Abstract
Although idiopathic hypogonadotropic hypogonadism (IHH) has traditionally been viewed as a life-long disease caused by a deficiency of gonadotropin-releasing hormone neurons, a portion of patients may gradually regain normal reproductive axis function during hormonal replacement therapy. The predictive factors for potential IHH reversal are largely unknown. The aim of our study was to investigate the incidence and clinical features of IHH male patients who had reversed reproductive axis function. In this retrospective cohort study, male IHH patients were classified into a reversal group (n = 18) and a nonreversal group (n = 336). Concentration of gonadotropins and testosterone, as well as testicle sizes and sperm counts, were determined. Of 354 IHH patients, 18 (5.1%) acquired normal reproductive function during treatment. The median age for reversal was 24 years old (range 21–34 years). Compared with the nonreversal group, the reversible group had higher basal luteinizing hormone (LH) (1.0 ± 0.7 IU l-1 vs 0.4 ± 0.4 IU l−1, P < 0.05) and stimulated LH (28.3 ± 22.6 IU l−1 vs 1.9 ± 1.1 IU l−1, P < 0.01) levels, as well as larger testicle size (5.1 ± 2.6 ml vs 1.5 ± 0.3 ml, P < 0.01), at the initial visit. In summary, larger testicle size and higher stimulated LH concentrations are favorite parameters for reversal. Our finding suggests that reversible patients may retain partially active reproductive axis function at initial diagnosis.
Keywords: hypothalamic-pituitary-gonadal axis, idiopathic hypogonadotropic hypogonadism, reversal
INTRODUCTION
Hypothalamic-pituitary-gonadal axis (HPGA) plays a major role in the regulation of the male reproductive endocrine system. During late fetal and early neonatal periods, gonadotropin secretion, which is stimulated by pulsatile hypothalamic gonadotropin-releasing hormone (GnRH), is necessary for Leydig and Sertoli cell proliferation as well as future spermatogenesis.1 Before puberty, HPGA is in a relatively inactive state. During puberty, HPGA reactivation triggers the development of secondary sexual characteristics and maintains sexual maturation.
Idiopathic hypogonadotropic hypogonadism (IHH) is traditionally considered to be a life-long disease due to an isolated deficiency or dysfunction of GnRH neurons. Mutations in genes such as KAL1 (300836),FGF8 (600483), FGFR1 (136350),PROK2/PROKR2 (607002, 607123), KISS1/KISSR (603286,604161), or TAC3/TACR3 (162330, 162332)2,3 can cause IHH. Patients with hyposmia or anosmia are defined as having Kallmann syndrome (KS), and those with normal olfactory status are defined as nIHH. IHH patients may share multiple of the above-mentioned gene mutations4 and may present with a wide spectrum of puberty development.5
No puberty development or virilization, delayed epiphysis closure, small testes and penis, and azoospermia are main clinical features of male IHH patients. Testosterone replacement therapy can promote sufficient virilization. When spermatogenesis is required, pulsatile GnRH infusion or gonadotropin therapy can be conducted. For most patients, hormonal replacement therapy is considered to be a life-long therapy. However, few patients may gradually recover from hypogonadism during treatment.6,7 Mutations in GnRHR (138850),6,8,9 CHD7 (608892),8 FGF8 (600483),FGFR1 (136350),6,8,10 PROK2/PROKR2 (607002, 607123),11 KISS1/KISSR (603286, 604161),TAC3/TACR3 (162330, 162332)12 and KAL1 (300836)11 have been found in some reversal patients. It is speculated that the effects of testosterone on GnRH neuron plasticity may be an underlying mechanism. Our knowledge on reversal and its predictive factors is still scarce due to limited cases.
We retrospectively investigated the clinical features of 18 IHH patients who had recovered from impaired HPGA function after hormonal therapies and follow-up for 4–15 years. Furthermore, several favorable predictive factors for reversal were identified.
MATERIALS AND METHODS
Study definition
A diagnosis of IHH includes the following three criteria: (1) no secondary sexual characteristic development at 18 years of age; (2) serum total testosterone levels below 100 ng dl−1 (3.47 nmol l−1) with isolated gonadotropin deficiency; (3) normal sellar magnetic resonance image (MRI).6
Complaints of anosmia in addition to olfactory bulb and tract dysplasia images in an MRI will lead to a KS diagnosis. Otherwise, the patient is diagnosed as nIHH. Additional subjective olfactory function tests were conducted by the by University of Pennsylvania Smell Identification Test,13 and objective tests (olfactory evoking electronic potential test14) were conducted in a small proportion of KS patients.
