Abstract
Lung injury and repair is a broad topic that includes many cell types and is relevant to the pathogenesis of most lung diseases. Here, we focus on injury and repair of the alveolus, the principal function of which is to achieve gas exchange. The many cell types and structures present in the alveolus are discussed, with emphasis on their interactions in both health and disease. We define injury as damage resulting in impaired gas exchange; physiologic repair, then, requires restoration of normal alveolar architecture and function. The role of inflammation in both injury and repair of structural alveolar cells, particularly epithelial cells, as well as mechanisms of resolution of inflammation will be addressed. Finally, emphasis is placed on the importance of addressing quantitatively the dynamic and complex multidirectional interactions between the many alveolar cell types and structures in three dimensions over time and in relating such mechanistic studies to physiologic outcomes and human disease.
Keywords: lung injury, lung repair, pulmonary fibrosis, inflammation, alveolar epithelium
The topic of lung injury and repair encompasses a wide variety of cell types and structures that can be injured, many causes and mechanisms of injury, and various forms of resolution and repair that can occur. Importantly, repair can be either physiologic, ultimately restoring normal lung structure and function, or pathologic, resulting in abnormal structure and function. The pathogenesis of many, if not all, lung diseases, including adult respiratory distress syndrome (ARDS), asthma, chronic obstructive pulmonary disease, and the many interstitial lung diseases, can be considered in terms of the cell type(s) injured, the cause and mechanism of injury, and the reparative responses, all of which may vary substantially between disease states. However, there are also common elements to these processes and diseases that deserve consideration. Moreover, subclinical injury and physiologic repair are likely occurring constantly in the healthy lung in response to daily inhalation of toxins, particles, and microorganisms. Here, we will present working definitions of injury and repair and discuss underlying mechanisms. Because of our particular interests, we will focus on injury and repair of the alveolus with emphasis on the role of inflammation and repair of the alveolar epithelium. However, the concepts put forth here also apply to other cells and structures of the respiratory tract.
The Alveolar Unit
The principal function of the lungs is gas exchange. A continuous column of air begins at the nares and mouth (sites often overlooked as part of the respiratory system) and extends through the larynx, trachea, and multiple generations of bronchi and bronchioles, which ultimately terminate in the alveoli. The alveolus is composed of multiple cell types and extracellular constituents (Figure 1) that function as a cohesive unit to provide a barrier between the external environment and the organism while promoting efficient gas exchange. The luminal surface of the alveolar unit is bounded by alveolar type I cells that cover more than 95% of the luminal surface and alveolar type II cells that produce surfactant, contribute to ion and fluid reabsorption, and serve as progenitors to replace injured type I cells. A human alveolus averages around 40 type I and 77 type II cells (Dallas Hyde, personal communication). Alveolar macrophages reside in the alveolar lumen, and perhaps also in the airways, and serve important roles in host defense and alveolar homeostasis by sensing and ingesting inhaled pathogens and particulates as well as endogenous substances such as surfactant and sloughed epithelial cells. Intriguingly, when viewed on perfusion-fixed lung sections (rather than standard airway fixed sections), the alveolar macrophage has a flattened, sessile appearance with a large area of contact with the epithelium, as shown in Figure 2. The basilar surfaces of the alveolar epithelial cells rest on a thin basement membrane and interstitial matrix components that are cooperatively maintained by fibroblasts, epithelial cells, and pulmonary capillary endothelial cells, which form a network of capillaries that, as discussed in more detail below, contain a substantial proportion of the body’s extramedullary neutrophils (1). The degree to which intravascular leukocytes contribute to homeostasis of the alveolus is unclear; however, on the basis of their sheer numbers, they must be included in any discussion of the alveolar unit and are certainly available for immediate recruitment.
Figure 1.
The alveolar unit is composed of the airspace and its contents, surrounding structural cells, and adjacent pulmonary capillaries. Alveolar macrophages are bathed in surfactant and may form physical connections with alveolar epithelial cells. Type I epithelial cells (TI Ep) cover most of the alveolar luminal surface. Type II alveolar epithelial cells (TII Ep) cover the remainder. These cells synthesize surfactant that is contained in lamellar bodies (LB) in the cytoplasm and released into the surface water layer lining the alveolar space by exocytosis. Endothelial cells form a network of pulmonary capillaries that surround the alveolus to enable efficient exchange of gas. When healthy, the luminal surface of the endothelium is covered with a glycocalyx of proteoglycans and glycosaminoglycans that extend into the lumen but is degraded during inflammation to permit leukocyte transmigration. Fibroblasts and pericytes occupy the interstitium and help maintain the health and function of the epithelium and endothelium, in part through maintenance of the basement membrane and interstitial matrix. Adapted by permission from Reference 43.
