Abstract
Male and female differ genetically by their respective sex chromosome composition, that is, XY as male and XX as female. Although both X and Y chromosomes evolved from the same ancestor pair of autosomes, the Y chromosome harbors male-specific genes, which play pivotal roles in male sex determination, germ cell differentiation, and masculinization of various tissues. Deletions or translocation of the sex-determining gene, SRY, from the Y chromosome causes disorders of sex development (previously termed as an intersex condition) with dysgenic gonads. Failure of gonadal development results not only in infertility, but also in increased risks of germ cell tumor (GCT), such as gonadoblastoma and various types of testicular GCT. Recent studies demonstrate that either loss of Y chromosome or ectopic expression of Y chromosome genes is closely associated with various male-biased diseases, including selected somatic cancers. These observations suggest that the Y-linked genes are involved in male health and diseases in more frequently than expected. Although only a small number of protein-coding genes are present in the male-specific region of Y chromosome, the impacts of Y chromosome genes on human diseases are still largely unknown, due to lack of in vivo models and differences between the Y chromosomes of human and rodents. In this review, we highlight the involvement of selected Y chromosome genes in cancer development in men.
Keywords: germ cell tumors, RBMY, Y-linked, somatic cancers, TSPY, Y chromosome
INTRODUCTION
Numerous studies have identified various sex differences in the risks, incidence and progression of various human diseases, such as asthma,1,2 autoimmune diseases,3,4 schizophrenia,5,6 autism spectrum disorders,7,8 cardiovascular disease,9,10 and non-sex-specific cancers such as liver cancer, bladder cancer, and lung cancer.11,12,13 According to the report by Cook and colleagues, 32 out of 36 cancer types showed male preference of cancer mortality in United States for the years between 1977 and 2006.14 However, the mechanisms responsible for such sex-differences are still largely unknown. The most significant genetic differences between men and women are genes on their sex chromosomes, that is, XY for men and XX for women. Men are prone to X-linked diseases caused by mutations on genes on their X chromosome while ectopic expression of the genes on their Y chromosome could have male-specific effects on normal development, physiology, and diseases. The human Y chromosome can be classified structurally into three regions: (i) male-specific region of the Y chromosome (MSY), (ii) pseudoautosomal regions (PAR1 and PAR2), and (iii) heterochromatin region on Yq (Figure 1).15 PARs contain 20 protein-coding genes (16 genes in PAR1 and 4 genes in PAR2) that are also present on the X chromosome.16 The MSY contains 23 protein-coding genes and numerous pseudogenes (Table 1).15,17,18 While genes in PARs are present in both X and Y chromosomes and undergo meiotic recombination similarly with autosomal genes, genes in MSY are excluded from meiotic recombination with a homologous chromosome partner. The MSY genes evolved during about 300 million years after beginning of X-Y differentiation.19 The MSY genes can be classified into two groups according to their expression patterns. Group-I genes are expressed almost ubiquitously, and Group-II genes are expressed specifically/predominantly in testis (Table 1).20,21,22 It is postulated that Group-I MSY genes function as broadly expressed regulators for gene expression and protein stability as maintaining the ancestral dosage of homologous X-Y gene pairs, e.g., DDX3Y, EIF1AY, KDM5D, RPS4Y, TBL1Y, USP9Y, UTY, and ZFY.21 The ubiquitous and/or somatic expressions of MSY genes suggest that the balanced expression between MSY genes and their X homologs could be crucial to maintain the healthy condition in men. In humans, 12 of 14 functional X-Y paired genes (86%) escape X-inactivation in female,21 thereby maintaining the dosage balance of X-Y paired genes. On the other hand, Group-II genes, including HSFY, SRY, RNA-binding motif protein, Y-linked (RBMY), and testis-specific protein, Y-encoded (TSPY), may play diverse functions from their X homologs.
Figure 1.
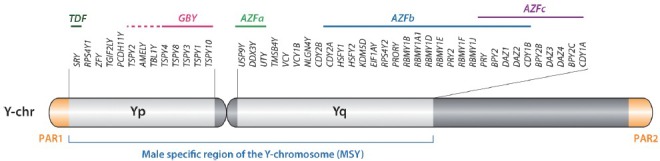
Schematic diagram of human Y chromosome indicating the protein-coding genes within the male-specific region of Y chromosome. AZFa-c: the deleted regions identified in azoospermia patients; GBY: the gonadoblastoma locus on Y chromosome; PAR1 and PAR2: the pseudoautosomal regions.
Table 1.
Protein coding MSY genes
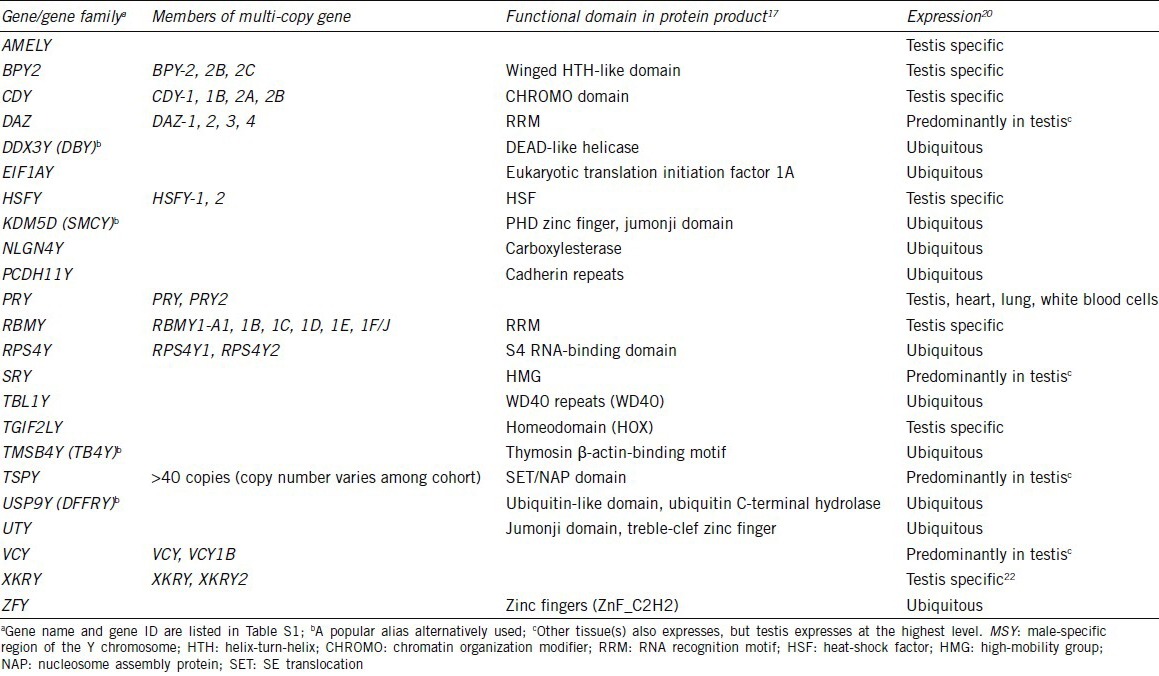
Table S1.
