Abstract
Mammalian embryo development is initiated by intracellular Ca2+ oscillations that result in oocyte activation following gamete membrane fusion. It is widely believed that oocyte Ca2+ oscillations are triggered by a sperm-specific protein, phospholipase C-zeta (PLCζ) that activates InsP3 production leading to repetitive Ca2+ release from intracellular stores. However, a recent report in the FASEB Journal by Aarabi et al. challenges this view by proposing postacrosomal WW domain-binding protein (PAWP) as another sperm-derived protein that can also initiate Ca2+ oscillations and zygotic development at fertilization. Here we discuss these new findings and examine the evidence suggesting PAWP as the “real” sperm factor.
At fertilization, the first event following the fusion of sperm and oocyte membranes is a series of transient rises in the intracellular-free Ca2+ concentration, termed Ca2+ oscillations.1,2,3 Over the past decade, mounting experimental and clinical evidence has been congruent with the hypothesis that the sperm factor responsible for the initiation of Ca2+ oscillations during mammalian fertilization is a testis-specific isoform of PLC-zeta (PLCζ).4,5,6,7,8 Since the discovery of PLCζ in 2002, many research laboratories across the world (Table 1) have reported experimental evidence compatible with the proposition that PLC, termed PLCζ is the sperm factor that causes Ca2+ oscillations at fertilization. Based upon numerous complementary studies, the current understanding of the molecular mechanism of PLCζ action in mammalian oocytes is summarized in Figure 1 (left side). Upon sperm-oocyte membrane fusion, PLCζ diffuses from the sperm head into the oocyte cytoplasm and hydrolyses phosphatidylinositol 4,5-bisphosphate (PIP2) located in an intracellular vesicle compartment. The resulting liberation of InsP3 stimulates opening of the InsP3 R Ca2+ release channel on the endoplasmic reticulum that results in Ca2+ oscillations, oocyte activation and embryo development.3
Table 1.
Academic institutions where independent research laboratories (as defined by the corresponding author's address) have published experimental evidence up to September 22, 2014, in peer-reviewed journals, that support either PAWP (left) or PLCζ (right), as the mammalian sperm factor responsible for initiating calcium oscillations and oocyte activation at fertilization
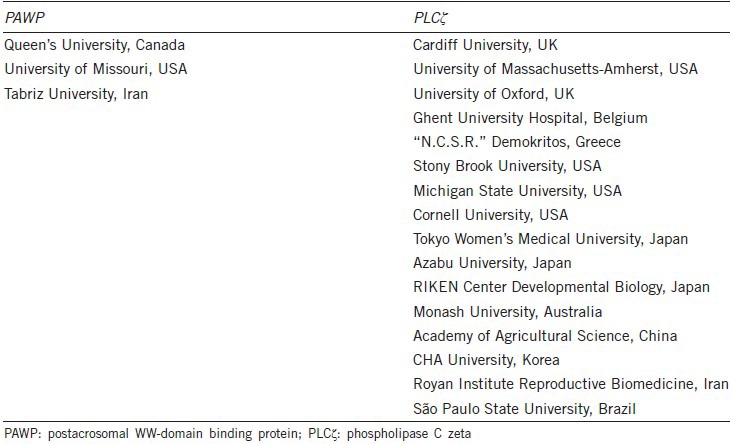
Figure 1.
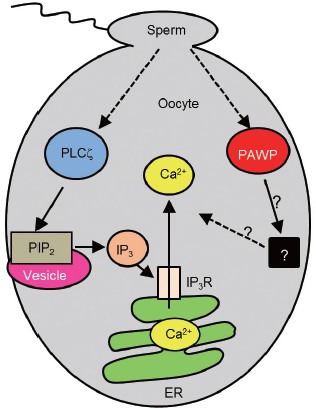
Schematic representation of known mechanisms of action of the sperm proteins, PLCζ (left side) and PAWP (right side) in mammalian oocytes. ER: endoplasmic reticulum; PIP2: phosphatidylinositol 4,5-bisphosphate; IP3: inositol 1,4,5-trisphosphate; PAWP: postacrosomal WW domain-binding protein; PLCζ: phospholipase C zeta.
The recent FASEB Journal paper by Aarabi et al.9 reports that PAWP, a sperm head protein that exclusively resides in the postacrosomal sheath region of the perinuclear theca, is able to produce Ca2+ oscillations and pronuclear formation in human and mouse oocytes similar to what is observed during intracytoplasmic sperm injection. This group previously identified PAWP10 as an alkaline-extractable protein with sequence homology to the N-terminal half of WW domain-binding protein 2, while the C-terminal half is rich in proline residues. Aarabi et al.9 also report that sperm-induced Ca2+ oscillations are blocked by co-injection of a competitive peptide inhibitor, derived from the WWI domain-binding motif of PAWP. This implies there is a requirement for PAWP binding to an oocyte-derived protein for successful fertilization to occur (Figure 1, right side).
