Abstract
Germ cells are the precursors of the sperm and oocytes and hence are critical for survival of the species. In mammals, they are specified during fetal life, migrate to the developing gonads and then undergo a critical period during which they are instructed, by the soma, to adopt the appropriate sexual fate. In a fetal ovary, germ cells enter meiosis and commit to oogenesis, whereas in a fetal testis, they avoid entry into meiosis and instead undergo mitotic arrest and mature toward spermatogenesis. Here, we discuss what we know so far about the regulation of sex-specific differentiation of germ cells, considering extrinsic molecular cues produced by somatic cells, as well as critical intrinsic changes within the germ cells. This review focuses almost exclusively on our understanding of these events in the mouse model.
Keywords: germ cells, meiosis, oocytes, sex determination processes, spermatozoa
INTRODUCTION
In mammals, the germ cell lineage is not preformed but instead arises during early development when a restricted group of epiblast cells responds to inductive signals, particularly BMP4, originating from the extra-embryonic ectoderm.1 In the mouse, a cohort of about 40 primordial germ cells (PGCs) is first identifiable at 6.25 to 7.25 days post coitum (dpc). These cells undergo considerable epigenetic reprogramming, including widespread chromatin modification that results in active down-regulation of key somatic marker genes, either maintenance or re-expression of pluripotency-associated genes (Oct4, Sox2, Nanog), and activation of genes characteristic of the germ cell lineage, particularly Prdm1 (also known as Blimp1) and Prdm14.2 The nascent germ cells then migrate through the embryo, proliferating as they go, and eventually colonize the gonad at about 10.5 dpc.3
When germ cells first enter the gonad, it is still “bi-potential” because somatic sex determination is yet to occur. If the somatic cells in the gonad are XX, an ovary tends to form. If the somatic cells are XY, on the other hand, the Y-chromosomally encoded male sex-determining gene Sry is expressed, peaking at around 11.5 dpc, and development is diverted from ovary to testis.4 Despite this dependence of gonadal somatic sexual fate on the presence or absence of the Y chromosome, it is critical to appreciate that, for germ cells, the initial steps toward ultimate sexual fate are dictated not by their own XX or XY chromosomal constitution but by the somatic environment in which they find themselves.5,6 Thus, germ cells in a fetal ovary will enter prophase of meiosis I, the first step toward oogenesis, and germ cells in a fetal testis will avoid entry into meiosis and instead halt their proliferation and begin to express markers characteristic of a commitment to spermatogenesis.3,7 Normally, of course, germ cells in an ovary will be XX and those in a testis will be XY; however, if they are not, whether by accident or experimental design, they will still align their sexual development with that of their somatic environment. Until recently, we had virtually no understanding of how somatic cells act to influence germ cell sexual fate. However, since 2006, when the meiosis-inducing role of retinoic acid (RA) in the fetal ovary was first demonstrated,8,9 significant progress has been made in our understanding of the intrinsic and extrinsic determinants of mammalian germ cell sexual fate.
PREPARING TO TAKE INSTRUCTION
Upon entering the gonad, PGCs undergo an almost complete global demethylation, including the erasure of genomic imprints and, for XX germ cells, X chromosome reactivation.2,10 Demethylation results in up-regulation of certain germ cell specific genes such as deleted in azoospermia-like (Dazl), mouse vasa homolog, also known as DEAD box polypeptide 4 (Mvh, Ddx4) and synaptonemal complex protein 3 (Sycp3).11,12 This stage of development is also characterized by additional chromatin modifications that confer a “ground state” pluripotency and, presumably, make the germ cells receptive to inductive signals that direct their fate13,14 (Figure 1).
Figure 1.
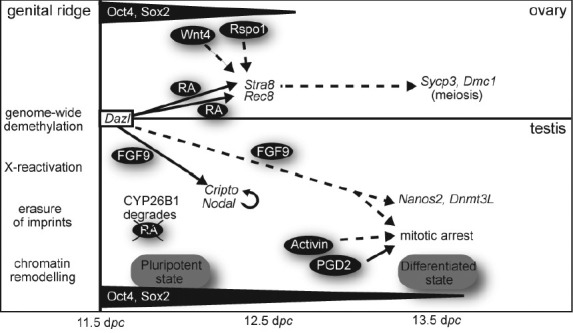
Regulation of sex-specific germ cell development in the mouse gonad. As germ cells enter the genital ridge (future gonad) they undergo genome-wide demethylation, including reactivation of the X chromosome, erasure of imprints as well as chromatin remodelling. In a developing ovary, RA induces expression of the pre-meiotic gene Stra8 as well as a meiosis-associated gene, Rec8. STRA8 is critical for germ cell meiosis that is marked by up-regulation of Sycp3 and Dmc1 at about 13.5 dpc. In the developing testis, endogenous RA is degraded by a P450 enzyme, CYP26B1. FGF9 is present and acts directly on testicular germ cells to up-regulate Cripto, triggering active cell autonomous Nodal signalling that appears to maintain the pluripotent state. FGF9 also influences expression of later male fate markers. Activin and PGD2 influence the timing of mitotic arrest. Germ cells do not differentate along either female or male pathways unless they first express Dazl. Signaling molecules are shown in black ovals; unbroken arrows indicate a direct effect and broken arrows indicate an incompletely characterised or likely indirect effect.