Gene name and Entrez gene ID of genes listed in Table 1
Transgenic mouse models using knockout strategies are useful tools to determine and infer the functions of respective genes in human health and diseases. However, only 9 of 17 ancestral genes in the human MSY are conserved in the mouse Y chromosome.21 Recent work by Soh et al.23 demonstrated that only 2.2% of mouse MSY sequence shares ancestry with the primate MSYs. Further, the mouse Y-chromosome long-arm harbors the highly amplified units (85–221 times) containing genes, such as Sly, Ssty1, Ssty2, or Srsy that are absent on the primates Y-chromosomes.23 Accordingly, mouse modeling of human MSY genes is difficult, and the impacts of MSY genes on human diseases are still largely unknown. Based on genetic mapping studies, three major loci have been assigned to the human MSY, that is, testis-determining factor (TDF), gonadoblastoma locus on Y chromosome (GBY) and azoospermia factor (AZF) (Figure 1). The SRY gene has been demonstrated to be the testis-determining gene,24,25 while a group of genes, that is, RBMY and DAZ, have been identified within the AZF locus on the long-arm.15,26,27 The gonadoblastoma (GBY) locus was initially mapped to a small region on the short arm of the Y chromosome proximal to the centromere, harboring a gene predisposing dysgenetic gonads of XY sex-reversed patients to develop gonadoblastoma.28 Subsequent studies showed that TSPY is the putative gene for this locus.29,30 The sex determination and genes for the AZF locus have recently been reviewed in details.27,31,32 The present review focuses on the MSY genes and their associations to human tumors, including gonadoblastoma.
EXPRESSION OF Y-LINKED GENES AND HUMAN CANCERS
Various studies have demonstrated that TSPY and RBMY are ectopically expressed in somatic cells under disease conditions, such as cancer, although they are normally expressed preferentially in testicular germ cells (see below). To explore the changes of MSY genes in somatic cancers, we had performed a data-mining study on hepatocellular carcinoma (HCC) using the RNA-Seq gene expression data on 27 pairs of male tumor-nontumor paired samples at the Cancer Genome Atlas (TCGA) project.33 Our results showed that, in addition to TSPY and RBMY, other MSY genes, that is, TGIF2LY and VCY, were consistently up-regulated in ~30% cases of liver cancer, while DDX3Y, ZFY, and DAZ1 were frequently down-regulated (~70%, Figure 2). Since the Y chromosome is unique to men, these observations suggest that the Y chromosome genes could potentially influence on the development, progression and outcomes of liver cancer in a male-specific manner(s).
Figure 2.
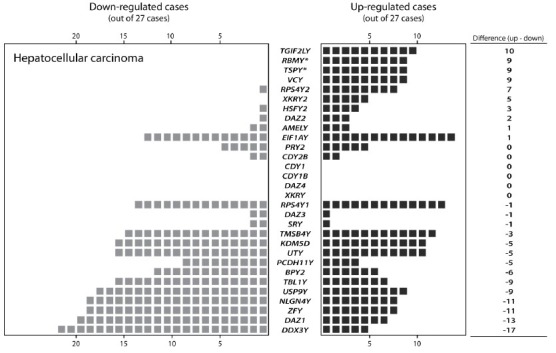
Gene expression profile of MYS protein-coding genes in male liver cancer cases. The case number showing either up-regulation or down-regulation in cancer specimens is indicated for each gene, according to the RNA-Seq gene expression data derived from the database of the Cancer Genome Atlas project. Twenty-seven pairs of tumor and corresponding nontumor tissue were analyzed. Each black or gray square indicates an up or down respectively the expression of the corresponding Y chromosome genes in the tumor to nontumor pairs.
Testis-specific protein, Y-encoded (TSPY)
The human TSPY gene was initially identified as a Y-linked gene specifically expressed in the testis.34,35 It is tandemly repeated in 20.3-kb highly homologous units, usually in the range of 21–35 copies, on the short arm of the Y chromosome.15,36,37,38 The human TSPY is expressed in gonocytes in the embryonic testis,39 spermatogonia, and prophase I spermatocytes at preleptotene to zygotene stages in adult testis.40 Deletion mapping has localized the TSPY repeat units to the critical region harboring the GBY locus on the short arm.29,30 It is postulated to serve normal functions in male germ cell differentiation, mitosis, and meiosis,41,42 but could promote gonadoblastoma development in patients with disorders of sex developments (DSDs) harboring Y chromosome materials including TSPY. Indeed, TSPY expression has been observed in gonadoblastoma, and various types of germ cell tumors (GCTs), including carcinoma in situ/intratubular germ cell neoplasia unclassified (CIS/ITGCNU) (the precursor for all testicular GCTs [TGCTs]), seminoma, and extragonadal intracranial GCT.43,44,45,46 In addition to GCTs, TSPY is frequently expressed in some somatic cancers including liver cancer,47,48 melanoma,49 and prostate cancer,50,51 suggesting that TSPY can be considered as a cancer-testis antigen (CT-antigen). CT-antigens are group of proteins that are predominantly/specifically in testis under normal conditions, but are ectopically expressed in somatic cancers.52 Although the biological functions of CT-antigens are currently uncertain, they have been proposed as diagnostic markers and therapeutic targets in cancers.48,52,53,54
Molecular functions of TSPY
TSPY is a member of SE translocation/nucleosome assembly protein 1 (SET/NAP1) superfamily harboring a highly homologous SET/NAP-domain, initially identified in the SET oncoprotein (also called template-activating factor I or TAF-I) and the NAP1.42,55 X-ray crystallography showed that the SET protein forms a homodimer with a headphone like structure, and the SET/NAP domain occupies the earmuff region, which could be important for protein-protein binding with interactive partners. The N-terminal alpha-helix region contains the binding site for the homodimerization.56,57,58 Members of the SET/NAP1 protein family could function as chaperones of histones. In particular, NAP1 plays crucial roles in shuttling and assembling core histones as a histone chaperone.57,59 SET forms inhibitor of histone acetyltransferase (INHAT) complex with its isoform TAF-Iα and pp32.60 INHAT complex associates with chromatin to inhibit the histone acetylation mediated by acetyltransferases, thereby suppressing the expression of targeted genes.60 SET also interacts with transactivators and enhances their respective target gene expression.54,61 These observations suggest that SET/NAP1 proteins serve a wide range of functions