Considering that testosterone production and spermatogenesis are two main indications of testicle function, our criteria for IHH reversal were: after discontinuation of any hormonal therapy for 6 months, either endogenous testosterone rises above 270 ng dl−1 (9.4 nmol l−1)6 or serum testosterone levels gradually increased above 150 ng dl−1 (5.2 nmol l−1) accompanied with increased testicle size, normal spontaneous sperm production, and normal erectile function and ejaculation.
Study population and data collection
A total of 354 IHH patients were included. Follow-up was conducted for these patients for more than 4 years at Peking Union Medical College Hospital between January 2005 and December 2011. Among them, 18 patients were classified into the reversal group and the remaining 336 patients were classified into the nonreversal group. For each patient, clinical presentation, past medical history (including cryptorchidism, micropenis and previous treatment) and family history were recorded during his first visit to our clinic. Follow-up information including virilization after testosterone therapy, gonadotropin-induced spermatogenesis, endogenous gonadotropins and testosterone, and testicle size (by Prader orchidometer methodology) were recorded in the medical profiles. The study was approved by the ethics committee of the Peking Union Medical College Hospital. Written informed consent was obtained from all of the participants.
Treatment and follow-up
Testosterone replacement therapy included 40–80 mg oral testosterone undecanoate three times per day or intramuscular 250 mg testosterone undecanoate injection per month. If spermatogenesis was required, intramuscular human chorionic gonadotropin (HCG) (2000 U) and human menopausal gonadotropin (HMG) (150 U) were administered twice weekly.
Regular follow-up was conducted at a 6–12 month interval. Secondary sexual characteristics, testicle size, gonadotropins and testosterone levels were measured on each visit. If a patient exhibited testicle growth and rising endogenous gonadotropins, semen analysis was conducted, and the hormonal therapy was suspended for 6 months. If the patient did not meet the definition of reversal on the next follow-up, testosterone or gonadotropins were resumed for 6–12 months. Then, the patient was reevaluated. For a patient with testicle size over 6 ml, his hormonal therapy was intermittently suspended to evaluate the capacity of endogenous testosterone production and spermatogenesis.
Hormone assay and triptorelin stimulating test
At baseline, a GnRH analog (triptorelin) stimulating test was conducted to evaluate the potential reservoir of HPGA function. After 100 mg triptorelin was intramuscularly injected, luteinizing hormone (LH) and follicular stimulating hormone (FSH) were measured basally and after 60 min. Our pervious data demonstrated that 92.3% of male IHH patients had LH60 min lower than 4 IU l−1, whereas this level is usually higher than 12 IU l−1 in normal male adults.15 FSH and LH levels were measured using commercial kits by chemiluminescence (ACS: 180 Automatic Chemiluminescence Systems, Bayer, Germany). The intra- and inter-assay coefficients of LH variation were 5.6% and 6.6%, respectively. The lowest measurable limit of LH was 0.02 IU l−1.
Statistical analysis
SPSS version 11.0 for Windows (SPSS, Chicago, IL, USA) was used for data analysis. Normal distributive data were expressed as the mean ± s.d., and nonnormal distribution data were expressed as medians. The paired-sample t-test was used to compare the differences before and after the reversal. Basal LH levels and basal testicle size between reversal and non-reversal groups were compared using an independent t-test. Comparison of the cryptorchidism rate between the two groups was performed by Chi-square test. Stimulated LH levels between the two groups were compared by univariate analysis of variance to control the influence of testicular size on the results. Statistical significance was set at P < 0.05.
RESULTS
Clinical features of idiopathic hypogonadotropic hypogonadism patients
At the initial visit to our clinic, the patients (n = 354) were generally in good conditions with normal routine blood and urine test results and normal liver and renal function. Systemic diseases such as malnutrition, depression, nephrotic syndrome and chronic diarrhea were excluded. In all IHH patients, 203 were KS and 151 were nIHH. Tests including a subjective olfactory function test and an olfactory evoking electronic potential test were performed in 112 and 48 KS patients, respectively. All of the patients who underwent the subjective olfactory function test had poor scores. For 48 KS patients who received the olfactory evoking electronic potential test, no electronic potential responses were observed.