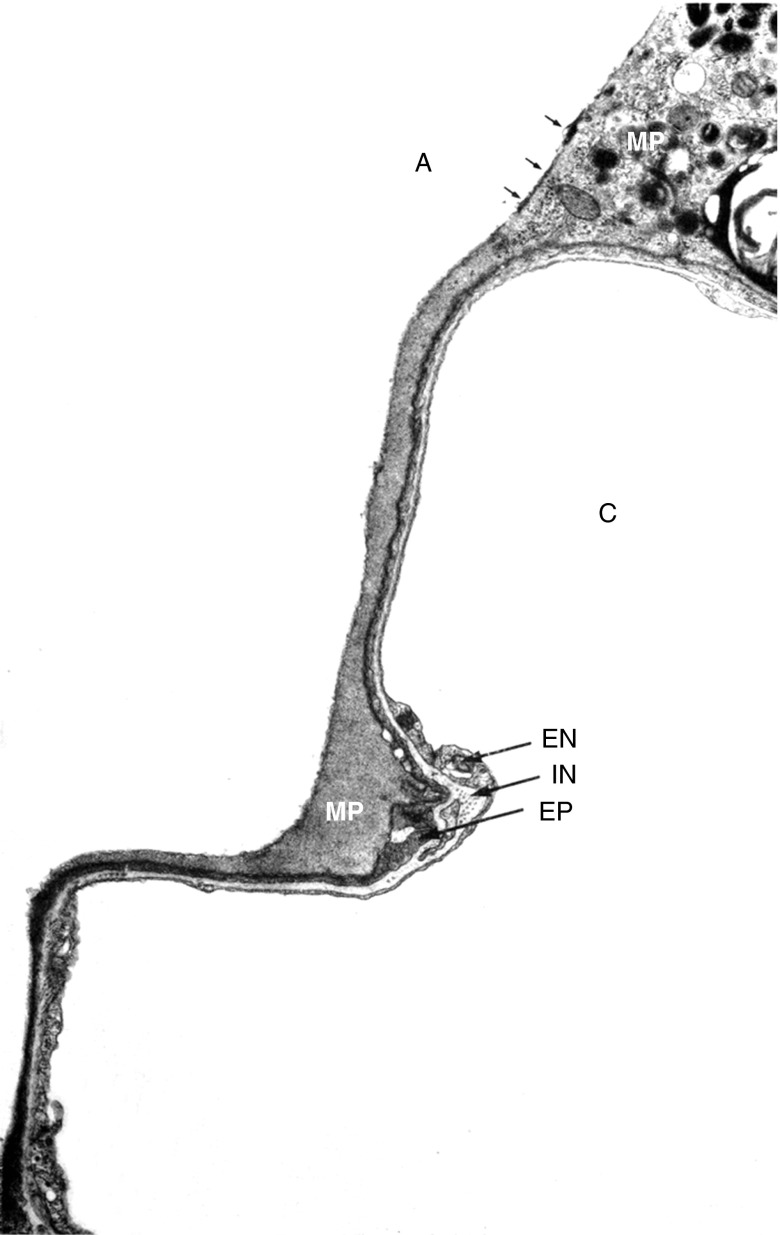
Figure 2.
Sessile alveolar macrophages contact alveolar epithelial cells. Electron micrograph prepared from perfusion-fixed lung section. A = airspace lumen; C = capillary lumen; EN = endothelial cell; EP = type I epithelial cell; IN = interstitium; MP = macrophage. Reprinted by permission from Reference 44.
Importantly, maintenance of the alveolar unit requires complex multidirectional interactions between the various cell types present. For example, fibroblasts communicate to endothelial cells and alveolar type II cells via gaps in the basement membranes and secretion of growth factors and thus serve as a “niche” for the alveolar epithelium (2, 3). Similarly, macrophages have been suggested to communicate with alveolar type II cells by mechanisms that include antiinflammatory signals transmitted through gap junctions (4) and secretion of cytokines that promote epithelial cell proliferation (5). Critically, each cell in the unit relies on the function and health of its neighbors, such that damage to any single cell type is inevitably accompanied by injury or a change in behavior to other cell types. This cellular interdependence is highlighted by experimental models of emphysema in which targeted destruction of the endothelium results in epithelial cell loss (6) and selective induction of epithelial death leads to effects on the capillary endothelium (7).