in many biological processes. Recently, we reported that TSPY binds the type B cyclins and enhances the kinase activity of the cyclin-B/CDK1 complex.62 Correlating with this function, overexpression of TSPY leads to shortening of the G2/M phase and acceleration of cell proliferation in TSPY-transfected HeLa and 3T3 cells in vitro and tumorigenicity in athymic mice in vivo.63 In contrast, its single-copy X-linked homolog TSPX (also termed as TSPY-like 2 [TSPYL2], differentially expressed nuclear TGF-β1 target [DENTT] or cell division autoantigen 1 [CDA1]) inhibits the kinase activity of cyclin-B/CDK1 complex.62 TSPX protein harbors a 250 amino acids aspartic acid/glutamic acid (D/E)-rich domain at its C-terminus, which is absent in TSPY. The inhibitory function of TSPX has been mapped on the C-terminal D/E-rich domain.62 Since the SET/NAP-domains of both proteins are well conserved, TSPY and TSPX could play contrasting roles on their common target molecules. Abrogated TSPX expression in lung cancer is associated with accelerated cancer progression.64 In vitro studies also demonstrated that overexpression of TSPX retards cell proliferation.65 Further, down-regulation of TSPX by nitric-oxide correlates with the glioma stem cell proliferation.66 These observations suggest that while TSPY and TSPX originated from the same ancestor gene, they have respectively evolved into two independent genes on the sex chromosomes, and play contrasting roles in human oncogenesis, that is, TSPY as a proto-oncogene and TSPX as a tumor suppressor gene.67,68
Yeast-two hybrid screening using the SET/NAP-domain of TSPY as bait has identified several novel TSPY binding proteins. The first one is the translation elongation factor 1A, eEF1A. The SET/NAP domain of TSPY binds to the domain-III of eEF1A, and enhances protein synthesis.69 TSPY also binds to the 40S ribosomal component RPS26 (unpublished data), suggesting that TSPY could be associated with the protein synthesis machinery in the cells. Recent studies suggest that protein synthesis is crucial in the regulation of cell proliferation and cancer progression.70,71,72,73 TSPY may normally support protein synthesis essential for the maintenance of germ cell proliferation, but when ectopically expressed, it could promote cancer growth under diseased conditions. Interestingly, we also showed that TSPY protein binds to the exon-1 of its structural gene and enhances its own expression in prostate cancer cells,74 suggesting that TSPY could intensify its functions by amplifying its own gene expression through a positive feedback loop. Hence, TSPY could play the role of a transcription regulator, by binding to the DNA/nuclear proteins on the chromatin of target genes. Since TSPY is located in the male-only Y chromosome, its functions in the protein synthetic machinery, cell cycle progression, histone chaperone/chromatin modification gene, and regulation could shed new insights on sex disparities associated with the development, progression and treatments responses among numerous somatic cancers, e.g., liver cancer and melanoma, with ectopic TSPY expression.
Expression of TSPY in germ cell tumors
Human GCTs can be classified into five types based on various parameters including age at clinical presentation, anatomical sites and histology; e.g., type-I, teratoma/yolk sac tumor; type-II, seminomatous/nonseminomatous GCTs; type-III, spermatocytic seminoma; type-IV, dermoid cyst; type-V, hydatidiform mole.75 TSPY expression is primarily detected in type-II TGCs and type-III spermatocytic seminoma.44 Type-II TCGs are further subdivided into nonseminomatous GCTs and seminomatous GCTs, including seminoma, dysgerminoma, germinoma, and gonadoblastoma.75,76 The type-II testicular germ cell tumors (TGCTs) are the most common malignancies among young men aged 15 to 34 years in United States, and its incidence is approximately 1.38–6.31 per 100 000 (years of 1973 to 2001).77 The incidence of TGCTs is globally increased during the past 70 years, especially among men of European ancestry, and the etiologies of such preference are uncertain.78,79
TGCTs, both seminoma and nonseminoma, are derived from CIS/ITGCNU.75,76,80,81 CIS/ITGCNU cells display a close phenotype to fetal germ cells, suggesting their origin is due to a developmental delay or failure of differentiation of early germ cells (Figure 3).75,82,83,84 TSPY is expressed in gonocytes85 and most CIS/ITGCNU cells with some minor exceptions (Figure 3).43,44 Upon further oncogenic progression, the seminoma cells maintain TSPY expression while, nonseminoma cells do not or rarely express TSPY (Figure 3).43,44,86 It has been speculated that the development of nonseminoma TGCT requires reprogramming to embryonic carcinoma state.75 Indeed, the global gene expression analysis using microarray hybridization strategy indicated that embryonic carcinoma cells showed significant similarities with human embryonic stem cells, while seminoma closely resemble transformed primordial germ cells.87 Accordingly, TSPY expression likely correlates with the germ cell lineage even in maturation-disturbed germ cells, but not with the reprogrammed cells like embryonic carcinoma with acquired pluripotency.
Figure 3.
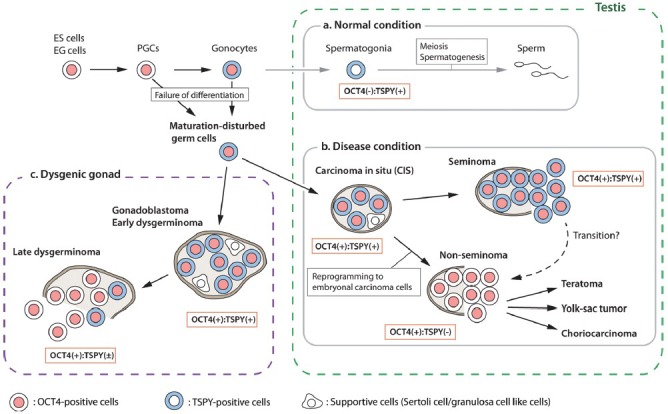
Schematic representation of expressions of testis-specific protein, Y-encoded (TSPY) and OCT3/4 in normal testis and type-II germ cell tumors. Germ cell expresses OCT3/4 until it reaches the maturation status. (Process-a) In normal testis, germ cell mature as spermatogonia, and lost OCT3/4 expression while it expresses TSPY. (Process-b) Failure of germ cell maturation causes development of carcinoma in situ (CIS) in the testis. The CIS cells are mostly OCT3/4-positive and TSPY-positive. It further develops into invasive seminoma (OCT3/4-positive and TSPY-positive) or nonseminoma via reprogramming to embryonal carcinoma status (OCT3/4-positive but TSPY-negative). (Process-c) In dysgenic gonad, maturation disturbed germ cells develop gonadoblastoma (OCT3/4-positive and TSPY-positive), and further progress into invasive dysgerminoma (OCT3/4-positive but TSPY-negative).