In total, 18 patients were identified as reversal (Figure 1). Of these, five were KS. The clinical characteristics including age of diagnosis, complicating diseases, testicle sizes and treatments were listed in Table 1. They were followed-up for a median of 5.6 years (range from 4 to 15 years). The median age at diagnosis and reversal was 20 years (range 17–31 years) and 24 years (range 21–34 years), respectively. In the reversal group, 44.4% of patients (8/18) had a history of HCG treatment, which may have increased testicular volumes at the initial visit to our hospital and promoted bone maturation (Table 1).
Figure 1.

Diagnosis, reversal time and hormonal therapies for the reversed idiopathic hypogonadotropic hypogonadism patients (n = 18).
Table 1.
The clinical features of IHH patients who had reversed reproductive axis function (n=18)
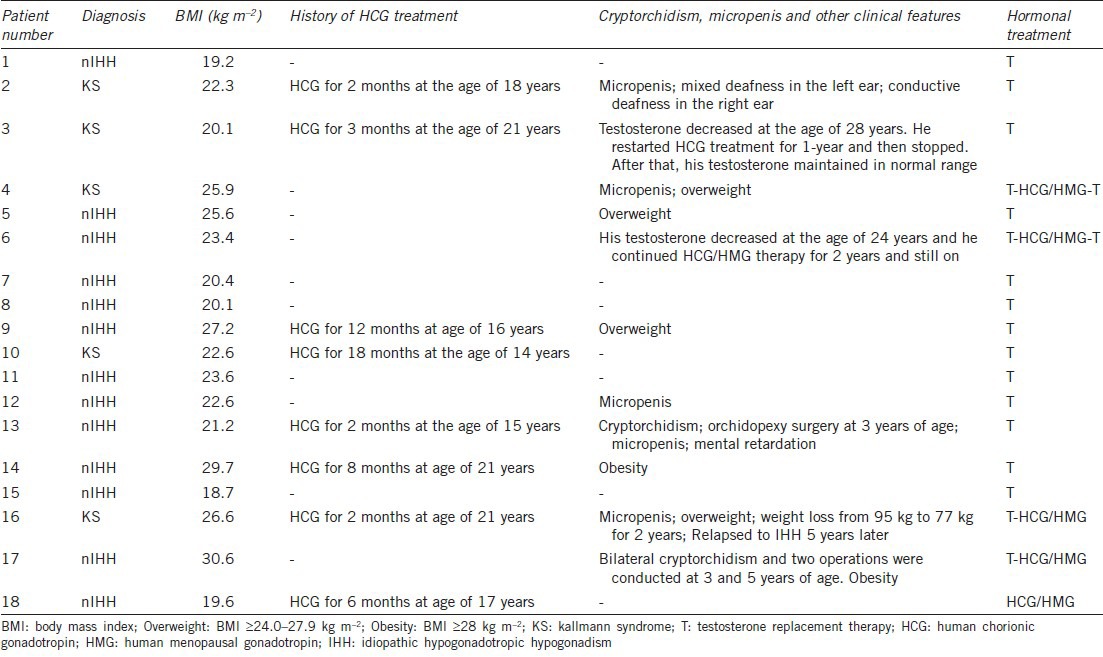
In the non-reversal group (n = 336), 59.0% of patients were KS. Of these, 26% had a history of HCG treatment. The median follow-up time was 5.2 years (range from 4 to 32 years). In the non-reversal group, 212 patients (63.1%) received gonadotropin (HCG + HMG) therapy for spermatogenesis during the follow-up.
Testicle sizes
In the reversible group, two patients (11.1%) had histories of cryptorchidism, and orchidopexy surgeries were performed at 3-5 years of age. At the initial visit, the reversible patients had larger testicle sizes than the nonreversible patients; 5.1 ± 2.6 ml versus 1.5 ± 0.3 ml (P < 0.05). During the follow-up, the testicle size in the reversible group increased from 5.1 ± 2.6 ml to 15.1 ± 3.8 ml; P < 0.01 (Figure 2).
Figure 2.

Changes in testosterone and testicular volume before and after reversal (n = 18). All of the reversal patients exhibited increased testosterone levels and testicle volumes.