Deleted in azoospermia-like, in particular, seems to be essential in the preparation of the germ cells for receiving instructions from extrinsic factors once in the gonad, at least in the C57BL/6 background. DAZL, an RNA-binding protein, is considered a germ-cell-intrinsic competence factor because germ cells must first express this if they are to later embark on either female or male sex-specific development.15,16 As yet we have no understanding of how expression of Dazl ensures that germ cells are responsive to feminizing or masculinizing cues from the somatic tissue other than that germ cells deleted for Dazl do not down-regulate the expression of PGC-characteristic pluripotency-associated markers such as OCT4, SOX2 and NANOG: possibly germ cells need to transition from the “primordial” state before they can differentiate.
EMBARKING ON OOGENESIS – THE ACTIONS OF RETINOIC ACID
In the ovary, germ cells stop proliferating, begin to condense their chromosomes and enter meiosis at 14.5 dpc and then arrest in late prophase of meiosis I until ovulation. Embarking upon meiosis during fetal life has traditionally been considered a commitment to oogenesis, although there is some evidence that ovarian germ cells that have never undergone meiosis can still differentiate into fertilization-competent oocyte-like cells.17 Until recent years various observations were interpreted as evidence that germ cells did not require a meiosis-inducing stimulus and that they would enter meiosis spontaneously and in a cell autonomous fashion unless a male-specific factor intervened to prevent this from occurring.18 However, it is now well accepted that the first steps toward meiosis are triggered by the presence of RA.8,9,18,19
Retinoic acid is present in the gonadal environment and is produced in abundance in the adjacent tissue, the mesonephros, although some may also be produced in the gonad itself.9,20 RA triggers the expression of a key premeiotic gene, stimulated by retinoic acid, gene 8 (Stra8), in ovarian germ cells: Stra8 is essential for meiosis in both sexes.21 The molecular mechanism by which Stra8 operates is unknown, although there is some evidence that the protein shuttles between nucleus and cytoplasm.22 STRA8 is essential for meiosis-specific DNA replication as well as for triggering later molecular events of meiotic prophase 1 such as the formation of DNA double stranded breaks and the up-regulation of SYCP3 and DMC1 (dosage suppressor of mck1 homolog, meiosis-specific homologous recombination [yeast]), first observed at about 13.5 dpc.21,23,24 Recently, a second meiosis-essential gene, Rec8 (which encodes a component of the cohesin complex that accumulates during meiotic S phase, REC8 meiotic recombination protein), was also found to be an RA target, activated independently of Stra8.23
NON-RA INFLUENCES ON THE EXPRESSION OF STIMULATED BY RETINOIC ACID, GENE 8
Retinoic acid acts directly on germ cells to induce Stra8 expression25,26 and, in responsive cell types, this occurs even when RA is present at extremely low concentrations25,27,28,29. Two RA response elements (RAREs) have been identified in the proximal promoter region of Stra830 and, at least in in vitro studies, these have been shown to direct Stra8 expression.31 ChIP-seq analysis in embryonic stem (ES) cells demonstrated direct binding of the RA/RA receptor (RAR) complex to the Stra8 promoter32 although this result has not yet been shown in fetal germ cells. However, several intrinsic germ cell factors appear to have some impact on the expression of Stra8. These include DMRT1: in the absence of this evolutionarily-conserved protein the expression of Stra8 is retarded in ovarian germ cells though, surprisingly, this effect varies substantially from cell to cell suggesting an element of stochasticity.33 The DMRT1 binding site detected by qChIP, carried out on mouse fetal ovary tissues, lies between the two proximal RAREs mentioned above. Interestingly, qChIP analysis did not detect DMRT1 binding to this site in fetal testis tissue even though DMRT1 is more abundant in XY germ cells than in XX germ cells.34 This result suggests that ovary-specific RA/RAR binding may facilitate DMRT1 binding to the Stra8 promoter that then enhances Stra8 transcription.
Other germ cell intrinsic factors that seem to have a bearing on the expression of Stra8 and, hence, meiosis initiation, are homeobox transcription factors MSX1 and MSX2. In the Msx1/Msx2 double knockout mutant fetal ovary, fewer germ cells than normal embark on meiosis, although those that do seem to progress through prophase of meiosis I correctly.35 In the F9 (mouse embryonal carcinoma) cell line MSX1 and MSX2 directly bind 3 distinct sequences upstream of the two RAREs in the Stra8 locus suggesting that they may have a direct effect on Stra8 activation. It seems however that the role of MSX1/2 is to maintain or augment rather than activate Stra8 expression: no abnormality in Stra8 expression is observed at 13.5 dpc, rather it is first seen at 14.5 dpc. Intriguingly, the germ cells that do enter meiosis tend to be found at the anterior end of the gonad, in agreement with the expectation that RA levels are higher at that end:18 possibly the higher levels of RA available in the region of the gonad closest to the mesonephric ducts are sufficient to achieve a meiosis-inducing level of Stra8 expression without assistance from MSX1/2.