Gonadoblastoma is a subclass of type-II TCGs preferentially developed in the dysgenetic gonads of XY females or individuals with DSD.75,76,88 The Y chromosome of gonadoblastoma patients frequently lacks sex determination region but retains common region of the short arm, termed GBY locus.28,29,30 The tandemly repeated units of TSPY gene are mapped within GBY critical region on the Y chromosome, hence TSPY is considered as a candidate gene for GBY, promoting gonadoblastoma development in the dysgenetic gonads of DSD patients.28,29,30 Gonadoblastoma morphologically resembles the CIS/ITGCNU in the TGCTs in the testis; gonadoblastoma cells are mixed with granulosa-like cells while TGCT cells are mixed with Sertoli cells in the seminiferous tubules enclosed by myoid cells.89 Most OCT4-positive gonadoblastoma cells strongly express TSPY as well as the germ cell/placental alkaline phosphatase (PLAP) and the proto-oncogene receptor c-Kit, similar to the testicular CIS.43,45,86,88 Noticeably, TSPY is rarely expressed in the dysgerminoma after progression from gonadoblastoma.86,88 Dysgerminoma is considered as a counterpart of seminoma based on morphology and expressed biomarkers.90,91,92 Loss of TSPY expression in dysgerminoma may indicate the dividing characteristics between dysgerminoma and seminoma.
Overall, TSPY is expressed differentially in a subset of GCTs positive for both OCT4 and PLAP biomarkers. Since TSPY is most frequently expressed in the CIS and gonadoblastoma, at early stages of germ cell tumorigenesis, it is postulated to play an important role in early stages of oncogenesis in the immature germ cell lineage. Accordingly, the risk of GCT development/progression is higher in TSPY positive than TSPY negative cases.93 TSPY could accelerate such progression of GCTs through its functions in cell cycle, protein synthesis, and histone/chromatin modification and gene regulation, as discussed above.
Expression of TSPY in somatic cancers
In addition to type-II and III GCTs, ectopic expression of TSPY has been frequently detected in various types of somatic cancers, including HCC,47,48 melanoma,49 and prostate cancer.50,51 Yin et al.48 reported that TSPY expression was detected in 50% cases of early stage HCC and 16% cases in undifferentiated stage (later stage of HCC). Our recent studies also demonstrated that TSPY was detected in 19.2% cases in tissue microarray and 46.9% cases in RNA samples isolated from fresh HCC specimens.47 Further immunohistochemical analysis showed that TSPY is expressed in the glypican 3-positive cells, a biomarker of the HCC.47,94 In the studies of prostate cancer, TSPY was immunohistochemically detected in the regions positive for alpha-methylacyl-CoA racemase, a biomarker of prostatic intraepithelial neoplasia and prostate cancer cells.50,95 TSPY expression was more frequently detected in clinical prostate cancer specimens (78%) than latent prostate cancer (47%) and noncancer prostate tissues (50%).50 These observations clearly indicate that TSPY is ectopically activated in somatic cancer cells.
While the correlation between TSPY expression and clinical outcome is still unclear, TSPY has been suggested as a prognostic biomarker and therapeutic target for immunotherapy.48 Further analysis incorporating clinical outcomes and TSPY expression would be important to elucidate the significance of TSPY expression on cancer progression and immunotherapy.
Rodent Tspy and human TSPY transgenic mouse models
Although TSPY is an evolutionarily conserved gene on the Y chromosomes of mammals including apes and bovines,96,97 the mouse Tspy gene is apparently nonfunctional as it contains multiple in-frame stop codons within the open reading frame.98 Rat Y chromosome harbors a single functional copy of Tspy gene,99 but its expression pattern is different from human TSPY, that is, the rat Tspy is expressed only in elongating spermatids while the human TSPY is primarily expressed in spermatogonia and spermatocytes,100 suggesting that the biological functions of the rat Tspy could be different from those of human TSPY. Accordingly, the gene knockout in rodents might not be a suitable strategy to explore the biological functions of human TSPY. To overcome this difficulty, Schubert et al.101 had generated a transgenic mouse line harboring 50 copies of human TSPY gene on Y chromosome of the mouse, designated as TgTSPY9. The 8.2-kb transgene contains 2.95-kb the promoter region, 2.8-kb structural gene and 2.45-kb 3’ flanking sequence of the human TSPY gene. It is predominantly expressed in spermatogonia and spermatocytes at early stages of spermatogenesis, similar to the pattern of TSPY in human testis.101 Expression of human TSPY transgene in testicular germ cells of TgTSPY9 mice does not show any significant effects in fertility or other physiology,101 consistent with the observation that the copy number of human TSPY gene varies among fertile men.38 By introducing the Y-located TSPY transgene of TgTSPY9 to the LADY mouse model of prostate cancer, we have demonstrated that the Y-located TSPY could be aberrantly activated during oncogenesis in the LADY model of prostate cancer.102 However, while TSPY is expressed in FoxA1-positive epithelial cells and prostate cancer cells in human clinical prostate cancer specimens, TgTSPY9 transgene was expressed in FoxA1-negative hypercellular stroma areas in the prostate of LADY mice.102 Such differential expression patterns suggest the potential limitations of current mouse models of prostate cancer in mimicking the ectopic expression of TSPY under disease conditions, such as during prostate cancer development.
Azoospermia factor (AZF) genes and cancer
RNA-binding motif protein, Y-linked (RBMY) isoforms are encoded by repetitive genes within the AZF region, frequently deleted in azoospermia patients (Figure 1). RBMY binds to the RNA stem-loops capped by a C[A/U] CAA pentaloop103 and may participate in the alternative splicing of various testis-specific gene transcripts.104 Indeed, deletion of RBMY resulted in the failure of meiosis.105 Abnormal expression of RBMY was observed in 36% cases of male liver HCC but not in normal liver tissues.106,107 Over-expression of RBMY caused tumorigenicity in mouse fibroblast 3T3 cells,106 and knock-down of RBMY in a liver cancer cell line HepG2 resulted in the reduction of transformation and anti-apoptotic efficiencies.107 Further, the liver-specific RBMY transgenic mice showed accelerated hepatic neoplastic changes in the diethylnitrosamine-induced hepatocarcinogenesis animal model.107 While the mechanism is still unclear, these observations suggest that the ectopic expression of RBMY genes could contribute to HCC development. On the contrary, multiple copies of BPY2, DAZ, and CDY1 genes are also mapped onto the microdeletion of AZFc region, and deletions of these AZF genes are associated with increased risks of seminoma.108,109 Consistently with these reports, our analysis of TCGA data showed that DAZ1 and BPY2 are frequently down-regulated in HCC (Figure 2). Although these observations are preliminary in nature, further studies on AZF genes could provide new insights into the roles of Y chromosome in cancer development and their usefulness as diagnostic biomarkers.
