Dear Editor,
There are two main causes of severe asthenozoospermia: ultrastructural defects (genetically inherited and congenital defects) of the sperm flagellum and necrozoospermia or sperm degeneration secondary to other pathological changes (see review by Ortega et al.1). Genetic-related absolute asthenozoospermia includes primary ciliary dyskinesia and dysplasia of the fibrous sheath (DFS), which result in 100% (or nearly) immotile spermatozoa.1,2,3
In 1987, Chemes et al.3 reported five subjects affected by severe asthenozoospermia (syndrome “DFS”), which showed rigid, short, thick, and/or irregular sperm tails. Electron microscopy revealed an abnormal development of the fibrous sheath (FS), which appeared hyperplastic and disorganized. The syndrome had been called short tails or stump tails besides DFS.4 Due to flagellar abnormalities, the name multiple morphological anomalies of the flagella (MMAF) seems more accurate.5 MMAF is assumed to be an autosomal recessive inheritance. Indeed, gene deletions in A-kinase anchoring proteins 3 (AKAP3) and AKAP4 binding domain were found in an infertile man with sperm FS dysplasia.6 More recently, dynein axonemal heavy chain 1 gene (DNAH1) mutations were found among North African MMAF subjects.5 However, the genetic origin and incidence of MMAF in China is still unknown.
Six Chinese patients were diagnosed with MMAF at our hospital from December 2012 to June 2014, with ages between 25 and 34. They had primary infertility for 1–7 years. Physical examination and reproductive hormone levels were normal. The patients’ lymphocyte karyotype was 46, XY. Patient 1 (P1) had chronic cough from childhood, and his parents were first-cousins. There was no consanguinity among the six patients. Signed informed consent was provided by each patient, and the ethics committee of our hospital approved the research.
Semen parameters were evaluated according to World Health Organization guidelines after 2–7 days of sexual abstinence, and repeated at least twice (Table 1). Volumes and pH values of all semen samples were in the normal range (7.2–7.7); sperm concentrations varied from 5.6 ×106 ml−1 to 39.4 ×106 ml−1; progressive motility (PR) of 0%–3.6%, motility of 0%–8.4%, and 9%–80% viability were observed. The flagella showed typical MMAF anomalies, with short, thick and irregular tails. Five of the six cases were diagnosed as complete form with 94.5%–99.5% affected spermatozoa, and the last patient had incomplete form with 79.5% affected spermatozoa, according to diagnosis criteria.7 It has been demonstrated that absolute asthenozoospermia in MMAF is not secondary to necrospermia.7
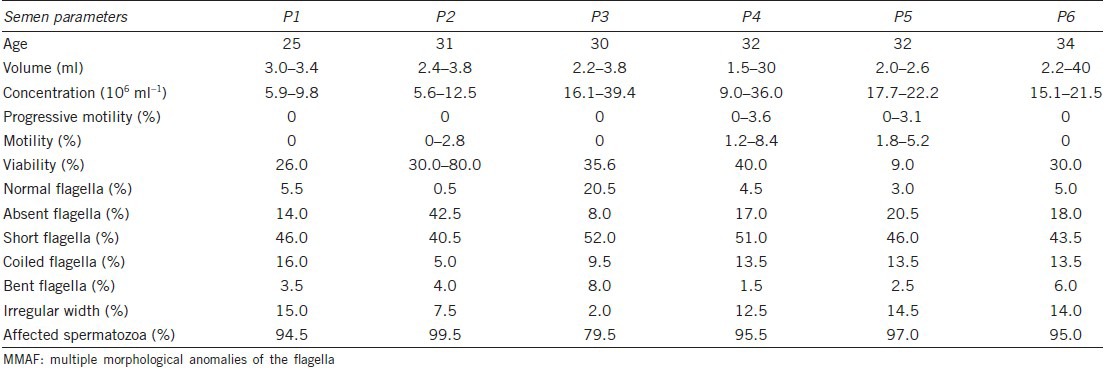
Table 1.
