Abstract
Transforming growth factor-β (TGF-β) plays a central role in driving tissue fibrosis. TGF-β is secreted in a latent form, held latent by noncovalent association of the active cytokine with a peptide derived from cleavage of the N-terminal domain of the same gene product, and needs to be activated extracellularly to exert any of its diverse biological effects. We have shown that two of the three mammalian isoforms of TGF-β, TGF-β1 and TGF-β3, depend on interactions with cell surface integrins for activation. We found that the integrin αvβ6 is highly induced on injured alveolar epithelial cells, potently induces TGF-β activation, and is critical for the development of pulmonary fibrosis and acute lung injury. However, although TGF-β drives fibrosis in virtually every anatomic site, αvβ6-mediated TGF-β activation is much more restricted. For example, αvβ6 is not induced on injured hepatocytes and plays little or no role in cirrhosis induced by repetitive hepatocyte injury. Fibroblasts are highly contractile cells that express multiple integrins closely related to αvβ6, which share the promiscuous αv subunit, so we reasoned that perhaps one or more of these αv integrins on fibroblasts might substitute for αvβ6 and activate the TGF-β required to drive liver fibrosis. Indeed, deletion of the αv subunit from activated fibroblasts protected mice from carbon tetrachloride–induced liver fibrosis. Importantly, these same mice were protected from bleomycin-induced pulmonary fibrosis and renal fibrosis caused by unilateral ureteral obstruction, despite the presence of epithelial αvβ6 in these mice. These results suggest that the generation and maintenance of sufficient quantities of active TGF-β to cause tissue fibrosis in multiple organs probably depends on at least two sources—TGF-β activation by injured epithelial cells that drives fibroblast expansion and activation and an amplification step that involves TGF-β activation by an αv integrin on activated fibroblasts. These results suggest that intervening at either of these steps could be useful for the treatment of fibrotic diseases.
Keywords: integrin, transforming growth factor-β, pulmonary fibrosis
Transforming growth factor-β has been long recognized to play a central role in tissue repair. Much work has focused on the well-characterized increase in TGF-β production at sites of injury, with the presumption that TGF-β production would be the critical axis for regulating this process. However, although each of the three mammalian TGF-β isoforms can produce potent biological effects at picomolar concentrations, most organs of healthy mammals constitutively contain stored extracellular TGF-β at concentrations several orders of magnitude higher. Work over the past 2 decades has helped to explain this apparent paradox. All of the TGF-β isoforms are secreted in a latent form that is sequestered in the extracellular matrix and on the surface of some cells (1). As a result, most of the regulation of TGF-β function occurs at the level of activation of these stored latent complexes.
The first evidence implicating members of the integrin family in activation of latent TGF-β came from observations of mice we generated lacking the epithelial integrin, αvβ6 (2). These mice develop normally and are born at the appropriate mendelian frequency but develop exaggerated inflammation in the skin and lungs in response to normally trivial injuries (3). Despite exaggerated inflammatory responses, these mice are dramatically protected from bleomycin- (2) and radiation-induced pulmonary fibrosis (4). Because inactivation of one of the three mammalian TGF-β isoforms, TGF-β1, leads to profound multiorgan inflammation, and TGF-β has long been known to be a central mediator of tissue fibrosis, these findings raised the possibility that TGF-β could be acting downstream of the αvβ6 integrin. Together with John Munger, we showed that this integrin binds to the linear tripeptide, arginine-glycine-aspartic acid, present within an amino terminal fragment of TGF-β1 (and TGF-β3) (2, 5) called the latency-associated peptide and through a pathway that requires actin polymerization, actin-myosin contraction and the generation of traction force alter the conformation of stored extracellular TGF-β to activate it (6). The recently solved crystal structure of latent TGF-β1 together with cryo-electron microscopy of latent TGF-β together with the secreted ectodomains of the αvβ6 integrin beautifully demonstrate how the latency-associated peptide prevents binding of TGF-β to its receptors and how physical force transmitted through the integrin unfolds the latent complex to release free TGF-β (7).