CONCLUSIONS AND FUTURE ASPECTS
In the past decades, associations between MSY genes and diseases have been identified. However, because of differences between human and rodents Y chromosomes21,110 and difficulties in generating knockout mice of Y chromosome genes,111 there are still limitations on investigating the roles of human MSY genes in vivo. As it has been suggested, most MSY genes may function as broadly expressed regulators for gene expression, protein stability and maintenance of the dosage of homologous XY gene pairs.21 Supporting this hypothesis, it was demonstrated that UTX and UTY could play comprehensive, but independent of their demethylase activities, during embryonic development.112 On the other hand, while TSPY displays proto-oncogenic properties,62,63,69 its X-linked homolog TSPX is a tumor suppressor and down-regulated in cancer.62,65,66 This is the first example for an MSY gene and its X-homologue possess distinct and opposing functions. Hence, it is important to establish respective in vivo models to elucidate the roles of human MSY genes in development and progression of diseases, including cancers.
Recently, an epidemiologic study reported that loss of Y chromosome (LOY) in peripheral blood cells significantly associated with shorter cancer survival and higher risk of cancer incidence in men.113 LOY in peripheral blood is frequently observed in elder men.114 According to a clinical study with >40 years of follow-up, Forsberg et al. found that LOY in peripheral blood associated with increased risk of both all-caused mortality and cancer mortality, particularly in nonhematological cancers.113 Further, transcriptome analysis of human peripheral blood samples detected the expression of some Y chromosome genes, e.g., EIF1AY, DDX3Y, KDM5D, CYorf15B, CYorf15A, and UTY.115 Although the mechanism linking LOY in human peripheral blood and cancer mortality remains to be elucidated, these observations strongly suggest that Y chromosome genes are involved in a wide variety biological processes that have not been fully explored. MSY genes play crucial roles in both hormonal regulation and the balance in gene expression and protein stability, as described above. Ectopic expression of one or a few of these Y chromosome genes, such as TSPY and RBMY, could exacerbate oncogenesis in the absence of proper counter-balance from the other MSY genes (Figure 4). Further studies of Y chromosome genes from the global aspects, including both coding and noncoding RNA genes, will shed new lights on their roles in health and diseases in men.
Figure 4.
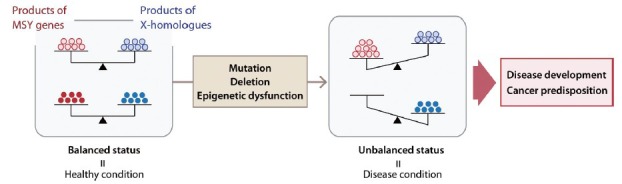
Conceptual illustration of gene balance between male-specific region of the Y chromosome genes and their X homologs in health maintenance and disease development, including cancer, in men.
AUTHOR CONTRIBUTIONS
TK performed the data-mining experiments. TK and YFCL co-wrote the manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was partially supported by a Merit-Reviewed grant and a Program Project award from the Department of Veterans Affairs to YFCL. YFCL is a Research Career Scientist of the Department of Veterans Affairs.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4(Suppl B):S133–46. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- 2.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–69. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Lockshin MD. Sex differences in autoimmune disease. Lupus. 2006;15:753–6. doi: 10.1177/0961203306069353. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JM, Cherkerzian S, Tsuang MT, Petryshen TL. Sex differences in the genetic risk for schizophrenia: history of the evidence for sex-specific and sex-dependent effects. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:698–710. doi: 10.1002/ajmg.b.32159. [DOI] [PubMed] [Google Scholar]
- 6.Wu YC, Hill RA, Gogos A, van den Buuse M. Sex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNF. Neuroscience. 2013;239:67–83. doi: 10.1016/j.neuroscience.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–53. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, et al. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 10.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–22. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura H, Ando K, Shinmyo T, Morita K, Mochizuki A, et al. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg. 2011;17:469–80. doi: 10.5761/atcs.oa.10.01637. [DOI] [PubMed] [Google Scholar]
- 12.Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol. 2011;29:457–63. doi: 10.1007/s00345-011-0709-9. [DOI] [PubMed] [Google Scholar]
- 13.Arbeev KG, Ukraintseva SV, Arbeeva LS, Yashin AI. Difference between male and female cancer incidence rates: how can it be explained? In: Nikulin MS, Commenges D, Huber-Carol C, editors. Probability, Statistics and Modelling in Public Health. New York: Springer; 2006. [Google Scholar]
- 14.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–37. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–37. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- 16.Helena Mangs A, Morris BJ. The Human Pseudoautosomal Region (PAR): origin, function and future. Curr Genomics. 2007;8:129–36. doi: 10.2174/138920207780368141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginalski K, Rychlewski L, Baker D, Grishin NV. Protein structure prediction for the male-specific region of the human Y chromosome. Proc Natl Acad Sci U S A. 2004;101:2305–10. doi: 10.1073/pnas.0306306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jangravi Z, Alikhani M, Arefnezhad B, Sharifi Tabar M, Taleahmad S, et al. A fresh look at the male-specific region of the human Y chromosome. J Proteome Res. 2013;12:6–22. doi: 10.1021/pr300864k. [DOI] [PubMed] [Google Scholar]
- 19.Lahn BT, Page DC. Four evolutionary strata on the human X chromosome. Science. 1999;286:964–7. doi: 10.1126/science.286.5441.964. [DOI] [PubMed] [Google Scholar]
- 20.The Ensembl Project. Human Body Map 2.0 data from Illumina. [Accessed on 2014 Oct 11]. Available from: http://www.ensembl.info/blog/2011/05/24/human-bodymap-2-0-data-from-illumina/
- 21.Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–9. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science. 1997;278:675–80. doi: 10.1126/science.278.5338.675. [DOI] [PubMed] [Google Scholar]
- 23.Soh YQ, Alföldi J, Pyntikova T, Brown LG, Graves T, et al. Sequencing the mouse Y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell. 2014;159:800–13. doi: 10.1016/j.cell.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–4. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 25.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–21. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 26.Vogt PH, Edelmann A, Hirschmann P, Köhler MR. The azoospermia factor (AZF) of the human Y chromosome in Yq11: function and analysis in spermatogenesis. Reprod Fertil Dev. 1995;7:685–93. doi: 10.1071/rd9950685. [DOI] [PubMed] [Google Scholar]