Germ cell extrinsic factors have also been reported to influence the levels of expression of Stra8. These include the signaling molecules WNT4 and RSPO1, both involved in the development of the ovarian soma.36,37 In Wnt4 null and Rspo1 null XX embryos germ cells enter meiosis normally but then die: it seems likely that the somatic environment is sufficiently abnormal in these mouse models as to impact on germ cell survival although it remains possible that one or both of these factors affects germ cell meiosis directly.38,39 FGF9, a signaling molecule produced by the Sertoli cells of the early fetal testis that is essential for normal somatic development in the testis,40 does appear to have a direct effect on germ cells.25,41 FGF9 signaling seems to affect the sensitivity of germ cells, be they XX or XY, to available levels of RA: in the presence of FGF9 Stra8 expression in response to a given level of RA is diminished.25 There is evidence that one key role for FGF9 is to push XY germ cells toward the male fate (see below), hence it is possible that FGF9 makes germ cells less susceptible to the effects of RA on Stra8 expression simply because those germ cells have already begun to differentiate along the male pathway.
TURNING OFF PLURIPOTENCY-ASSOCIATED GENES AT MEIOTIC ONSET
At specification and during migration to the fetal gonads both XX and XY germ cells are highly pluripotent and express pluripotency marker genes Oct4, Sox2 and Nanog, also common to ES cells. The bona fide pluripotency of these cells is evident in their ability to seed colonies of pluripotent embryonic germ (EG) cells - pluripotent cell lines that mimic ES cells.42,43,44 In the ovary, these markers are substantially down-regulated by 13.5 dpc (just prior to entry into meiosis).45,46,47
Recent studies have investigated whether germ cell nuclear factor (GCNF) might be involved in repressing Oct4 expression in the ovary. GCNF is an orphan nuclear factor and a known direct transcriptional repressor of Oct4 in somatic cells in vivo48 and in ES cells.49 GCNF is expressed in an ovarian-specific manner in germ cells, making it a good candidate for involvement in down-regulation of Oct4 in fetal ovarian germ cells. However, two recent studies differ markedly in their conclusions as to the role of GCNF in ovarian germ cells and further studies are clearly required. One study found that the absence of GCNF is indeed associated with a loss of Oct4 repression and that, moreover, GCNF is involved in activation of Stra8 expression and initiation of meiosis;50 the other showed that GCNF is not required for either down-regulation of Oct4 expression or meiotic onset.51
WHY IS STIMULATED BY RETINOIC ACID, GENE 8 INDUCTION BY RA SPECIFIC TO GERM CELLS?
Retinoic acid, the meiotic inducer, is widely available during development so it is unclear why most cells are completely resistant to Stra8 induction by RA while others, such as fetal germ cells and premeiotic spermatogonia, are highly sensitive. It seems likely that long-term and efficient epigenetic silencing mechanisms are involved in silencing the Stra8 locus in most cell types and that these mechanisms are relaxed at certain stages of the germ cell cycle. Possibly the highly naive status of germ cells shortly after they colonize the gonad makes them particularly sensitive to RA.
The idea of epigenetic silencing of the Stra8 locus was borne out by recent studies that demonstrated that activity of the transcriptional repressor Polycomb repressive complex 1 (PRC1) has a bearing on when gonadal germ cells are free to commence meiosis.52 When components of PRC1 are genetically deleted, Stra8 expression and entry into meiosis occur earlier than normal. Importantly, the absence of PRC1 components does not lead to the expression of Stra8 in XY germ cells indicating that the absence of PRC1 is permissive but not instructive for Stra8 expression. It seems that direct binding of PRC1, and possibly PRC2, to the Stra8 promoter makes germ cells less sensitive to RA: at 11.5 dpc the Stra8 promoter is in a repressed, but poised ‘bivalent’ state characterized by detectable H3K27me3 (repressive mark) and H3K4me3 (active mark). Presumably this mechanism influences the timing of Stra8 expression by controlling the threshold of RA required to trigger Stra8 expression. Interestingly, other cell types with the potential to respond to RA by up-regulating Stra8, such ES and EG cells are also characterized by this bivalent epigenetic state.14,53
An earlier study also suggested that the Stra8 locus is under epigenetic control. In vitro, treatment with trichostatin-A (TSA, a histone deacetylase [HDAC] inhibitor) promoted Stra8 expression in a synergistic fashion with RA and, in vivo, TSA treatment of 13.5 dpc litters resulted in up-regulation of Stra8 in testicular germ cells.31 These results suggest the possibility that Stra8 expression is held silent at least partially by an HDAC-dependent epigenetic mechanism. This idea correlates well with the fact that HDACs are well known to be recruited by unbound RARs, which work in some circumstances in a repressive capacity.54
THE ALTERNATIVE FATE – SPERMATOGENESIS
Germ cells in a fetal ovary, cultured without the mesonephros, do not enter meiosis but nor do they develop into spermatogonia.55 Similarly, in the Stra8 KO, when XX germ cells cannot enter meiosis, they do not adopt the spermatogenic fate by default: they maintain a premeiotic morphology and then, by birth, they die.21 Studies have shown that a signal from the Sertoli cells, operating by 12.5 dpc, is required to induce male fate in germ cells28,56 although a later diffusible signal from peritubular and/or Leydig cells at 13.5 dpc has also been implicated.57 Although the details of how male germ cell fate is determined are far from settled, it seems clear that a signal/s from somatic cells are involved rather than that male germ cell fate occurs spontaneously in the absence of RA.
In the testicular environment germ cells differentiate into prespermatogonia in a process characterized by certain events that generally occur nonsynchronously over a period of several days. In chronological order these include: activation of the Nodal/Cripto signaling pathway (12.5 dpc–14.5 dpc), exit from the mitotic cell cycle (12.5 dpc–15.5 dpc), up-regulation of late male fate markers (13.5 dpc onward), down-regulation of pluripotency markers (13.5 dpc onward) and establishment of de novo methylation (14.5 dpc onward).