In the non-reversal group, 76 patients (22.6%) had cryptorchidism, which was significantly higher than the reversal group (11.1%P < 0.01). In this group, testicle sizes increased only in patients receiving gonadotropin therapy. Once they stopped gonadotropin therapy, testicle sizes stopped increasing, and testosterone levels dropped to near zero.
Baseline luteinizing hormone, testosterone and gonadotropin-releasing hormone analog stimulating test
At the initial visit, basal LH levels in the reversal group were significantly higher than the nonreversal group; 1.0 ± 0.7 IU l−1 versus 0.4 ± 0.4 IU l−1 (P < 0.05). At the initial visit, serum testosterone levels between the two groups were similar. After follow-up for 4–15 years, the testosterone concentration in the reversible group gradually increased from 41 ± 23 ng dl−1 to 353 ± 171 ng dl−1.
At the time of the initial visit, LH levels in the reversal group after GnRHa (triptorelin) stimulation were 28.3 ± 22.6 IU l−1 (n = 14), which was significantly higher than the nonreversal group 1.9 ± 1.1 IU l−1 (n = 163) (P < 0.01,Figure 3). Of the reversible patients, 86% (11/14) had LH60 min above 12 IU l−1, while in the nonreversal group, 4% of them achieved such a high level.
Figure 3.
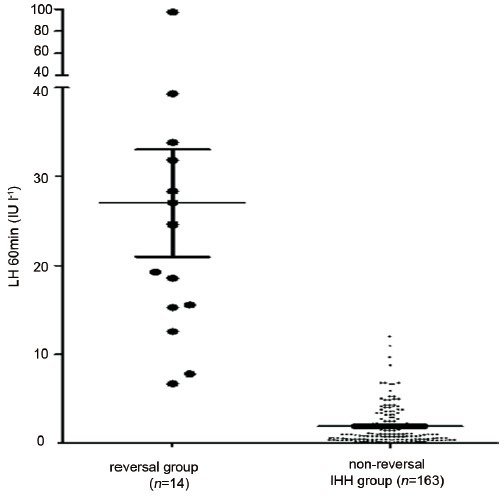
Serum luteinizing hormone (LH) levels after triptorelin stimulation were remarkably higher in the reversal group than in the nonreversal group (P< 0.01). At the time of diagnosis, 78.6% of the reversible patients (11/14) had LH60 min levels above 12 IU l−1 after triptorelin stimulation, while in the nonreversal group, only 4% of them achieved such a high level. These results indicate that reversible patients may retain partial reproductive axis function at initial diagnosis.
Spermatogenesis in the reversal group
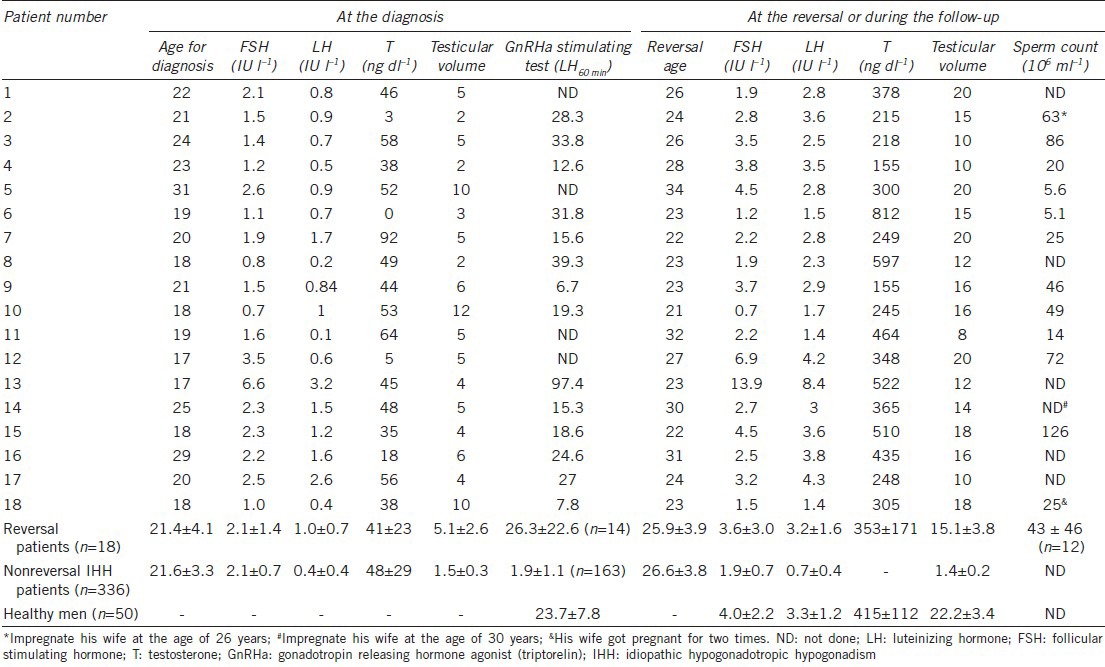
Semen was successfully collected from twelve patients (12/18), and the median sperm count was 25 million ml−1 (range from 5.1 to 126 million ml−1,Table 2). Other patients were not able to provide semen samples. In the reversible group, three patients impregnated their wives spontaneously.