- 27.Vogt PH, Falcao CL, Hanstein R, Zimmer J. The AZF proteins. Int J Androl. 2008;31:383–94. doi: 10.1111/j.1365-2605.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 28.Page DC. Hypothesis: a Y-chromosomal gene causes gonadoblastoma in dysgenetic gonads. Development. 1987;101(Suppl):151–5. doi: 10.1242/dev.101.Supplement.151. [DOI] [PubMed] [Google Scholar]
- 29.Salo P, Kääriäinen H, Petrovic V, Peltomäki P, Page DC, et al. Molecular mapping of the putative gonadoblastoma locus on the Y chromosome. Genes Chromosomes Cancer. 1995;14:210–4. doi: 10.1002/gcc.2870140309. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya K, Reijo R, Page DC, Disteche CM. Gonadoblastoma: molecular definition of the susceptibility region on the Y chromosome. Am J Hum Genet. 1995;57:1400–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Svingen T, Koopman P. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013;27:2409–26. doi: 10.1101/gad.228080.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krausz C, Hoefsloot L, Simoni M, Tüttelmann F. European Academy of Andrology. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state-of-the-art 2013. Andrology. 2014;2:5–19. doi: 10.1111/j.2047-2927.2013.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The TCGA Research Network. [Downloaded on 31 Oct 2014]. Available from: http://www.cancergenome.nih.gov/
- 34.Arnemann J, Epplen JT, Cooke HJ, Sauermann U, Engel W, et al. A human Y-chromosomal DNA sequence expressed in testicular tissue. Nucleic Acids Res. 1987;15:8713–24. doi: 10.1093/nar/15.21.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang JS, Yang-Feng TL, Muller U, Mohandas TK, de Jong PJ, et al. Molecular isolation and characterization of an expressed gene from the human Y chromosome. Hum Mol Genet. 1992;1:717–26. doi: 10.1093/hmg/1.9.717. [DOI] [PubMed] [Google Scholar]
- 36.Manz E, Schnieders F, Brechlin AM, Schmidtke J. TSPY-related sequences represent a microheterogeneous gene family organized as constitutive elements in DYZ5 tandem repeat units on the human Y chromosome. Genomics. 1993;17:726–31. doi: 10.1006/geno.1993.1393. [DOI] [PubMed] [Google Scholar]
- 37.Giachini C, Nuti F, Turner DJ, Laface I, Xue Y, et al. TSPY1 copy number variation influences spermatogenesis and shows differences among Y lineages. J Clin Endocrinol Metab. 2009;94:4016–22. doi: 10.1210/jc.2009-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nickkholgh B, Noordam MJ, Hovingh SE, van Pelt AM, van der Veen F, et al. Y chromosome TSPY copy numbers and semen quality. Fertil Steril. 2010;94:1744–7. doi: 10.1016/j.fertnstert.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 39.Honecker F, Stoop H, de Krijger RR, Chris Lau YF, Bokemeyer C, et al. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol. 2004;203:849–57. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- 40.Lau YF, Li Y, Kido T. Role of the Y-located putative gonadoblastoma gene in human spermatogenesis. Syst Biol Reprod Med. 2011;57:27–34. doi: 10.3109/19396368.2010.499157. [DOI] [PubMed] [Google Scholar]
- 41.Schnieders F, Dörk T, Arnemann J, Vogel T, Werner M, et al. Testis-specific protein, Y-encoded (TSPY) expression in testicular tissues. Hum Mol Genet. 1996;5:1801–7. doi: 10.1093/hmg/5.11.1801. [DOI] [PubMed] [Google Scholar]
- 42.Lau YF. Gonadoblastoma, testicular and prostate cancers, and the TSPY gene. Am J Hum Genet. 1999;64:921–7. doi: 10.1086/302353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kersemaekers AM, Honecker F, Stoop H, Cools M, Molier M, et al. Identification of germ cells at risk for neoplastic transformation in gonadoblastoma: an immunohistochemical study for OCT3/4 and TSPY. Hum Pathol. 2005;36:512–21. doi: 10.1016/j.humpath.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Tabatabai ZL, Lee TL, Hatakeyama S, Ohyama C, et al. The Y-encoded TSPY protein: a significant marker potentially plays a role in the pathogenesis of testicular germ cell tumors. Hum Pathol. 2007;38:1470–81. doi: 10.1016/j.humpath.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Vilain E, Conte F, Rajpert-De Meyts E, Lau YF. Testis-specific protein Y-encoded gene is expressed in early and late stages of gonadoblastoma and testicular carcinoma in situ. Urol Oncol. 2007;25:141–6. doi: 10.1016/j.urolonc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Lau Y, Chou P, Iezzoni J, Alonzo J, Kömüves L. Expression of a candidate gene for the gonadoblastoma locus in gonadoblastoma and testicular seminoma. Cytogenet Cell Genet. 2000;91:160–4. doi: 10.1159/000056838. [DOI] [PubMed] [Google Scholar]
- 47.Kido T, Lo RC, Li Y, Lee J, Tabatabai ZL, et al. The potential contributions of a Y-located protooncogene and its X homologue in sexual dimorphisms in hepatocellular carcinoma. Hum Pathol. 2014;45:1847–58. doi: 10.1016/j.humpath.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Yin YH, Li YY, Qiao H, Wang HC, Yang XA, et al. TSPY is a cancer testis antigen expressed in human hepatocellular carcinoma. Br J Cancer. 2005;93:458–63. doi: 10.1038/sj.bjc.6602716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallagher WM, Bergin OE, Rafferty M, Kelly ZD, Nolan IM, et al. Multiple markers for melanoma progression regulated by DNA methylation: insights from transcriptomic studies. Carcinogenesis. 2005;26:1856–67. doi: 10.1093/carcin/bgi152. [DOI] [PubMed] [Google Scholar]
- 50.Kido T, Hatakeyama S, Ohyama C, Lau YF. Expression of the Y-Encoded TSPY is associated with progression of prostate cancer. Genes (Basel) 2010;1:283–93. doi: 10.3390/genes1020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau YF, Lau HW, Kömüves LG. Expression pattern of a gonadoblastoma candidate gene suggests a role of the Y chromosome in prostate cancer. Cytogenet Genome Res. 2003;101:250–60. doi: 10.1159/000074345. [DOI] [PubMed] [Google Scholar]
- 52.Fratta E, Coral S, Covre A, Parisi G, Colizzi F, et al. The biology of cancer testis antigens: putative function, regulation and therapeutic potential. Mol Oncol. 2011;5:164–82. doi: 10.1016/j.molonc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mengus C, Schultz-Thater E, Coulot J, Kastelan Z, Goluza E, et al. MAGE-A10 cancer/testis antigen is highly expressed in high-grade non-muscle-invasive bladder carcinomas. Int J Cancer. 2013;132:2459–63. doi: 10.1002/ijc.27914. [DOI] [PubMed] [Google Scholar]