FGF9 IS AN EARLY INSTRUCTOR FOR MALENESS
At the time of germ cell colonization of the gonads FGF9 is a predominant signaling factor in the testis, but not the ovary. FGF9, produced by newly-specified Sertoli cells, is maximally expressed at 11.5 dpc25 and is critical for testis development as it antagonises the default ovarian pathway.58 Although the important requirement for FGF9 in somatic testis development make it difficult to study its role with respect to germ cell sexual differentiation, there is evidence that it acts directly on testicular germ cells to maintain expression of pluripotency markers, to induce differentiation markers and to provide a back-up mechanism to ensure germ cells do not enter meiosis.25,41
EARLY MARKERS OF MALE GERM CELL FATE – SWITCHING ON CRIPTO/NODAL
One early role for FGF9 is to up-regulate Cripto expression in testicular germ cells.59 Cripto, which encodes an obligate co-receptor for the TGFβ signaling molecule Nodal, is currently the earliest marker of male germ cell fate and is expressed male-specifically in germ cells from 12.5 to 14.5 dpc.59,60 Expression of Cripto is transient, suggesting that loss of Cripto expression may simply reflect the loss of FGF9 after about 12.5 dpc. Shortly after Cripto is expressed, the gene encoding its ligand, Nodal, is up-regulated, followed also by genes encoding downstream transcriptional targets and repressors of Nodal/Cripto signaling, Lefty1 and Lefty2. These transcriptional changes suggested that cell autonomous Nodal/Cripto signaling is activated in testicular germ cells shortly after their colonization of the gonad and this was confirmed by demonstration of phosphorylated SMAD2 in male germ cells from 12.5 dpc.59
Nodal/Cripto signaling plays important roles during both early embryonic development and in stem cell biology: it is responsible for mesoderm and endoderm specification during gastrulation and initiation of left-right asymmetry as well as patterning of the nervous system reviewed by.61 Moreover, the Nodal co-receptor, Cripto, is required in stem cells for self-renewal and pluripotency and is commonly overexpressed in many cancers.62 Given these known roles for Nodal/Cripto signaling, its activation in germ cells of the fetal testis is intriguing. In the context of male fetal germ cells, it seems that the primary role of Cripto is akin to its role in stem cells - to maintain pluripotency for a defined period of time.59,63 In a model of suppressed Nodal signaling (Nodalflox/flox hypomorphant), expression of pluripotency markers was decreased, which correlated with a reduced ability of these cells to seed EG cells ex vivo and was associated with precocious expression of male fate markers.59 In culture, blocking of TGFβ/Nodal/Activin signaling using chemical inhibitor to ALK4/5/7 (SB431542) in ex vivo culture from 12.5 dpc led to down-regulation of a pluripotency marker, Nanog, previously noted as a downstream target of Nodal.63,64
A role for Nodal/Cripto signaling in the suppression of meiosis in male germ cells has also been postulated. Again using the Alk4/5/7 inhibitor SB431542 at 11.5 dpc in ex vivo culture, robust germ cell entry into meiosis was observed using markers Stra8, Rec8, Dmc1, γH2AX and SYCP3.60,63,65 It should be noted however that under these conditions SB431542 causes major disruption to Sertoli cell proliferation, organization and differentiation such that cords do not form, indicating that TGFβ/Activin/Nodal signaling also acts on the soma in the developing testis.63,65 Hence, given the fact that the testis soma is abnormal, it is impossible to conclude from these in vitro studies that autocrine Activin/Nodal signaling in germ cells functions to suppress meiosis. Caution on this point seems warranted also because the use of the Alk4/5/7 inhibitor SB431542 one day later at 12.5 dpc results in normal testis cord morphology and, with rare exception, germ cells did not enter meiosis.63 In addition, no ectopic meiosis was observed in the Nodal hypomorphic model mentioned above.59 Conditional deletion of Cripto and/or Nodal in germ cells will be required to determine the precise roles Nodal/Cripto signaling plays with respect to pluriptotency, meisois suppression and differentiation.
LOCKING-IN MALE GERM CELL IDENTITY
Germ cells isolated from testes at 12.5 dpc are considered already committed to spermatogenesis66 yet recent experiments indicate that XY germ cells remain susceptible to meiotic induction until about 14.5 dpc26 or even 15.5 dpc.67 This suggests that the male fate signal from the soma acts early, by at least 12.5 dpc, but that germ cells remain bipotential for a time, only irreversibly committing to their male identity several days later.
