoangioblasts appear to lack the full potential to generate ectodermal and endodermal derivatives such as skin epidermis and prostatic epithelium seen with SSCs. These studies showing limited developmental capability of mesoangioblasts contrast with the ability of SSCs to give rise to tissues of all germ layers in this tissue recombination system.8
SUMMARY AND CONCLUSIONS
Work from many laboratories over the past decade has demonstrated the remarkable capacity of SSCs from both humans and rodents to form a wide variety of other tissue types under the correct environmental conditions. This capacity of SSCs to make tissues derived from all germ layers contrasts with another stem cell type, mesoangioblasts, and suggests that SSCs may have unique attributes not shared by other stem cell types, which may make them valuable in regenerative medicine. Additional work is clearly required to address basic questions such as the exact mechanism by which SSCs are converted into another cell type, as well as the mechanistic basis of why other stem cell types may not share this capacity. The promise of stem cells in regenerative medicine is immense, and SSCs may have a role to play as this exciting scientific field continues to develop.
One limitation to the use of SSCs as a source of pluripotent tissues that has been frequently discussed is that if this approach were used only for autologous grafts where the stem cell donor and recipient are the same individual, this methodology would only be applicable to males. The recent demonstration that female germ-line stem cells obtained from neonatal mouse ovaries manifest the same pluripotent characteristics as SSCs under certain experimental conditions54 suggests that both male and female germ cells may have potential in the field of regenerative medicine.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–26. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–49. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dym M, He Z, Jiang J, Pant D, Kokkinaki M. Spermatogonial stem cells: unlimited potential. Reprod Fertil Dev. 2009;21:15–21. doi: 10.1071/rd08221. [DOI] [PubMed] [Google Scholar]
- 4.Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, et al. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–26. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protoc. 2013;8:2098–104. doi: 10.1038/nprot.2013.138. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal L, Usmani A, Dalal SN, Majumdar SS. Generation of transgenic mice by exploiting spermatogonial stem cells in vivo. Methods Mol Biol. 2014;1194:327–37. doi: 10.1007/978-1-4939-1215-5_18. [DOI] [PubMed] [Google Scholar]
- 7.Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–7. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- 8.Simon L, Ekman GC, Kostereva N, Zhang Z, Hess RA, et al. Direct transdifferentiation of stem/progenitor spermatogonia into reproductive and nonreproductive tissues of all germ layers. Stem Cells. 2009;27:1666–75. doi: 10.1002/stem.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning L, Goossens E, Geens M, Van Saen D, Van Riet I, et al. Mouse spermatogonial stem cells obtain morphologic and functional characteristics of hematopoietic cells in vivo. Hum Reprod. 2010;25:3101–9. doi: 10.1093/humrep/deq269. [DOI] [PubMed] [Google Scholar]
- 10.He Z, Kokkinaki M, Jiang J, Zeng W, Dobrinski I, et al. Isolation of human male germ-line stem cells using enzymatic digestion and magnetic-activated cell sorting. Methods Mol Biol. 2012;825:45–57. doi: 10.1007/978-1-61779-436-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26:1296–306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- 12.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 14.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–9. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 19.Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, et al. Generation of functional human pancreatic ß cells in vitro. Cell. 2014;159:428–39. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–81. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–63. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 23.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–77. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 24.Kooreman NG, Wu JC. Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J R Soc Interface. 2010;7(Suppl 6):S753–63. doi: 10.1098/rsif.2010.0353.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 26.Hou P, Li Y, Zhang X, Liu C, Guan J, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–4. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 27.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen J. The first successful allogeneic bone-marrow transplant: georges Mathé. Transfus Med Rev. 2005;19:246–8. doi: 10.1016/j.tmrv.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 30.Sejpal K, Ali MH, Maddileti S, Basu S, Ramappa M, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131:731–6. doi: 10.1001/jamaophthalmol.2013.2308. [DOI] [PubMed] [Google Scholar]