In a broad sense, the concept of tissue injury can be defined at the organ, cellular, or molecular level. Here, we define injury as structural damage to the alveolus such that lung function (e.g., gas exchange, barrier integrity) is impaired. In the case of the alveolar epithelium, for example, this injury may include disassembly of the tight junctions, holes in the plasma membranes, or loss of cells. In turn, repair can be simply defined as restitution of damaged structures such that alveolar function is restored. For example, after alveolar epithelial type I cell death, type II cells spread, proliferate, and transdifferentiate to replace lost type I cells, restoring barrier function and permitting efficient gas exchange. Critically, repair requires coordinated responses by multiple cells types (and the matrix) that can occur through paracrine signaling or physical juxtaposition. Repair of the alveolar epithelium is discussed in more detail below.
The Paradox of Inflammation
Inflammation is a beneficial, nonspecific response of tissues to infection and injury that promotes immunity and generally drives repair. However, inflammation itself also induces cell and tissue injury and is associated with the vast majority of pulmonary (and other) diseases. In considering these paradoxical processes, it is clear that in the context of the pulmonary alveolus “inflammation,” in addition to “injury” and “repair,” must be defined. Traditionally, inflammation meant the accumulation and various actions of inflammatory leukocytes migrating from the blood, with associated alterations to the local vasculature and blood flow. More recently, the presence of one or more of the myriad “mediators” of inflammation (cytokines, chemokines, peptides, hormones, biologically active lipids, and the like) that promote these tissue changes and the accumulation of inflammatory cells has itself come to be equated with the term inflammation. We would argue here that, just as for discussion of “injury,” more precision in describing the ongoing processes in inflammation is required. For example, determining whether the injurious effects of inflammation result directly from accumulated inflammatory cells or from direct effects of inflammatory mediators on tissue cells (or both) is important.
The simplistic concept of the “acute inflammatory response” may thus be characterized by initial generation of mediators that alter local hemodynamics as well as attract neutrophils as the usual first migrating responder. A substantial proportion of the neutrophils, and other leukocytes, in the circulation are at any one time found in the pulmonary capillaries (the so-called marginating pool). This occurs because leukocytes must undergo physical deformation to pass through these capillaries (as a consequence of size disparity and local rheological features of pulmonary blood flow), therefore leading to slower passage through the pulmonary capillary bed (1). In essence, as noted above, leukocytes found in the pulmonary capillaries can be considered part of the overall alveolar unit and constitute a reservoir for immediate response to alveolar injury and tissue cell stimulation. Importantly, unlike the predominant migration site through the postcapillary venules for leukocytes in the systemic circulation (including the bronchial vasculature), migration into the alveolar unit occurs through the capillaries (8) and thence out into the airspaces through the thick side of the alveolus and predominantly through the type I/type II epithelial cell junctions (9, 10).
During inflammation, neutrophils can auto-induce further neutrophil accumulation as well as the commonly seen acute inflammatory sequence where monocytes and later lymphocytes are attracted, followed by maturation of migrating monocytes toward various functional subtypes including macrophages (distinguished from monocytes by their large size and greatly enhanced phagocytic capability). As noted below, the monocytes and macrophages are highly responsive to the local environment and develop highly variable programming states that early on contribute to the inflammatory (protective and potentially injurious) state and later play critical roles in turning off and resolving the inflammation and driving repair of the tissue. An additional important component of this sequence is the generation of antigen-processing cells (monocytes and dendritic cells) with induction of adaptive immune responses to foreign stimuli or, in some cases, against self-antigens exposed during the injurious phases. Thus, the resolving inflammatory process normally maintains a critical balance between selectively enhanced protective immunity while controlling autoimmune responses.