Nanos homolog 2 (Nanos2) (Drosophila) encodes an RNA-binding protein that appears to be essential for the maintenance of the male germ cell fate.68 In the developing testis, where RA is absent and FGF9 present, germ cells begin to express Nanos2 at 13.5 dpc.25,41,69 Nanos2 null germ cells die, but if they are rescued (by removal of the pro-apoptotic gene Bax1) they eventually express low levels of Stra8 at 14.5 dpc, presumably concomitant with the loss of RA-degrading CYP26B1 activity at that time.68 Nanos2 makes germ cells resistant to meiotic induction at least in part because it binds (in conjunction with the CCR3-NOT deadenylation complex) and sequesters meiosis-associated transcripts such as Stra8 and Sycp3 in RNA degradation centers known as P-bodies.70
In terms of “locking in” male germ cell fate, Nanos2 is also necessary for the up-regulation of the de novo methylase-associated protein DNA methyltransferase 3-like (DNMT3L) at about 13.5 to 14.5 dpc.68 As mentioned above, the period of germ cell migration and gonad colonization is characterized by erasure of virtually all methylation marks in germ cells.2,10 Female germ cells enter meiosis in this epigenetically ‘naive’ state, and it is not until after birth and entry into meiosis that sex-specific DNA methylation patterns are established. Conversely, male germ cells undergo progressive de novo methylation during the 13.5 dpc–18.5 dpc period of fetal development. Methyltransferases DNMT3A, DNMT3B and the noncatalytic but critical modulatory protein, DNMT3L, are responsible for establishing male-specific remethylation at this time.71,72 Expression of Dnmt3a and Dnmt3b is observed at low levels in both male and female fetal germ cells prior to 13.5 dpc; Dnmt3l, however, is absent in germ cells until it is robustly and male-specifically up-regulated at 14.5 dpc.47 Owing to this expression pattern, Dnmt3l is considered a definitive marker of a germ cell that is committed to the male germ cell fate.
CONTROLLING PLURIPOTENCY – OCT4/SOX2/NANOG
In the male germ cells, repression of pluripotency markers Sox2 and Nanog occurs at the transcriptional level by 14.5 dpc–15.5 dpc and strong remethylation of the Sox2 and Nanog promoter regions is observed in male germ cells by 17.5 dpc.47 Interestingly, Oct4 transcripts are still detectable in 15.5 dpc male germ cells and promoter regions remain unmethylated yet OCT4 protein is absent from germ cells by 15.5 dpc, indicating posttranscriptional regulation must be involved in loss of this key pluripotency-associated protein.47 It is hypothesized that suppression of latent germ cell pluripotency is critical during fetal life in the male embryo if the development of germ cell tumors is to be avoided in early adult life.73 The aberrations in fetal germ cell development that are likely to underlie the formation of carcinoma in situ, the precursor of germ cell tumor, are beyond the scope of this review but have been considered extensively elsewhere.74,75
MALE GERM CELL MITOTIC ARREST
During migration and genital ridge colonization, germ cells are actively proliferating so that the founding population of around 40 cells increases to more than 25 000 per gonad by 12.5 dpc.76 Beginning at 12.5 dpc, and occurring over several days, germ cells in a testis cease mitotic proliferation and exit the cell cycle at the G1/G0 transition: this arrest is maintained until after birth.7 By 14.5 dpc, approximately 95% of germ cells are arrested as assessed by flow cytometry,77 though there are some reports of mitotically active germ cells as late at 16.5 dpc.78 Recent studies have found that germ cells use cell cycle machinery common to somatic cells to achieve this arrest: cyclin-dependent kinases inhibitors p21Cip1, p57Kip2, p27kip1 and p15INK4b and G1/S phase checkpoint protein retinoblastoma 1 are all involved in maintaining G1/G0 arrest during this developmental window.77,79
There is evidence that removal of endogenous RA by CYP26B1 is necessary not just to ensure XY germ cells avoid meiosis but, also, so that normal mitotic arrest can occur.24,80 In Cyp26b1−/−; Stra8−/− XY gonads, where endogenous RA is not degraded but ectopic meiosis is prevented, mitotic arrest was delayed in some germ cells.24 The fact that excess RA can impede the normal processes of mitotic arrest is perhaps not surprising given that RA is known to act as a potent germ cell mitogen.81 Based on the incomplete nature of the phenotype in this mouse mutant, however, it seems likely that it is not just the absence of RA but also the presence of certain factors, produced by Sertoli or Leydig cells, which ensures XY germ cells arrest at the appropriate time. To date members of the TGFβ signaling family, as well as Prostaglandin D2 (PGD2), have been implicated; it is possible that a complex and redundant system is involved.
From 12.5 dpc expression of Activin subunits, Inhba and Inhbb, is up-regulated in male somatic cells and expression of Activin receptors (Alk4, Alk7, Acvr2a, Acvr2b) is observed in both germ and somatic cells, peaking by 15.5 dpc–16.5 dpc.59,63,82,83 Pharmacological studies suggest that signaling through the Activin/Nodal receptors, ALK4 and ALK7 is required for promoting both differentiation of male germ cells and their entry into mitotic arrest63 and, in the Inhba knockout, germ cell numbers are increased at 15.5 dpc, suggesting a role for Activin in initiation and/or maintenance of G1/G0 arrest.82
The TGFβ morphogen Nodal also uses the common Activin receptors ALK4, ALK7 and ACVR2B. Importantly, however, the expression of the obligate co-receptor Cripto specifically by male germ cells should theoretically restrict Nodal signaling to this population. In other systems, the presence of Cripto on a cell surface renders cells resistant to the effects of Activins because it physically interacts with them, disrupting their ability to bind and activate the Activin type I-typeII receptor complex.84 Therefore, it is possible that the timing of male germ cell mitotic arrest is regulated by expression of Cripto: once Cripto expression falls, germ cells become susceptible to the effects of Activins. In line with this, proliferation was slightly decreased in a hypomorphic Nodal mutant, though this did not manifest a significant difference in overall germ cell number.59 It will be necessary to test this idea in a Cripto-null germ cell model.