Table 2.
Hormone levels and testicular volumes in the reversal IHH patients (n=18)
Follow-up after hypothalamic-pituitary-gonadal axis reversal
In general, testosterone concentrations in the reversible group were 353 ± 171 ng dl−1 after they discontinued any hormonal therapies for 6 months, which was significantly lower than the healthy group 415.4 ± 112.4 ng dl−1 (P < 0.05).
Patient 3 had a reversal at 26 years of age. His testosterone was 218 ng dl−1, and his sperm count was 86 million ml−1. During the next 2 years of follow-up, his testosterone level gradually decreased to 49 ng dl−1. At 28 years of age, he restarted gonadotropin therapy (HCG + HMG), and his testosterone level increased to 285 ng dl−1. After 1 year, he stopped therapy again; since then, his testosterone and spermatogenesis maintained at normal levels.
Serum testosterone levels also decreased in patient 6. He had a reversal at 23 years of age with testosterone level of 812 ng dl−1 and sperm count of 5.1 million ml−1. He resumed testosterone replacement therapy 1 year later. The testosterone levels and sperm counts in patients 4 and 9, although relatively low, were sustained during the follow-up.
Patient 16 was admitted to our hospital when he was 29 years old. He was 189 cm tall and weighed 95 kg. He received testosterone replacement therapy for 2 years. During this period, he lost weight to 77 kg by exercise and diet control. He was diagnosed as a reversal at 31 years old when his testosterone increased to 435 ng dl−1 without sperm count measurement (“ND” in Table 2). After 5 years, he came to our hospital due to erectile dysfunction. His weight was maintained at 80 kg. His testosterone level was 26 ng dl−1, and his sperm count was 2.3 million ml−1. Gonadotropin therapy commenced, and normal sexual function and sperm counts were acquired.
For the other patients, gonadotropin and testosterone levels were in the normal range, and no erectile dysfunction was reported.
To identify any predictive factors for relapse to hypogonadism, the reversible patients were further divided into two subgroups. Patients 3, 4, 6, 9, 16 were included in group one due to their low testosterone levels or hypogonadism relapse. The other patients were included in group 2. No significant differences were observed in the basal and stimulated gonadotropins or in testicle size between the two subgroups.
DISCUSSION
This retrospective study identified that 5.1% (18/354) of IHH patients restored their gonadal function after discontinuation of hormonal therapy. Of the patients, fifteen out of 18 restored their HPGA function before 30 years old. Compared with nonreversal patients, they had higher basal and stimulated LH levels and larger baseline testicle size.
The definition of reversal from IHH has not been clearly determined. One study defined it as endogenous total testosterone levels above 270 ng dl−1 after discontinuation of all hormonal therapy for 6 months.6 Another study defined the reversal based on spontaneous testicular growth and normal reproductive hormone levels without any symptoms of hypogonadism.8 Our definition was made considering the following: first, testosterone levels in healthy male adults vary greatly from 260 to 1180 ng dl−1 and are influenced by many factors suchas obesity16 and SHBG levels.17 There is trend of overweight and obesity in IHH patients, who have a PROK2/PROK2R mutation.18 Thus, the reversed obese patients may have relatively lower total testosterone levels. Second, spermatogenesis, which does not always parallel endogenous testosterone, is an important indicator of normal HPGA function and should be included in the definition. Finally, it is noted that in reversal group, restoration of HPGA function may take several years.6 Therefore, the trend of increasing endogenous gonadotropins, testosterone and testicle size is more reliable than transient testosterone measurement. Considering these facts, we designed new criteria for reversal in this study: endogenous testosterone increases above 270 ng dl−1 (9.4 nmol l−1) after discontinuation of any hormonal therapy for 6 months or gradually increasing serum testosterone levels above 150 ng dl−1 (5.2 nmol l−1) accompanied by increased testicle size, normal spontaneous sperm count and absence of symptoms of hypogonadism. By this definition, 18 patients were recruited.