Inflammation as it applies to the alveolar unit, as well as for other tissues, is usually thought of as injurious (so called “collateral damage”)—even when it is also associated with its defensive properties in combatting infection. Transmigration of neutrophils from the alveolar capillary to the airspace is generally associated with a key alteration of alveolar function (one form of “injury”), namely disrupting the maintenance of a dry lung (i.e., the prevention of plasma and interstitial fluid leak into the airspace) (11) (Figure 3). Direct effects of the neutrophils on endothelial and epithelial barrier integrity are implied and, for example, shown elegantly in a recent intravital microscopic examination of endothelial permeability initiated by simple application of neutrophil-directed chemotactic chemokines in the cremaster muscle (12). A similar, extremely rapid increase in overall alveolar permeability (i.e., alteration of both endothelial and epithelial barriers) may be seen after instillation of neutrophil chemokines into the lung (11). It should be noted here that there is some evidence that neutrophil transmigration can under some circumstances occur without major barrier disruption (13) and that dual neutrophil stimulation, so-called priming plus activation, may be required for the barrier-altering effects. However, it also seems likely that these are nearly always present in cases of inflammatory neutrophil emigration and that completely benign migration is not the norm. In most cases, the neutrophil migration into the airspaces appears to be accompanied by actual physical damage to the type I epithelial cell, as noted for example in the early ultrastructural studies of ARDS lungs by Bachofen and Weibel (14) and in numerous experimental models. In addition, direct damage to the epithelia of the airways and alveolus (as occurs, for example, after aspiration of acidic stomach [15] contents or inhalation of ozone [16, 17]) also induces inflammation (i.e., inflammatory mediators and accumulation of the sequence of inflammatory cells), which thereby can enhance the initial injury.
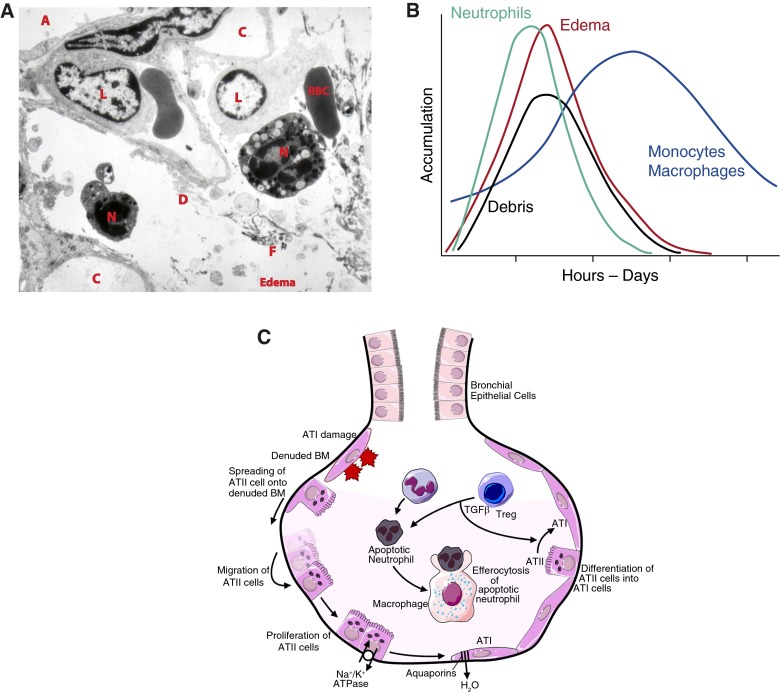
Figure 3.
Inflammatory lung injury. (A) During inflammatory lung injury, neutrophils (N) migrate from the intravascular space (C, capillary) across the endothelium and epithelium into the airspaces (A). During transmigration, neutrophils release toxic mediators, which cause injury to the endothelium and epithelium. Epithelial injury includes death and sloughing of cells, which contribute to airspace debris (D). Injury to the alveolocapillary barrier results in permeability and the influx of edema fluid and fibrin (F) from the vasculature into the airspaces. As inflammation progresses, mononuclear leukocytes (L), including monocytes and lymphocytes, migrate from the circulation to sites of injury. (B) Representative time course illustrating the kinetics of leukocyte migration, debris accumulation, and edema formation in the injured alveolus. (C) Reepithelialization of the injured alveolar epithelium occurs in stages. Type I cells are the most susceptible to injury. Surviving type II (ATII) cells (1) spread and migrate onto the denuded basement membrane (BM), (2) proliferate, and (3) differentiate into type I (ATI) cells to restore a normal alveolar architecture. Meanwhile, active ion transport by the Na+/K+ ATPase as well as passive ion uptake through ion channels (ENaC and CFTR, not shown) creates an osmotic gradient. Water reabsorption follows through aquaporin channels. The resolution of inflammation depends on apoptosis of neutrophils followed by the uptake of apoptotic neutrophils by macrophages (efferocytosis), a process that is enhanced by transforming growth factor (TGF)-β released from T-regulatory lymphocytes (Tregs). Reprinted by permission from Reference 45.