Recent studies suggest that PGD2, produced by Sertoli cells, acts through the D2 receptor on germ cells to influence male germ cell fate. The actions of PGD2 appear to be associated with the onset of mitotic arrest specifically by activation of CDK inhibitor p21Cip1.85
CONCLUSIONS
The study of germ cells during the fetal period of development is intrinsically fascinating: it allows us to focus on a defined developmental system that hides secrets regarding the critical balance between proliferation and differentiation. Although the sex-specific behavior of germ cells during fetal life has been recognized for many decades, it is only in recent years that some of the specific factors responsible for directing germ cells toward their oogenic or spermatogenic fate have been identified. Progress has been rapid yet a great deal remains unclear and, undoubtedly, many more extrinsic and intrinsic participants are yet to be revealed.
One key question that remains relates to the function of STRA8 – what sort of a protein is it and how does it harness the meiotic machinery so effectively? Puzzles remain also in terms of how this critical meiosis-inducing gene comes to be regulated: how is the Stra8 gene so sensitive to RA at certain times and in certain cells whilst so resistant in most situations? In the fetal testis, we are yet to resolve exactly which soma-produced signals are involved in triggering spermatogenic fate and how mitotic arrest and up-regulation of the key male fate gene, Nanos2, are interrelated. The role of Nodal and its receptor Cripto is still unclear but, given the importance of these factors in other systems, is likely to be key to our understanding of the control of latent pluripotency in germ cells.
As more germ cell fate markers have become available, a number of instances where germ cell fate is disrupted have been reported in mouse mutants. In interpreting the evidence, in such situations, it is essential to not lose sight of the long-accepted dogma that the soma controls germ cell development. It is vital to design and perform experiments that will clarify whether the factor in question affects germ cell development directly or via its effects on testicular or ovarian development.
As the community builds the necessary tools to study mouse fetal germ cells, building on discovery after discovery, the entire field is gaining in momentum. An upsurge in knowledge relevant to our understanding of the subtleties of optimal reproductive capacity, the formation, prevention and treatment germ cell tumors, and the principles of stem cell biology more generally can only follow.
AUTHOR CONTRIBUTIONS
CMS and JB wrote the manuscript, reviewed it and approved its final form.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–6. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, et al. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol. 2005;278:440–58. doi: 10.1016/j.ydbio.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 3.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 4.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–21. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 5.Evans EP, Ford CE, Lyon MF. Direct evidence of the capacity of the XY germ cell in the mouse to become an oocyte. Nature. 1977;267:430–1. doi: 10.1038/267430a0. [DOI] [PubMed] [Google Scholar]
- 6.McLaren A. Sex reversal in the mouse. Differentiation. 1983;23(Suppl):S93–8. doi: 10.1007/978-3-642-69150-8_16. [DOI] [PubMed] [Google Scholar]
- 7.Hilscher B, Hilscher W, Bülthoff-Ohnolz B, Krämer U, Birke A, et al. Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974;154:443–70. doi: 10.1007/BF00219667. [DOI] [PubMed] [Google Scholar]
- 8.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–9. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 10.Sato S, Yoshimizu T, Sato E, Matsui Y. Erasure of methylation imprinting of Igf2r during mouse primordial germ-cell development. Mol Reprod Dev. 2003;65:41–50. doi: 10.1002/mrd.10264. [DOI] [PubMed] [Google Scholar]
- 11.Maatouk DM, Kellam LD, Mann MR, Lei H, Li E, et al. DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development. 2006;133:3411–8. doi: 10.1242/dev.02500. [DOI] [PubMed] [Google Scholar]
- 12.Hackett JA, Reddington JP, Nestor CE, Dunican DS, Branco MR, et al. Promoter DNA methylation couples genome-defence mechanisms to epigenetic reprogramming in the mouse germline. Development. 2012;139:3623–32. doi: 10.1242/dev.081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 14.Hajkova P. Epigenetic reprogramming in the germline: towards the ground state of the epigenome. Philos Trans R Soc Lond B Biol Sci. 2011;366:2266–73. doi: 10.1098/rstb.2011.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill ME, Hu YC, Lin Y, Page DC. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc Natl Acad Sci U S A. 2011;108:7443–8. doi: 10.1073/pnas.1104501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–7. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 17.Dokshin GA, Baltus AE, Eppig JJ, Page DC. Oocyte differentiation is genetically dissociable from meiosis in mice. Nat Genet. 2013;45:877–83. doi: 10.1038/ng.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134:3401–11. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- 19.Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod. 2012;86:35. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu X, Wen J, Guo M, Wang J, Li G, et al. Retinoic acid derived from the fetal ovary initiates meiosis in mouse germ cells. J Cell Physiol. 2013;228:627–39. doi: 10.1002/jcp.24172. [DOI] [PubMed] [Google Scholar]