- 31.Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–7. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;87:1–26. doi: 10.1002/bdrc.20142. [DOI] [PubMed] [Google Scholar]
- 33.Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–33. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
- 34.Simon L, Hess RA, Cooke PS. Spermatogonial stem cells, in vivo transdifferentiation and human regenerative medicine. Expert Opin Biol Ther. 2010;10:519–30. doi: 10.1517/14712591003614731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser T, Opitz T, Kischlat T, Konang R, Sasse P, et al. Adult germ line stem cells as a source of functional neurons and glia. Stem Cells. 2008;26:2434–43. doi: 10.1634/stemcells.2008-0163. [DOI] [PubMed] [Google Scholar]
- 36.Izadyar F, Pau F, Marh J, Slepko N, Wang T, et al. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771–84. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]
- 37.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, et al. Generation of functional multipotent adult stem cells from GPR125+germline progenitors. Nature. 2007;449:346–50. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 39.Streckfuss-Bömeke K, Vlasov A, Hülsmann S, Yin D, Nayernia K, et al. Generation of functional neurons and glia from multipotent adult mouse germ-line stem cells. Stem Cell Res. 2009;2:139–54. doi: 10.1016/j.scr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Baba S, Heike T, Umeda K, Iwasa T, Kaichi S, et al. Generation of cardiac and endothelial cells from neonatal mouse testis-derived multipotent germline stem cells. Stem Cells. 2007;25:1375–83. doi: 10.1634/stemcells.2006-0574. [DOI] [PubMed] [Google Scholar]
- 41.Guan K, Wagner S, Unsöld B, Maier LS, Kaiser D, et al. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–25. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Cao J, Ji P, Zhang D, Ma L, et al. Oocyte-like cells induced from mouse spermatogonial stem cells. Cell Biosci. 2012;2:27. doi: 10.1186/2045-3701-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, et al. Nk×3.1, a murine homolog of Ddrosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219:248–60. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1054>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Signoretti S, Pires MM, Lindauer M, Horner JW, Grisanzio C, et al. p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci U S A. 2005;102:11355–60. doi: 10.1073/pnas.0500165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, et al. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–78. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–83. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 47.Berry SE, Liu J, Chaney EJ, Kaufman SJ. Multipotential mesoangioblast stem cell therapy in the mdx/utrn-/- mouse model for Duchenne muscular dystrophy. Regen Med. 2007;2:275–88. doi: 10.2217/17460751.2.3.275. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Kamath A, Frye J, Iwamoto GA, Chun JL, et al. Aorta-derived mesoangioblasts differentiate into the oligodendrocytes by inhibition of the Rho kinase signaling pathway. Stem Cells Dev. 2012;21:1069–89. doi: 10.1089/scd.2011.0124. [DOI] [PubMed] [Google Scholar]
- 49.Tagliafico E, Brunelli S, Bergamaschi A, De Angelis L, Scardigli R, et al. TGFbeta/BMP activate the smooth muscle/bone differentiation programs in mesoangioblasts. J Cell Sci. 2004;117:4377–88. doi: 10.1242/jcs.01291. [DOI] [PubMed] [Google Scholar]
- 50.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–92. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 51.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–9. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 52.Chun JL, O’Brien R, Song MH, Wondrasch BF, Berry SE. Injection of vessel-derived stem cells prevents dilated cardiomyopathy and promotes angiogenesis and endogenous cardiac stem cell proliferation in mdx/utrn-/- but not aged mdx mouse models for duchenne muscular dystrophy. Stem Cells Transl Med. 2013;2:68–80. doi: 10.5966/sctm.2012-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon L, Cooke PS, Berry SE. Aorta-derived mesoangioblasts can be differentiated into functional uterine epithelium, but not prostatic epithelium or epidermis, by instructive mesenchymes. Cells Tissues Organs. 2013;198:169–78. doi: 10.1159/000354900. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Jiang M, Bi H, Chen X, He L, et al. Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol. 2014;6:164–71. doi: 10.1093/jmcb/mju004. [DOI] [PubMed] [Google Scholar]