The inflammatory response, and in particular for this example, the neutrophil accumulation, also appears to play an important role in the repair of the epithelium, even while also contributing early on to the actual epithelial damage and removal. It has been suggested that part of this reparative function may reside in the clearance of epithelial debris (debridement of the damaged cells), thus creating a clean matrix for the necessary reepithelialization (17). In addition to removal of the initial stimuli (whether organic or inorganic) and/or the potentially toxic and proinflammatory debris generated by the original injury, neutrophils can also directly activate reparative responses of the epithelium (11).
Resolving Inflammation and the Initiation of Repair—Mononuclear Phagocytes Clean Up the Mess
Almost immediately after the onset of acute inflammation, the alveoli and surrounding tissues become filled with debris (Figure 3A). The nature of this debris is highly complex but at a minimum includes vesicles and microparticles released from leukocytes and tissue cells, matrix materials, surfactant components, pathogens (either dead or alive), apoptotic bodies, and intact apoptotic cells—all of which must be cleared to resolve inflammation and restore function of the alveolar unit (18, 19). The primary cells involved in the clearance of debris and apoptotic cells are mononuclear phagocytes (i.e., macrophages, monocytes, and dendritic cells), although tissue cells such as endothelial cells, fibroblasts, and epithelial cells can also have, or develop, the ability to recognize and ingest apoptotic cells and extracellular materials (20, 21).
The mononuclear phagocyte system in the lung is complex, and the lack of a consistent nomenclature has been a barrier to progress in the field. We suggest, at a minimum, that mononuclear phagocytes should be categorized according to the ontogeny (or lineage) of the phagocyte as well as the compartment in which it resides. In this context, perhaps the easiest cell to define is the resident alveolar macrophage. This cell has embryonic origin and is embryonically derived from fetal liver monocytes that migrate to the airspaces and mature into macrophages on birth (22). During health, these embryonically derived alveolar macrophages are maintained in constant number through local self-renewal; however, in response to inflammation they may proliferate locally to transiently increase their numbers (23). Monocytes (derived from the bone marrow) can be found in significant numbers in the pulmonary vasculature during health (1). During inflammation, circulating monocytes migrate to the tissues and alveoli, where they mature into “recruited” macrophages that can markedly increase the total number of macrophages in the lungs (24). As inflammation resolves, these bone marrow–derived macrophages undergo programmed cell death and are ingested locally by the macrophages that remain (24). Some may also be cleared via the mucociliary escalator. Return of the macrophage pool to its original size and programming state is essential for complete resolution of inflammation and for the repair of the alveolar unit. Failure or delay of macrophage death may lead to fibrosis and chronic inflammation.
The programing state (or activation state) of mononuclear phagocytes is largely driven by the environment in which they reside. Accordingly, at very early points in the inflammatory cascade, pathogen-associated molecular pathogens as well as cell-derived microvesicles and debris produced as “collateral damage” from inflammation can render macrophages proinflammatory and may amplify the inflammatory cascade. However, at nearly the same time, apoptotic cells (or even vesicles released from dying cells—so-called apoptotic bodies) can lead to profound antiinflammatory programming of the macrophages (19, 25). Accordingly, the environmental cues that drive pro- and antiinflammatory macrophage programming represent a balance that occurs on a temporal continuum. In this context, phosphatidylserine (PS) deserves special mention. PS is normally confined to the inner cell membrane but is rapidly exposed on the cell surface during early apoptosis (26). Similarly, PS may be exposed on cell-derived microvesicles or microparticles that lack the cellular machinery to maintain phospholipid asymmetry (27). Last, activated neutrophils can also expose phosphatidylserines before undergoing programmed cell death and in this way can initiate resolution and repair processes even while potentially also inducing injury (26). Accordingly, sloughed epithelial cells, dying neutrophils, and microvesicles comprise a rich depot of PS during the height of inflammation. Recognition of PS structures by mononuclear phagocytes can reprogram them to an antiinflammatory and prorepair state characterized by production of mediators such as transforming growth factor-β, IL-10, vascular endothelial growth factor, hepatocyte growth factor, and insulinlike growth factor-1 (18, 19, 25). Intriguingly, several of these molecules are also associated with fibrosis, and it is enticing to speculate that if the macrophages persist in this activation state, they may become drivers of fibrotic lung diseases such as idiopathic pulmonary fibrosis (28).