- 21.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, et al. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–4. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 22.Tedesco M, La Sala G, Barbagallo F, De Felici M, Farini D. STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J Biol Chem. 2009;284:35781–93. doi: 10.1074/jbc.M109.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koubova J, Hu YC, Bhattacharyya T, Soh YQ, Gill ME, et al. Retinoic acid activates two pathways required for meiosis in mice. PLoS Genet. 2014;10:e1004541. doi: 10.1371/journal.pgen.1004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saba R, Wu Q, Saga Y. CYP26B1 promotes male germ cell differentiation by suppressing STRA8-dependent meiotic and STRA8-independent mitotic pathways. Dev Biol. 2014;389:173–81. doi: 10.1016/j.ydbio.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, et al. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell. 2010;19:440–9. doi: 10.1016/j.devcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Ohta K, Lin Y, Hogg N, Yamamoto M, Yamazaki Y. Direct effects of retinoic acid on entry of fetal male germ cells into meiosis in mice. Biol Reprod. 2010;83:1056–63. doi: 10.1095/biolreprod.110.085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tedesco M, Desimio MG, Klinger FG, De Felici M, Farini D. Minimal concentrations of retinoic acid induce stimulation by retinoic acid 8 and promote entry into meiosis in isolated pregonadal and gonadal mouse primordial germ cells. Biol Reprod. 2013;88:145. doi: 10.1095/biolreprod.112.106526. [DOI] [PubMed] [Google Scholar]
- 28.Ohta K, Yamamoto M, Lin Y, Hogg N, Akiyama H, et al. Male differentiation of germ cells induced by embryonic age-specific Sertoli cells in mice. Biol Reprod. 2012;86:112. doi: 10.1095/biolreprod.111.095943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oulad-Abdelghani M, Bouillet P, Décimo D, Gansmuller A, Heyberger S, et al. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–77. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuili G, Tomljenovic A, Labrecque N, Oulad-Abdelghani M, Rassoulzadegan M, et al. Murine spermatogonial stem cells: targeted transgene expression and purification in an active state. EMBO Rep. 2002;3:753–9. doi: 10.1093/embo-reports/kvf149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–49. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahony S, Mazzoni EO, McCuine S, Young RA, Wichterle H, et al. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011;12:R2. doi: 10.1186/gb-2011-12-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, et al. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol. 2011;356:63–70. doi: 10.1016/j.ydbio.2011.05.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei N, Hornbaker KI, Rice DA, Karpova T, Agbor VA, et al. Sex-specific differences in mouse DMRT1 expression are both cell type- and stage-dependent during gonad development. Biol Reprod. 2007;77:466–75. doi: 10.1095/biolreprod.106.058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bouffant R, Souquet B, Duval N, Duquenne C, Hervé R, et al. Msx1 and Msx2 promote meiosis initiation. Development. 2011;138:5393–402. doi: 10.1242/dev.068452. [DOI] [PubMed] [Google Scholar]
- 36.Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–9. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 37.Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–9. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 38.Naillat F, Prunskaite-Hyyryläinen R, Pietilä I, Sormunen R, Jokela T, et al. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum Mol Genet. 2010;19:1539–50. doi: 10.1093/hmg/ddq027. [DOI] [PubMed] [Google Scholar]
- 39.Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, et al. RSPO1/ß-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS One. 2011;6:e25641. doi: 10.1371/journal.pone.0025641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmahl J, Kim Y, Colvin JS, Ornitz DM, Capel B. Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development. 2004;131:3627–36. doi: 10.1242/dev.01239. [DOI] [PubMed] [Google Scholar]
- 41.Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, et al. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–80. doi: 10.1242/jcs.057968. [DOI] [PubMed] [Google Scholar]
- 42.Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–7. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 43.Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–1. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 44.Durcova-Hills G, Surani A. Reprogramming primordial germ cells (PGC) to embryonic germ (EG) cells. Curr Protoc Stem Cell Biol. 2008;(Chapter 1) doi: 10.1002/9780470151808.sc01a03s5. Unit1A3. [DOI] [PubMed] [Google Scholar]
- 45.Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68:422–8. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- 46.Pesce M, Wang X, Wolgemuth DJ, Schöler H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 47.Western PS, van den Bergen JA, Miles DC, Sinclair AH. Male fetal germ cell differentiation involves complex repression of the regulatory network controlling pluripotency. FASEB J. 2010;24:3026–35. doi: 10.1096/fj.09-151555. [DOI] [PubMed] [Google Scholar]
- 48.Fuhrmann G, Chung AC, Jackson KJ, Hummelke G, Baniahmad A, et al. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev Cell. 2001;1:377–87. doi: 10.1016/s1534-5807(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 49.Gu P, LeMenuet D, Chung AC, Mancini M, Wheeler DA, et al. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol Cell Biol. 2005;25:8507–19. doi: 10.1128/MCB.25.19.8507-8519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 50.Sabour D, Xu X, Chung AC, Le Menuet D, Ko K, et al. Germ cell nuclear factor regulates gametogenesis in developing gonads. PLoS One. 2014;9:e103985. doi: 10.1371/journal.pone.0103985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okumura LM, Lesch BJ, Page DC. The ligand binding domain of GCNF is not required for repression of pluripotency genes in mouse fetal ovarian germ cells. PLoS One. 2013;8:e66062. doi: 10.1371/journal.pone.0066062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokobayashi S, Liang CY, Kohler H, Nestorov P, Liu Z, et al. PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature. 2013;495:236–40. doi: 10.1038/nature11918. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 54.Weston AD, Blumberg B, Underhill TM. Active repression by unliganded retinoid receptors in development: less is sometimes more. J Cell Biol. 2003;161:223–8. doi: 10.1083/jcb.200211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byskov AG. Does the rete ovarii act as a trigger for the onset of meiosis? Nature. 1974;252:396–7. doi: 10.1038/252396a0. [DOI] [PubMed] [Google Scholar]