αvβ6 is expressed at low levels on alveolar epithelial cells and plays an important role in the basal low-level activation of TGF-β that is needed to suppress the activity of alveolar macrophages and maintain normal alveolar homeostasis (8). However, in response to epithelial injury, epithelial cells are induced to contract their subcortical actin-myosin cytoskeletons, exerting increased amounts of deforming physical force on latent TGF-β, thereby increased the amount of activated TGF-β. Because TGF-β itself is the most potent inducer of αvβ6 expression, this process triggers a feed-forward loop of increasing integrin expression and further increases in TGF-β activation (9, 10). As pathologic extracellular matrix accumulates in the fibrosing lung, the lung becomes stiffer, making it easier for any degree of epithelial cell contraction to activate more TGF-β, further increasing αvβ6 expression. One consequence of this feed-forward loop is that αvβ6 protein expression is dramatically up-regulated in epithelial cells overlying regions of lung fibrosis (11), an effect we have consistently seen in tissue samples from more than 50 patients with a variety of fibrotic lung diseases. Based on this observation, multiple groups have developed methods to image αvβ6 expression in vivo, which could allow noninvasive evaluation of pulmonary fibrosis and potentially identify patients who are likely to be especially responsive to inhibition of αvβ6 or other steps in this pathway (12).
We generated potent blocking antibodies that inhibit the function of αvβ6 in multiple species, and we and others have used these to demonstrate the potential therapeutic usefulness of targeting this pathway in murine models of bleomycin and radiation-induced fibrosis (4, 11), in renal fibrosis induced by unilateral ureteral obstruction or a genetic model of Alport syndrome (13), and in biliary fibrosis induced by ligation of the common bile duct (14). β6 knockout mice and our blocking antibodies have also allowed us to identify an important role for this integrin in alveolar flooding in multiple models of acute lung injury (15). A humanized version of one of these antibodies is currently in early phase 2 clinical trials for treatment of pulmonary fibrosis.
However, it is clear that the αvβ6 integrin is not the only important activator of TGF-β that contributes to tissue fibrosis. For example, liver fibrosis in response to hepatocyte injury can be inhibited by blocking TGF-β but proceeds normally in mice lacking the β6 subunit (16). We therefore sought to identify other relevant mechanisms of pathologic TGF-β activation, using carbon tetrachloride–induced liver fibrosis as a model. In vitro work from Boris Hinz’s laboratory showed that fibroblasts have the capacity to use integrins to activate TGF-β (17), but this effect is clearly not dependent on αvβ6, because αvβ6 is never expressed on fibroblasts. After trying a number of mouse lines expressing cre recombinase under the control of putative fibroblast targeting promoters and failing to observe efficient recombination in liver fibroblasts, we settled on a line originally designed to target pericytes that expressed cre under the control of the platelet-derived growth factor receptor (PDGFR)-β promoter. We chose this line because resting hepatic stellate cells, the major source for collagen-producing liver fibroblasts, closely resemble pericytes in other organs and because PDGFR-β is highly expressed on activated fibroblasts. Although PDGFRβ expression is not restricted to fibroblasts, this line resulted in very efficient recombination in activated stellate cells in fibrotic livers. Based on evidence from our laboratory and others that multiple integrins that share the αv subunit can activate TGF-β in vitro, we deleted this whole family of integrins in activated fibroblasts by crossing the PDGFRβ-cre allele into mice designed for conditional deletion of αv (αv f/f mice).
αv f/f × PDGFRβ-cre mice were significantly protected from CCl4-induced liver fibrosis (16). We then sought to determine which αv-containing integrins are expressed on activated liver fibroblasts and found that these cells express moderate amounts of αvβ1, αvβ3, and αvβ5; minimal amounts of αvβ8; and no αvβ6. Mice globally lacking αvβ3, αvβ5, or αvβ6 or mice conditionally lacking αvβ8 on activated fibroblasts all had normal fibrotic responses to CCl4. Unfortunately, because the β1 subunit is present in 12 different integrins, and deletion of β1 with PDGFR-β results in perinatal mortality, we could not use mutant mice to directly examine the role of αvβ1 in this process. These results suggest that either there is redundancy among fibroblast αv integrins in driving liver fibrosis or that the major integrin responsible for this effect is αvβ1.