Semen analysis in MMAF patients under light microscopy
Scanning electron microscopy analysis (Stereoscan260, Cambridge, UK) confirmed the flagellar anomalies with improved resolution (Figure 1a). Both transversal and cross sections under transmission electron microscopy (TECNAI-10; Philips, Amsterdam, Netherlands) showed marked hypertrophy and hyperplasia of the FS. Besides the serious FS distortion in the affected spermatozoa, a mid-piece was not formed, and mitochondria were poorly assembled or abnormally localized (Figure 1b). Normal centrioles were seen. In order to assess the microtubules and dynein arms, at least 50 cross sections were analyzed for each subject. Interestingly, the six patients presented different percentages of central pair absence, ranging from 41% to 81%. In the mid-piece sections, central pairs were seldom observed. Some axonemes were completely disorganized, and no central pair was identified (Figure 1c). The absence of outer dense fibers (ODFs) always accompanied that of peripheral tubules. However, the number of ODFs in P2 doubled in several sections (Figure 1d).
Figure 1.
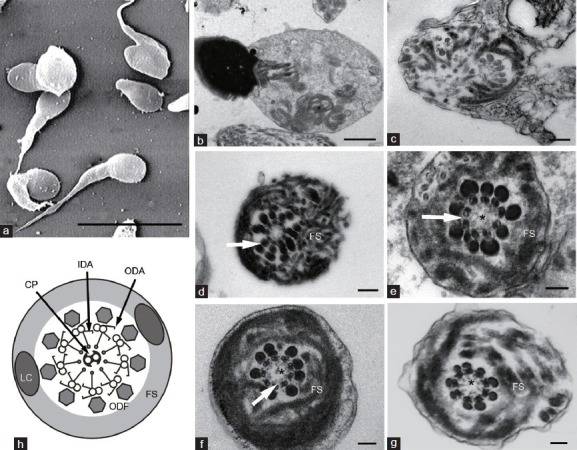
Electron microscopy analysis of spermatozoa. (a) Scanning electron microscopy micrograph of spermatozoa with multiple morphological anomalies of the flagella (MMAF) showing short, thick and irregular tails. Transmission electron microscopy micrographs of sperm flagellum with MMAF (b–g), showing hypertrophy of the fibrous sheath (FS): (b) a longitudinal section of a spermatozoon showing disarrangement of flagellar component including mitochondria (asterisk). (c) Completely distorted flagella, with randomly distributed microtubules and outer dense fibers (ODFs). (d) The number of ODFs doubled (arrow). (e) Absence of central pair (asterisk) and both outer dynein arms (ODA) and inner dynein arms (IDA) (arrow). (f) Absence of IDA (arrow) and central pair (asterisk). (g) Absence of central pair (asterisk), with intact dynein arms. Scale bars = 10 μm (a), 1 m (b), 0.2 m (c and d), and 0.1 m (e–g). (h) Schematic diagram of the normal flagella at principal piece (replicated image). 8 The axoneme is surrounded by ODFs and FS, which is composed of two longitudinal columns and circumferential ribs
Dynein arms could only be clearly confirmed in a limited number of sections, which were completely vertical to the axoneme. P1 revealed the absence of inner and outer dynein arms (IDA and ODA) (Figure 1e), but intact dynein arms were observed in one section. Absence or decreased number of IDA was observed at available sections in P2 (Figure 1f). The majority of P3 sections showed no IDA or ODA, or only IDA was affected in a few sections. In the available sections, intact dynein arms were observed in P4 and P5 (Figure 1g), who possessed motile spermatozoa in all semen samples. The observation for dynein arms was not available for P6. The absolute asthenozoospermia in P1 and P3 was in accordance with a complete absence of dynein arms. When only IDA was affected, scarce nonprogressive motile spermatozoa were observed in some P2 semen samples.