In the reversal group, 11 of 18 were included due to testosterone levels above 270 ng dl−1. Another 7 of 18 are included with testosterone levels above 150 ng dl−1 along with normal sperm production and increased testicular volume. Reversal incidence was 5.1% in our study, which is lower than 10%6 and 8%8 reported by the other two studies. If the testosterone level above 270 ng dl−1 were the only diagnostic set point, reversal incidence would be 3.1%, which is much lower than the previously reported data. A reasonable explanation for the low incidence of reversal is that the participants in our study generally had smaller testicle volumes, indicating more severe defects in HPGA reservation. In Raivio et al.'s study,6 the average testicle size in the nonreversal IHH patients was 4 ± 3ml, while it was 1.5 ± 0.3 ml in our study. The reversal incidence in our study was statistically more powerful due to the larger sample size.
Baseline testicle size in the reversal group was 5 ± 3 ml, which was significantly larger than the nonreversal group. This phenomenon was also documented by other studies.6,8 In their studies, baseline testicle volume in the reversal group was 8 ± 5 ml (n = 15)6 and 5 ± 2 ml (n = 6),8 respectively. In IHH patients, cryptorchidism is a sign of severe impairment of HPGA function, while larger testes indicate a better potential of HPGA function.5 Larger testicular volume is also a consistent favorable predictor for gonadotropin-induced spermatogenesis.19,20,21,22 Therefore, testicle size reflects the degree of exposure to endogenous gonadotropins and provides a reliable and simple predictor for possible reversal. Conversely, as observed in our study, previous HCG treatment may also contribute to a larger testicle size. Therefore, the compound influence of endogenous and exogenous gonadotropins on testicle size should be carefully weighed.
Another interesting finding was that the reversal group initially had a higher basal LH level than the nonreversal group. This difference was further magnified using a GnRHa (triptorelin) stimulating test in which stimulated LH levels substantially increased in the reversible patients. Of these patients, 78.6% had stimulated LH levels above 12 IU l−1, while in the nonreversal group, stimulated LH levels were usually less than 4 IU l−1 (1.9 ± 1.1 IU l−1). Our previous data have demonstrated that 92% of IHH patients (n = 57) and 81% of pan-hypopituitarism patients (n = 31) had a stimulated LH level below 4 IU l−1, while 48% of boys with constitutional delayed puberty (n = 29) and 96% of postpuberty adolescence (n = 28) had stimulated LH levels above 12 IU l−1.15 Therefore, stimulated LH levels above 12 IU l−1 indicate a good gonadotropin reserve and partially activated HPGA (more information is available in the supplement).
The above clinical features indicate that for most reversal patients, HPGA function is partially or subnormally activated when the IHH diagnosis was initially made. Although it is difficult to qualitatively evaluate HPGA function, testicular volume, penis size, basal and stimulated levels of LH, inhibin B and Mullerian inhibitory substance may reflect the degree of HPGA dysfunction severity.5 Among these factors, testicular volume and stimulated LH measurement is mostly available. Pulsatile LH monitoring is a good parameter for evaluating HPGA function, and pulsatile LH restoration was demonstrated in reversal IHH patients.6 However, it is seldom used in clinical practice due to its inconvenience.