- 56.Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–64. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 57.Guerquin MJ, Duquenne C, Lahaye JB, Tourpin S, Habert R, et al. New testicular mechanisms involved in the prevention of fetal meiotic initiation in mice. Dev Biol. 2010;346:320–30. doi: 10.1016/j.ydbio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Jameson SA, Lin YT, Capel B. Testis development requires the repression of Wnt4 by Fgf signaling. Dev Biol. 2012;370:24–32. doi: 10.1016/j.ydbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiller CM, Feng CW, Jackson A, Gillis AJ, Rolland AD, et al. Endogenous Nodal signaling regulates germ cell potency during mammalian testis development. Development. 2012;139:4123–32. doi: 10.1242/dev.083006. [DOI] [PubMed] [Google Scholar]
- 60.Souquet B, Tourpin S, Messiaen S, Moison D, Habert R, et al. Nodal signaling regulates the entry into meiosis in fetal germ cells. Endocrinology. 2012;153:2466–73. doi: 10.1210/en.2011-2056. [DOI] [PubMed] [Google Scholar]
- 61.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–34. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 62.Klauzinska M, Castro NP, Rangel MC, Spike BT, Gray PC, et al. The multifaceted role of the embryonic gene Cripto-1 in cancer, stem cells and epithelial-mesenchymal transition. Semin Cancer Biol. 2014;29C:51–58. doi: 10.1016/j.semcancer.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miles DC, Wakeling SI, Stringer JM, van den Bergen JA, Wilhelm D, et al. Signaling through the TGF beta-activin receptors ALK4/5/7 regulates testis formation and male germ cell development. PLoS One. 2013;8:e54606. doi: 10.1371/journal.pone.0054606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 65.Wu Q, Kanata K, Saba R, Deng CX, Hamada H, et al. Nodal/activin signaling promotes male germ cell fate and suppresses female programming in somatic cells. Development. 2013;140:291–300. doi: 10.1242/dev.087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–13. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 67.Li H, MacLean G, Cameron D, Clagett-Dame M, Petkovich M. Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS One. 2009;4:e7501. doi: 10.1371/journal.pone.0007501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–5. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, et al. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–41. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki A, Igarashi K, Aisaki K, Kanno J, Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc Natl Acad Sci U S A. 2010;107:3594–9. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bourc’his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–9. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- 72.Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, et al. Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet. 2007;16:2272–80. doi: 10.1093/hmg/ddm179. [DOI] [PubMed] [Google Scholar]
- 73.Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 74.Looijenga LH, Gillis AJ, Stoop H, Biermann K, Oosterhuis JW. Dissecting the molecular pathways of (testicular) germ cell tumour pathogenesis; from initiation to treatment-resistance. Int J Androl. 2011;34:e234–51. doi: 10.1111/j.1365-2605.2011.01157.x. [DOI] [PubMed] [Google Scholar]
- 75.Spiller CM, Bowles J, Koopman P. Nodal/Cripto signaling in fetal male germ cell development: implications for testicular germ cell tumors. Int J Dev Biol. 2013;57:211–9. doi: 10.1387/ijdb.130028pk. [DOI] [PubMed] [Google Scholar]
- 76.Tam PP, Snow MH. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol. 1981;64:133–47. [PubMed] [Google Scholar]
- 77.Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells. 2008;26:339–47. doi: 10.1634/stemcells.2007-0622. [DOI] [PubMed] [Google Scholar]
- 78.Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–43. doi: 10.1530/jrf.0.0930233. [DOI] [PubMed] [Google Scholar]
- 79.Spiller CM, Wilhelm D, Koopman P. Retinoblastoma 1 protein modulates XY germ cell entry into G1/G0 arrest during fetal development in mice. Biol Reprod. 2010;82:433–43. doi: 10.1095/biolreprod.109.078691. [DOI] [PubMed] [Google Scholar]
- 80.Trautmann E, Guerquin MJ, Duquenne C, Lahaye JB, Habert R, et al. Retinoic acid prevents germ cell mitotic arrest in mouse fetal testes. Cell Cycle. 2008;7:656–64. doi: 10.4161/cc.7.5.5482. [DOI] [PubMed] [Google Scholar]
- 81.Koshimizu U, Watanabe M, Nakatsuji N. Retinoic acid is a potent growth activator of mouse primordial germ cells in vitro. Dev Biol. 1995;168:683–5. doi: 10.1006/dbio.1995.1113. [DOI] [PubMed] [Google Scholar]
- 82.Mendis SH, Meachem SJ, Sarraj MA, Loveland KL. Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol Reprod. 2011;84:379–91. doi: 10.1095/biolreprod.110.086231. [DOI] [PubMed] [Google Scholar]
- 83.Mithraprabhu S, Mendis S, Meachem SJ, Tubino L, Matzuk MM, et al. Activin bioactivity affects germ cell differentiation in the postnatal mouse testis in vivo. Biol Reprod. 2010;82:980–90. doi: 10.1095/biolreprod.109.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagaoka T, Karasawa H, Castro NP, Rangel MC, Salomon DS, et al. An evolving web of signaling networks regulated by Cripto-1. Growth Factors. 2012;30:13–21. doi: 10.3109/08977194.2011.641962. [DOI] [PubMed] [Google Scholar]
- 85.Moniot B, Ujjan S, Champagne J, Hirai H, Aritake K, et al. Prostaglandin D2 acts through the Dp2 receptor to influence male germ cell differentiation in the foetal mouse testis. Development. 2014;141:3561–71. doi: 10.1242/dev.103408. [DOI] [PubMed] [Google Scholar]


