Abstract
The recent manuscript in New England Journal of Medicine by Antonarakis et al.1 has important clinical implications. This study evaluates mRNA expression of a particular androgen receptor splice variant-7 (AR-V7), in circulating tumor cells (CTCs) from metastatic castrate-resistant prostate cancer (mCRPC) patients receiving enzalutamide or abiraterone. The findings were striking, none of the 18 patients with detectable AR-V7 in CTCs had prostate-specific antigen (PSA) responses. Further, the median time to PSA progression after enzalutamide or abiraterone treatment was only 1.3–1.4 months in AR-V7-positive patients as compared to 5.3–6.1 months in AR-V7 negative patients. AR-V7 in CTCs was also associated with shorter survival.
The prevalence of detectable AR-V7 in CTCs, which was 9%–15% before treatment with enzalutamide or abiraterone, was increased to ~ 50% after progression on either agent. These results clearly indicate that AR-V7 detection in CTCs represents an important new predictive biomarker for response to enzalutamide and/or abiraterone, and that increased AR-V7 expression may represent one of the mechanisms of resistance to these agents.
Various AR-Vs were initially described in 2008,2 but their clinical relevance has been unclear until now. There are many AR-Vs, at least 15 have been described, 3 but AR-V7 is the most abundant and the only one to which a specific antibody is available commercially. Its protein expression has been definitively demonstrated in clinical specimens. This variant is composed of the first three exons of the AR (out of 8) fused with a “cryptic exon” that is ordinarily not translated (Figure 1). The cryptic exon contains a stop codon that prematurely truncates the AR in a manner that deletes exons 4–8. These exons ordinarily code for the hinge region and the ligand-binding domain of the full-length AR (AR-FL) and comprise the C-terminal end of the translated protein (Figure 1). Other AR-Vs utilize other cryptic exons, which also encode premature stop codons, and some AR-Vs are derived from aberrant exon-skipping mRNA splicing.2,4,5,6,7
Figure 1.
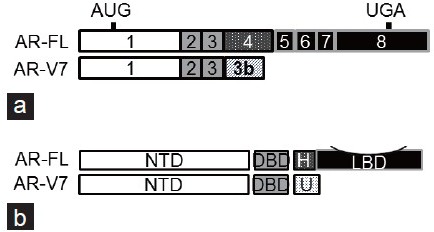
Schematic representation of AR-FL and AR-V7 structure. (a) AR transcripts. The solid and hatched boxes denote exons and cryptic exons, respectively. AR-FL: full-length androgen receptor; AR-V7: androgen receptor splice variant-7. (b) AR proteins. NTD: N-terminal domain; DBD: DNA-binding domain; H: hinge region; LBD: ligand-binding domain; U: unique C-terminal sequence.
It is important to understand the methodology of the AR-V7 detection and the clinical cohort in order to understand the study by Antonarakis et al.1 First, note that this is not a tissue-based marker. The assays were performed on CTCs derived from the blood of men with advanced prostate cancer. This means that if there were no CTCs, those samples would not be evaluable for AR-V7 status. Second, the assay has some arbitrary cut-offs for sensitivity that may vary in the future. As a positive control, an AR-V7-expressing prostate cancer cell line (VCaP) was used to “spike” normal blood. Polyadenylated mRNA was isolated from the CTCs, and after reverse transcription (RT), polymerase chain reaction (PCR) with specific primers was performed to assess both AR-FL and AR-V7. Patients examined in these assays all had far advanced cancer, mCRPC, and many had undergone multiple therapies at the time the assays were performed. All patients with AR-V7 also expressed AR-FL. Unlike prior manuscripts where the AR-V mRNAs were expressed in very low quantity compared to the AR-FL in human tumors,7,8 the AR-V7 was expressed in reasonable proportions to the AR-FL. In quantitative RT-PCR assays, the AR-V7 was expressed between 1.8% and 208% of the AR-FL transcript.
The transcriptional programs of AR-FL and AR-Vs have been studied in detail.4,6,9,10 AR-Vs were shown to regulate both the canonical AR-FL targets, such as the PSA, and a distinct set of genes enriched for cell-cycle function, such as ubiquitin-conjugating enzyme E2C. Despite the fact that AR-Vs are now ascertained as being clinically relevant, there is much more work to be done. The assay itself, though elegant, needs to be qualified for biomarker detection and cleared by the FDA. That will take larger multi-institutional studies. The current focus on AR-V7 might or might not be optimal. Other AR-Vs may also be clinically important, especially ARv567es, which is also commonly detected in clinical specimens.8,11 The questions of how AR-V7 is induced by enzalutamide and abiraterone and how AR-V7 influences overall survival need more exploration. Emerging clinical data provided evidence for a high degree of cross-resistance of mCRPC to enzalutamide and abiraterone.12,13,14,15 AR-V upregulation may represent a plausible mechanism of cross-resistance. Moreover, early stages of prostate cancer need to be studied as AR-Vs have been shown to be expressed in a subset of benign prostate and primary cancer.4,5,6,8
REFERENCES
- 1.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Zhan Y, Liu X, Qi Y, Zhang G, et al. Splicing variants of androgen receptor in prostate cancer. Am J Clin Exp Urol. 2013;1:18–24. [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Z, Yang X, Sun F, Jiang R, Linn DE, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hörnberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Lonergan PE, Nacusi LP, Wang L, Schmidt LJ, et al. The cistrome and gene signature of androgen receptor splice variants in castration – Resistant prostate cancer cells. J Urol. 2014 doi: 10.1016/j.juro.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Wang BE, Leong KG, Yue P, Li L, et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;72:527–36. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 12.Bianchini D, Lorente D, Rodriguez-Vida A, Omlin A, Pezaro C, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50:78–84. doi: 10.1016/j.ejca.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, et al. Antitumour activity of abiraterone acetate against metastatic castration – Resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–12. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 14.Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration – Resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–7. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 15.Schmid SC, Geith A, Böker A, Tauber R, Seitz AK, et al. Enzalutamide after docetaxel and abiraterone therapy in metastatic castration – Resistant prostate cancer. Adv Ther. 2014;31:234–41. doi: 10.1007/s12325-014-0092-1. [DOI] [PubMed] [Google Scholar]