The recent indication of PAWP-induced Ca2+ signalling9 in oocytes comes 7 years after this group's initial data showing that PAWP promotes meiotic resumption and pronuclear development during fertilization.10 Based on their studies, microinjection of PAWP protein into porcine, bovine, macaque, and Xenopus oocytes resulted in pronuclear formation, an indicative event of successful oocyte activation.10 However, to date, no other research groups have independently verified PAWP's ability to activate oocytes and/or cause Ca2+ oscillations. Recently, we have investigated whether mouse PAWP can initiate Ca2+ oscillations and oocyte activation in the mouse.11 We microinjected into mouse oocytes the recombinant mouse PAWP protein, or the complementary RNA encoding either untagged PAWP, or YFP-PAWP, or PAWP-luciferase, but we consistently failed to observe any Ca2+ increases. In addition, PAWP was unable to hydrolyse PIP2 in vitro and also did not act as a generic activator of PLC activity.11 To the best of our knowledge, this is the first attempt to independently confirm the key findings on PAWP by the Oko group, but we could not verify that PAWP has any ability to mobilize intracellular Ca2+ or activate mouse oocytes.
Aarabi et al.9 also state that PAWP and an oocyte-derived WWI domain protein substrate is required for successful fertilization, because sperm-induced Ca2+ oscillations were blocked by co-injection of a 16-amino acid proline-rich peptide, derived from the PAWP WWI domain binding motif. Interestingly, the negative control peptide that was used had only a single amino acid substitution (Tyr/Phe) and this did not affect the sperm-induced Ca2+ oscillations in oocytes. The results with these PAWP peptides has led to the hypothesis that PAWP mediates its effects in oocytes via interaction with other proteins, such as the yes-associated protein, that may then lead to activation of PLCγ. However, previous studies using SH2 domain-derived peptides have suggested that PLCγ does not mediate Ca2+ oscillations in fertilizing mouse oocytes.12 Hence, if PAWP mediates any potential effects via PLCγ, then its precise role in physiological activation during fertilization remains to be clarified. As with the data they obtained using the recombinant PAWP protein, it will be important for other groups to independently investigate these specific claims for the potent inhibitory effects of these PAWP-derived proline-rich peptides, since these claims have important implications for our understanding of the mechanism of Ca2+ release at fertilization.
It is worthwhile to reflect that over the last few decades there have been various sperm-derived molecules implicated in activating the oocyte by causing Ca2+ release. These previous “sperm factor” candidates include a 33 kDa protein,13 nitric oxide,14 and tr-kit, a truncated form of the c-kit receptor.15 None of these molecules stood the test of time, mainly because subsequent research either could not validate or else did not build upon, the original data. In contrast, when PLCζ was first shown to cause Ca2+ oscillations in mouse oocytes, two independent verification and extensions of the original findings were reported within 2 years.6,16 In the following decade, many other groups have confirmed that PLCζ causes Ca2+ oscillations in oocytes from a range of different species (Table 1).3 Interestingly, immunolocalization analysis has indicated that PLCζ, like PAWP, is also present in the perinuclear matrix of the sperm.16 At present, unfortunately, there remains no published data from mouse “knockout” models for either PLCζ or PAWP. The availability of such mice should provide the conclusive evidence of a direct physiological role for these mammalian sperm proteins in the Ca2+ signaling that is essential for oocyte activation.
So despite the intriguing new data presented in the paper by Aarabi et al.9 there is a need for further independent verification of these important results so that PAWP can then also be considered a candidate for the “real” sperm factor, which mobilizes the physiological Ca2+ signal that triggers oocyte activation and early embryo development.
REFERENCES
- 1.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–76. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- 3.Nomikos M, Swann K, Lai FA. Starting a new life: sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development: an essential phospholipase C with implications for male infertility. Bioessays. 2012;34:126–34. doi: 10.1002/bies.201100127. [DOI] [PubMed] [Google Scholar]
- 4.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, et al. PLC zeta: a sperm-specific trigger of Ca (2+) oscillations in eggs and embryo development. Development. 2002;129:3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 5.Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, et al. Sperm phospholipase C zeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–23. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- 6.Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, et al. Recombinant phospholipase C zeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–12. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- 7.Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca (2+) release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118:3671–81. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nomikos M, Yu Y, Elgmati K, Theodoridou M, Campbell K, et al. Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility. Fertil Steril. 2013;99:76–85. doi: 10.1016/j.fertnstert.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, et al. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014;28:4434–40. doi: 10.1096/fj.14-256495. [DOI] [PubMed] [Google Scholar]
- 10.Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2007;282:12164–75. doi: 10.1074/jbc.M609132200. [DOI] [PubMed] [Google Scholar]
- 11.Nomikos M, Sanders JR, Theodoridou M, Kashir J, Matthews E, et al. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol Hum Reprod. 2014;20:938–47. doi: 10.1093/molehr/gau056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase C gamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol. 1998;203:221–32. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- 13.Parrington J, Swann K, Shevchenko VI, Sesay AK, Lai FA. Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature. 1996;379:364–8. doi: 10.1038/379364a0. [DOI] [PubMed] [Google Scholar]
- 14.Kuo RC, Baxter GT, Thompson SH, Stricker SA, Patton C, et al. NO is necessary and sufficient for egg activation at fertilization. Nature. 2000;406:633–6. doi: 10.1038/35020577. [DOI] [PubMed] [Google Scholar]
- 15.Sette C, Bevilacqua A, Bianchini A, Mangia F, Geremia R, et al. Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development. 1997;124:2267–74. doi: 10.1242/dev.124.11.2267. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, et al. Mammalian phospholipase C zeta induces oocyte activation from the sperm perinuclear matrix. Dev Biol. 2004;274:370–83. doi: 10.1016/j.ydbio.2004.07.025. [DOI] [PubMed] [Google Scholar]