Repair of the Alveolar Epithelium
Repair of the alveolar epithelium requires reepithelialization of the denuded basement membrane and reassembly of tight junctions. The present discussion focuses on reepithelialization of the denuded basement membrane. As mentioned above, during lung injury, alveolar type I cells are particularly susceptible to injury; they die and slough off, resulting in permeability that allows the influx of edema fluid. Reepithelialization of the denuded basement membrane is accomplished in large part by alveolar type II cells, which are relatively resistant to injury, although other progenitor cell populations have recently been identified (3, 11, 29–32). Type II cells spread, proliferate, and transdifferentiate into type I cells to restore normal alveolar structure and barrier function (Figure 3C) (3). Various signaling pathways have been identified that promote type II cell spreading (33, 34), proliferation (5, 11), and transdifferentiation (32, 35). Type II cell proliferation is necessary to replace lost cells, but when overexuberant can result in hyperplasia. Soluble mediators implicated in type II cell proliferation include KGF and HGF, and these are likely secreted by the fibroblast, which forms the type II cell niche (3). B-catenin signaling (11) and FoxM1 signaling (31) also induce type II cell proliferation during repair. However, much of this work has been done in cell culture; the reparative mechanisms identified in vitro must be validated in animal models of lung injury. Characterization of additional pathways and additional progenitor cells are active areas of investigation. Under certain circumstances, epithelial injury and ineffective repair can promote the activation of fibroblasts, resulting in fibrotic lung disease. In addition, fundamental questions remain regarding the timing and relative importance of different reparative mechanisms during varying forms and severity of injury as well as the heterogeneity of type II cells, particularly regarding their progenitor function. Finally, the role of inflammation in repair of the alveolar epithelium merits further study.
Challenges to the Study of Lung Injury and Repair
The alveolar unit consists of many cell types in close proximity. Although often studied in isolation, the behavior of each cell type is intimately dependent on signals from neighboring cells. In vitro studies of single cell types, critical for dissection of intracellular signaling pathways, should be integrated with coculture (5, 11) and in vivo approaches that reproduce the complex interactions of many cell types of the alveolar unit. For example, as highlighted above, mononuclear phagocytes adapt rapidly to their environment, and therefore information derived from macrophage culture should be validated by coculture and/or in vivo approaches. Similarly, a thorough understanding of the role of extracellular structures in the function of the alveolar unit will come from research using complex model systems. Examples include the appropriate extracellular matrix and the endothelial glycocalyx, which strongly influence cell behavior during lung injury and repair (36, 37).
In addition to considering the multiple constituents of the alveolar unit, investigations on lung injury and repair will need to reproduce the three-dimensional structure of the lung. Standard lung tissue sections only provide information in two dimensions and when analyzed qualitatively can provide misleading or even erroneous information (see Reference 38 for examples). Hence, stereologic approaches, enabling quantitative assessment of three-dimensional structures from two-dimensional sections, should be performed whenever possible (39). In this context, recent three-dimensional studies of the lung, both in vivo and ex vivo, have begun to illuminate the critical events that occur in three dimensions during lung injury and repair (40, 41). Similarly, whereas traditional methods generally evaluate tissues or cells at a single point in time, the events that occur during lung injury and repair are dynamic. Recent advances such as intravital microscopy and live cell imaging now enable assessment of these events in real time (37, 42). As these tools become more developed, quantitative assessment of the entire organ must be made (39).
In sum, if our investigations on lung injury and repair are to illuminate physiologic processes during homeostasis and disease, they must quantitatively analyze the entire alveolar unit, in three dimensions, over time. The contrasting effects and processes occurring simultaneously, as a temporal continuum (Figure 4) with changing balance between overall functions, must be recognized. This makes analysis challenging—for example, separating the simultaneous ongoing injury and repair functions of a given cell type and recognizing that at any one point in time a given cell type may exhibit potentially opposing activities. Similarly, the pleotropic effects of a single signaling molecule can be in opposition. Moreover, although studies in mice have advantages, rats and larger animals may more closely approximate the physiology of humans. Finally, whereas animal models are critical for experimental work, they are mere models of human disease; studies using human tissue should complement experimental work whenever possible. Studies of the complete alveolar unit, including complex multicellular and cell-structure interactions, with quantitative assessments of dynamic three-dimensional processes that are related to physiologic outcomes of organ function, with complementary studies in experimental animals and human tissue, will propel our understanding of lung injury and repair during homeostasis and disease.