Although mice lacking the αvβ6 integrin are protected from pulmonary and renal fibrosis, fibrosis in those organs is also characterized by accumulation of contractile fibroblasts. Because pathologic fibrosis requires a sustained and substantial increase in active TGF-β, we reasoned that loss of either αvβ6-mediated activation by epithelial cells (as shown) or of αv integrin–mediated TGF-β activation by fibroblasts might protect against lung or kidney fibrosis. We therefore evaluated the efficiency of PDGFR-β–mediated recombination on activated fibroblasts in the lung and kidney and found it to be equally effective to what we observed in the liver. αv f/f × PDGFR-β-cre mice were also protected against bleomycin-induced pulmonary fibrosis and unilateral ureteral obstruction–induced renal fibrosis. Finally, to determine whether fibroblast αv integrins could be reasonable therapeutic targets for fibrotic diseases, we examined the effects of a broadly active small molecule inhibitor of αv integrins, CWHM-12, administered therapeutically beginning either on Day 21 after the start of CCl4 administration or on Day 14 after treatment with intratracheal bleomycin. In both cases we found similar reductions in fibrosis to what we observed in αv f/f × PDGFR-β-cre mice.
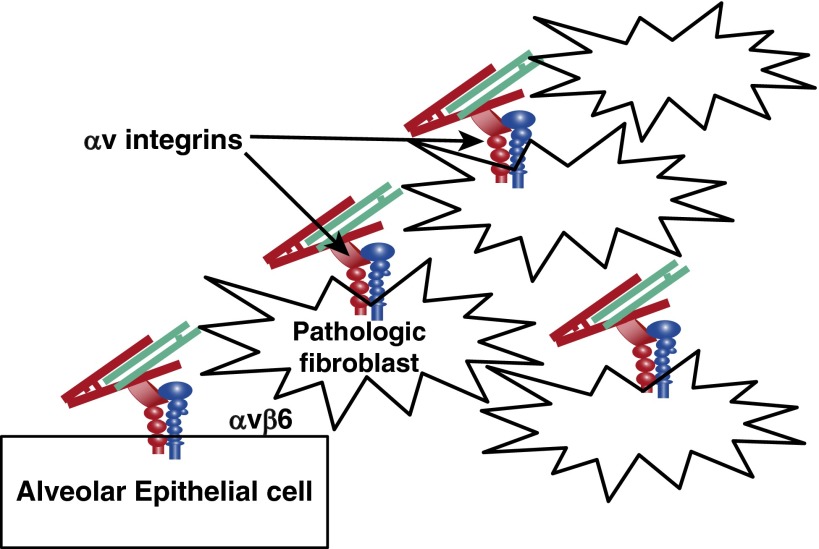
The combined results of our studies with inhibitors and knockouts of αvβ6 on epithelial cells and αv integrins on fibroblasts support a model in which epithelial injury and ongoing epithelial cell dysfunction leads to up-regulation of the αvβ6 integrin and persistent TGF-β activation (Figure 1). TGF-β activation on the surface of epithelial cells drives differentiation of adjacent cells into collagen-producing pathologic fibroblasts. TGF-β activation by other αv integrins on these fibroblasts provides an important amplification loop, leading to further expansion of the population of pathologic fibroblasts. Because there are potent homeostatic pathways to bring this system back into a healthy balance, pathologic fibrosis requires ongoing high-level TGF-β activity, which explains why inhibition of each of these complementary pathways can substantially inhibit pathologic pulmonary fibrosis. These results provide encouragement that inhibition of either epithelial TGF-β activation or fibroblast TGF-β activation could provide significant therapeutic benefit and would ultimately provide a rationale for combining these approaches.
Figure 1.
Model of how distinct αv integrins expressed on epithelial cells and pathologic fibroblasts each contribute to fibroblast activation and pathologic tissue fibrosis.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 2.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 3.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 2002;511:65–68. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 6.Giacomini MM, Travis MA, Kudo M, Sheppard D. Epithelial cells utilize cortical actin/myosin to activate latent TGF-β through integrin α(v)β(6)-dependent physical force. Exp Cell Res. 2012;318:716–722. doi: 10.1016/j.yexcr.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-β structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 9.Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am J Respir Cell Mol Biol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard D, Cohen DS, Wang A, Busk M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992;267:17409–17414. [PubMed] [Google Scholar]
- 11.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 12.John AE, Luckett JC, Tatler AL, Awais RO, Desai A, Habgood A, Ludbrook S, Blanchard AD, Perkins AC, Jenkins RG, et al. Preclinical SPECT/CT imaging of αvβ6 integrins for molecular stratification of idiopathic pulmonary fibrosis. J Nucl Med. 2013;54:2146–2152. doi: 10.2967/jnumed.113.120592. [DOI] [PubMed] [Google Scholar]
- 13.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, et al. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]