- 54.Sommermeyer D, Conrad H, Krönig H, Gelfort H, Bernhard H, et al. NY-ESO-1 antigen-reactive T cell receptors exhibit diverse therapeutic capability. Int J Cancer. 2013;132:1360–7. doi: 10.1002/ijc.27792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozbun LL, You L, Kiang S, Angdisen J, Martinez A, et al. Identification of differentially expressed nucleolar TGF-beta1 target (DENTT) in human lung cancer cells that is a new member of the TSPY/SET/NAP-1 superfamily. Genomics. 2001;73:179–93. doi: 10.1006/geno.2001.6505. [DOI] [PubMed] [Google Scholar]
- 56.Muto S, Senda M, Akai Y, Sato L, Suzuki T, et al. Relationship between the structure of SET/TAF-Ibeta/INHAT and its histone chaperone activity. Proc Natl Acad Sci U S A. 2007;104:4285–90. doi: 10.1073/pnas.0603762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Arcy S, Martin KW, Panchenko T, Chen X, Bergeron S, et al. Chaperone Nap1 shields histone surfaces used in a nucleosome and can put H2A-H2B in an unconventional tetrameric form. Mol Cell. 2013;51:662–77. doi: 10.1016/j.molcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park YJ, Luger K. The structure of nucleosome assembly protein 1. Proc Natl Acad Sci U S A. 2006;103:1248–53. doi: 10.1073/pnas.0508002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loyola A, Almouzni G. Histone chaperones, a supporting role in the limelight. Biochim Biophys Acta. 2004;1677:3–11. doi: 10.1016/j.bbaexp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Seo SB, McNamara P, Heo S, Turner A, Lane WS, et al. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–30. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 61.Chae YC, Kim KB, Kang JY, Kim SR, Jung HS, et al. Inhibition of FoxO1 acetylation by INHAT subunit SET/TAF-Iß induces p21 transcription. FEBS Lett. 2014;588:2867–73. doi: 10.1016/j.febslet.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Lau YF. TSPY and its X-encoded homologue interact with cyclin B but exert contrasting functions on cyclin-dependent kinase 1 activities. Oncogene. 2008;27:6141–50. doi: 10.1038/onc.2008.206. [DOI] [PubMed] [Google Scholar]
- 63.Oram SW, Liu XX, Lee TL, Chan WY, Lau YF. TSPY potentiates cell proliferation and tumorigenesis by promoting cell cycle progression in HeLa and NIH3T3 cells. BMC Cancer. 2006;6:154. doi: 10.1186/1471-2407-6-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kandalaft LE, Zudaire E, Portal-Núñez S, Cuttitta F, Jakowlew SB. Differentially expressed nucleolar transforming growth factor-beta1 target (DENTT) exhibits an inhibitory role on tumorigenesis. Carcinogenesis. 2008;29:1282–9. doi: 10.1093/carcin/bgn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tu Y, Wu W, Wu T, Cao Z, Wilkins R, et al. Antiproliferative autoantigen CDA1 transcriptionally up-regulates p21(Waf1/Cip1) by activating p53 and MEK/ERK1/2 MAPK pathways. J Biol Chem. 2007;282:11722–31. doi: 10.1074/jbc.M609623200. [DOI] [PubMed] [Google Scholar]
- 66.Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delbridge ML, Longepied G, Depetris D, Mattei MG, Disteche CM, et al. TSPY, the candidate gonadoblastoma gene on the human Y chromosome, has a widely expressed homologue on the X-implications for Y chromosome evolution. Chromosome Res. 2004;12:345–56. doi: 10.1023/B:CHRO.0000034134.91243.1c. [DOI] [PubMed] [Google Scholar]
- 68.Lau YF, Kido T, Li Y. The TSPT gene family. In: Lau YF, Chan WY, editors. The Y Chromosome and Male Germ Cell Biology in Health and Diseases. Hackensack: World Scientific Publishers; 2007. pp. 73–90. [Google Scholar]
- 69.Kido T, Lau YF. The human Y-encoded testis-specific protein interacts functionally with eukaryotic translation elongation factor eEF1A, a putative oncoprotein. Int J Cancer. 2008;123:1573–85. doi: 10.1002/ijc.23697. [DOI] [PubMed] [Google Scholar]
- 70.Wang W, Nag S, Zhang X, Wang MH, Wang H, et al. Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med Res Rev. 2015;35:225–85. doi: 10.1002/med.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang YW, Traugh JA. Phosphorylation of elongation factor 1 and ribosomal protein S6 by multipotential S6 kinase and insulin stimulation of translational elongation. J Biol Chem. 1997;272:28252–7. doi: 10.1074/jbc.272.45.28252. [DOI] [PubMed] [Google Scholar]
- 72.Sonenberg N. Translation factors as effectors of cell growth and tumorigenesis. Curr Opin Cell Biol. 1993;5:955–60. doi: 10.1016/0955-0674(93)90076-3. [DOI] [PubMed] [Google Scholar]
- 73.Spilka R, Ernst C, Mehta AK, Haybaeck J. Eukaryotic translation initiation factors in cancer development and progression. Cancer Lett. 2013;340:9–21. doi: 10.1016/j.canlet.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Kido T, Lau YF. The Y-located gonadoblastoma gene TSPY amplifies its own expression through a positive feedback loop in prostate cancer cells. Biochem Biophys Res Commun. 2014;446:206–11. doi: 10.1016/j.bbrc.2014.02.083. [DOI] [PubMed] [Google Scholar]
- 75.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–22. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 76.Hersmus R, de Leeuw BH, Wolffenbuttel KP, Drop SL, Oosterhuis JW, et al. New insights into type II germ cell tumor pathogenesis based on studies of patients with various forms of disorders of sex development (DSD) Mol Cell Endocrinol. 2008;291:1–10. doi: 10.1016/j.mce.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 77.McGlynn KA, Devesa SS, Graubard BI, Castle PE. Increasing incidence of testicular germ cell tumors among black men in the United States. J Clin Oncol. 2005;23:5757–61. doi: 10.1200/JCO.2005.08.227. [DOI] [PubMed] [Google Scholar]
- 78.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol. 2003;170:5–11. doi: 10.1097/01.ju.0000053866.68623.da. [DOI] [PubMed] [Google Scholar]
- 79.McGlynn KA, Cook MB. The epidemiology of testicular cancer. In: Foulkes WD, Cooney KA, editors. Male Reproductive Cancers. New York: Springer; 2010. [Google Scholar]
- 80.Skakkebaek NE. Possible carcinoma-in-situ of the testis. Lancet. 1972;2:516–7. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- 81.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–23. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- 82.Kristensen DM, Sonne SB, Ottesen AM, Perrett RM, Nielsen JE, et al. Origin of pluripotent germ cell tumours: the role of microenvironment during embryonic development. Mol Cell Endocrinol. 2008;288:111–8. doi: 10.1016/j.mce.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 83.Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 84.Oosterhuis JW, Looijenga LH, van Echten J, de Jong B. Chromosomal constitution and developmental potential of human germ cell tumors and teratomas. Cancer Genet Cytogenet. 1997;95:96–102. doi: 10.1016/s0165-4608(96)00275-0. [DOI] [PubMed] [Google Scholar]
- 85.Rajpert-De Meyts E, Bartkova J, Samson M, Hoei-Hansen CE, Frydelund-Larsen L, et al. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS. 2003;111:267–78. doi: 10.1034/j.1600-0463.2003.11101301.x. [DOI] [PubMed] [Google Scholar]