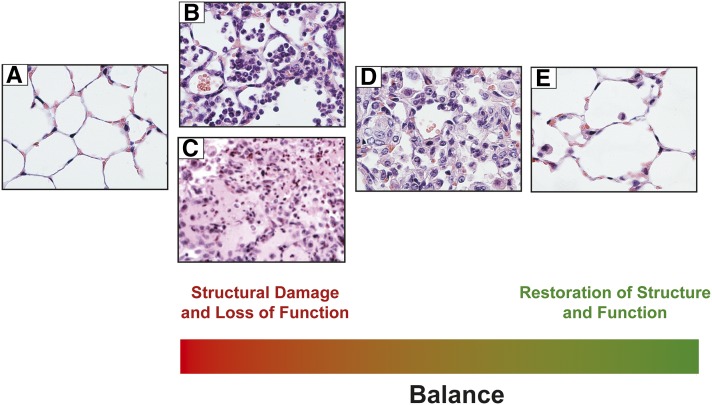
Figure 4.
Temporal balance of injury and repair. Opposing effects of injury and repair occur simultaneously and vary over time. (A) Naive mouse lung. (B) Neutrophils in the airspaces and interstitium 24 hours after intratracheal LPS. (C) Edema fluid in the alveoli and tissues representing impaired barrier function. (D) Accumulation of mononuclear phagocytes during resolving inflammation. (E) Restoration of lung structure and function in a mouse lung 9 days after LPS administration.
Acknowledgments
Acknowledgment
The authors thank Boyd Jacobson for assistance with graphics.
Footnotes
Supported by grants HL109517 (W.J.J.), HL114381 (P.M.H.), and HL103772 (R.L.Z.); the Boettcher Foundation (R.L.Z.); and the American Heart Association (R.L.Z.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Doerschuk CM, Downey GP, Doherty DE, English D, Gie RP, Ohgami M, Worthen GS, Henson PM, Hogg JC. Leukocyte and platelet margination within microvasculature of rabbit lungs. J Appl Physiol. 1990;68:1956–1961. doi: 10.1152/jappl.1990.68.5.1956. [DOI] [PubMed] [Google Scholar]
- 2.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993;92:969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, Lohmeyer J, Herold S. Macrophage tumor necrosis factor-alpha induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med. 2009;180:521–532. doi: 10.1164/rccm.200812-1837OC. [DOI] [PubMed] [Google Scholar]
- 6.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003;28:555–562. doi: 10.1165/rcmb.2002-0090OC. [DOI] [PubMed] [Google Scholar]
- 8.Downey GP, Doherty DE, Schwab B, III, Elson EL, Henson PM, Worthen GS. Retention of leukocytes in capillaries: role of cell size and deformability. J Appl Physiol. 1990;69:1767–1778. doi: 10.1152/jappl.1990.69.5.1767. [DOI] [PubMed] [Google Scholar]
- 9.Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res. 1995;50:397–416. doi: 10.1006/mvre.1995.1067. [DOI] [PubMed] [Google Scholar]
- 10.Behzad AR, Chu F, Walker DC. Fibroblasts are in a position to provide directional information to migrating neutrophils during pneumonia in rabbit lungs. Microvasc Res. 1996;51:303–316. doi: 10.1006/mvre.1996.0029. [DOI] [PubMed] [Google Scholar]
- 11.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA. 2011;108:15990–15995. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massena S, Christoffersson G, Hjertstrom E, Zcharia E, Vlodavsky I, Ausmees N, Rolny C, Li JP, Phillipson M. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood. 2010;116:1924–1931. doi: 10.1182/blood-2010-01-266072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 15.Zambelli V, Di Grigoli G, Scanziani M, Valtorta S, Amigoni M, Belloli S, Messa C, Pesenti A, Fazio F, Bellani G, et al. Time course of metabolic activity and cellular infiltration in a murine model of acid-induced lung injury. Intensive Care Med. 2012;38:694–701. doi: 10.1007/s00134-011-2456-1. [DOI] [PubMed] [Google Scholar]
- 16.Kleeberger SR, Hudak BB. Acute ozone-induced change in airway permeability: role of infiltrating leukocytes. J Appl Physiol. 1992;72:670–676. doi: 10.1152/jappl.1992.72.2.670. [DOI] [PubMed] [Google Scholar]
- 17.Hyde DM, Miller LA, McDonald RJ, Stovall MY, Wong V, Pinkerton KE, Wegner CD, Rothlein R, Plopper CG. Neutrophils enhance clearance of necrotic epithelial cells in ozone-induced lung injury in rhesus monkeys. Am J Physiol. 1999;277:L1190–L1198. doi: 10.1152/ajplung.1999.277.6.L1190. [DOI] [PubMed] [Google Scholar]