The reversal mechanisms are still undetermined. First, the reversal is an extreme model of delayed puberty and is on the end of the wide spectrum of clinical manifestations of IHH. In a family with the same FGFR1 mutation,10 brothers reportedly presented with normal, delayed or absent puberty, indicating that delayed puberty is a variant clinical manifestation of IHH. However, a recent study did not find any coding mutations in the FGFR1,GNRHR,TAC3 and TACR3 genes in 64 boys with delayed puberty, indicating that other factors may be involved in puberty timing.23 Elks and her colleagues identified 32 new loci associated with time of menarche by a meta-analysis of 32 genome-wide association studies. Among these loci, four were associated with body mass index (BMI) (FTO, SEC16B, TRA2B and TMEM18), three were involved in energy homeostasis (BSX, CRTC1 and MCHR2) and three were involved in hormonal regulation (INHBA, PCSK2 and RXRG).24 Therefore, pubertal development is elaborately regulated by multiple genetic factors as well as ethnic and social economic status. Second, exposure to high testosterone concentrations may promote GnRH neuron maturation and activate HPGA function as posited by Raivio et al.6 This theory is also supported by the facts that central precocious puberty may be induced in patients with 21-hydoxlase deficiency after the adrenal-derived androgen was suppressed by glucocorticoids.17,25
It is important to note that reversal patients had a greater risk of hypogonadism relapse or were sustained at low testosterone levels for a long period such as patients 3, 4, 6, 9 and 16. This phenomenon was also observed and investigated by several other studies.6,8,26 A recent study conducted by Sidhoum et al.26 demonstrated that 5 of 44 male patients could not sustain their reversal and again developed hypogonadotropism. Therefore, it is reasonable to assume that for some reversible patients, GnRH neurons may need more time to synchronize for gonadotropin secretion, or HPGA function is only partially activated due to the limited number of GnRH neurons.
In the reversal group, 6 of 18 patients (33.3%) were overweight or obese (BMI 25.6-30.6 kg m−2). Of six patients who relapsed into hypogonadism or sustained low total testosterone levels, three were overweight or obese (patient 4, 9 and 16). Lower levels of total testosterone are associated with obesity in men, especially for severe obesity,27 due to lower SHBG, excessive aromatase activity28 and reduced LH secretion.17,25 In our study, the patients were of mild adiposity, and therefore their body weights did not remarkably influence total testosterone levels.
Our study had some limitations. First, although the follow-up in this study was above 4 years, it is possible that with a longer tracking time, more reversal cases may occur. Second, the frequency and amplitude of pulsatile LH secretion was not measured. Intermittent LH peaks at night is the first sign of puberty initiation and may provide more information about HPGA function. We believed that the triptorelin stimulation test may be able to substitute for pulsatile LH measurement and may accurately reflect HPGA function.
CONCLUSION
Our study demonstrated that reversal incidence in IHH patients was approximately 5%, and most reversal occurred in patients with partially activated HPGA function. Testicle size and GnRHa (triptorelin)-stimulated LH levels, which reflect HPGA defect severity, are two reliable predictive factors for reversal. Because overtreatment should be avoided for patients with potential for reversal, the two predictors may guide us identify these patients and plan their therapeutic regimens accordingly.
AUTHOR CONTRIBUTIONS
JFM and HLX collected clinical data and wrote the article; JD, RRC, LL, and BL performed genetic investigation and mutation analysis; MN performed patient follow-up and collected clinical data. LM, HBZ and XYW designed the study, collected patient information, and polished the article.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (No: 81100416).
REFERENCES
- 1.Bouvattier C, Maione L, Bouligand J, Dodé C, Guiochon-Mantel A, et al. Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2011;8:172–82. doi: 10.1038/nrendo.2011.164. [DOI] [PubMed] [Google Scholar]
- 2.Bonomi M, Libri DV, Guizzardi F, Guarducci E, Maiolo E, et al. New understandings of the genetic basis of isolated idiopathic central hypogonadism. Asian J Androl. 2012;14:49–56. doi: 10.1038/aja.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quaynor SD, Kim HG, Cappello EM, Williams T, Chorich LP, et al. The prevalence of digenic mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Fertil Steril. 2011;96:1424–30.e6. doi: 10.1016/j.fertnstert.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topaloglu AK, Kotan LD. Molecular causes of hypogonadotropic hypogonadism. Curr Opin Obstet Gynecol. 2010;22:264–70. doi: 10.1097/GCO.0b013e32833bb425. [DOI] [PubMed] [Google Scholar]