- 86.Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, et al. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer. 2007;6:12. doi: 10.1186/1476-4598-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003;100:13350–5. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cools M, Stoop H, Kersemaekers AM, Drop SL, Wolffenbuttel KP, et al. Gonadoblastoma arising in undifferentiated gonadal tissue within dysgenetic gonads. J Clin Endocrinol Metab. 2006;91:2404–13. doi: 10.1210/jc.2005-2554. [DOI] [PubMed] [Google Scholar]
- 89.Rajpert-De Meyts E, Ottesen AM, Hoei-Hansen CE, Sonne SB, Leffers H, et al. Origin of testicular germ cell neoplasia: the role of sex chromosomes. In: Lau YF, Chan WY, editors. The Y Chromosome and Male Germ Cell Biology in Health and Diseases. Hackensack: World Scientific; 2007. pp. 289–308. [Google Scholar]
- 90.Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, et al. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr Rev. 2013;34:339–76. doi: 10.1210/er.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cossu-Rocca P, Zhang S, Roth LM, Eble JN, Zheng W, et al. Chromosome 12p abnormalities in dysgerminoma of the ovary: a FISH analysis. Mod Pathol. 2006;19:611–5. doi: 10.1038/modpathol.3800576. [DOI] [PubMed] [Google Scholar]
- 92.Ulbright TM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol. 2005;18(Suppl 2):S61–79. doi: 10.1038/modpathol.3800310. [DOI] [PubMed] [Google Scholar]
- 93.Pleskacova J, Hersmus R, Oosterhuis JW, Setyawati BA, Faradz SM, et al. Tumor risk in disorders of sex development. Sex Dev. 2010;4:259–69. doi: 10.1159/000314536. [DOI] [PubMed] [Google Scholar]
- 94.Shirakawa H, Kuronuma T, Nishimura Y, Hasebe T, Nakano M, et al. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int J Oncol. 2009;34:649–56. doi: 10.3892/ijo_00000190. [DOI] [PubMed] [Google Scholar]
- 95.Luo J, Zha S, Gage WR, Dunn TA, Hicks JL, et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–6. [PubMed] [Google Scholar]
- 96.Vogel T, Dechend F, Manz E, Jung C, Jakubiczka S, et al. Organization and expression of bovine TSPY. Mamm Genome. 1997;8:491–6. doi: 10.1007/s003359900482. [DOI] [PubMed] [Google Scholar]
- 97.Hughes JF, Skaletsky H, Pyntikova T, Graves TA, van Daalen SK, et al. Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature. 2010;463:536–9. doi: 10.1038/nature08700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schubert S, Dechend F, Skawran B, Kunze B, Winking H, et al. Silencing of the Y-chromosomal gene tspy during murine evolution. Mamm Genome. 2000;11:288–91. doi: 10.1007/s003350010054. [DOI] [PubMed] [Google Scholar]
- 99.Mazeyrat S, Mitchell MJ. Rodent Y chromosome TSPY gene is functional in rat and non-functional in mouse. Hum Mol Genet. 1998;7:557–62. doi: 10.1093/hmg/7.3.557. [DOI] [PubMed] [Google Scholar]
- 100.Kido T, Lau YF. The rat Tspy is preferentially expressed in elongated spermatids and interacts with the core histones. Biochem Biophys Res Commun. 2006;350:56–67. doi: 10.1016/j.bbrc.2006.08.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schubert S, Skawran B, Dechend F, Nayernia K, Meinhardt A, et al. Generation and characterization of a transgenic mouse with a functional human TSPY. Biol Reprod. 2003;69:968–75. doi: 10.1095/biolreprod.103.016501. [DOI] [PubMed] [Google Scholar]
- 102.Kido T, Schubert S, Hatakeyama S, Ohyama C, Schmidtke J, et al. Expression of a Y-located human proto-oncogene TSPY in a transgenic mouse model of prostate cancer. Cell Biosci. 2014;4:9. doi: 10.1186/2045-3701-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skrisovska L, Bourgeois CF, Stefl R, Grellscheid SN, Kister L, et al. The testis-specific human protein RBMY recognizes RNA through a novel mode of interaction. EMBO Rep. 2007;8:372–9. doi: 10.1038/sj.embor.7400910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dreumont N, Bourgeois CF, Lejeune F, Liu Y, Ehrmann IE, et al. Human RBMY regulates germline-specific splicing events by modulating the function of the serine/arginine-rich proteins 9G8 and Tra2-{beta} J Cell Sci. 2010;123:40–50. doi: 10.1242/jcs.055889. [DOI] [PubMed] [Google Scholar]
- 105.Elliott DJ, Millar MR, Oghene K, Ross A, Kiesewetter F, et al. Expression of RBM in the nuclei of human germ cells is dependent on a critical region of the Y chromosome long arm. Proc Natl Acad Sci U S A. 1997;94:3848–53. doi: 10.1073/pnas.94.8.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsuei DJ, Hsu HC, Lee PH, Jeng YM, Pu YS, et al. RBMY, a male germ cell-specific RNA-binding protein, activated in human liver cancers and transforms rodent fibroblasts. Oncogene. 2004;23:5815–22. doi: 10.1038/sj.onc.1207773. [DOI] [PubMed] [Google Scholar]
- 107.Tsuei DJ, Lee PH, Peng HY, Lu HL, Su DS, et al. Male germ cell-specific RNA binding protein RBMY: a new oncogene explaining male predominance in liver cancer. PLoS One. 2011;6:e26948. doi: 10.1371/journal.pone.0026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nathanson KL, Kanetsky PA, Hawes R, Vaughn DJ, Letrero R, et al. The Y deletion gr/gr and susceptibility to testicular germ cell tumor. Am J Hum Genet. 2005;77:1034–43. doi: 10.1086/498455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Linger R, Dudakia D, Huddart R, Easton D, Bishop DT, et al. A physical analysis of the Y chromosome shows no additional deletions, other than Gr/Gr, associated with testicular germ cell tumour. Br J Cancer. 2007;96:357–61. doi: 10.1038/sj.bjc.6603557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Graves JA. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–14. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 111.Kato T, Miyata K, Sonobe M, Yamashita S, Tamano M, et al. Production of Sry knockout mouse using TALEN via oocyte injection. Sci Rep. 2013;3:3136. doi: 10.1038/srep03136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shpargel KB, Sengoku T, Yokoyama S, Magnuson T. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet. 2012;8:e1002964. doi: 10.1371/journal.pgen.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Forsberg LA, Rasi C, Malmqvist N, Davies H, Pasupulati S, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet. 2014;46:624–8. doi: 10.1038/ng.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiktor AE, Van Dyke DL, Hodnefield JM, Eckel-Passow J, Hanson CA. The significance of isolated Y chromosome loss in bone marrow metaphase cells from males over age 50 years. Leuk Res. 2011;35:1297–300. doi: 10.1016/j.leukres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 115.Jansen R, Batista S, Brooks AI, Tischfield JA, Willemsen G, et al. Sex differences in the human peripheral blood transcriptome. BMC Genomics. 2014;15:33. doi: 10.1186/1471-2164-15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]