- 18.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henson PM, Bratton DL. Antiinflammatory effects of apoptotic cells. J Clin Invest. 2013;123:2773–2774. doi: 10.1172/JCI69344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandivier RW, Richens TR, Horstmann SA, deCathelineau AM, Ghosh M, Reynolds SD, Xiao YQ, Riches DW, Plumb J, Vachon E, et al. Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am J Physiol Lung Cell Mol Physiol. 2009;297:L677–L686. doi: 10.1152/ajplung.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–1095. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 26.Frasch SC, Bratton DL. Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog Lipid Res. 2012;51:199–207. doi: 10.1016/j.plipres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L364–L381. doi: 10.1152/ajplung.00354.2011. [DOI] [PubMed] [Google Scholar]
- 28.Redente EF, Keith RC, Janssen W, Henson PM, Ortiz LA, Downey GP, Bratton DL, Riches DW. Tumor necrosis factor-alpha accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am J Respir Cell Mol Biol. 2014;50:825–837. doi: 10.1165/rcmb.2013-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, Zhao YY, Malik AB. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med. 2011;208:1473–1484. doi: 10.1084/jem.20102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L, Yee M, O’Reilly MA. Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-beta and BMP signaling. Am J Physiol Lung Cell Mol Physiol. 2013;305:L409–L418. doi: 10.1152/ajplung.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L163–L169. doi: 10.1152/ajplung.00396.2001. [DOI] [PubMed] [Google Scholar]
- 34.Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, Lau LF, Kahn M, Downey GP. Role of β-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;304:L415–L427. doi: 10.1152/ajplung.00180.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhaskaran M, Kolliputi N, Wang Y, Gou D, Chintagari NR, Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor beta 1 through the Smad pathway. J Biol Chem. 2007;282:3968–3976. doi: 10.1074/jbc.M609060200. [DOI] [PubMed] [Google Scholar]
- 36.Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henson PM, Downey GP, Irvin CG. It’s much more than just pretty pictures. Am J Respir Cell Mol Biol. 2010;42:515–516. doi: 10.1165/rcmb.2010-0085ED. [DOI] [PubMed] [Google Scholar]
- 39.Hsia CC, Hyde DM, Ochs M, Weibel ER. ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alanis DM, Chang DR, Akiyama H, Krasnow MA, Chen J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat Commun. 2014;5:3923. doi: 10.1038/ncomms4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med. 2006;174:654–658. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornton EE, Krummel MF, Looney MR. Live imaging of the lung. Curr Protoc Cytom. 2012;Chapter 12:Unit12.28. doi: 10.1002/0471142956.cy1228s60. [DOI] [PubMed] [Google Scholar]
- 43.Sirianni FE, Chu FS, Walker DC. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med. 2003;168:1532–1537. doi: 10.1164/rccm.200303-371OC. [DOI] [PubMed] [Google Scholar]
- 44.Gil J, Weibel ER. Improvements in demonstration of lining layer of lung alveoli by electron microscopy. Respir Physiol. 1969;8:13–36. doi: 10.1016/0034-5687(69)90042-5. [DOI] [PubMed] [Google Scholar]
- 45.Zemans RL, Downey GP.Injury and repair of the lungInBroaddus VC, Mason RJ, Murray JF, Nadel JA, King TE, Jr, Ernst J, Lazarus SC, Slutsky AS.editorsTextbook of respiratory medicine, 6th edPhiladelphia: Elsevier; In press [Google Scholar]