In our analysis, peripheral microtubules and ODFs were less affected, while central pairs were largely impacted. FS and two central microtubules assembled from distal to proximal, while ODF assembly was shown to follow the opposite direction.9 All flagellar components are transported from cell body to the location of flagellum assembly through intra-flagellar transport (IFT); indeed, knockout of a gene responsible for IFT and intramanchette transport (IMT) severely impaired sperm tail formation.10 Therefore, it is reasonable to assume that IFT or IMT impairment contributes to MMAF occurrence. Ultrastructural study of the testis revealed that flagellar anomalies in MMAF occur during late spermiogenesis, when flagella are elongating in spermatids.11 Testicular sperm of P2 was obtained for intra-cytoplasmic sperm injection (ICSI), but the spermatozoa were completely immotile and mostly tailless. This confirmed that flagellar anomalies arose from the testis, and were not secondary to epididymal hostile environment.
A-kinase anchoring proteins 4 play a major role in FS assembly. Indeed, targeting the AKAP4 gene in mice resulted in sharply decreased sperm motility and shortened flagellum. Recently, DNAH1 mutations confirmed it as a causal gene in some MMAF cases, with IDA partially missing:5 DNAH1 encodes the heavy chain of IDA, and its mutations were associated with morphological characteristics. Then, we sequenced the three genes using genomic DNA from peripheral blood. The DNAH1 sequencing of exons 23, 31, 74, 78 and their intron-exon boundaries covering the reported mutations was carried out using previously described protocols.5 Meanwhile, all AKAP3 and AKAP4 exons were sequenced. No pathogenic mutations were found by gene sequencing. Therefore, the genetic origin of Chinese MMAF remains unknown, but likely not involving mutations in AKAP3, AKAP4 and the four DNAH1 exons.
As reviewed, no spontaneous pregnancy has been reported in patients with MMAF, but a fertilization rate of 63% ±16% and 10 pregnancies were achieved in 12 couples by ICSI.4 However, the transmission risk of genetic defects by ICSI should be evaluated.
AUTHOR CONTRIBUTIONS
SMY designed the study, participated in the whole process of this study, and drafted the manuscript. HBL carried out the genetic studies. JXW participated in morphology assessment. YCS carried out an assessment of viability and offered helpful suggestions for the study. HBC offered helpful suggestions for genetic study and carried out the semen analysis. WW and HL carried out the artificial reproduction to the affected couples. JQH participated in study design. HL and DGW participated in study design and revised the manuscript critically for important intellectual content. All authors have read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was funded by Suzhou Youth Project of Science and Education for Medicine (KJXW2013025) from the Suzhou municipal bureau of healthy.
REFERENCES
- 1.Ortega C, Verheyen G, Raick D, Camus M, Devroey P, et al. Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update. 2011;17:684–92. doi: 10.1093/humupd/dmr018. [DOI] [PubMed] [Google Scholar]
- 2.Chemes HE. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21:799–808. [PubMed] [Google Scholar]
- 3.Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48:664–9. doi: 10.1016/s0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 4.Chemes HE, Alvarez Sedo C. Tales of the tail and sperm headaches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl. 2012;14:14–23. doi: 10.1038/aja.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Khelifa M, Coutton C, Zouari R, Karaouzène T, Rendu J, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baccetti B, Collodel G, Estenoz M, Manca D, Moretti E, et al. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum Reprod. 2005;20:2790–4. doi: 10.1093/humrep/dei126. [DOI] [PubMed] [Google Scholar]
- 7.Chemes HE, Olmedo SB, Carrere C, Oses R, Carizza C, et al. Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod. 1998;13:2521–6. doi: 10.1093/humrep/13.9.2521. [DOI] [PubMed] [Google Scholar]
- 8.De Jonge CJ, Barratt CL. Cambridge: Cambridge University Press; 2006. The Sperm Cell: Production, Maturation, Fertilization, Regeneration; p. 110. [Google Scholar]
- 9.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 10.Lehti MS, Kotaja N, Sironen A. KIF3A is essential for sperm tail formation and manchette function. Mol Cell Endocrinol. 2013;377:44–55. doi: 10.1016/j.mce.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Barthelemy C, Tharanne MJ, Lebos C, Lecomte P, Lansac J. Tail stump spermatozoa: morphogenesis of the defect. An ultrastructural study of sperm and testicular biopsy. Andrologia. 1990;22:417–25. doi: 10.1111/j.1439-0272.1990.tb02020.x. [DOI] [PubMed] [Google Scholar]