- 5.Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, et al. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:152–60. doi: 10.1210/jcem.87.1.8131. [DOI] [PubMed] [Google Scholar]
- 6.Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–73. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- 7.Quinton R, Cheow HK, Tymms DJ, Bouloux PM, Wu FC, et al. Kallmann's syndrome: is it always for life? Clin Endocrinol (Oxf) 1999;50:481–5. doi: 10.1046/j.1365-2265.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- 8.Caunt CJ, Perett RM, Fowkes RC, McArdle CA. Mechanisms of GnRH-induced extracellular signal-regulated kinase nuclear localization. PLoS One. 2012;7:e40077. doi: 10.1371/journal.pone.0040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitteloud N, Boepple PA, DeCruz S, Valkenburgh SB, Crowley WF, Jr, et al. The fertile eunuch variant of idiopathic hypogonadotropic hypogonadism: spontaneous reversal associated with a homozygous mutation in the gonadotropin-releasing hormone receptor. J Clin Endocrinol Metab. 2001;86:2470–5. doi: 10.1210/jcem.86.6.7542. [DOI] [PubMed] [Google Scholar]
- 10.Pitteloud N, Acierno JS, Jr, Meysing AU, Dwyer AA, Hayes FJ, et al. Reversible kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab. 2005;90:1317–22. doi: 10.1210/jc.2004-1361. [DOI] [PubMed] [Google Scholar]
- 11.Sinisi AA, Asci R, Bellastella G, Maione L, Esposito D, et al. Homozygous mutation in the prokineticin-receptor2 gene (Val274Asp) presenting as reversible Kallmann syndrome and persistent oligozoospermia: case report. Hum Reprod. 2008;23:2380–4. doi: 10.1093/humrep/den247. [DOI] [PubMed] [Google Scholar]
- 12.Root AW. Reversible isolated hypogonadotropic hypogonadism due to mutations in the neurokinin B regulation of gonadotropin-releasing hormone release. J Clin Endocrinol Metab. 2010;95:2625–9. doi: 10.1210/jc.2010-0733. [DOI] [PubMed] [Google Scholar]
- 13.Jagtap VS, Sarathi V, Lila AR, Nair S, Bukan A, et al. An objective olfactory evaluation and its correlation with magnetic resonance imaging findings in Asian Indian patients with idiopathic hypogonadotropic hypogonadism. Endocr Pract. 2013;19:669–74. doi: 10.4158/EP13008.OR. [DOI] [PubMed] [Google Scholar]
- 14.Rombaux P, Collet S, Martinage S, Eloy P, Bertrand B, et al. Olfactory testing in clinical practice. B-ENT. 2009;5(Suppl 13):39–51. [PubMed] [Google Scholar]
- 15.Wu XY, Nie M, Lu SY, Mao JF. Clinical values of triptorelin stimulating test in assessing hypothalamus-pituitary-gonad axis function in male patients with hypothalamus-pituitary-gonad axis disorders. Zhonghua Yi Xue Za Zhi. 2011;91:679–82. Article in Chinese. [PubMed] [Google Scholar]
- 16.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 17.Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, et al. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66:103–9. [PubMed] [Google Scholar]
- 18.Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab. 2008;93:3551–9. doi: 10.1210/jc.2007-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuse H, Akashi T, Kazama T, Katayama T. Gonadotropin therapy in males with hypogonadotropic hypogonadism: factors affecting induction of spermatogenesis after gonadotropin replacement. Int Urol Nephrol. 1996;28:367–74. doi: 10.1007/BF02550500. [DOI] [PubMed] [Google Scholar]
- 20.Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, et al. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94:801–8. doi: 10.1210/jc.2008-1648. [DOI] [PubMed] [Google Scholar]
- 21.Warne DW, Decosterd G, Okada H, Yano Y, Koide N, et al. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril. 2009;92:594–604. doi: 10.1016/j.fertnstert.2008.07.1720. [DOI] [PubMed] [Google Scholar]
- 22.Miyagawa Y, Tsujimura A, Matsumiya K, Takao T, Tohda A, et al. Outcome of gonadotropin therapy for male hypogonadotropic hypogonadism at university affiliated male infertility centers: a 30-year retrospective study. J Urol. 2005;173:2072–5. doi: 10.1097/01.ju.0000158133.09197.f4. [DOI] [PubMed] [Google Scholar]
- 23.Vaaralahti K, Wehkalampi K, Tommiska J, Laitinen EM, Dunkel L, et al. The role of gene defects underlying isolated hypogonadotropic hypogonadism in patients with constitutional delay of growth and puberty. Fertil Steril. 2011;95:2756–8. doi: 10.1016/j.fertnstert.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 24.Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010;42:1077–85. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, et al. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab. 1990;71:929–31. doi: 10.1210/jcem-71-4-929. [DOI] [PubMed] [Google Scholar]
- 26.Sidhoum VF, Chan YM, Lippincott MF, Balasubramanian R, Quinton R, et al. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99:861–70. doi: 10.1210/jc.2013-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 28.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–8. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]


